Abstract
Codonopsis lanceolata (Campanulaceae) traditionally have been used as a tonic and to treat patients with lung abscesses. Recently, it was proposed that the extract and some compounds isolated from C. lanceolata reversed scopolamine-induced memory and learning deficits. The purpose of this study was to evaluate the improvement of cognitive enhancing effect of C. lanceolata by steam and fermentation process in scopolamine-induced memory impairment mice models by passive avoidance test and Morris water maze test. The extract of C. lanceolata or the extract of steamed and fermented C. lanceolata (SFCE) was orally administered to male mice at the doses of 100 and 300 mg/kg body weight. As a result, mice treated with steamed and fermented C. lanceolata extract (SFCE) (300 mg/kg body weight, p.o.) showed shorter escape latencies than those with C. lanceolata extract or the scopolamine-administered group in Morris water maze test. Also, it exerted longer step-through latency time than scopolamine treated group in passive avoidance test. Furthermore, neuroprotective effect of SFCE on glutamate-induced cytotoxicity was assessed in HT22 cells. Only SFCE-treated cells showed significant protection at 500 μg/ml. Interestingly, steamed C. lanceolata with fermentation contained more phenolic acid including gallic acid and vanillic acid than original C. lanceolata. Collectively, these results suggest that steam and fermentation process of C. lanceolata increased cognitive enhancing activity related to the memory processes and neuroprotective effect than original C. lanceolata.
Keywords: Codonopsis lanceolata, Steaming, Fermentation, Morris water maze test, Passive avoidance test, Cognitive-enhancing activity, Neuroprotective effect
INTRODUCTION
Neurodegenerative disorder such as dementia is a serious loss of cognitive ability and memory deficits that result in loss of brain function and neuronal cells death (Rubinsztein, 2006; Richard et al., 2012). Alzheimer’s disease (AD) is the most common form of dementia and accounts for approximately 50 to 80% of dementia cases (Brookmeyer et al., 1998; Murray et al., 2007). Although the causes of AD have become increasingly clear, they are not yet fully understood.
The accumulation of protein called amyloid plaques and neurofibrillary tangles, the loss of acetylcholine, and the induction of oxidative stress are considered as the causes associated with the processes of AD (Coyle et al., 1983; Brion, 1998; Tam and Pasternak, 2012). Especially, oxidative damage and accumulation of reactive oxygen species (ROS) exerts an important role for pathogenesis of AD and also associated with amyloid plaques and neurofibrillary tangles (Bains and Shaw, 1997).
Acetylcholine is one of the neurotransmitter involved in memory and cognition processes. The loss in the acetylcholine concentration in the cholinergic neuron in AD patients leads to the degree of cognitive dysfunction (McGleenon et al., 1999). Thus, scopolamine-induced memory impairments model, which blocks cholinergic neurotransmission, was widely adopted as a model to test cognitive functions including learning and memory (Izquierdo, 1989).
Codonopsis lanceolata (C. lanceolata), which belongs to the Campanulaceae family, is used as a food resource in East Asia, including Korea, China and Japan. C. lanceolata has been used as an effective medical plant for treatment of several lung inflammatory diseases such as asthma, tonsillitis and pharyngitis (Yongxu and Jicheng, 2008; Byeon et al., 2009). C. lanceolata is composed of saponins, alkaloids, tannins and polysaccharides (Ushijima et al., 2008). Several studies have reported that fermented C. lanceolata by various processes enhanced bioactivity of C.lanceolanta such as antioxidant, antimicrobial and antibiofilm activity by increasing the concentration of compounds during fermentation (He et al., 2011).
Thus, the aim of our study was to examine whether the steam and fermentation process could increase the neuroprotective and cognitive enhancing activity of C. lanceolata. In the present study, we found that the cognitive active of steamed and fermented C. lanceolata extract (SFCE) on spatial learning and memory performance were significantly enhanced than the C. lanceolata extract as assessed via Morris water maze and passive avoidance test in scopolamine-induced memory impaired mice. In addition, the neuroprotective effect of the SFCE against glutamate-induced neuronal cell damage on HT22 cells was significanly increased as compared with C. lanceolata extract. Interestingly, the contents of several phenolic compounds in C. lanceolata were significanly increased by the steam and fermentation process as measured by HPLC-DAD method. Collectively, these results suggested that the steam and fermentation process of C. lanceolata altered the components of it and siginificanlty increased the neuroprotective and cognitive enhancing activity.
MATERIALS AND METHODS
Plant materials
The roots of C. lanceolata were obtained from a Heongseong, Gangwon, Korea. The steaming process modified previously method (Weon et al., 2013). C. lanceolata was steamed using a steam device (Dechang stainless, Seoul, Korea) at 3 consecutive step, 50, 60 and 90℃ for 2 hours, respectively and steamed additionally at 100℃. Above process was conducted in triplicate.
Fermentation and extract of C. lanceolata
The steamed C. lanceolata was aseptically inoculated with approximately 106 CFU/g of B. longum, L. acidophilus and L. mesenteroidesdes and the inoculated C. lanceolatas were fermented and the cultures were harvested by spinning at 5,000 rpm for 10 min at 4℃. Fermented C. lanceolata was extracted in 70% ethanol (100 kg/10,000 L) for 24 hours of reflux extraction at 80 and 100℃ respectively.
C. lanceolata were washed with tap water and dried at 20-30℃ for 2 days. Dried C. lanceolata was extracted in 70% ethanol (100 kg/10,000 L) for 24 hours of reflux extraction at 80 and 100℃ respectively.
Animals
ICR mice weighing between 25-30 g (male, 5 weeks of age) were used in the present study (Dae Han Biolink Co., Eumseong, Korea). The mice housed 7 per cage, in a room under a 12/12 h light-dark cycle and controlled temperature (20 ± 3℃) with free access commercial pellet feed and water ad libitum and were used after 1 week adaptation period. All procedures in this study of the animal experiments procedures were conducted according to the guidelines of the Kangwon National University IACUC (KIACUC).
Morris water maze test
The water maze test was performed by the Morris methods with some modifications to access the spatial learning and memory in mice. The water maze consisted of circle pool (diameter: 90 cm, height: 40 cm) filled with water and maintained at 20 ± 1℃. The maze was divided into four equal quadrants. A white escape platform (10 cm in diameter and 26 cm high) was located in the centre of one quadrant and submerged at a depth about 1 cm below water level. All swimming behavior of mice were monitored and analyzed by Smart (ver. 2.5.21) video-tracking system. The escape latency, the time to locate the platform was used as measures for development of spatial memory. In first day, mice were given 60s to swim in the absence of the platform the day prior to test. The mice received two trial sessions per day for 4 consecutive days, with inter-trial interval of 20 min. Location of the platform were unchanged between trials 1 and trials 2 during test period but the starting point was changed on each day. Once the mouse reached the platform, it was allowed 10 s to stay on the platform. If mouse failed to locate the platform within 120 s, the trial was stopped and mouse remained on the platform for 10 s. The escape latency was recorded as 120 s. Probe trial was performed for a time period of 60 s without platform on the last day, to investigate the time spent in the target quadrant. The time spent in the correct quadrant was recorded as a measure of spatial memory. 0.5% carboxymethylcellulose (CMC; control group), SFCE and C. lanceolata (100 and 300 mg/kg, dissolved in CMC) and donepezil (1 mg/kg) were oral administered 120 min before the testing, respectively. After 90 min, control group was administered subcutaneously with normal saline and all the other groups were given subcutaneously with scopolamine (1 mg/kg, dissolved in saline) to induce amnesia. Test was performed 30 min after scopolamine treatment.
Passive avoidance test
Passive avoidance apparatus (Gemini, San Fransisco, USA) consisted of two compartments, equally sized light and dark compartments (17×12×10 cm), which equipped with an electrifiable grid floor. A guillotine door was made in the centre of the partition between the two compartments. A training trial was performed on the first day; the mice were initially placed in the light compartment. The door between the two compartments was opened 20 s after. When mice move in the dark compartment, the guillotine door closed automatically and electric foot-shock (0.1 mA/10 g body weight) of 2 s duration was delivered through grid floor. During each trial, time taken to enter the dark compartment was record as the training latency. Mice that not enter the dark compartment within 180 s, were excluded from experiment. 0.5% carboxymethylcellulose (CMC) (control group), SFCE and C.lanceolata (500 mg/kg body weight) were orally administered for 120 min prior to start of training trial. After 90 min, amnesia was induced by scopolamine (1 mg/kg body weight) given subcutaneously. Twenty hours after the training, the mice replaced in the light compartment and the latency time was measured.
Cell viability
Cell viability was determined by MTT assay. The mouse hippocampal HT22 cells were provided by Seoul National University, Korea. HT22 cells were seed at a density of 6.7×104/well (48 wells plate) in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS), 1% penicillin/streptomycin, NaHCO3 (2 mg/ml) and 15 mM HEPES and incubated at 37℃ at 5% CO2 for 24 h. After incubation of HT22 cells with glutamate in the presence or absence of 10, 100 and 500 μg/ml of SFCE, C. lanceolata and Trolox (50 μg/ml, positive control) for 24 h, MTT solution (1 mg/ml) was added to each well and the incubated for 3 h. Dimethyl sulfoxide (DMSO) was added and the optical density of the solubilized formazan product in each well was measured at 570 nm using an ELISA reader.
Analysis of compounds in C. lanceolata extract
The contents of seven phenolic compounds, gallic acid, 4-hydroxybenzoic acid, caffeic acid, vanillic acid, 4-coumaric acid, trans-feulic acid, caffecine in SFCE were analyzed using HPLC system (Aglient 1260 series, Aglient technologies., California, USA) equipped with diode array UV/VIS detector. The analysis of compounds was conducted on a ZORBAX Eclipse XDB-C18 (5 μm, 4.60 mm I.D.×250 mm) at 35℃. The mobile phase consisted of 10% acetonitrile with 0.1% formic acid (TFA) (A) and 0.1% formic acid in 40% acetonitrile and 40% methanol (B) at a flow rate of 1 ml /min. The mobile phase system used was a gradient of solvent A and solvent B as follows; 0-15 min, 95% A; 15-23 min, 60% A; 23-33 min, 60% A; 33-42 min, 0% A; 42-45 min, 95% A; 45-50 min, 95% A. According to the maximal UV absorption of each four compounds, UV wavelength of DAD detector was set. The UV wavelength was set at 280 nm. The sample injection volume was 20 μl.
Statistical analysis
Each data value of Morris water maze, passive avoidance test and MTT assay were expressed as the means ± SD. The probe test data of the water maze test, the latency of the passive avoidance test and data of MTT assay were analyzed by one-way ANOVA. The escape latencies of water maze test were analyzed by two-way ANOVA. Statistical significance was set at a value of p<0.05 or less.
RESULTS
Effect of SFCE and C. lanceolata in the Morris water maze test
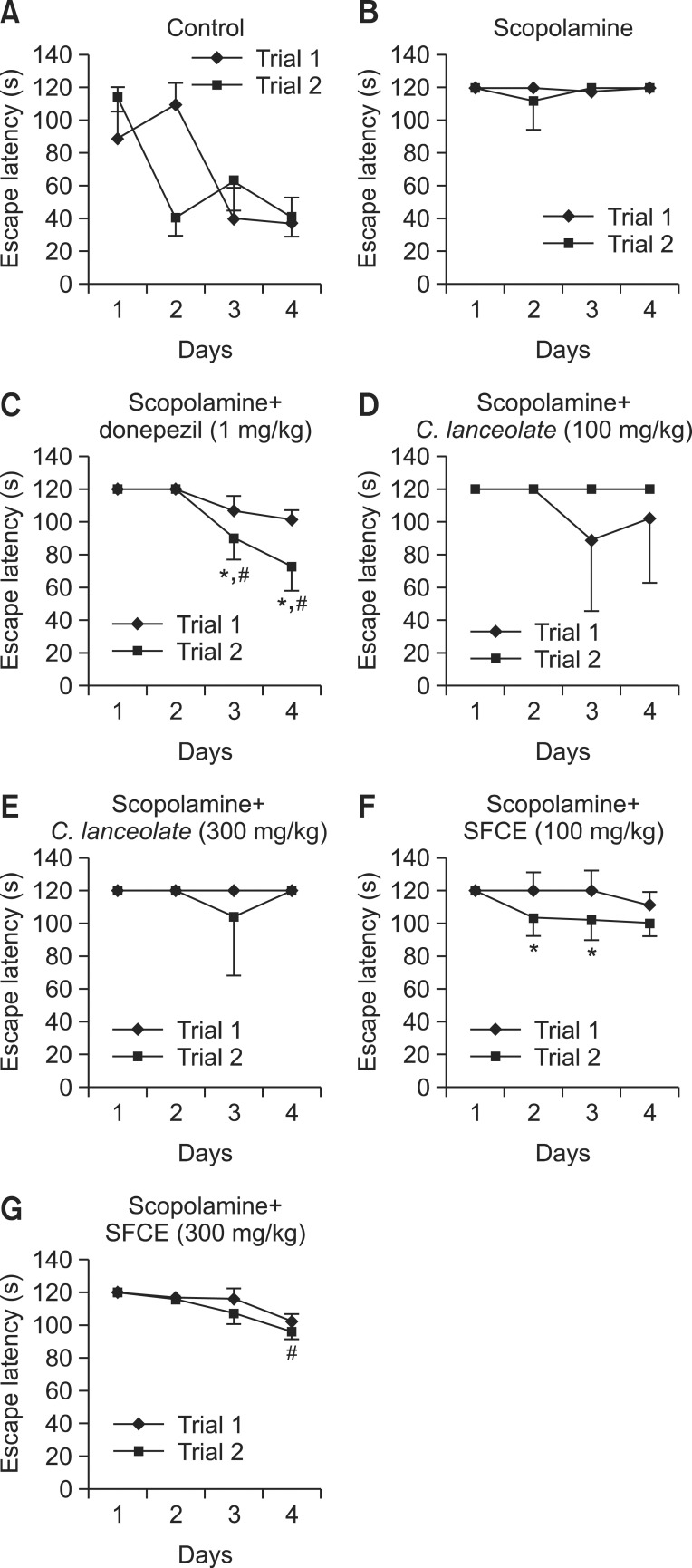
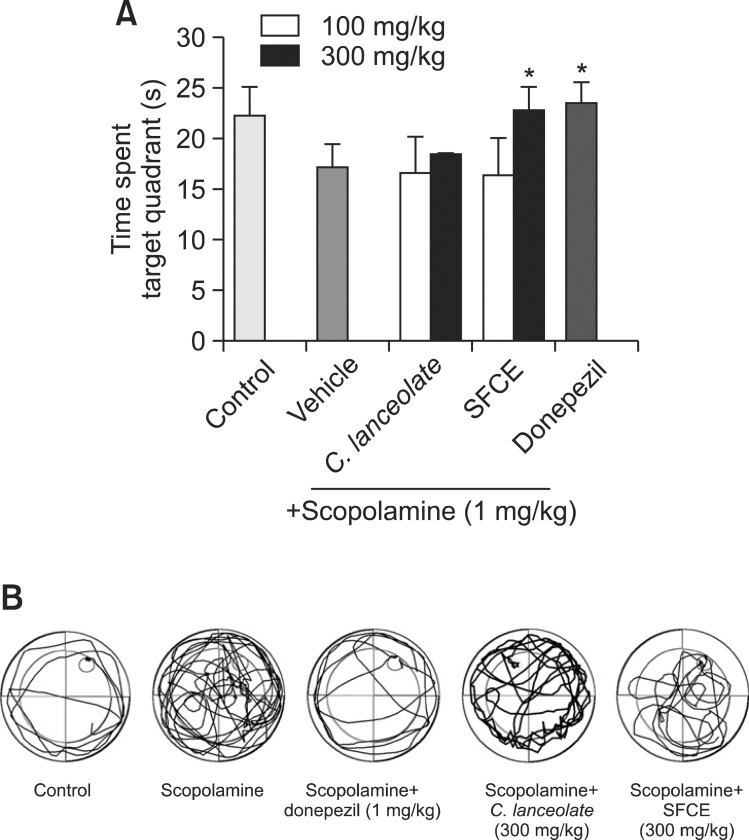
Morris water maze test was performed to observe the improvement effects of SFCE on spatial memory process. The results of Morris water maze are summarized in Fig. 1. In Morris water maze test, the escape latency of scopolaminetreated group was longer than control group. In contrast, scopolamine-treated group with SFCE (100 mg/kg) and C. lanceolata (trial 2 of 100 and 300 mg/kg) treatment exhibited shorter escape latency than scopolamine-treated group albeit there was no significant difference. Interestingly, mice treated with SFCE (300 mg/kg) significantly decreased the escape latency time of trial 2 in day 4 as compared with mice treated with scopolamine, but the treatment of C.lanceolata extract did not significantly decrease the latency time. Further, the escape latency in the SFCE group was significantly decreased as compared to mice treated with C.lanceolata extract. In the probe trial, the swimming time of SFCE (300 mg/kg)-treated group within the quadrant where the platform was significantly increased and also took longer time than the C. lanceolata (Fig. 2A). In the Morris water maze test, the effects of SFCE treated group were significant with respect to treatment [F(5,144)=2.26, p<0.001], with respect to days [F(3,144)=2.06, p<0.05], and with respect to interaction between the treatment and day [F(15,144)=1.49, p=0.89]. Fig. 2B shows the swimming routes of mice at last day in this test. Pattern of scopolamine treated mouse show near the wall of the pool. SFCE decreased distance for search platform of mice induced memory impairment by scopolamine during the water maze test and shorter distance than C. lanceolata. Collectively, these results suggested that steam and fermentation process of C.lanceolata increased the cognitive enhancing activity of it.
Fig. 1. Escape latency in the Morris water maze during four days. The Morris water maze test of mice treated with (A) control, (B) scopolamine, (C) donepezil, (D) scopolamine+SFCE (100 mg/kg), (E) scopolamine+SFCE (300 mg/kg), (F) scopolamine+C. lanceolata (100 mg/kg) and (G) scopolamine+C. lanceolata (300 mg/kg), respectively. The values shown are the mean escape latency ± SD (n=7). Escape latency of the trial 2 significantly differ from the value in trial 1: *p<0.05, **p<0.01 and ***p<0.001. #p<0.05, ##p<0.01 and ###p<0.001 compared with scopolamine group. Results were analyzed by two-way ANOVA.
Fig. 2. (A) The time spent in the quadrant where the platform was once placed in probe test. Results are expressed as mean ± SD (n=7). *p<0.05, **p<0.01 and ***p<0.001 compared with scopolamine group. Results were analyzed by one-way ANOVA. (B) The typical swimming routes of each group in Morris water maze test. control group, scopolamine treated group, scopolamine+donepezil treated group,scopolamine+C. lanceolata (300 mg/kg), scopolamine+SFCE (300 mg/kg).
Effect of SFCE and C. lanceolata on passive avoidance test
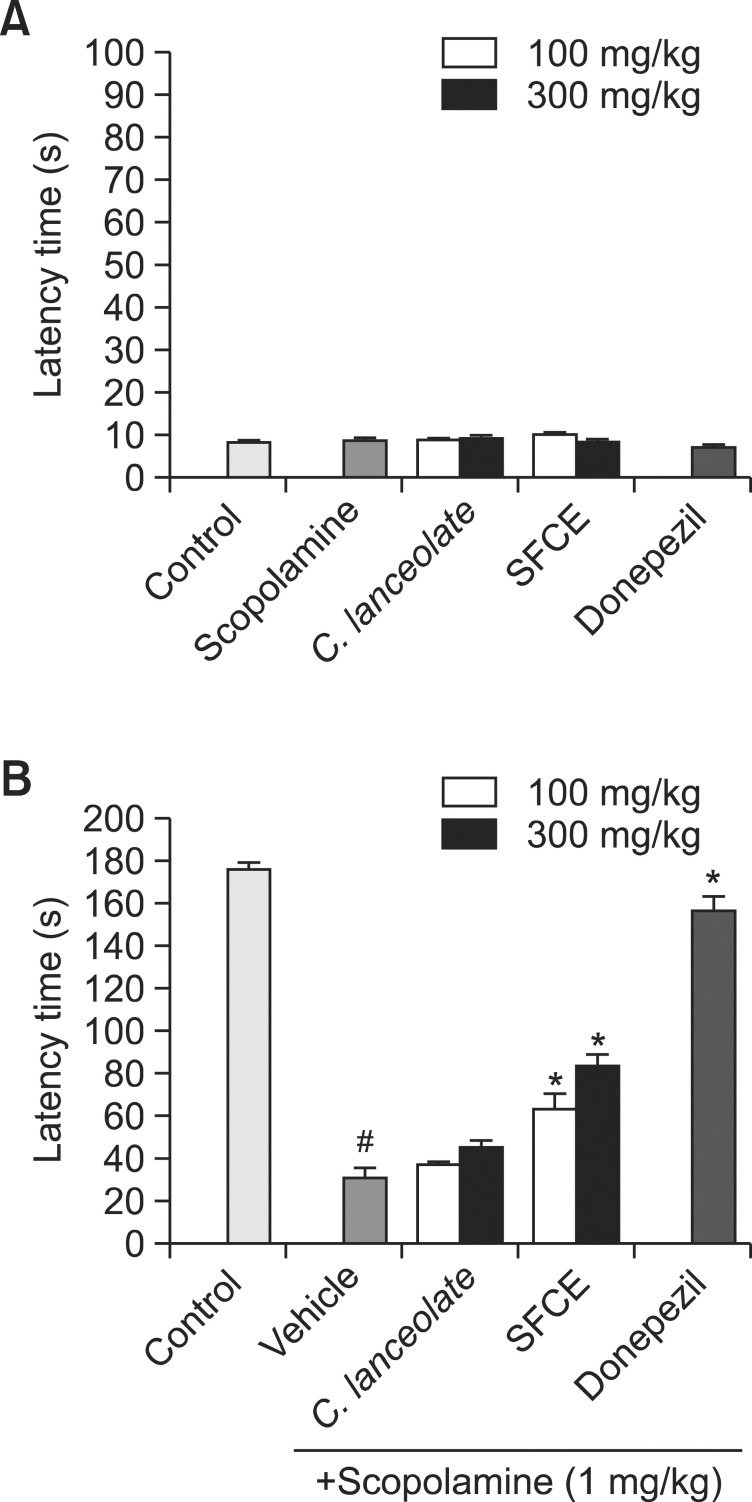
Passive avoidance test is one of behavioral tasks to evaluate the memory ability (Fig. 3). Significant differences in the step-through latency time were not observed between groups during the training trial (Fig. 3A). In Fig. 3B, the step through latency of scopolamine treated group with vehicle was shorter than control group. SFCE and C. lanceolata significantly increased the latency compared to the scopolamine-treated group and SFCE was more effective than the C. lanceolata as the mean latency time of SFCE (300 mg/kg) was 84.0 ± 5.3 s, while C. lanceolata (300 mg/kg) was 45.5 ± 3.4 s. However, SFCE showed partial inhibitory effect in passive avoidance test as compared with donepezil (1 mg/kg)-treated mice. Collectively, mice treated with SFCE (300 mg/kg) showed significant improvement in passive avoidance test over C. lanceolata (300 mg/kg) after scopolamine-treatment.
Fig. 3. Effect of SFCE on scopolamine-induced memory impairment mice in Passive avoidance test: (A) training trial, (B) test trial. The values shown the mean latency time (s) ± SD (n=7). #p<0.05 compared with control group. *p<0.05 compared with scopolamine group.
Effect of SFCE and C. lanceolata on glutamate-induced neurotoxicity
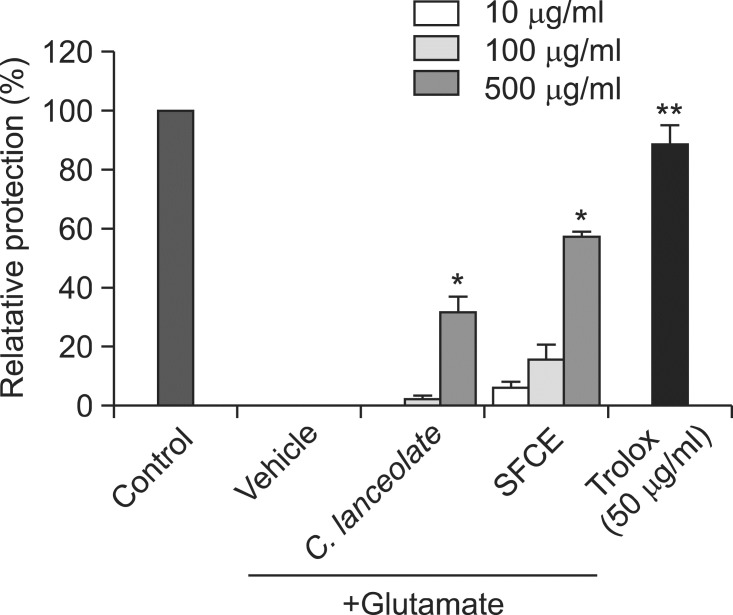
Neuroprotective effect of SFCE extract on the glutamateinduced HT22 cells death was examined by MTT assay. SFCE (500 μg/ml) showed relative protection of 56.99 ± 2.05% against glutamate-induced cell death of HT22 cells only treated with vehicle (Fig. 4). Interestingly, SFCE extract resulted in significanly more neuroprotective effect as compared with cells treated with C. lanceolata extract.
Fig. 4. The neuroprotective effects of SFCE and C. lanceolata against glutamate-induced cytotoxicity in neuronal HT22 cells. Each bar represents the mean relative protection ± SD of three independent experiments. *p<0.05, **p<0.01 and ***p<0.001 vs. glutamate-injured cells.
Analysis of compounds in SFCE and C. lanceolata
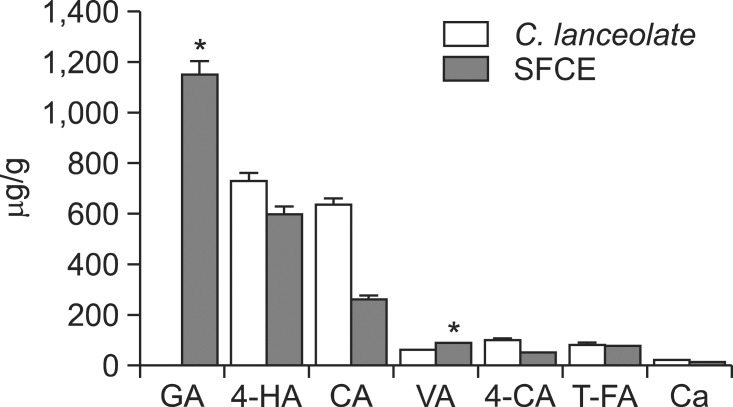
To determine of contents of seven phenolic compounds (gallic acid, 4-hydroxybenzoic acid, caffeic acid, vanillic acid, 4-coumaric acid, trans-feulic acid, caffecine) in SFCE, HPLC system was used and compared to extract of C.lanceolata. The contents of compounds depicted in Fig. 5. Among seven compounds, contents of gallic acid, vanillic acid in C. lanceolata was significanly increased by steam and fermentation process (Fig. 5).
Fig. 5. The contents of phenolic compounds in the SFCE and C. lanceolata. Significantly different from the C. lanceolata (*p<0.05).
DISSCUSION
In this study, we evaluated the effect of SFCE on scopolamine- induced memory impairments in the Morris water maze test and passive avoidance test.
Scopolamine is very widely used in screening of memory enhancing activity in experimental animals (Flood and Cherkin, 1986). Muscarinic cholinergic antagonist scopolamine, blocks cholinergic neurotransmission and impairs cognitive functions including learning and memory (Izquierdo, 1989). In Morris water maze test, SFCE on scopolamine-injected mice showed that decreased escape latency time during three days (day 2-day 4), which indicated the SFCE improved the long-term memory. SFCE-treated group increased time spent in the swimming quadrant without platform. In swim route of water maze test, three different patterns were detected spatial persistent (target directed), nonspatial persistent (semi-target directed), nonspatial random (not target directed). Scopolamine-treated group showed nonsptial random pattern but SFCE treat group exhibited as a non spatial persistent pattern.
SFCE at dose of 500 mg/kg also significantly increased the memory retrieval in passive test. The step-through latency time during the training trial was not affect by SFCE sample, which indicated that SFCE ameliorates memory impairment without affecting exercise capacity of mouse. Morris water maze test and passive avoidance test are used to assess hippocampal- dependent spatial learning and memory and memory retention based on the natural tendency of animals formed between an aversive stimulus, respectively (Friedman et al., 1983; Jäkälä et al., 1992). SFCE showed the most potent anti-amnesic activity in Morris water maze test compared to passive avoidance test. This result indicated that SFCE affect nonmnemonic cognitive function (Whishaw, 1989; Puumala et al., 1996).
In HT22 cell model, SFCE showed neuroprotective effect against glutamate induced cell death. Glutamate is an excitatory neurotransmitter in the central nervous system. At high concentrations, glutamate is able to oxidative stress by inhibition of cystein uptake through the cystein/glutamate antiporter and lead to accumulation of intracellular Ca2+ via N-methyl-D-aspartate (NMDA) receptor activation (Murphy et al., 1989; Randall and Thayer, 1992; Fukui et al., 2009). As mentioned in introduction, Oxidative stress or accumulation of ROS and intracellular Ca2+ concentration is thought contribute to important role to pathogenesis of neurodegenerative diseases.
We observed higher content of gallic acid and vanillic acid in SFCE than original C. lanceolata by HPLC analysis. And the contents of total phenolic compounds also were increased. Gallic acid and vanillic acid are known to antioxidant (Yena et al., 2002; Kumar et al., 2011). Further, gallic acid also reported to have AchE inhibitory activity and inhibit elevation of glutamate release, Ca2+ and ROS generation associated with the neuroprotective effect. (Ban et al., 2008; Ghayur et al., 2011; Hong et al., 2012). In previously study, vanillic acid showed neuroprotective activity against glutamate induced cell death in HT22 cells and neuroprotective activities against serum deprivation induced SH-SYSY-JNK3 cell apoptosis (Gan et al., 2011; Weon et al., 2012). Since scopolamine increases AchE activity in the cortex and hippocampus and glutamate induce neuronal cell death, the effect of fermented C. lanceolata may be associated with inhibitor of AchE activity, antioxidant activity and neuroprotective activity of gallic acid and vanillic acid.
Previous study reported that fermentation enhanced antioxidant activity and increased concentration of isoflavone aglycones in soybean (Pyo et al., 2005). We also observed that neuroprotective effect of traditional medicine such as Hwangryunhaedok-tang and Insampaedok-san in HT22 cells improved through fermentation (Weon et al., 2011; Yang et al., 2011). Our results have shown that and.
Hence, we considered that steamed and fermented process increased contents of phenolic acid such as gallic acid and total phenolic compounds in C. lanceolata and it may be involved improvement of cognitive enhancing activity and neuroprotective effect of C. lanceolata.
In this study, we increased temperature sequentially from 50℃ to 100℃. This method steamed C. lanceolata rapidly compared to previous method and improve activity of C. lanceolata similarly in steaming process.
Brain of AD patients observed cholinergic dysfunction and increased neuron degeneration and neuronal cell death by oxidative stress. Based on results in this study, SFCE may not only afford memory improvements and the amelioration of cholinergic function in AD but also may provide neuroprotective activity (Markesbery, 1997; Francis et al., 1999). We hypothesized that SFCE ameliorates cognitive impairment through the AchE inhibition and neuroprotective effect via accumulation of ROS inhibition. To demonstrate these hypotheses, further study is being done on the mechanism of activity of SFCE.
In conclusion, SFCE attenuates cognitive impairments in mice injected with scopolamine-induced dementia mouse model and protects neuronal cell. Cognitive enhancing and neuroprotective effect of SFCE were associated with the increase of total phenolic compounds, anti-oxidantive activity and AchE inhibitory activity of gallic acid and vanilic acid. Collectively, these data suggest that SFCE could be useful in the therapy of AD. However, further study will be required to identify the exact mechanism of SFCE.
Acknowledgments
This work was carried out with the support of "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009001)" Rural Development Administration, Republic of Korea.
References
- 1.Bains J. S., Shaw C. A. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res.Brain Res. Rev. (1997);25:335–358. doi: 10.1016/S0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 2.Ban J. Y., Nguyen H. T., Lee H. J., Cho S. O., Ju H. S., Kim J. Y., Bae K., Song K. S., Seong Y. H. Neuroprotective properties of gallic acid from Sanguisorbae radix on amyloid beta protein (25--35)-induced toxicity in cultured rat cortical neurons. Biol. Pharm. Bull. (2008);31:149–153. doi: 10.1248/bpb.31.149. [DOI] [PubMed] [Google Scholar]
- 3.Brion J. P. Neurofibrillary tangles and Alzheimer's disease. Eur. Neurol. (1998);40:130–140. doi: 10.1159/000007969. [DOI] [PubMed] [Google Scholar]
- 4.Brookmeyer R., Gray S., Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am. J. Public Health. (1998);88:1337–1342. doi: 10.2105/AJPH.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byeon S. E., Choi W. S., Hong E. K., Lee J., Rhee M. H., Park H. J., Cho J. Y. Inhibitory effect of saponin fraction from Codonopsis lanceolata on immune cell-mediated inflammatory responses. Arch. Pharm. Res. (2009);32:813–822. doi: 10.1007/s12272-009-1601-7. [DOI] [PubMed] [Google Scholar]
- 6.Coyle J. T., Price D. L., DeLong M. R. Alzheimer's disease: a disorder of cortical cholinergic innervations. Science. (1983);219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 7.Flood J. F., Cherkin A. Scopolamine effects on memory retention in mice: a model of dementia? Behav. Neural Biol. (1986);45:169–184. doi: 10.1016/S0163-1047(86)90750-8. [DOI] [PubMed] [Google Scholar]
- 8.Francis P. T., Palmer A. M., Snape M., Wilcock G. K. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. (1999);66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman E., Lerer B., Kuster J. Loss of cholinergic neurons in the rat neocortex produces deficits in passive avoidance learning. Pharmacol. Biochem. Behav. (1983);19:309–312. doi: 10.1016/0091-3057(83)90057-6. [DOI] [PubMed] [Google Scholar]
- 10.Fukui M., Song J. H., Choi J., Choi H. J., Zhu B. T. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur. J. Pharmacol. (2009);617:1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 11.Gan M., Lin S., Zhang Y., Zi J., Song W., Hu J., Chen N., Wang L., Wang X., Shi J. Liposoluble constituents from Iodes cirrhosa and their neuroprotective and potassium channel-blocking activity. Zhongguo Zhong Yao Za Zhi. (2011);36:1183–1189. [PubMed] [Google Scholar]
- 12.Ghayur M. N., Kazim S. F., Rasheed H., Khalid A., Jumani M. I., Choudhary M. I., Gilani A. H. Identification of antiplatelet and acetylcholinesterase inhibitory constituents in betel nut. Zhong Xi Yi Jie He Xue Bao. (2011);9:619–625. doi: 10.3736/jcim20110607. [DOI] [PubMed] [Google Scholar]
- 13.He X., Zou Y., Yoon W. B., Park S. J., Park D. S., Ahn J. Effects of probiotic fermentation on the enhancement of biological and pharmacological activities of Codonopsis lanceolata extracted by high pressure treatment. J. Biosci. Bioeng. (2011);112:188–193. doi: 10.1016/j.jbiosc.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Hong S. Y., Jeong W. S., Jun M. Protective effects of the key compounds isolated from Corni fructus against β-amyloidinduced neurotoxicity in PC12 cells. Molecules. (2012);17:10831–10845. doi: 10.3390/molecules170910831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izquierdo I. Mechanism of action of scopolamine as an amnestic. Trends Pharmacol. Sci. (1989);10:175–177. doi: 10.1016/0165-6147(89)90231-9. [DOI] [PubMed] [Google Scholar]
- 16.Jäkälä P., Sirviö J., Jolkkonen J., Riekkinen P. Jr, Acsady L., Riekkinen P. The effects of p-chlorophenylalanine-induced serotonin synthesis inhibition and muscarinic blockade on the performance of rats in a 5-choice serial reaction time task. Behav. Brain Res. (1992);51:29–40. doi: 10.1016/S0166-4328(05)80309-2. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S., Prahalathan P., Raja B. Antihypertensive and antioxidant potential of vanillic acid, a phenolic compound in LNAME-induced hypertensive rats: a dose-dependence study. Redox Rep. (2011);16:208–215. doi: 10.1179/1351000211Y.0000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markesbery W. R. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. (1997);23:134–147. doi: 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 19.McGleenon B. M., Dynan K. B., Passmore A. P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. (1999);48:471–480. doi: 10.1046/j.1365-2125.1999.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy T. H., Miyamoto M., Sastre A., Schnaar R. L., Coyle J. T. Glutamate toxicity in neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. (1989);2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 21.Murray M. E., Knopman D. S., Dickson D. W. Vascular dementia: clinical, neuroradiologic and neuropathologic aspects. Panminerva Med. (2007);49:197–207. [PubMed] [Google Scholar]
- 22.Pyo Y. H., Lee T. -C., Lee Y. -C. Effect of lactic acid fermentation on enrichment of antioxidant properties and bioactive isoflavones in soybean. J. Food Sci. (2005);70:S215–220. doi: 10.1111/j.1365-2621.2005.tb07160.x. [DOI] [Google Scholar]
- 23.Puumala T., Sirviö J., Ruotsalainen S., Riekkinen P. Sr. Effects of St-587 and prazosin on water maze and passive avoidance performance of scopolamine-treated rats. Pharmacol. Biochem. Behav. (1996);55:107–115. doi: 10.1016/0091-3057(95)02231-7. [DOI] [PubMed] [Google Scholar]
- 24.Randall R. D., Thayer S. A. Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J. Neurosci. (1992);12:1882–1895. doi: 10.1523/JNEUROSCI.12-05-01882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richard E., Schmand B., Eikelenboom P., Westendorp R. G., Van Gool W. A. The Alzheimer myth and biomarker research in dementia. J. Alzheimers Dis. (2012);31:S203–209. doi: 10.3233/JAD-2012-112216. [DOI] [PubMed] [Google Scholar]
- 26.Rubinsztein D. C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. (2006);443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 27.Tam J. H., Pasternak S. H. Amyloid and Alzheimer's disease: inside and out. Can. J. Neurol. Sci. (2012);39:286–298. doi: 10.1017/s0317167100013408. [DOI] [PubMed] [Google Scholar]
- 28.Ushijima M., Komoto N., Sugizono Y., Mizuno I., Sumihiro M., Ichikawa M., Hayama M., Kawahara N., Nakane T., Shirota O., Sekita S., Kuroyanagi M. Triterpene glycosides from the roots of Codonopsis lanceolata. Chem. Pharm. Bull. (2008);56:308–314. doi: 10.1248/cpb.56.308. [DOI] [PubMed] [Google Scholar]
- 29.Weon J. B., Kim C. Y., Yang H. J., Ma C. J. Neuroprotective compounds isolated from Cynanchum paniculatum. Arch. Pharm. Res. (2012);35:617–621. doi: 10.1007/s12272-012-0404-4. [DOI] [PubMed] [Google Scholar]
- 30.Weon J.B., Ma J. Y., Yang H. J., Ma C. J. Quantitative analysis of compounds in fermented Insampaedok-san and their neuroprotective activity in HT22 Cells. Nat. Prod. Sci. (2011);17:58–63. [Google Scholar]
- 31.Weon J. B., Yun B. -R., Lee J., Eom M. R., Kim J. S., Lee H. Y., Park D. S., Chung H. -C., Chung J. Y., Ma C. J. The ameliorating effect of steamed and fermented Codonopsis lanceolata on scopolamine-induced memory impairment in mice. Evid Based Complement. Alternat. Med. (2013);2013:464576. doi: 10.1155/2013/464576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whishaw I. Q. Dissociating performance and learning deficits on spatial navigation tasks in rats subjected to cholinergic muscarinic blockade. Brain Res. Bull. (1989);23:347–358. doi: 10.1016/0361-9230(89)90221-9. [DOI] [PubMed] [Google Scholar]
- 33.Yang H. J., Weon J.B., Lee B., Ma C. J. The alteration of components in the fermented Hwangryunhaedok-tang and its neuroprotective activity. Pharmacogn. Mag. (2011);7:207–212. doi: 10.4103/0973-1296.84234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yena G. -C., Duhb P. -D., Tsaia H. -L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. (2002);79:307–313. doi: 10.1016/S0308-8146(02)00145-0. [DOI] [Google Scholar]
- 35.Yongxu S., Jicheng L. Structural characterization of a water- soluble polysaccharide from the roots of Codonopsis pilosula and its immunity activity. Int. J. Biol. Macromol. (2008);43:279–282. doi: 10.1016/j.ijbiomac.2008.06.009. [DOI] [PubMed] [Google Scholar]