Abstract
The purpose of this study was to examine whether ginsenoside Rg3 (GRg3) could improve learning and memory impairments and inflammatory reactions induced by injecting lipopolysaccharide (LPS) into the brains of rats. The effects of GRg3 on proinflammatory mediators in the hippocampus and the underlying mechanisms of these effects were also investigated. Injection of LPS into the lateral ventricle caused chronic inflammation and produced deficits in learning in a memory-impairment animal model. Daily administration of GRg3 (10, 20, and 50 mg/kg, i.p.) for 21 consecutive days markedly improved the LPS-induced learning and memory disabilities demonstrated on the step-through passive avoidance test and Morris water maze test. GRg3 administration significantly decreased expression of pro-inflammatory mediators such as tumor necrosis factor-α, interleukin-1β, and cyclooxygenase-2 in the hippocampus, as assessed by reverse transcription-polymerase chain reaction analysis and immunohistochemistry. Together, these findings suggest that GRg3 significantly attenuated LPS-induced cognitive impairment by inhibiting the expression of pro-inflammatory mediators in the rat brain. These results suggest that GRg3 may be effective for preventing or slowing the development of neurological disorders, including Alzheimer’s disease, by improving cognitive and memory functions due to its anti-inflammatory activity in the brain.
Keywords: Lipopolysaccharide, Memory, Inflammation, Ginsenoside Rg3, Morris water maze, Cyclooxygenase-2
INTRODUCTION
Neuroinflammation, which includes inflammation of the cen tral nervous system (CNS), has been implicated as a common cause of various neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and ischemic stroke (Zipp and Aktas, 2006; Mrak, 2009). Much evidence suggests that neuroinflammatory and neurodegenerative disorders and sustained increases in various pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in the CNS are closely correlated with the neuronal damage and cognitive dysfunction primarily associated with progression of AD pathogenesis (Schwab and McGeer, 2008; Mrak, 2009). For example, in AD, the entorhinal cortex and hippocampus appear to be vulnerable to chronic neuroinflammation. However, these regions exhibit a high degree of glia cell activation in the early disease stage but show a great extent of atrophy with disease progression (Deng et al., 2012). It is considered that synaptic damage may occur during the early phase of chronic neurodegeneration and may lead to cognitive impairment and loss of other neuronal function (Deng et al., 2012). Lipopolysaccharide (LPS), a non-infectious component of the outer membranes of gramnegative bacteria, induces a neuroinflammatory response, impairs memory function, and increases oxidative stress by increasing TNF-α and IL-1β mRNA levels in the hippocampus when administered by intraventricular microinjection or chronic infusion (Kitazawa et al., 2005). LPS is a potent stimulator of microglia to produce proinflammatory cytokines within the brain (Sayyah et al., 2003). Cytokine receptors are distributed throughout the brain with high densities in the hippocampus (Yin et al., 2013). Thus, the hippocampus is thought to be particularly vulnerable to immune-related alterations (Bilbo et al., 2005). It is likely that the interaction of proinflammatory cyto-kines with neuronal elements during development may alter the brain in a manner that makes it more susceptible to LPSinduced hippocampus-dependent memory impairment or synaptic plasticity in rats (Min et al., 2009; Hwang et al., 2011; Yin et al., 2013). The memory dysfunction caused by LPS-induced inflammation has been hypothesized to play an important role in the pathogenesis of the neurodegenerative changes and cognitive and memory impairments are closely associated with AD (Lukiw and Bazan, 2000; Cunningham et al., 2009). In fact, it is well established that LPS-induced inflammation in the hippocampus produces severe learning and memory deficits in a variety of behavioral tasks (Frank-Cannon et al., 2009). Thus, many studies have suggested that chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) prevents cognitive decline in elderly and other individuals diagnosed with AD dementia (Szekely et al., 2008). However, long-term treatment using NSAIDs can cause gastrointestinal side effects and even occasional liver and kidney toxicity (Graupera et al., 2003). These side effects have encouraged the development of new NSAIDs that are safer for long-term treatment (Kelloff et al., 2000). Recent studies have suggested the use of herbal medicines or natural products for treating Alzheimer’s type-dementia-related disorders exhibiting cognitive memory impairment and neuroinflammation (Ho et al., 2011).
Panax ginseng C.A. Mayer and its constituents are frequently used in Korean traditional herbal medicines to help patients recover from fatigue, enhance resistance capabilities against various neurodegenerative disorders and chronic inflammatory diseases, provide various benefits against memory impairment and to strengthen the immune system (Kim et al., 2011). Many studies have been conducted on the mechanisms of action of Panax ginseng and its processed product, Korean red ginseng (RG), and these substances possess multiple pharmacological and anti-AD activities (Tode et al., 1999; Lee et al., 2010). Ginsenoside, the most effective ingredient in ginseng, is responsible for the pharmacological effects of ginseng (Attele et al., 1999). Ginsenoside prevents memory loss by upregulating the plasticity-related proteins in the hippocampus (Zhao et al., 2011; Lee et al., 2012) and improving learning in mice. Several studies have shown that ginsenoside Rb1 may regulate the inflammatory response by stimulating cyclooxygenase-2 (COX-2) activity against amyloid beta-peptide (Aβ) 1-42-induced memory impairment (Wang et al., 2011) and attenuate the symptoms of scopolamine-induced dementia, as shown by improved cholinergic function and increased cognitive function on behavioral tests (Wang et al., 2010). Ginsenosdie Rg3 (GRg3), which is the main component of RG, plays a role in modulating inflammatory processes in the brain (Kang et al., 2007). For example, GRg3 reduces COX-2, inducible nitric oxide synthase (iNOS), and pro-inflammatory cytokine expression, including TNF-α and IL-1β, induced by LPS or Aβ 1-42 stimulation in vitro (Bae et al., 2006b; Joo et al., 2008). These studies suggest that the protopanaxadiol (PD) type of RG ginsenoside may be useful for suppressing inflammation in neurodegenerative diseases. Thus, PD-type GRg3 may be effective for alleviating learning deficits in a memory impaired or neuroinflammatory animal models.
The aim of the present study was to evaluate the anti-inflammatory effects of GRg3 on learning and memory functions in rats exposed to LPS-induced neuroinflammation as measured by performance on the step-through passive avoidance test (PAT) and the Morris water maze (MWM) test. We also examined how these effects were related to the molecular modulation of neuroinflammation in terms of the neural mechanisms underlying the memory-enhancing activity of GRg3.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley (SD) rats weighing 200-220 g (6 weeks-old) were obtained from Samtako Animal Co. (Seoul, Korea). The rats were housed in a limited access rodent facility with up to five rats per polycarbonate cage. The room controls were set to maintain the temperature at 22 ± 2℃ and the relative humidity at 55 ± 15%. Cages were lit by artificial light for 12 h each day. Sterilized drinking water and standard chow diet were supplied ad libitum to each cage during the experiments. The animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), revised in 1996, and were approved by the Kyung Hee University Institutional Animal Care and Use Committee. All animal experiments began at least 7 days after the animals arrived.
Lesion generation and LPS administration
To develop learning and memory deficits, male rats were induced with a bilateral intracranial injection (right and left side) of a small dose of LPS, according to procedures described previously by Guo et al. (2010), with a slight modification. The entire operation proceeded with the aid of a stereotaxic apparatus (Stoelting Co., Wood Dale, IL, USA) under anesthesia with sodium pentobarbital (50 mg/kg, i.p.). Fifty micrograms LPS dissolved in 10 μl cerebrospinal fluid (CSF) (Sigma- Aldrich Co., St. Louis, MO, USA) was microinjected into the lateral ventricle in the rat brains in all lesion groups. Sham animals as a vehicle control received microinjection of artificial CSF instead of LPS as a vehicle. Artificial CSF consists of 140 mM NaCl, 3.0 mM KCl, 2.5 mM CaCl2 and 1.2 mM Na2HPO4, and maintained at pH 7.4. The lateral ventricle in the stereotaxic coordinate was designated according to the Pa xinos and Watson brain atlas (AP: -0.2, L: ±0.3, DV: -6.2 referenced to the bregma; Paxinos and Watson, 1986). Artificial CSF or LPS solution was injected for 5 min at a flow rate of 2 μl/min using 22-gauge Hamilton syringe and microinjection pump (Pump 22; Harvard Apparatus Inc., Holliston, MA, USA). LPS (Escherichia coli; 055:B5) and ibuprofen were purchased from Sigma- Aldrich Co. (St. Louis, MO, USA). Administration of GRg3 was started 24 h after the lesion generation.
Experimental groups
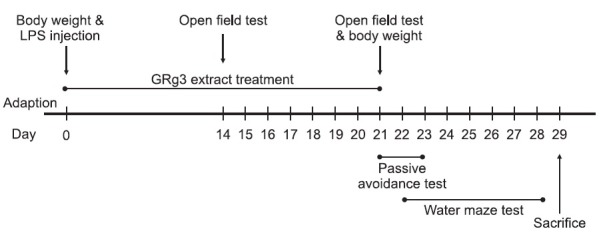
Different rats in an experimental group were subjected to either behavioral testing or immunohistochemistry. Rats were randomly divided into seven groups of six individuals as follows: CSF-injected sham group being regarded as normal (SAL group, n=6), CSF-injected plus 50 mg/kg GRg3-treated group (GRg3 group, n=6), LPS-injected plus saline-treated group (LPS group as a negative control, n=6), LPS-injected plus 10 mg/kg GRg3-treated group (LPS+GRg3-10 group, n=6), LPSinjected plus 20 mg/kg GRg3-treated group (LPS+GRg3-20 group, n=6), LPS-injected plus 50 mg/kg GRg3-treated group (LPS+GRg3-50 group, n=6), and LPS-injected plus 40 mg/kg ibuprofen-treated (LP S+IBU group as a positive control, n=6). According to the protocols of Kim et al. (2009), ginsenoside Rg3 from RG was isolated and kindly provided by Korea Ginseng and Tobacco Research Institute (Daejeon, Korea). Ibuprofen, a centrally acting cholinesterase inhibitor, was used as a positive control. The rats were intraperitoneally administrated with GRg3 and ibuprofen for 21 days, and GRg3 and ibuprofen were dissolved in 0.9% physiological saline before use. Body weight was measured on the 1st day prior to the LPS injection, and on the 21th day prior to behavioral test. All rats sequentially performed to take the PAT on the 21th day after starting the LPS injection, and to take the MWM test on the 22th day after starting the LPS injection. After the behavioral testing and body weighting, rats were sacrificed and brain tissues were immediately collected for experiments or stored at -70℃ for later use. The entire experimental schedule of all drug administration and behavioral examinations are shown in Fig. 1.
Fig. 1. Experimental schedule of lesion generation, GRg3 administration, and behavioral tests in rats. GRg3: ginsenoside Rg3; LPS: lipopolysaccharide.
Open field test
Prior to water maze testing, the rats were individually housed in a rectangular container that was made of black polyethylene (60×60×30 cm) to provide the best contrast to the white rats in a dimly lit room equipped with a video camera above the center of the room, and their locomotor activities (animal’s movements) were then measured. The locomotor activity indicated by the speed and distance of movements was monitored by a computerized video-tracking system using the S-MART program (Panlab Co., Barcelona, Spain). Tests were performed in the breeding room from 8:30 to 16:00 on the 14th and 21th day after starting LPS injection. The animals were allowed to adapt to the container for 5 min before testing to acclimatize to the new environment. The individual rats were placed in the middle of the chamber for each trail. After 5 min adaptation, the distance they traveled in the container was recorded for another 5 min. The locomotor activity was expressed in centimeters. The floor surface of each chamber was thoroughly cleaned with 70% ethanol between tests. The number of rearing events of the rats was also recorded in order to analyze locomotor activity in the open field test (OPT).
Step-through passive avoidance test
The test was basically performed according to the stepthrough method. The Gemini Avoidance System (SD Instruments., San Diego, CA, USA) was used for this experiment. Basically, the step-through passive avoidance apparatus (PAA) consists of a tilting floor acrylic box divided into twocompartments, a lightened compartment connected to a darkened compartment, by an automatic guillotine door and a control unit generating electric shock (Behbood Pardaz Co., Ghaem, Iran). The electric shock can be delivered to the grid floor, made of stainless steel rods (3 mm diameter) spaced 1 cm apart, in both compartments. First, the rats were give trials to acquisition test in the apparatus. In the training session, a rat was placed in a lightened compartment of the PAA facing away from the entrance to the dark compartment, and then the guillotine door was opened. Because of intrinsic preference to the dark environment, the rat immediately entered the dark compartment and the door was closed. During the acquisition test, the latency time before entry into the dark compartment was recorded for each rat. After 30 min, the rats were placed in the lightened compartment once again. After entering the dark compartment, the guillotine door was closed, and subsequently a mild electrical shock (0.5 mA) was applied for 3 s. The retention test was started 24 h after the acquisition trial for training. The rat was again placed in the lightened compartment and the guillotine door was opened. In the retention test, the rat was placed in the PAA as previously described and the time required for the rat to enter the dark compartment was measured for a maximum period of 3 min as in the same method with the acquisition test. The rats that did not enter the dark compartment within this period received a latency time of 180 s.
Morris water maze test
Morris water maze apparatus: The MWM test was performed in a small circular pool (2.0 m in diameter and 0.35 m deep) made of polypropylene and internally painted white. The pool was half-filled with water to a depth of 30 cm. The water in the pool was made opaque by adding 1 kg skim milk powder and continuously maintained at 22 ± 2℃. The pool was divided into four quadrants of equal area. During the MWM test, an escape platform (15 cm in diameter) was located in one of the four sections of the pool, being hidden 1.5 cm below the water surface and approximately 50 cm away from the sidewalls. Several visual cues were placed around the pool in plain sight of the animals. A digital camera was mounted to the ceiling straight above the center of the pool and was connected to a computerized recording system equipped with a tracking program (S-MART: Panlab Co., Barcelona, Spain), which permitted on- and off-line automated tracking of the paths taken by the rats.
Hidden platform trial for the acquisition test: The MWM test was initiated on the 21st day after the LPS and GRg3 administration commenced. The animals received three trials per day. The rats were trained to find the hidden platform, which remained in a fixed location throughout the test. The trials lasted for a maximum of 180 s, and the escape latency was expressed by the swimming time to find the submerged platform in the pool. The animals were tested with three trials per day for 5 days, and they received a 60-s probe trial on the sixth day. Finding the platform was defined as staying on it for at least 4 s before the acquisition time of 180 s ended. When the rat failed to find the platform in the limited time in first trial of hidden platform test, the rats should be placed on the platform for 20 s and assigned a latency of 180 s. Between one trial and the next, the water in the pool was stirred to remove olfactory traces of previous swim patterns. The entire schedule proceeded for 6 days and each animal had three trials for training per day with 30-40 min inter-trial interval.
Probe trial for the retention test: For the probe trial, a rat was placed in the quadrant located diagonally from the target quadrant and allowed to swim to the quadrant from which the escape platform had been removed for a maximum of 60 s. The probe trial was expressed by the ratio of the time spent (or the distance traveled) in searching for the platform in the target quadrant to the total duration spent swimming in the pool.
Immunohistochemistry for proinflammatory markers
For immunohistochemical studies, three rats in each group were deeply anesthetized with sodium pentobarbital (80 mg/ kg, by intraperitoneal injection) and perfused through the ascending aorta with normal saline (0.9%), followed by 300 ml (per rat) of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were removed in a randomized order, post-fixed overnight, and cryoprotected with 20% sucrose in 0.1 M PBS at 4℃. Coronal sections 30 μm thick were serially cut through the hippocampus using a cryostat (Leica CM1850; Leica Microsystems Ltd., Nussloch, Germany). The sections were obtained according to the rat atlas of Paxinos and Watson (hippocampus; between bregma -2.6 and -3.6; Paxinos and Watson, 1986). The sections were immunostained for TNF-α, IL-1β and COX-2 expression using the avidin-biotinperoxidase complex (ABC) method. The sections were incubated with primary rabbit anti-TNF-α antibody (1:200 dilution; Novus Biochemicals LLC., Littleton, CO, USA), rabbit anti-IL- 1β antibody (1:200 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and goat anti-COX-2 antibody (1:200 dilution; Cambridge Research Biochemicals Co., Bellingham, UK) in PBST (PBS plus 0.3% Triton X-100) for 48 h at 4℃, respectively. The sections were incubated for 120 min at room temperature with secondary antibody. The secondary antibodies were obtained from Vector Laboratories Co. (Burlingame, CA, USA) and diluted 1:200 in PBST containing 2% normal serum. To visualize immunoreactivity, the sections were incubated for 90 min in ABC reagent (Vectastain Elite ABC kit; Vector Labs. Co., Burlingame, CA, USA), and incubated in a solution containing 3,3′-diaminobenzidine (DAB; Sigma) and 0.01% H2O2 for 1 min. Images were captured using an AxioVision 3.0 imaging system (Carl Zeiss, Inc., Oberkochen, Germany) and processed using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA). The sections were viewed at 200× magnification, and the numbers of cells within 100×100- μm2 grids were counted by observers blinded to the experimental groups. Counting immunopositive cells was performed in at least three different hippocampal sections per rat brain. Stained sections were randomly chosen from equal levels of serial sections along the rostral-caudal axis. The TNF-α-, IL- 1β- and COX-2-immunopositive cells were only counted when their densities reached a defined darkness above the background level. Distinct brown spots for TNF-α, IL-1β and COX-2 were observed in the cytoplasms and in the membranes of the cone-shaped cells of the hippocampus. The cells were anatomically localized according to the stereotactic rat brain atlas of Paxinos and Watson (Paxinos and Watson, 1986). The brightness and contrast between images were not adjusted to exclude any possibility of subjective selection of immuoreactive cells.
Total RNA preparation and RT-PCR analysis
The expression levels of TNF-α, IL-1β and COX-2 mRNAs were determined by the reverse transcription-polymerase chain reaction (RT-PCR). The brain hippocampus was isolated from three rats per group. The total RNAs were prepared from the brain tissues using a TRIzol® reagent (Invitrogen Co., Carlsbad, CA, USA) according to the supplier’s instructions. Complementary DNA was first synthesized from total RNA using a reverse transcriptase (Takara Co., Shiga, Japan). PCR was performed using a PTC-100 programmable thermal controller (MJ Research, Inc., Watertown, MA, USA). All primers were designed using published mRNA sequences of those cytokines and a primer designing software, Primer 3, offered by the Whitehead Institute for Biomedical Research (Cambridge, MA, USA; htp://primer3.wi.mit.edu) on the website. The PCR products were separated on 1.2% agarose gels and stained with ethidium bromide. The density of each band was quantified using an image-analyzing system (i-MaxTM, CoreBio System Co., Seoul, Korea). The expression levels were compared each other by calculating the relative density of target band, such as TNF-α, IL-1β and COX-2, to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical analysis
All measurements were performed by an independent investigator blinded to the experimental conditions. Results in figures are expressed as mean ± standard error of means (SE). Differences within or between normally distributed data were analyzed by analysis of variance (ANOVA) using SPSS (Version 13.0; SPSS, Inc., Chicago, IL, USA) followed by Tukey’s post hoc test. Statistical significance was set at p<0.05. For statistical analysis of behavioral data was assessed using a one or two-way ANOVA followed by the appropriate Tukey’s post hoc analysis. Immunohistochemical data and PCR analysis were also analyzed by one-way ANOVA followed by Tukey’s post hoc test.
RESULTS
Effect of GRg3 on LPS-induced body weight loss
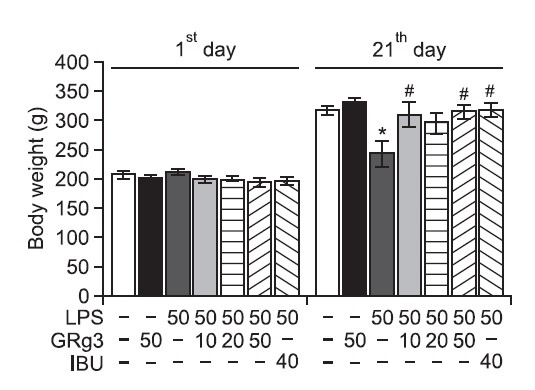
Rats administered to the single administration of LPS begin to lose body weight on the first day of LPS infusion, and this LPS-induced initial reduction in body weight was sustained for 21 days in the absence of return to the normal level and was even exacerbated in some cases (Gaab et al., 2005). We measured the body weight of each rat in each group on days 1 and 21 (Fig. 2). The LPS-injected rats gradually gained less body weight over the treatment period than did the sham control rats. Analysis of body weight values revealed no significant differences among the seven groups on the day prior to starting the LPS infusion. However, a significant reduction in body weight gain was observed in the LPS group compared to the SAL group during the 21 days of treatment (p<0.05). Interestingly, the body weight of rats treated with 10 or 50 mg/ kg GRg3 showed exhibited a significant inhibition of the reduction in body weight gain compared to that of rats in the LPS group during the 21 days of treatment (p<0.05), indicating that recovery of body weight in the 10 mg/kg or 50 mg/kg GRg3-treated groups were closely associated with that in the LPS+IBU group.
Fig. 2. Effects of GRg3 on body weight gain on the first day prior to LPS injection and on day 21 after LPS injection. *p<0.05 vs. SAL group; #p<0.05 vs. LPS group.
Effect of GRg3 on LPS-induced locomotor disability in the open field test
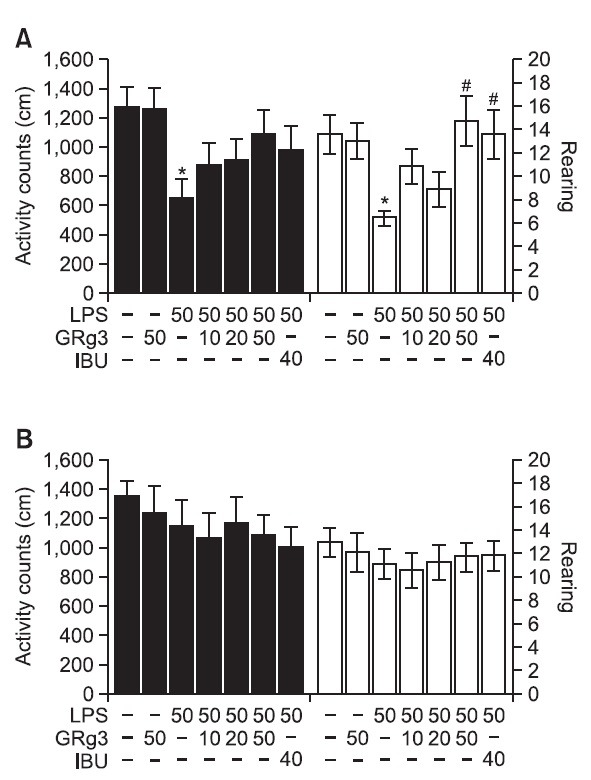
Open field activity was used to evaluate locomotor activity and exploratory behavior among the rats that receiving LPS injection into the lateral ventricle for 14 and 21 days (Fig. 3). The results indicated that rats infused with LPS showed significantly reduced locomotor activity (motor function) and total rearing activity (exploratory activity) compared to the rats in the SAL group during 14 days (p<0.05; Fig. 3A). It suggested that the rats treated with LPS subsequently developed impairments in the locomotor activity and exploratory activity in the OPT that were closely associated with motor functions. However, the GRg3-treated rats (50 mg/kg) demonstrated slightly increased locomotor activity compared to the LPS group, altho ugh these differences did not reach statistical significance (p=0.243). Also, after 14 days of treatment, rats treated with 50 mg/kg of GRg3 showed a remarkable enhancement in the active responses required for rearing (p<0.05), indicating that the GRg3 treatment restored exploratory behavior and accordingly alleviated LPS-induced impairment in the motor behavior of the rats. However, the results indicated that the rats in all groups had no effects on their locomotor activity and total rearing activity in the OPT during 21 days (Fig. 3B). Because any significant differences of locomotor activities and total numbers of rearing were not observed between all groups in the OPT, it could be suggested that observed impairment of memory of the rats with LPS injection were not attributed to the differences of their locomotion activities. It is may reflect an active responses and water avoidance stress when the animal is confronted with a WMW test. However, our results suggest that rats in all groups displayed no anxiolyticlike behaviors in the OPT after a pretest stress exposure in the MWM test. This indicated that the administration of GRg3 did not affect the active responses or psychomotor function as measured by the rat’s performance in the MWM test during the 21 days of treatment.
Fig. 3. Effects of GRg3 on locomotor activity and total learing behaviors during the open field test on days 14 (A) and 21 (B). *p<0.05 vs. SAL group; #p<0.05 vs. LPS group.
Effect of GRg3 on LPS-induced step-through latency defi-cit in the passive avoidance test (PAT)
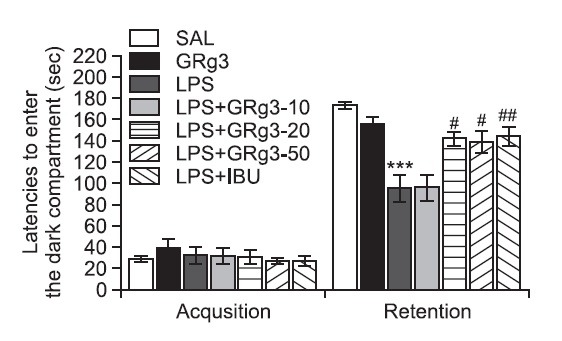
GRg3 was administered to rats with LPS-induced memory impairment, and their cognitive functions were examined with the PAT to determine whether GRg3 promotes the recovery of memory dysfunction (Fig. 4). It was verified that the rats in all groups had no physiological defect (i.e. motor function) or intrinsic cognitive impairments through acquisition trials without electric challenge. During the time for acquisition trials, indicated by latencies for entering the dark compartment, there were no significant differences among all groups. After acquisition trials, the effect of GRg3 on the retention latency was examined 24 h after applying electric shock in the dark box in the PAT. In the retention, it was shown that the rats in the 20 mg/kg and 50 mg/kg GRg3-treated groups had significantly increased latencies to enter the dark compartment for retention as compared to those in the LPS group (p<0.05). This study indicated that the LPS injection severely impaired long-term memory, and the treatment of GRg3 significantly attenuated LPS-induced memory deficit in the PAT. It also indicated that the restoration of memory function in the 20 mg/kg and 50 mg/kg GRg3-treated groups were almost close to that in the LPS+IBU group. The present study showed that LPS infusion significantly shortened the step-through latency of the retention trial and severely impaired long-term memory. Our results demonstrated that administration of GRg3 significantly increased the step-through latency in the memory retention trial, which had been shortened by LPS injection, in the PAT.
Fig. 4. Effects of GRg3 on the latencies preceding entrance into the dark compartment during the acquisition trial and on the retention test during the passive avoidance test. ***p<0.001 vs. SAL group; #p<0.05 and ##p<0.01 vs. LPS group.
Effect of GRg3 on LPS-induced memory impairment in the water maze test
The effect of the GRg3 treatment on swimming to reach the submerged platform in the MWM test is elucidated in Fig. 5. Rats in the SAL group rapidly learned the location of the submerged hidden platform and reached it within 20 s on day 5 of the trials. The LPS group showed marked retardation in escape latency, probably due to memory deficits resulting from LPS-induced impairment of learning and memory. The analysis of escape latency revealed that the rats in the 50 mg/kg GRg3-treated group had significantly reduced swimming latency compared with that in the LPS group (p<0.05 on days 4 and 5; Fig. 5A). The distance swum in each group was closely associated with the escape latency during this task. Analysis of searching distance values revealed that rats in the 50 mg/kg GRg3-treated group had significantly reduced swimming distances compared with that of rats in the LPS group (p<0.01 on day 4, p<0.05 on day 5; Fig. 5B). The 50 mg/kg GRg3-treatment group showed improved learning ability and memory function, as evidenced by decreased in escape latency and searching distance throughout the training period. To investigate the effect on memory, the performance in the probe trial on day 6 was examined by analyzing the percentages of time and distance required to swim to the expected position of the platform, respectively, to investigate the effect on memory (Fig. 5C and D). The swimming times and distances were decreased in the rats that swam directly and without confusion to the target quadrant where the platform had been located. The rats infused with LPS showed severely impaired performance on the MWM (p<0.01). The rats in the 50 mg/kg GRg3-treated group spent more time around on (p<0.05) and the rats in the 20 mg/kg and 50 mg/kg GRg3-treated groups were more distant from (p<0.05) the platform area than those in the LPS group. The LPS group was not significantly different from the other groups in terms of the mean swimming speed, as calculated by dividing the total swimming distance by the latency (Fig. 5E). Based on these results, rats treated with 50 mg/kg GRg3 were suggested to show greater improvement in acquisition during the hidden platform trial and, accordingly, reached the platform quicker than the LPS-treated rats. Administration of GRg3 significantly attenuated the LPS-induced defi cits in learning and memory demonstrated in the MWM test. Thus, GRg3-treated rats showed significant amelioration on the memory retention test because they spent more time and distance in the quadrant in which the platform was formerly located and swam to the platform location more frequently. It also indicated that the swimming latencies of the LPS-injected rats receiving 50 mg/kg GRg3 was similar to that of rats receiving 40 mg/kg IBU.
Fig. 5. Effects of GRg3 on latency of escaping from water (A) and swimming distance (B) during acquisition trials using a submerged platform, the percentages of time (C) and distance (D) in a probe trial without a platform, and swimming speed (E) in the Morris water maze test. *p<0.05 and **p<0.01 vs. SAL group; #p<0.05 and ##p<0.01 vs. LPS group.
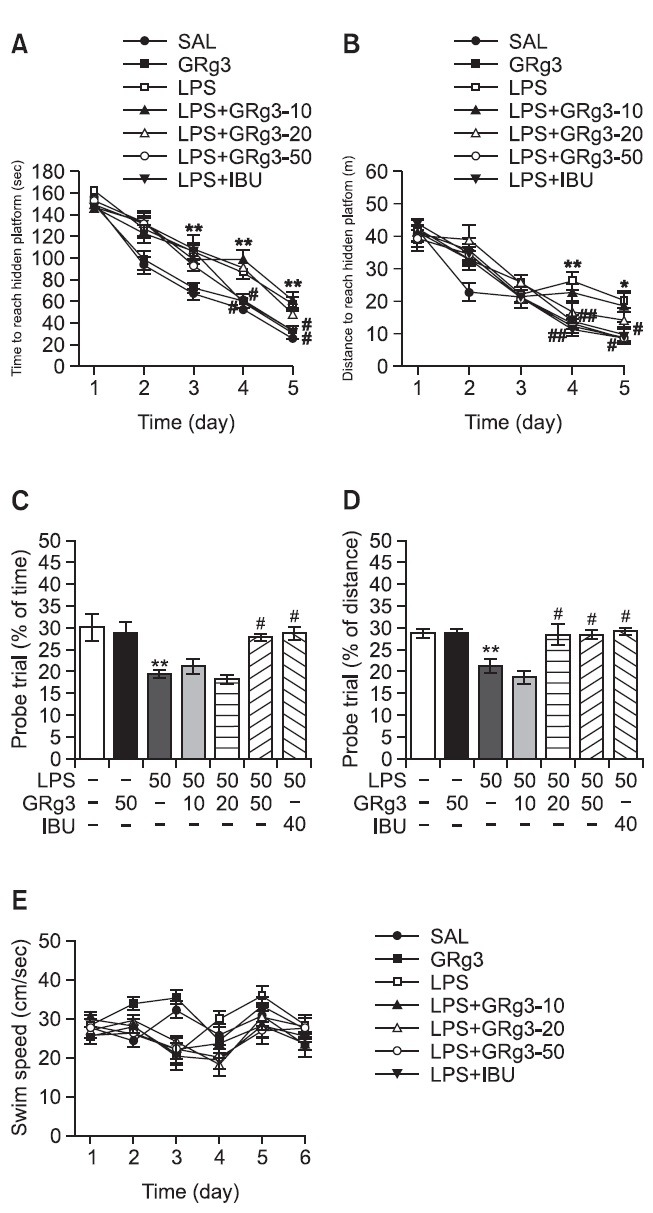
Effects of GRg3 on LPS-induced immunohistochemical changes in inflammatory mediators
Following the behavioral tasks, brain tissue samples from the rats were analyzed using immunohistochemistry to investigate the effect of administering GRg3 on the expression of the pro-inflammatory markers activated by LPS-induced inflammation in the rat brains (Fig. 6A). Distinct yellow stains for TNF-α, IL-1β and COX-2 were observed in the cytoplasm and membranes of the cone-shaped cells of the hippocampus. The immunoreactivity analysis showed the deepest yellow staining in the rat hippocampus. The brain immunohistochemistry of the LPS group showed a significant increase in TNF-α expression in the hippocampus of this group compared with that in the hippocampus of the SAL group (p<0.01; Fig. 6B). TNF-α immunoreactive cells decreased significantly in the hippocampal regions in the LPS+GRg3-50 group as compared with the LPS group (p<0.05). Similarly, IL-1β expression in the hippocampus of the LPS group increased significantly compared with that in the SAL group (p<0.01). IL-1β immunoreactive cells decreased significantly in the hippocampal region in the LPS+GRg3-20 and LPS+GRg3-50 groups (p<0.05). Similarly, brain sections taken from the LPS group showed significantly increased COX-2 expression in the hippocampus compared with those taken from the SAL group (p<0.05). Despite the appearance of COX-2-immunoreactive cells in the hippocampal regions in the LPS+GRg3-50 group, no significant difference was observed when this group was compared with the LPS group is this regard (p=0.079). Increases in pro-inflammatory mediators such as IL-1β by LPS-induced inflammation were significantly inhibited by GRg3 and the restoration was similar to that observed in the LPS+IBU group.
Fig. 6. Effects of GRg3 on the mean number of tumor necrosis factor-α (TNF-α)-, interleukin-1β (IL-1β)-, and cyclooxygenase-2 (COX-2)-stained hippocampal areas after the Morris water maze test. Representative photographs and the relative percentage values are indicated in (A) and (B), respectively. Sections were cut coronally at 30 μm. Scale bar indicates 50 μm. *p<0.05 and **p<0.01 vs. SAL group; #p<0.05 vs. LPS group.
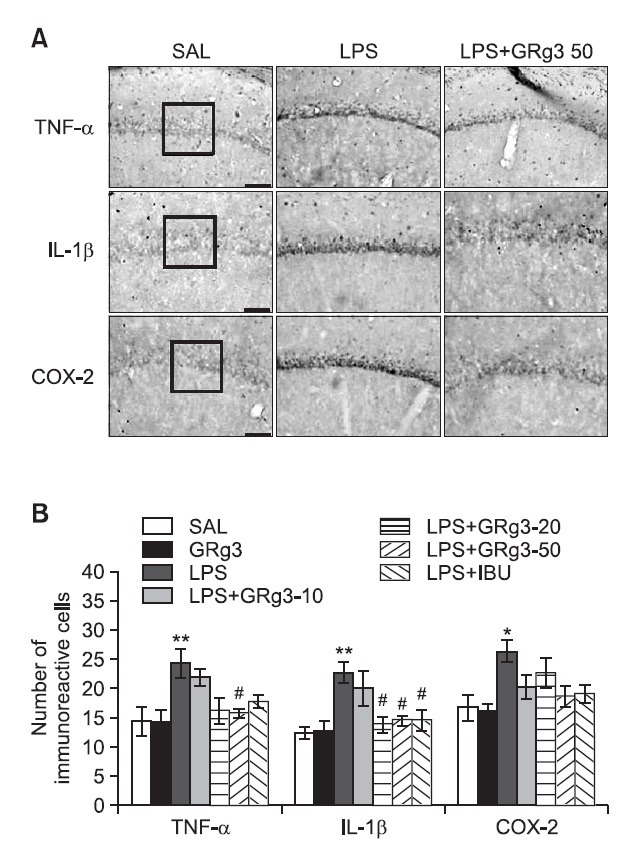
Effects of GRg3 on LPS-induced expression of TNF-α, IL-1 β and COX-2 mRNAs in the hippocampus
The effect of GRg3 administration on LPS-induced expression of TNF-α, IL-1β, and COX-2 mRNAs in the rat hippocampus was investigated using RT-PCR analysis (Fig. 7). Hippocampal expression of TNF-α, IL-1β and COX-2 mRNA in the LPS group were significantly increased compared with that in the SAL group (TNF-α and IL-1β; p<0.01, COX-2; p<0.05). The increased TNF-α mRNA expression in the LPS group was significantly restored in the LPS+GRg3-50 group (p<0.01). The increased expression of IL-1β mR NA in the LPS group was significantly restored by administering 20 and 50 mg/ kg GRg3 (p<0.01). However, the LPS-induced expression of COX-2 mRNA in the hippocampus was slightly inhibited by administration of 50 mg/kg GRg3 (p=0.472). The restored levels of these inflammatory mediators (e.g., TNF-α and IL-1β) were similar to those observed in the LPS+IBU group.
Fig. 7. Effects of GRg3 on the expression of tumor necrosis factor- α (TNF-α), interleukin-1β (IL-1β), and cyclooxygenase-2 (COX- 2) mRNAs in rats with LPS-induced hippocampal impairment. PCR bands on agarose gel and their relative intensities are indicated in (A) and (B), respectively. The expression levels of TNF-α, IL- 1β and COX-2 mRNAs were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. *p<0.05 and **p<0.01 vs. LPS group; ##p<0.01 vs. LPS group.
DISCUSSION
RG is one of several processed versions of Panax ginseng, and its saponin fraction possesses a variety of neurodegenerative effects, such as anti-AD and anti-stress activities (Jin et al., 1999). Ginsenosides isolated from RG are major active components with pharmacological and biological properties (Wang et al., 2010; Wang et al., 2011; Zhao et al., 2011; Lee et al.,2012). The composition of ginsenoside in ginseng is different based on various factors, such as the species, age and processing method (Gum and Cho, 2013). Interestingly, different ginsenoside profiles are responsible for diverse pharmacological effects. Indeed, ginsenosides effects as the improvement of memory function supported by the GRb1 and GRg1 which represent anti-inflammation activities, whereas GRe and GRb3 are found to be antidepressant-like effect (Wang et al., 2010; Lee et al., 2012). Rg3 produced by the steaming process from raw ginseng is major active component of the red ginseng specific components, suggesting that Rg3 in ginsenoside plays a key role for the improvement of memory function. GRg3 plays an important role modulating in vivo and in vitro inflammatory processes (Park et al., 2012). Some studies have shown that GRg3 is an effective neuroprotectant and anti-inflammatory agent against cerebral ischemia-induced injury in the rat brain by reducing lipid peroxides, scavenging free radicals, and improving energy metabolism (Tian et al., 2005). Additionally, GRg3 effectively attenuates pro-inflammatory cytokines and microglial activation in the brain induced by systemic LPS treatment in rats (Park et al., 2012). GRg3 effectively suppresses proinflammatory cytokines, including TNF-α, IL-1β and IL-6, in LPS-stimulated BV-2 microglial cells (Bae et al., 2006a) in Aβ1-42-treated BV-2 microglial cells (Joo et al., 2008) and in oxazolone-treated anaphylactic mice (Bae et al., 2006a). These results may suggest several therapeutic strategies, associated with the anti-inflammatory effect of GRg3 and understandable in the context of an animal model, for inhibiting the microglial activation in neurodegenerative diseases. However, it is currently unknown whether treatment with PD-type GRg3 can improve cognitive impairment induced by LPS injection in the rats. Therefore, we selected the PTtype GRg3 for the current study.
We examined the dose-dependent activity of GRg3 (10, 20, or 50 mg/kg), and found that 50 mg/kg was most effective for inhibiting LPS-induced harmful effects, such as memory deficits, in the PAT and MWM tests. The optimum dose determined in this study was reported previously (Lee et al., 2012).
Our results clearly demonstrate that administrating GRg3 significantly improves the learning and memory impairments induced by LPS infusion into the bilateral ventricles, as evidenced by behaviors during the PAT and MWM tests. GRg3 also attenuated the increase in pro-inflammatory markers such as TNF-α, IL-1β, and COX-2 in the hippocampus, as assessed by RT-PCR analysis and immunohistochemistry. Accordingly, our results demonstrate the GRg3 is an effective anti- inflammatory agent and probably a useful neuroprotectant as it inhibited the production of pro-inflammatory substances.
Recent evidence indicates that chronic and complex neuroinflammation may play an important role in the pathogenesis of various neurodegenerative changes and in the cognitive memory impairment closely associated with AD or senile CNS dysfunction (Lukiw and Bazan, 2000). Injections of an Aβ-peptide (25-35) or LPS produce sustained increases in the hippocampus inflammatory response and impair the cognitive memory and other brain functions (Stepanichev et al., 2003; Cunningham et al., 2009). As described in background, several studies reported that single injection of LPS into the lateral ventricle in the rat brains induces production of proinflammatory cytokines such as IL-1α, IL-1β, TNF-α IL-6 and COX-2 in mouse hippocampus for 3 weeks (Guo et al., 2010; Miwa et al., 2011). These increases in the expression levels of proinflammatory cytokines peaked about 6-9 hr after LPS injection. Choi et al., reported that i.c.v. injection of LPS induces neuronal damage and activation of microglia and astrocytes in hippocampus 24 hr after LPS injection (Choi et al., 2008). Consistent with previous findings (Lee et al., 2008; Gong et al., 2010), our results demonstrate that infusion of LPS into the lateral ventricle of rat brains caused significant increases in the pro-inflammatory mediators in the hippocampus and produced severe cognitive memory defi cits. We found that administering GRg3 after an LPS infusion into the lateral ventricle markedly restored body weight gain, suggesting that GRg3 inhibited the physiological changes caused by LPS-induced neuroinflammation (Castanon et al., 2001). GRg3 enhanced resistance against stress and various psychosomatic disorders and was associated with various benefits, such as strengthening the immune system. Furthermore, although sickness behavior is usually assessed within 21 days of induction, we conducted behavioral experiments 21-29 days after LPS induction. Consistent with the results of other investigations, no sickness-like behavior was observed on these days; therefore, we think that the effects of LPS and GRg3 relate to memory function rather than to other phenomena. Taken together, our results suggest that GRg3 had a preventive effect on the LPS-induced memory impairment caused by the neuroinflammatory response.
We used the PAT and MWM to identify the effects of GRg3 on cognitive memory and learning, respectively. Our results demonstrated that the administering of GRg3 significantly increased step-through latency on a the memory retention trial, which was shortened by LPS infusion, in the PAT (Jain et al., 2002). Administering GRg3 during the MWM trial sessions resulted in a significant reduction in escape latency, enhanced cognitive performance, and ameliorated memory deficits associated with LPS injection. These results show that LPS infusion into the lateral ventricle of rat brains retarded escape latency on the MWM test, indicating deficits in learning ability and reference memory. The MWM test escape latency score is considered to reflect long-term memory ability (Lee et al., 2010). In this study, administering of GRg3 shortened escape latency without affecting swimming velocity and extended the time spent swimming in the place at which the platform was previously located. This indicated that GRg3 significantly improved the long-term memory deficit in rats suffering from LPS-induced memory impairment. We conclude that a chronic inflammatory response played an important role in learning acquisition and synaptic plasticity during the PAT and MWM tests (Jain et al., 2002).
Our results also showed that LPS infusion into the lateral ventricle significantly increased expression of TNF-α and IL- 1β in the hippocampus, ultimately leading to a chronic neuroinflammatory response in the brain. Neuronal damage in the hippocampus generally results in reduced in learning ability and is involved in the consolidation of declarative memory in humans and animals (Guo et al., 2010). Thus, LPS-stimulated sustained increases in the expression of pro-inflammatory cytokines have been directly linked to neurodegenerative disorders associated with decreased working memory (Frank- Cannon et al., 2009). TNF-α and IL-1β mRNA expression is dynamically regulated by various immune cells during the hippocampal inflammatory response (Collister and Albensi, 2005). GRg3 continuously decreased LPS-induced expression of TNF-α and IL-1β mRNAs, which eventually resulted in recovery from the chronic inflammation and persistent brain dysfunction (Park et al., 2012). According to the inflammation hypothesis, memory impairment in patients with senile dementia is due to selective and irreversible dysfunction and chronic inflammation in the brain (Cunningham et al., 2009). Thus, we propose that the anti-inflammatory effects of GRg3 significantly reversed both impaired in memory retention and increased expression of pro-inflammatory cytokines such as TNF-α and IL-1β.
Much experimental evidence has suggested that the inducible gene encoding COX-2 is a key element that modulates the generation of proinflammatory mediators including various prostaglandins (Fehér et al., 2010). Expression of COX-2 and the synthesis of prostaglandin E2 (PGE2), one of its products, increase in the hippocampus of patients with AD (Fujimi et al., 2007), which may be related to the pathogenesis of the degenerative changes and cognitive impairment (Hwang et al., 2002). These results suggest that inflammatory responses to LPS infusion significantly stimulated COX-2 protein and mRNA expression in the hippocampus by modulating the nuclear factor kappa B (NF-κB) pathway (Gong et al., 2011). The increase in COX-2 expression by activating NF-κB can accelerate inflammatory responses and subsequently contribute to learning and memory deficits. Thus, treatment with longlasting COX-2 inhibitors during the initial stages of AD, before clinical symptoms of dementia appear, may suppress inflammatory responses and the synthesis of pro-inflammatory mediators in the brain (Kumar et al., 2006). In the present study, GRg3 slightly decreased LPS-stimulated behavioral changes and memory disturbances by inhibiting COX-2 mRNA expression in spite of little statistical significance. However, these suppressive effect in COX-2 expression suggest that GRg3 inhibited the pro-inflammatory cytokines induced by LPS injection. Our results may help to explain that GRg3 may be associated with intracellular NF-κB, which is a major transcription factor that regulates genes responsible for both innate and adaptive immune responses (Joo et al., 2008).
In summary, we demonstrated that GRg3 significantly improved learning and memory ability in rats with LPS-induced brain dysfunction, as evidenced by performance on the PAT and MWM tests. GRg3 also suppressed LPS-simulated mRNA expression of pro-inflammatory mediators such as TNF-α, IL- 1β, and COX-2 in the hippocampus. Thus, GRg3 may be useful as an alternative medicine for treating or retarding the development of neurodegenerative diseases including AD.
Acknowledgments
This research was supported by a grant the National Research Foundation of Korea Grant funded by the Korean Government (MEST)(2010-0003678) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2005-0049404).
References
- 1.Attele A. S., Wu J. A., Yuan C. S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem. Pharmacol. (1999);58:1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 2.Bae E. A., Han M. J., Shin Y. W., Kim D. H. Inhibitory effects of Korean red ginseng and its genuine constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive cutaneous anaphylaxis reaction and contact dermatitis models. Biol. Pharm. Bull. (2006a);29:1862–1867. doi: 10.1248/bpb.29.1862. [DOI] [PubMed] [Google Scholar]
- 3.Bae E. A., Kim E. J., Park J. S., Kim H. S., Ryu J. H., Kim D. H. Ginsenosides Rg3 and Rh2 inhibit the activation of AP-1 and protein kinase A pathway in lipopolysaccharide/interferongamma- stimulated BV-2 microglial cells. Planta Med. (2006b);72:627–633. doi: 10.1055/s-2006-931563. [DOI] [PubMed] [Google Scholar]
- 4.Bilbo S. D., Biedenkapp J. C., Der-Avakian A., Watkins L. R., Rudy J. W., Maier S. F. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J. Neurosci. (2005);25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castanon N., Bluthé R. M., Dantzer R. Chronic treatment with the atypical antidepressant tianeptine attenuates sickness behavior induced by peripheral but not central lipopolysaccharide and interleukin-1beta in the rat. Psychopharmacology (Berl) (2001);154:50–60. doi: 10.1007/s002130000595. [DOI] [PubMed] [Google Scholar]
- 6.Choi S. H., Langenbach R., Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide- induced inflammatory response and brain injury. FASEB J. (2008);22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collister K. A., Albensi B. C. Potential therapeutic targets in the NF-kappaB pathway for Alzheimer's disease. Drug News Perspect. (2005);18:623–629. doi: 10.1358/dnp.2005.18.10.959576. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham C., Campion S., Lunnon K., Murray C. L., Woods J.F., Deacon R. M., Rawlins J. N., Perry V. H. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol. Psychiatry . (2009);65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng X. H., Ai W. M., Lei D. L., Luo X. G., Yan X. X., Li Z. Lipopolysaccharide induces paired immunoglobulin-like receptor B (PirB) expression, synaptic alteration, and learning-memory deficit in rats. Neuroscience. (2012);209:161–170. doi: 10.1016/j.neuroscience.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Fehér A., Juhász A., Rimanóczy A., Kálmán J., Janka Z. Association study of interferon-γ, cytosolic phospholipase A2, and cyclooxygenase-2 gene polymorphisms in Alzheimer disease. Am. J. Geriatr. Psychiatry . (2010);18:983–987. doi: 10.1097/JGP.0b013e3181e70c05. [DOI] [PubMed] [Google Scholar]
- 11.Frank-Cannon T. C, Alto L. T, McAlpine F. E., Tansey M. G. Does neuroinfl ammation fan the flame in neurodegenerative diseases? Mol. Neurodegener. (2009);4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimi K., Noda K., Sasaki K., Wakisaka Y., Tanizaki Y., Iida M., Kiyohara Y., Kanba S., Iwaki T. Altered expression of COX-2 in subdivisions of the hippocampus during aging and in Alzheimer's disease: the Hisayama Study. Dement. Geriatr. Cogn. Disord. (2007);23:423–431. doi: 10.1159/000101957. [DOI] [PubMed] [Google Scholar]
- 13.Gaab J., Rohleder N., Heitz V., Engert V., Schad T., Schürmeyer T.H., Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. (2005);30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Gong Q. H., Pan L. L., Liu X. H., Wang Q., Huang H., Zhu Y. Z. S-propargyl-cysteine (ZYZ-802), a sulphur-containing amino acid, attenuates beta-amyloid-induced cognitive deficits and pro-inflammatory response: involvement of ERK1/2 and NF-κB pathway in rats. Amino Acids. (2011);40:601–610. doi: 10.1007/s00726-010-0685-1. [DOI] [PubMed] [Google Scholar]
- 15.Gong Q. H., Wang Q., Pan L. L., Liu X. H., Huang H., Zhu Y. Z. Hydrogen sulfide attenuates lipopolysaccharide-induced cognitive impairment: a pro-inflammatory pathway in rats. Pharmacol. Pharmacol. Biochem. Behav. (2010);96:52–58. doi: 10.1016/j.pbb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Graupera M., García-Pagán J. C., Abraldes J. G., Peralta C., Bragulat M., Corominola H., Bosch J., Rodés J. Cyclooxygenase-derived products modulate the increased intrahepatic resistance of cirrhotic rat livers. Hepatology . (2003);37:172–181. doi: 10.1053/jhep.2003.50004. [DOI] [PubMed] [Google Scholar]
- 17.Gum S. I., Cho M. K. Korean red ginseng extract prevents APAP-induced hepatotoxicity through metabolic enzyme regulation: the role of ginsenoside Rg3, a protopanaxadiol. Liver Int. (2013);33:1071–1084. doi: 10.1111/liv.12046. [DOI] [PubMed] [Google Scholar]
- 18.Guo J., Li F., Wu Q., Gong Q., Lu Y., Shi J. Protective effects of icariin on brain dysfunction induced by lipopolysaccharide in rats. Phytomedicine. (2010);17:950–955. doi: 10.1016/j.phymed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Ho Y. S., So K. F., Chang R. C. Drug discovery from Chinese medicine against neurodegeneration in Alzheimer's and vascular dementia. Chin. Med. (2011);6:15. doi: 10.1186/1749-8546-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang D. Y., Chae K. R., Kang T. S., Hwang J. H., Lim C. H., Kang H. K., Goo J. S., Lee M. R., Lim H. J., Min S. H., Cho J. Y., Hong J. T., Song C. W., Paik S. G., Cho J. S., Kim Y. K. Alterations in behavior, amyloid beta 1-42, caspase-3, and COX- 2 in mutant PS2 transgenic mouse model of Alzheimer's disease. FASEB J. (2002);16:805–813. doi: 10.1096/fj.01-0732com. [DOI] [PubMed] [Google Scholar]
- 21.Hwang Y. K., Ma J., Choi B. R., Cui C. A., Jeon W. K., Kim H., Kim H. Y., Han S. H., Han J. S. Effects of Scutellaria baicalensis on chronic cerebral hypoperfusion-induced memory impairments and chronic lipopolysaccharide infusion-induced memory impairments. J. Ethnopharmacol. (2011);137:681–689. doi: 10.1016/j.jep.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Jain N. K., Patil C. S., Kulkarni S. K., Singh A. Modulatory role of cyclooxygenase inhibitors in aging- and scopolamine or lipopolysaccharide-induced cognitive dysfunction in mice. Behav. Brain Res. (2002);133:369–376. doi: 10.1016/S0166-4328(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 23.Jin S. H., Park J. K., Nam K. Y., Park S. N., Jung N. P. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J. Ethnopharmacol. (1999);66:123–129. doi: 10.1016/S0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]
- 24.Joo S. S., Yoo Y. M., Ahn B. W., Nam S. Y., Kim Y. B., Hwang K. W., Lee I. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol. Pharm. Bull. (2008);31:1392–1396. doi: 10.1248/bpb.31.1392. [DOI] [PubMed] [Google Scholar]
- 25.Kang K. S., Kim H. Y., Yamabe N., Park J. H., Yokozawa T. Preventive effect of 20(S)-ginsenoside Rg3 against lipopolysaccharide-induced hepatic and renal injury in rats. Free Radic. Res. (2007);41:1181–1188. doi: 10.1080/10715760701581740. [DOI] [PubMed] [Google Scholar]
- 26.Kelloff G. J., Crowell J. A., Steele V. E., Lubet R. A., Malone W. A., Boone C. W., Kopelovich L., Hawk E. T., Lieberman R., Lawrence J. A., Ali I., Viner J. L., Sigman C. C. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J. Nutr. (2000);130:467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- 27.Kim J. H., Kang S. A., Han S. M., Shim I. Comparison of the antiobesity effects of the protopanaxadiol-and protopanaxatriol-type saponins of red ginseng. Phytother. Res. (2009);23:78–85. doi: 10.1002/ptr.2561. [DOI] [PubMed] [Google Scholar]
- 28.Kim N. H., Kim K. Y., Jeong H. J., Kim H. M. Antidepressant-like effect of altered Korean red ginseng in mice. Behav. Med. (2011);37:42–46. doi: 10.1080/08964289.2011.566591. [DOI] [PubMed] [Google Scholar]
- 29.Kitazawa M., Oddo S., Yamasaki T. R., Green K. N., LaFerla F. M. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J. Neurosci. (2005);25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A., Seghal N., Padi S. V., Naidu P. S. Differential effects of cyclooxygenase inhibitors on intracerebroventricular colchicine-induced dysfunction and oxidative stress in rats. Eur. J. Pharmacol. (2006);551:58–66. doi: 10.1016/j.ejphar.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 31.Lee J. W., Lee Y. K., Yuk D. Y., Choi D. Y., Ban S. B., Oh K. W., Hong J. T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflammation. (2008);5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee B., Park J., Kwon S., Park M. W., Oh S. M., Yeom M. J., Shim I., Lee H. J., Hahm D. H. Effect of wild ginseng on scopolamine-induced acetylcholine depletion in the rat hippocampus. J. Pharm. Pharmacol. (2010);62:263–271. doi: 10.1211/jpp.62.02.0015. [DOI] [PubMed] [Google Scholar]
- 33.Lee B., Shim I., Lee H., Hahm D. H. Effect of ginsenoside Re on depression-and anxiety-like behaviors and cognition memory deficit induced by repeated immobilization in rats. J. Microbiol. Biotechnol. (2012);22:708–720. doi: 10.4014/jmb.1112.12046. [DOI] [PubMed] [Google Scholar]
- 34.Lukiw W. J., Bazan N. G. Neuroinflammatory signaling upregulation in Alzheimer's disease. Neurochem. Res. (2000);25:1173–1184. doi: 10.1023/A:1007627725251. [DOI] [PubMed] [Google Scholar]
- 35.Min S. S., Quan H. Y., Ma J., Han J. S., Jeon B. H., Seol G. H. Chronic brain inflammation impairs two forms of long-term potentiation in the rat hippocampal CA1 area. Neurosci. Lett. . (2009);456:20–24. doi: 10.1016/j.neulet.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 36.Miwa M., Tsuboi M., Noguchi Y., Enokishima A., Nabeshima T., Hiramatsu M. Effects of betaine on lipopolysaccharide-induced memory impairment in mice and the involvement of GABA transporter 2. J. Neuroinflammation. (2011);8:153. doi: 10.1186/1742-2094-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mrak R. E. Neuropathology and the neuroinflammation idea. J. Alzheimers Dis. (2009);18:473–481. doi: 10.3233/JAD-2009-1158. [DOI] [PubMed] [Google Scholar]
- 38.Park S. M., Choi M. S., Sohn N. W., Shin J. W. Ginsenoside Rg3 attenuates microglia activation following systemic lipopolysaccharide treatment in mice. Biol. Pharm. Bull. (2012);35:1546–1552. doi: 10.1248/bpb.b12-00393. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G., Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: (1986). pp. 54–85. [Google Scholar]
- 40.Sayyah M., Javad-Pour M., Ghazi-Khansari M. The bacterial endotoxin lipopolysaccharide enhances seizure susceptibility in mice: involvement of proinflammatory factors: nitric oxide and prostaglandins. Neuroscience. (2003);122:1073–1080. doi: 10.1016/j.neuroscience.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 41.Schwab C., McGeer P. L. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J. Alzheimers Dis. (2008);13:359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- 42.Stepanichev M. Y., Zdobnova I. M., Yakov A. A., Onufriev M. V., Lazareva N. A., Zarubenko II., Gulyaeva N. V. Effects of tumor necrosis factor-alpha central administration on hippocampal damage in rat induced by amyloid beta-peptide (25-35). J. Neurosci. Res. (2003);71:110–120. doi: 10.1002/jnr.10469. [DOI] [PubMed] [Google Scholar]
- 43.Szekely C. A., Breitner J. C., Fitzpatrick A. L., Rea T. D., Psaty B.M., Kuller L. H., Zandi P. P. NSAID use and dementia risk in the cardiovascular health study: role of ApoE and NSAID type. Neurology. (2008);70:17–24. doi: 10.1212/01.wnl.0000284596.95156.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian J., Fu F., Geng M., Jiang Y., Yang J., Jiang W., Wang C., Liu K. Neuroprotective effect of 20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci. Lett. (2005);374:92–97. doi: 10.1016/j.neulet.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 45.Tode T., Kikuchi Y., Hirata J., Kita T., Nakata H., Nagata I. Effect of Korean red ginseng on psychological functions in patients with severe climacteric syndromes. Int. J. Gynaecol. Obstet. (1999);67:169–174. doi: 10.1016/S0020-7292(99)00168-X. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Liu J., Zhang Z., Bi P., Qi Z., Zhang C. Antineuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci. Lett. (2011);487:70–72. doi: 10.1016/j.neulet.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q., Sun L. H., Jia W., Liu X. M., Dang H. X., Mai W. L., Wang N., Steinmetz A., Wang Y. Q., Xu C. J. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother. Res. (2010);24:1748–1754. doi: 10.1002/ptr.3130. [DOI] [PubMed] [Google Scholar]
- 48.Yin P., Li Z., Wang Y. Y., Qiao N. N., Huang S. Y, Sun R. P., Wang J. W. Neonatal immune challenge exacerbates seizure-induced hippocampus-dependent memory impairment in adult rats. Epilepsy Behav. (2013);27:9–17. doi: 10.1016/j.yebeh.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 49.Zhao H. F., Li Q., Li Y. Long-term ginsenoside administration prevents memory loss in aged female C57BL/6J mice by modulating the redox status and up-regulating the plasticity-related proteins in hippocampus. Neuroscience. (2011);183:189–202. doi: 10.1016/j.neuroscience.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 50.Zipp F., Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. . (2006);29:518–527. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]


