Abstract
Phosphatidylserine (PS) exposure in red blood cells (RBCs) from sickle cell disease (SCD) patients is increased compared to levels in normal individuals and may participate in the anaemic and ischaemic complications of SCD. Exposure is increased by deoxygenation and occurs with elevation of intracellular Ca2+ to low micromolar levels. The Ca2+ entry step has not been defined but a role for the deoxygenation-induced pathway, Psickle, is postulated. Partial Psickle inhibitors 4-acetamido-4′-isothiocyanostilbene-2,2′-disulphonic acid (SITS), 4,4′-dithiocyano-2,2′-stilbene-disulphonic acid (DIDS) and dipyridamole inhibited deoxygenation-induced PS exposure (DIDS IC50, 118 nM). Inhibitors and activators of other pathways (including these stimulated by depolarisation, benzodiazepines, glutamate and stretch) were without effect. Zn2+ and Gd3+ stimulated PS exposure to high levels. In the case of Zn2+, this effect was independent of oxygen (and hence HbS polymerisation and RBC sickling) but required extracellular Ca2+. The effect was completely abolished when Zn2+ (100 μM) was added to RBCs suspended in autologous plasma, implying a requirement of high levels of free Zn2+.
Keywords: Sickle cell disease, Red blood cell, Phosphatidylserine, Deoxygenation, Calcium, Cation channel
Introduction
Phosphatidylserine (PS) is an aminophospholipid which is normally confined to the inner leaflet of lipid bilayers [40]. In red blood cells (RBCs), this is particularly important because its exposure is prothrombotic, encourages phagocytosis and increases RBC adherence [7, 29, 59, 63, 64]. In healthy individuals, usually <1 % RBCs expose PS [40]. Programmed externalisation is thought to occur in damaged or senescent RBCs to aid their removal from the circulation. This process has been termed eryptosis [42, 45] and is analogous to the apoptosis of nucleated cells. Elevated PS exposure is also seen in a number of disease states, including sickle cell disease (SCD) [17, 66].
In SCD, patients show various complications which fall into two main groups: a chronic anaemia and acute vaso-occlusive disorders (such as pain, stroke, acute chest syndrome and osteonecrosis). PS exposure is observed in a high, but variable, percentage of RBCs (2–10 % is often quoted [15, 17, 40, 41, 66, 67]). PS may therefore contribute to both the anaemic and ischaemic complications of SCD [29]. The mechanism by which it becomes externalised has therefore received considerable attention [1, 17, 40].
Asymmetrical distribution of PS is usually maintained by high activity of an ATP-dependent aminophospholipid translocase (or flippase) in conjunction with relatively low activity of a Ca2+-activated scrambling process (or scramblase activity) [4, 25]. The presence of the abnormal haemoglobin, HbS, in patients’ RBCs somehow perturbs this equilibrium to favour PS exposure. HbS molecules are able to aggregate on deoxygenation forming rigid polymers which distort RBC shape, alter rheology and stimulate a number of other deleterious sequelae [29]. Deoxygenation also stimulates PS exposure [6, 49, 66]. Damage to the cytoskeleton may free PS from anchor sites resulting in its higher mobility [22, 26, 52]. In addition, however, deoxygenation-induced PS exposure is Ca2+ dependent requiring elevation of the concentration of intracellular Ca2+ ([Ca2+]i) to low micromolar levels [66]. The high cation permeability of HbS-containing RBCs may be involved in this process [24, 31, 46]. In particular, a role has been postulated for the deoxygenation-induced cation conductance (sometimes termed Psickle) which is activated upon deoxygenation, HbS polymerisation and RBC shape change [32, 46, 50]. This pathway is permeable to divalent cations including Ca2+ and can therefore mediate Ca2+ entry. Elevated [Ca2+]i both inhibits the flippase and also activates the scramblase [25]. A number of other RBC cationic permeabilities have also been described, however, including conductances activated by depolarisation, glutamate, benzodiazepines and stretch, as well as following infection with intraerythrocytic parasites like malaria [3, 35] (although the latter is more widely considered to increase activity of anion-selective pathways [39]). One or more of these, instead of, or as well as Psickle, may contribute to the Ca2+ entry involved in deoxygenation-induced PS exposure. Clear identification of the pathway(s) involved would be beneficial as it would direct the search for potential inhibitors amenable to clinical use for amelioration of the complications of SCD.
In this study, we use various inhibitors and agonists of RBC pathways in an attempt to clarify the Ca2+ entry step which results in deoxygenation-induced PS exposure in RBCs from SCD patients. Results indicate that partial inhibitors of Psickle reduced deoxygenation-induced PS exposure, consistent with mediation of Ca2+ entry via this pathway, but those inhibitors and activators of several other potential entry pathways were without effect.
Materials and methods
Solutions and chemicals
The main experimental saline comprised (in millimolar): NaCl 140, KCl 4, CaCl2 1.1, MgCl2 0.15, N-2-hydroxyethylpiperzine-N′-2-ethanesulphonic acid (HEPES) 10 and inosine 10 with a pH of 7.4 at 37 °C (low potassium-containing saline, LK HBS). For washing red blood cells (RBCs), a high potassium-containing saline (HK HBS) was usually used, comprising (in millimolar) NaCl 54, KCl 90, MgCl2 0.15, HEPES 10 and inosine 10 with a pH of 7.4 at room temperature. For experiments carried out in plasma, the RBC wash solution comprised NaHCO3 24 mM and NaCl 137 mM equilibrated with air and 5 % CO2, pH 7.4. The ice-cold wash solution for K+ flux measurements comprised (in millimolar) MgCl2 107, 3-(N-morpholino)-propanesulphonic acid (MOPS) 10, pH 7.4 at 0 °C. Fluorescein isothiocyanate-conjugated lactadherin (LA-FITC)-binding buffer consisted of LK HBS with 1 mM vanadate and 16 nM LA-FITC. Osmolality of all solutions was 290 ± 5 mOsm kg−1. LA-FITC was obtained from Haematologic Technologies Inc. (VT, USA) supplied via Cambridge Bioscience (Cambridge, UK), phycoerythrin (PE)-conjugated anti-glycophorin A from Becton Dickinson Biosciences (CA, USA), HEPES and 4,4′-dithiocyano-2,2′-stilbene-disulphonic acid (DIDS) from Calbiochem (Merck, Darmstadt, Germany) and 86Rb+ from PerkinElmer (MA, USA). Diazepam and PK11195 were gifts from Dr. Guillaume Bouyer (CNRS, Roscoff, France). All other chemicals came from Sigma-Aldrich (Poole, Dorset, UK).
RBC preparation
Blood samples were collected from HbSS SCD patients, with consent and ethical permission (11/LO/0065), in the anticoagulant EDTA, except for the experiments in which RBCs were incubated in autologous plasma. In this case, heparin was used as an anticoagulant to prevent chelation of Ca2+ and/or Zn2+ (Fig. 6). In Fig. 6, only RBCs from normal (HbAA) individuals were used. RBCs were washed three times into HK HBS for 3 min at 600 g and then subsequently twice into LK HBS, unless otherwise indicated, to give a final haematocrit (Hct) of 5 %. RBC suspensions were then deoxygenated for 20 min in Eschweiler tonometers flushed with warmed humidified N2, by which time PS exposure was unchanged relative to that measured in untreated RBCs (as shown previously [66]). They were then diluted into test tubes also pre-equilibrated with N2 (final Hct 0.5 %) in the absence or presence of potential inhibitors/activators of PS exposure. Incubation was carried out at 37 °C for up to 60 min in the presence of 1 mM vanadate to inhibit both the flippase and the plasma membrane calcium pump. Solutions of DIDS (stock 5–50 mM in DMSO) were made up fresh every other day as stability decreased after 3 days resulting in strong stimulation of PS exposure, probably through the action of a breakdown product(s). Controls were exposed to the same final concentration of solvent (DMSO or H2O, 0.2 %) but otherwise treated in the same way. For experiments with glutamate receptor agonists, N-methyl-d-aspartate (NMDA) and homocysteine, RBCs were kept oxygenated and incubated in Eppendorf tubes (up to 60 min, 37 °C). For experiments to test the effect of plasma on the action of Zn2+, RBCs were re-suspended in autologous plasma after washing, equilibrated with air and 5 % CO2 delivered using a Wösthoff gas mixer (Bochum, Germany) at 37 °C to give a final pH 7.4. Normal total plasma Zn2+ levels are about 10–15 μM [16, 28, 48]. This was not measured, rather additional Zn2+ at the concentration indicated was added directly to plasma and thus represented the minimum total concentration present.
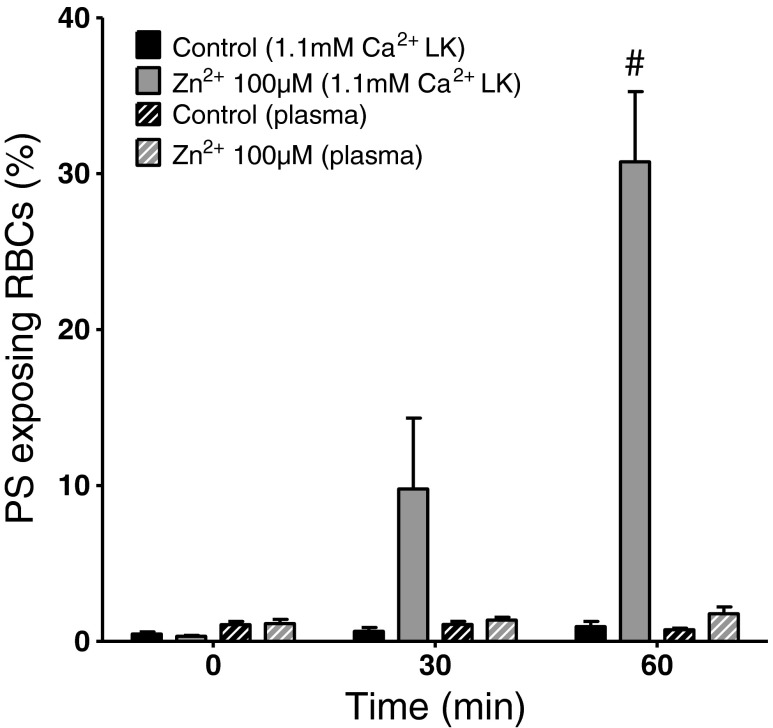
Fig. 6.
Effect of Zn2+ and autologous plasma on PS exposure in RBCs from normal (HbAA) individuals. RBCs were incubated under oxygenated conditions in LK saline (solid histograms) or in autologous plasma (hatched histograms), in which case pH was maintained by flushing with warm humidified CO2 (5 %). Percentage PS exposure is shown at time 0, just before addition of Zn2+ (100 μM), and after 30 and 60 min. Histograms represent means ± S.E.M., n = 4 (paired samples). # p < 0.008 compared to values in plasma
Labelling of PS exposure
Samples of 5 μl aliquots (105 RBCs) were placed in 250 μl of LA-FITC binding buffer. PS labelling was carried out in the dark, because of the high sensitivity of FITC to light, at room temperature for 10 min. RBCs were then pelleted by centrifugation for 10 s at 16,100×g and washed once into LK HBS to remove unbound LA-FITC. Unlike annexin-V, LA-FITC binds to PS in a Ca2+-independent manner [14, 61]. Control experiments showed that binding was irreversible. Samples were then kept on ice until analysed by flow cytometry (FACS). Inhibitors/activators were tested (at 100 μM) for self-fluorescence using unlabelled RBCs. The percentage of RBCs exposing PS is usually normalised to values measured in control RBCs prior to addition of inhibitors, as the absolute magnitude of exposure varied between samples. These control values are given in the figure legends.
FACS acquisition and analysis
Externalised PS was measured in the FL-1 channel, with an emission wavelength for FITC of 519 nm, of a fluorescence-activated flow cytometer (FACSCalibur, Becton Dickinson, BD) and analysed with BD CellQuest Pro software using the protocol as previously published [66]. In control experiments, forward scatter (FSC, size) and side scatter (SSC, granularity) gates for RBCs were identified using a PE-labelled anti-glycophorin A assay. FSC was set with threshold at 512. Measurements were taken under logarithmic gain with voltages set at FSC, E00; SSC, 235; FL-1, 688; FL-2, 630; and FL-3, 590. Compensation was set as FL-1, 2.0 % of FL-2; FL-2, 19.6 % FL-1; FL-2, 0.0 % FL-3; and FL-3, 8.5 % FL-2 to minimise the effect of over spill of fluorescence to adjacent channels. For each measurement 10,000 events were gated. All gated LA-FITC-labelled cells were additionally cross checked against overlap into FL-2 and FL-3 fluorescent channels and for spill out of the size gate and if these occurred events were excluded from analysis. The percentage of such resolved events was noted and for most experiments was ≤1 %. On FL-1/FL-2 dot plot graph, the cut-off quadrants, for negative fluorescent gate, were set using unlabelled cells as x = 20 and y = 20. The PS-positive cells were represented as the percentage of all gated RBCs with sufficient externalised PS to appear positive fluorescently in the FL-1 channel [14] and thus fallen into the quadrant (x > 20 and y < 20).
K+ flux measurements
Potassium fluxes were measured using 86Rb+ as a tracer for K+ [18, 62]. In these experiments, RBCs (Hct about 20 %) were first equilibrated in Eschweiler tonometers (Kiel, Germany) in air or N2 at 37 °C, pH 7.4, in LK saline in which Cl− was substituted with NO3 − and MOPS for HEPES. Cl− substitution prevented K+ transport through the Na+-K+-2Cl− cotransporter and K+-Cl− cotransporter. Aliquots were then diluted tenfold into test tubes containing the same saline and also pre-equilibrated with air or N2 for flux measurements. Bumetanide (10 μM), ouabain (100 μM) and clotrimazole (5 μM) were all also present to inhibit K+ transport through the Na+-K+-2Cl− cotransport, Na+/K+ pump and Ca2+-activated K+ channel. 86Rb+ was then added into a KCl solution to give a final [K+] of 7.5 mM. Influx was performed over 10 min, after which RBCs were washed four times in ice-cold isotonic MgCl2 wash solution, lysed using Triton X-100 (0.1 %) and protein was precipitated with trichloroacetic acid (5 %) and 5 min centrifugation at 13,000×g. 86Rb+ (K+) in the supernatant was then counted by liquid scintillation as Čerenkov radiation (Tri-Carb 2100TR, PerkinElmer, MA, USA). The difference in K+ influx at the two gas tensions provides a measure of the deoxygenation-induced cation conductance, Psickle.
RBC morphology
Suspensions of RBCs (0.2 % Hct) were fixed in saline in the presence of glutaraldehyde (0.3 %) and viewed under a phase field mode on Leica DM6000B Microscope (Leica Microsystems Ltd, UK). From each sample, at least eight different frames (×20) were saved using a digital camera (AF600DFC) and Leica LAS AF Lite software to determine the percentage of sickled cells. At least 500 cells were counted for each sample.
Statistics
Unless otherwise indicated, data are presented as means ± S.E.M. for n individuals. Statistical significance was tested with paired Student’s t test; p < 0.05 was accepted as significant. All graphs were made using GraphPad Prism 5 (San Diego, CA). Samples from at least five individuals were collected for each of experiments. Calculations were made either using Microsoft Office Professional 2010 Excel software or GraphPad Prism 5.
Results
Effect of oxygen tension on PS exposure in RBCs from SCD patients
In control experiments, PS exposure in RBCs from SCD patients was 3.3 ± 0.2 % at time 0, increasing after deoxygenation for 30 and 60 min to 7.5 ± 0.4 and 10.5 ± 0.6 % (means ± S.E.M., n = 124), respectively. The lowest PS exposure values were 0.5, 1.7 and 1.9 % at time 0 and after 30 and 60 min deoxygenation, respectively, while the highest values were 14.3, 27.5 and 26.2 %. In separate experiments, PS exposure in RBCs kept oxygenated did not change (3.1 ± 0.5 % at time 0 and 3.8 ± 0.7 and 4.1 ± 0.6 % after 30 and 60 min of incubation, respectively, n = 13). We have previously reported similar findings [66].
Effects of inhibitors of Psickle
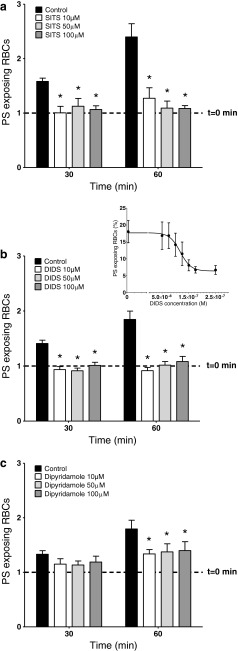
Although there are no specific inhibitors of Psickle, stilbenes [30] and the pyrimidine derivative dipyridamole [33] have been shown to inhibit the deoxygenation-induced cation permeability of RBCs from SCD patients. In the first series of experiments, therefore, the effects of 4-acetamido-4′-isothiocyanostilbene-2,2′-disulphonic acid (SITS), 4,4′-dithiocyano-2,2′-stilbene-disulphonic acid (DIDS) and dipyridamole were tested on deoxygenation-induced PS exposure (Fig. 1). Each of the three reagents significantly inhibited the deoxygenation-induced PS exposure at both 30 and 60 min, at all concentrations used (10, 50 and 100 μM). The effect of DIDS was explored in more detail. Significant inhibition was observed by 100 nM (p < 0.005), with an IC50 of 118 ± 10 nM (n = 9), and was largely complete by 250 nM.
Fig. 1.
Effect of Psickle inhibitors on deoxygenation-induced phosphatidylserine (PS) exposure in red blood cells (RBCs) from SCD patients. a PS exposure is normalised to exposure in RBCs at time 0, just before addition of SITS, which was 2.8 ± 0.8 % (indicated by the dotted line), and after 30 and 60 min deoxygenation in the absence (solid black histograms) or presence (lighter histograms) of SITS at 10, 50 and 100 μM. Data represent means ± S.E.M., n = 5. *p < 0.05 compared to deoxygenated RBCs in the absence of SITS. b PS exposure is normalised to exposure in RBCs at time 0, just before addition of DIDS, which was 4.3 ± 0.5 % (indicated by the dotted line), and after 30 and 60 min deoxygenation in the absence (solid black histograms) or presence (lighter histograms) of DIDS at 10, 50 and 100 μM. Data represent means ± S.E.M., n = 8. *p < 0.002 compared to deoxygenated RBCs in the absence of DIDS. Inset represents the concentration dependence of DIDS inhibition of deoxygenation-induced PS exposure in RBCs from SCD patients. Percentage PS exposure is shown after 60 min deoxygenation in RBCs treated with DIDS at the concentrations indicated. PS exposure at time 0, just before addition of DIDS, was 4.8 ± 0.7 %. IC50 for DIDS was 118 ± 10 nM. Data represent means ± S.E.M., n = 9. c PS exposure is normalised to exposure in RBCs at time 0, just before addition of dipyridamole, which was 3.8 ± 0.4 % (indicated by the dotted line), and after 30 and 60 min deoxygenation in the absence (solid black histograms) or presence (lighter histograms) of dipyridamole at 10, 50 and 100 μM. Data represent means ± S.E.M., n = 7. *p < 0.02 compared to deoxygenated RBCs in the absence of dipyridamole
It was possible that these reagents acted indirectly through inhibition of HbS polymerisation and sickling. This was tested using morphological studies to assess the percentage of RBCs which underwent sickling following deoxygenation. After 60 min deoxygenation, in the absence and presence of DIDS (100 μM), sickling was 61 ± 8 and 62 ± 5 % (means ± S.E.M., n = 4, not significant (N.S.)), respectively. A possible explanation for the effect of DIDS on PS exposure via inhibition of sickling was therefore discounted.
Effect of inhibitors of the non-specific cation channels of RBCs
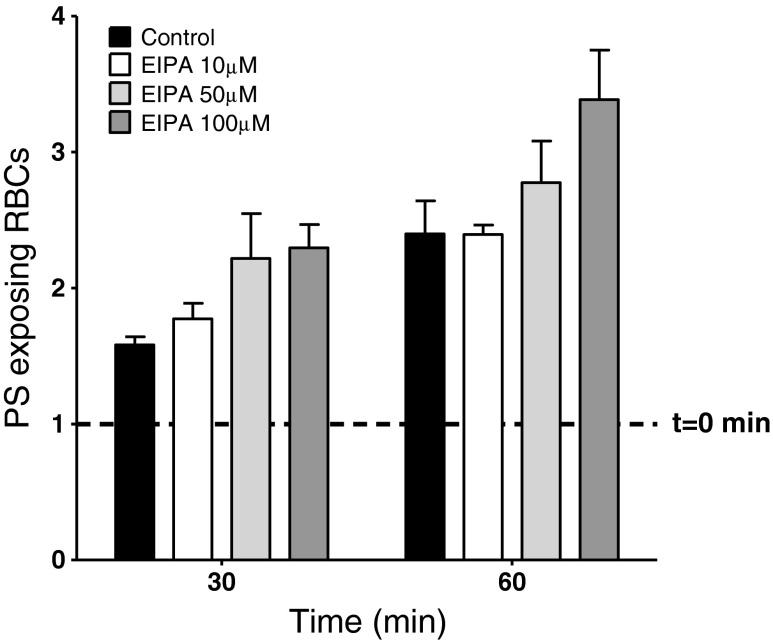
A non-selective cation conductance has been described in RBCs from normal individuals, activated by a number of manoeuvres including osmotic or oxidative shock, or Cl− substitution with gluconate (which will also cause RBC shrinkage) [19, 43]. Amiloride derivatives represent effective inhibitors of this pathway [44]. The effect of ethylisopropylamiloride (EIPA) was therefore tested on the deoxygenation-induced PS exposure of RBCs from patients with SCD but no significant effect was observed (Fig. 2).
Fig. 2.
Effect of EIPA on deoxygenation-induced PS exposure in RBCs from SCD patients. PS exposure is normalised to exposure in RBCs at time 0, just before addition of EIPA, which was 2.8 ± 0.8 % (indicated by the dotted line), and after 30 and 60 min deoxygenation in the absence (solid black histograms) or presence (lighter histograms) of EIPA at 10, 50 and 100 μM. Data represent means ± S.E.M., n = 5. All N.S. compared to deoxygenated RBCs in the absence of EIPA
Effects of inhibitors of unusual RBC cation pathways
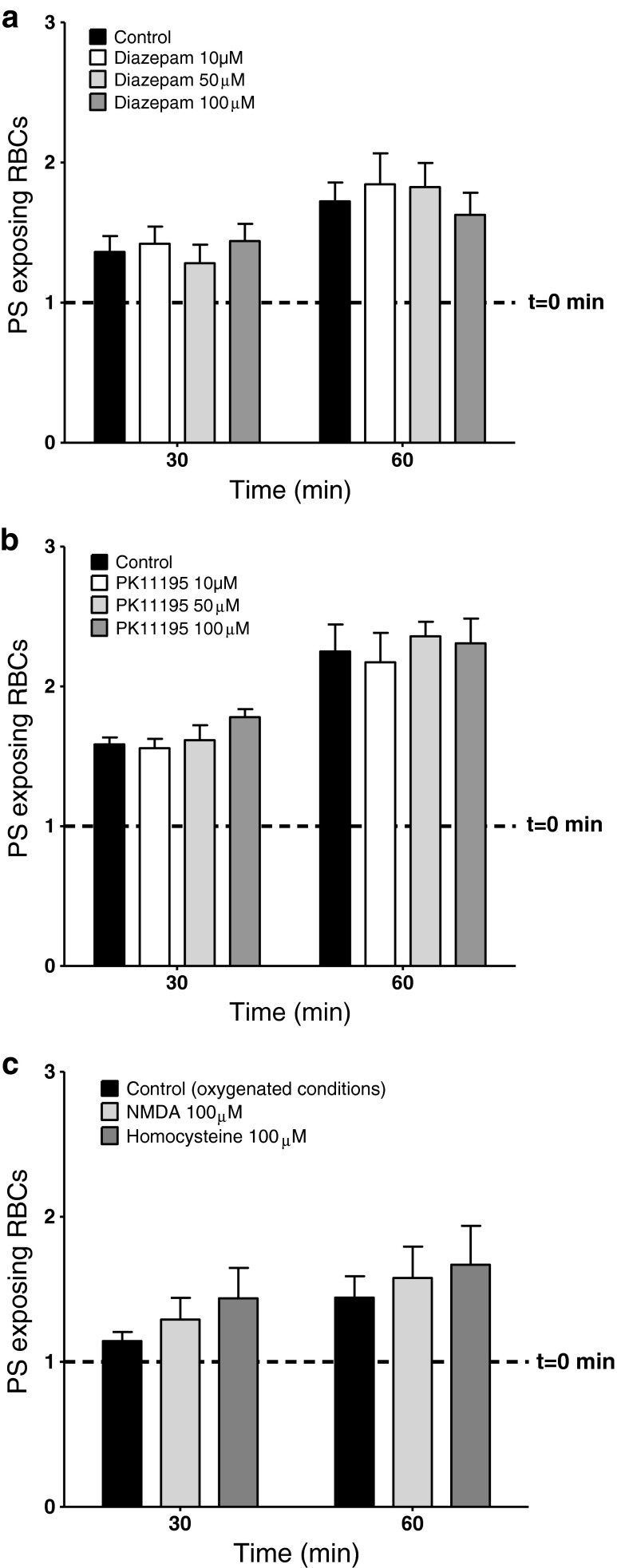
Recently, it has been proposed that RBCs express a conductance mediated via the peripheral benzodiazepine receptor (PBR) [9]. This pathway has multiple conductance states and is permeable to a number of ions including anions. It has also been postulated that RBCs respond to glutamate receptor agonists, such as NMDA and homocysteine, with the possibility that these ligand-gated channels may also be present [51]. Various agonists and antagonists of these pathways were investigated (Fig. 3). In experiments on deoxygenation-induced PS exposure, however, neither the PBR agonist diazepam (10–100 μM; Fig. 3a) nor its antagonist PK11195 (10–100 μM; Fig. 3b) had any significant effects. When tested on oxygenated RBCs, the glutamate receptor agonists NMDA and homocysteine (both 100 μM; Fig. 3c) did not affect PS exposure. Similarly, the NMDA receptor antagonist (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (MK-801, 50 μM) had no effect on the deoxygenation-induced PS exposure (data not shown).
Fig. 3.
Effect of modulators of novel RBC cation channels on PS exposure in RBCs from SCD patients. a PS exposure is normalised to exposure in RBCs at time 0, just before addition of diazepam, which was 5.3 ± 1.0 % (indicated by the dotted line), and after 30 and 60 min deoxygenation in the absence (solid black histograms) or presence (lighter histograms) of diazepam at 10, 50 and 100 μM. Data represent means ± S.E.M., n = 6. All N.S. compared to deoxygenated RBCs in the absence of diazepam. b PS exposure is normalised to exposure in RBCs at time 0, just before addition of PK11195, which was 3.0 ± 0.5 % (indicated by the dotted line), and after 30 and 60 min deoxygenation in the absence (solid black histograms) or presence (lighter histograms) of PK11195 at 10, 50 and 100 μM. Data represent means ± S.E.M., n = 7. All N.S. compared to deoxygenated RBCs in the absence of PK11195. c PS exposure is normalised to exposure in oxygenated RBCs at time 0, just before addition of NMDA or homocysteine, which was 4.0 ± 0.9 % (indicated by the dotted line), and after 30 and 60 min of incubation in the absence (black histograms) or presence (lighter histograms) of NMDA or homocysteine (both 100 μM). Data represent means ± S.E.M., n = 6. All N.S. compared to oxygenated RBCs in the absence of NMDA or homocysteine
Effects of inhibitors of stretch-activated channels
Under certain conditions, the aminoglycoside antibiotics, streptomycin and gentamicin [13], and the heavy metals Zn2+ and Gd3+ [10, 13, 50] reduce the permeability of RBCs from SCD patients, possibly acting as stretch-activated channel blockers [60]. Long-term treatment with Zn2+ has also been observed to induce PS exposure in normal human RBCs [38]. Their effects on deoxygenation-induced PS exposure were investigated (Figs. 4 and 5). In the presence of streptomycin, PS exposure increased, but not significantly (Fig. 4).
Fig. 4.
Effect of streptomycin on deoxygenation-induced PS exposure in RBCs from SCD patients. PS exposure is normalised to exposure in RBCs at time 0, just before addition of streptomycin, which was 5.7 ± 0.8 % (indicated by the dotted line), and after 30 and 60 min deoxygenation in the absence (solid black histograms) or presence (lighter histograms) of streptomycin at 10, 50 and 100 μM. Data represent means ± S.E.M., n = 9. All N.S. compared to deoxygenated RBCs in the absence of streptomycin
Fig. 5.
Effect of heavy metals, Zn2+ and Gd3+, on deoxygenation-induced PS exposure in RBCs from SCD patients. a PS exposure at time 0, just before addition of Zn2+, was 3.8 ± 0.4 % (indicated by the dotted line), and is also shown after 30 and 60 min deoxygenation in the absence (solid black histograms) or presence (lighter histograms) of Zn2+ at 10, 50 and 100 μM. Data represent means ± S.E.M., n = 7. # p < 0.001, + p < 0.03 compared to deoxygenated RBCs in the absence of Zn2+. b PS exposure at time 0, just before addition of Gd3+, was 3.5 ± 0.6 % (indicated by the dotted line), and is also shown after 30 and 60 min deoxygenation in the absence (solid black histograms) or presence (lighter histograms) of Gd3+ at 10, 25 and 50 μM. Data represent means ± S.E.M., n = 5. # p < 0.002, + p < 0.02 compared to deoxygenated RBCs in the absence of Gd3+
Both Zn2+ (up to 100 μM) and Gd3+ (up to 50 μM) markedly increased PS exposure (Fig. 5a, b). Higher concentrations of Gd3+ (100 μM) caused RBC aggregation and its effects could not be investigated using FACS. The action of Zn2+ (100 μM) was studied in more detail (Table 1). Zn2+ significantly increased PS exposure in both oxygenated and deoxygenated RBCs. Its effect was therefore different to the deoxygenation-induced PS exposure, requiring HbS polymerisation and sickling. The effect, however, was also dependent on the presence of extracellular Ca2+ (1.1 mM) and largely abolished when it was omitted (Table 1). Additionally, similar effects were observed for both the RBCs from SCD patients and from healthy individuals (cf Figs. 5a (HbSS)) and Fig. 6 (HbAA). In corresponding K+ flux measurements, Zn2+ increased the deoxygenation-induced K+ influx from 0.59 ± 0.14 to 1.02 ± 0.13 mmol (l cells h)−1 (means ± S.E.M., n = 3; p < 0.002) thus almost doubling the activity of the Psickle-like activity.
Table 1.
Effect of Zn2+ on phosphatidylserine (PS) exposure in red blood cells (RBCs) from patients with sickle cell disease. All RBCs were incubated in low potassium-containing (LK) saline and treated with Zn2+ (100 μM) for the duration indicated. Extracellular Ca2+ was also present at 1.1 mM or omitted from saline (with addition of 2 mM EGTA). RBCs were equilibrated with nitrogen (deoxygenated) or air (oxygenated). PS exposures are given as percentage of positive cells. Values represent means ± S.E.M. (n = 4). Statistics show p values for RBCs treated with Zn2+ compared to paired controls without Zn2+ or for Zn2+-treated RBCs in the presence or absence of Ca2+
| 1.1 mM [Ca2+]o | 0 mM [Ca2+]o | |||||
|---|---|---|---|---|---|---|
| Control | 100 μM Zn2+ | Control | 100 μM Zn2+ | |||
| Time of incubation (min) | Mean ± S.E.M. | Mean ± S.E.M. | Student’s t test vs control | Mean ± S.E.M. | Mean ± S.E.M. | Student’s t test vs 1.1 mM [Ca2+]o |
| Deoxygenated conditions | ||||||
| 0 | 4.1 ± 1.4 | 4.5 ± 1.4 | 4.7 ± 1.4 | 4.1 ± 1.4 | ||
| 30 | 5.2 ± 1.4 | 45.9 ± 10.9 | p < 0.02 | 5.8 ± 1.1 | 6.6 ± 1.8 | p < 0.02 |
| 60 | 7.9 ± 1.4 | 53.0 ± 13.6 | p < 0.02 | 7.1 ± 1.0 | 10.5 ± 4.2 | p < 0.01 |
| Oxygenated conditions | ||||||
| 0 | 3.3 ± 0.6 | 6.2 ± 1.4 | 4.4 ± 1.0 | 4.9 ± 1.4 | ||
| 30 | 3.8 ± 0.8 | 52.7 ± 18.2 | p < 0.05 | 5.1 ± 1.2 | 8.1 ± 3.3 | p < 0.04 |
| 60 | 5.1 ± 1.0 | 71.7 ± 12.9 | p < 0.008 | 5.0 ± 0.8 | 20.6 ± 11.0 | p < 0.001 |
As negatively charged plasma proteins are likely to chelate Zn2+, in the final series of experiments, the effect of Zn2+ was determined in RBCs (in this case from normal HbAA individuals suspended in autologous plasma, Fig. 6). In these experiments, PS exposure was measured in the absence and presence of added Zn2+ (100 μM). In saline, as in Fig. 5a, Zn2+ resulted in considerable PS exposure. In plasma, however, an increase in the percentage of RBCs showing PS exposure remained modest even after 60 min of incubation. Similar findings were observed with RBCs from SCD patients (data not shown).
Discussion
The present findings further define the Ca2+ entry step responsible for deoxygenation-induced PS exposure in RBCs from SCD patients. In particular, partial Psickle inhibitors (the stilbenes, SITS and DIDS and the pyrimidine derivative dipyridamole) were similarly active against PS externalisation. By contrast, modulators of other pathways (including inhibitors of the non-specific cation channel and agonists or blockers of glutamate- and benzodiazepine-gated channels) were without effect. Heavy metals Zn2+ and Gd3+, rather than inhibiting PS exposure, caused increased levels of externalisation, an effect which in the case of Zn2+ was shown to be Ca2+ dependent but independent of oxygen tension, HbS polymerisation and RBC sickling.
RBCs from SCD patients have been known to show increased solute permeability for over 50 years. Seminal experiments by Tosteson and colleagues showed that deoxygenated RBCs from SCD patients lost K+ at greater rates than they gained Na+, resulting in net solute loss and shrinkage [65]. This is particularly important as the lag time to HbS polymerisation following deoxygenation is inversely proportional to a very high power of the concentration of HbS [20]. Modest dehydration will markedly increase the likelihood of sickling as RBCs traverse hypoxic areas of the vasculature. Since then, the nature of the altered permeability has been much studied and several pathways have been identified [23, 31, 46]. Amongst these are the KCl cotransporter (likely KCC1 and KCC3 isoforms) and the Ca2+-activated K+ channel (or Gardos channel). In addition to increased permeability to univalent cations, RBCs from SCD patients also show elevated permeability to both Ca2+ and Mg2+ [21, 55, 58]. The pathway(s) responsible for passage of these ions remains less certain, but a pre-eminent role for the deoxygenation-induced cation conductance termed Psickle has been proposed [31, 46].
Although the permeability characteristics of Psickle have been extensively investigated, its molecular identity remains enigmatic [31, 32]. As a flux pathway, its activation is associated with deoxygenation, HbS polymerisation and RBC shape change [31, 53, 58]. It is permeable to both univalent and divalent cations including Ca2+, shows a distinct pH dependence for both activation and permeation [34] and appears randomly (or “stochastically”) activated upon deoxygenation [47]. As for its molecular nature, identification of specific inhibitors has proved elusive. Stilbenes [30] and dipyridamole [33], however, are partial inhibitors, with the latter having been used in clinical trials [12].
Deoxygenation of RBCs from SCD patients is known to result in increased PS exposure [6, 49, 66]. The effect is Ca2+ dependent, requiring physiological levels of extracellular Ca2+ and elevation of intracellular Ca2+ to low micromolar levels [66]. Psickle is therefore an obvious candidate for mediation of Ca2+ entry with subsequent PS exposure following from inhibition of the aminophospholipid translocase (or flippase) and stimulation of the RBC scramblase—both now known to occur at low micromolar [Ca2+] [5, 66]. Notwithstanding, various other RBC cation pathways have been described [35] and it is possible that one or more of these are also involved.
The present results support a role for the Psickle-like pathway in deoxygenation-induced PS exposure. Thus, stilbene derivatives and dipyridamole were active against deoxygenation-induced PS exposure, with no effect on morphological sickling (as also observed previously [30]) implying that they do not act via reduction in HbS polymerisation and subsequent RBC shape change. Inhibition by DIDS, in particular, occurred at low concentrations (IC50 118 nM), more similar to those required to inhibit anion flux through the anion exchanger AE1, rather than monovalent cation flux through the Psickle pathway described previously [30]. Altered AE1 behaviour has been postulated to play a role in the conductance of deoxygenated sickle cells [46] and has been shown to mediate a cation permeability [11] or alter the activity of other cation transporters [8], at least when mutated, which may be relevant to the present findings. DIDS may also directly inhibit the scrambling transporter, as well as Ca2+ entry, but this occurs at much lower affinity with an IC50 of about 10 μM [37]. Here, 250 nM reduced PS exposure during deoxygenation to oxygenated levels.
By contrast, none of the other channel inhibitors/agonists, including those for the more established non-specific cation channel [2, 3, 27] or for the more controversial PBR- and glutamate-gated pathways [9, 51], had any effect. Streptomycin, which might act as a stretch-activated channel blocker [13, 60], was also without effect.
Zn2+ and Gd3+, which also inhibit the cation conductance of RBCs from SCD patients [10, 50], did not inhibit PS exposure. Instead, they caused greatly increased levels of externalisation—an effect previously described for Zn2+ in RBCs from normal individuals following longer term (24 h) exposure [38]. This was not dependent on oxygen tension and appears to be mediated via a different mechanism than the deoxygenation-induced PS exposure under study. Their stimulation also required extracellular Ca2+, as described previously [38]. It would appear that both destabilise the RBC permeability, in a similar way to that described previously for lead and aluminium [45]. A possible action is via titration of the surface negative charges. K+ influx measurements, however, also showed a rise in the activity of Psickle-like pathway which may also provide increased entry of Ca2+ for PS scrambling. Notwithstanding, experiments in plasma suggest that chelation of Zn2+ would reduce its free concentration below values required for the rapid PS exposure described here. This effect may be of some significance as some SCD patients are Zn2+ deficient and supplementation is sometimes given [56, 57]. Ensuring that free plasma [Zn2+] does not rise excessively is an obvious caveat in these cases.
Finally, although the present work is concerned with Ca2+ entry and deoxygenation-induced PS exposure in RBCs from SCD patients, it is also important to note that in RBCs from normal individuals other pathways may be significant. In this context, it is important to note that the present results concern RBCs from SCD patients whose permeability is altered by the presence of polymerised HbS. Most previous reports on PS exposure have been carried out on RBCs from normal individuals (e.g. [44]). It is likely that Ca2+ entry, which triggers subsequent PS exposure, can occur via several different routes. Thus, in addition to the Psickle pathway of sickle cells, normal RBCs show marked Ca2+ elevation in response to lysophosphatidic acid [54, 63, 68], possibly via the non-selective cation channel [35, 36, 44]. An alternative pathway for PS exposure involving protein kinase C has also been proposed [54, 63], as well as the Ca2+-mediated one. The role of these pathways in RBCs from SCD patients remains to be investigated; however, the lack of effect of EIPA on deoxygenation-induced PS exposure argues against the involvement of the non-specific cation channel.
In summary, we show that PS exposure in RBCs from SCD patients is stimulated by deoxygenation, via a pathway likely consistent with mediation via Psickle. Future experiments will be aimed at establishing the molecular identity of this pathway and the establishment of inhibitors with potential for clinical use.
Acknowledgments
UMC is supported by a BBSRC studentship. We also thank the British Heart Foundation and the Medical Research Council for financial support for the work.
References
- 1.Barber LA, Palascak MB, Joiner CH, Franco RS. Aminophospholipid translocase and phospholipid scramblase activities in sickle erythrocyte subpopulations. Br J Haematol. 2009;146:447–455. doi: 10.1111/j.1365-2141.2009.07760.x. [DOI] [PubMed] [Google Scholar]
- 2.Bennekou P. The voltage-gated non-selective cation channel from human red cells is sensitive to acetylcholine. Biochim Biophys Acta. 1993;1147:165–167. doi: 10.1016/0005-2736(93)90328-W. [DOI] [PubMed] [Google Scholar]
- 3.Bennekou P, Christophersen P. Ion channels. In: Bernhardt I, Ellory JC, editors. Red cell membrane in health and disease. Berlin: Springer; 2003. pp. 139–152. [Google Scholar]
- 4.Bevers EM, Williamson PL. Phospholipid scrambling: an update. FEBS Lett. 2010;584:2724–2730. doi: 10.1016/j.febslet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Bitbol M, Fellmann P, Zachowski A, Devaux PF. Ion regulation of phosphatidylserine and phosphatidylethanolamine outside-inside translocation in human erythrocytes. Biochim Biophys Acta. 1987;904:268–282. doi: 10.1016/0005-2736(87)90376-2. [DOI] [PubMed] [Google Scholar]
- 6.Blumenfeld N, Zachowski A, Galacteros F, Beuzard Y, Devaux PF. Transmembrane mobility of phospholipids in sickle erythrocytes: effect of deoxygenation on diffusion and asymmetry. Blood. 1991;77:849–854. [PubMed] [Google Scholar]
- 7.Boas FE, Forman L, Beutler JA. Phosphatidylserine exposure and red cell viability in red cell ageing and in hemolytic anemia. Proc Natl Acad Sci USA. 1998;95:3077. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanova A, Goede JS, Weiss E, Bogdanov N, Bennekou P, Bernhardt I, Lutz HU. Cryohydrocytosis: increased activity of cation carriers in red cells from a patient with a band 3 mutation. Haematologica. 2010;95:189–198. doi: 10.3324/haematol.2009.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouyer G, Cueff A, Egée S, Kmiecik J, Maksimova Y, Glogowska E, Gallagher PG, Thomas SL. Erythrocyte peripheral benzodiazepine receptor/voltage-dependent anion channels are upregulated by Plasmodium falciparum. Blood. 2011;118:2305–2312. doi: 10.1182/blood-2011-01-329300. [DOI] [PubMed] [Google Scholar]
- 10.Browning JA, Robinson HC, Ellory JC, Gibson JS. Deoxygenation-induced non-electrolyte pathway in red cells from sickle cell patients. Cell Physiol Biochem. 2007;19:165–174. doi: 10.1159/000099204. [DOI] [PubMed] [Google Scholar]
- 11.Bruce LJ, Robinson HC, Guizouarn H, Borgese F, Harrison P, King MJ, Goede JS, Coles SE, Gore DM, Lutz HU, Layton DM, Iolascon A, Ellory JC, Stewart GW. Monovalent cation leaks in human red cells caused by single amino-acid substitutions in the transport domain of the band 3 chloride-bicarbonate exchanger, AE1. Nat Genet. 2005;37:1258–1263. doi: 10.1038/ng1656. [DOI] [PubMed] [Google Scholar]
- 12.Charneski L, Congdon HB. Effects of antiplatelet and anticoagulant medications on the vasoocclusive and thrombotic complications of sickle cell disease: a review of the literature. Am J Health Syst Pharmacol. 2010;67:895–900. doi: 10.2146/ajhp090229. [DOI] [PubMed] [Google Scholar]
- 13.Dalibalta S, Ellory JC, Browning JA, Wilkins RJ, Rees DC, Gibson JS. Novel permeability characteristics of red blood cells from sickle cell patients. Blood Cells Mol Dis. 2010;45:46–52. doi: 10.1016/j.bcmd.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta SK, Guchhait P, Thiagarajan P. Lactadherin binding and phosphatidylserine expression on a cell surface—comparison with annexin A5. Transl Res. 2006;148:19–25. doi: 10.1016/j.lab.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta SK, Thiagarajan P. The role of lactadherin in the phagocytosis of phosphatidylserine-expressing sickle red blood cells by macrophages. Haematologica. 2005;90:1267–1268. [PubMed] [Google Scholar]
- 16.Davies IJT, Musa M, Dormandy TL. Measurements of plasma zinc. J Clin Pathol. 1968;21:359–365. doi: 10.1136/jcp.21.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong K, Larkin SK, Styles LA, Bookchin RM, Kuypers FA. Characterization of the phosphatidylserine-exposing subpopulations of sickle cells. Blood. 2001;98:860–867. doi: 10.1182/blood.V98.3.860. [DOI] [PubMed] [Google Scholar]
- 18.Dunham PB, Ellory JC. Passive potassium transport in low potassium sheep red cells: dependence upon cell volume and chloride. J Physiol. 1981;318:511–530. doi: 10.1113/jphysiol.1981.sp013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duranton SM, Huber SM, Lang F. Oxidation induces a Cl-dependent cation conductance in human red blood cells. J Physiol. 2002;539:847–855. doi: 10.1113/jphysiol.2001.013040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton JW, Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987;70:1245–1266. [PubMed] [Google Scholar]
- 21.Etzion Z, Tiffert T, Bookchin RM, Lew VL. Effects of deoxygenation on active and passive Ca2+ transport and on the cytoplasmic Ca2+ levels of sickle cell anemia red cells. J Clin Investig. 1993;92:2489–2498. doi: 10.1172/JCI116857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franck PFH, Bevers EM, Lubin BH, Comfurius P, Chiu DT-Y, Op den Kamp JAF, Zwaal RF, van Deenen LLM, Roelofsen B. Uncoupling of the membrane skeleton from the lipid bilayer. J Clin Investig. 1985;75:183–190. doi: 10.1172/JCI111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson JS. Oxygen-sensitive cation transport in sickle cells. Blood Cells Mol Dis. 2001;27:112–120. doi: 10.1006/bcmd.2000.0361. [DOI] [PubMed] [Google Scholar]
- 24.Gibson JS, Khan A, Speake PF, Ellory JC. O2 dependence of K+ transport in sickle cells: the effects of different cell populations and the substituted benzaldehyde, 12C79. FASEB J. 2001;15:823–832. doi: 10.1096/fj.00-0177com. [DOI] [PubMed] [Google Scholar]
- 25.Haest CWM. Distribution and movement of membrane lipids. In: Bernhardt I, Ellory JC, editors. Red cell membrane transport in health and disease. Berlin: Springer; 2003. pp. 1–25. [Google Scholar]
- 26.Haest CWM, Plasa G, Kamp D, Deuticke B. Spectrin as a stabilizer of the phospholipid asymmetry in the human erythrocyte membrane. Biochim Biophys Acta. 1978;509:21–32. doi: 10.1016/0005-2736(78)90004-4. [DOI] [PubMed] [Google Scholar]
- 27.Halperin JA, Brugnara C, Tosteson MT, Van Ha T, Tosteson DC. Voltage-activated cation channels in human erythrocytes. Am J Physiol. 1989;257:C986–C996. doi: 10.1152/ajpcell.1989.257.5.C986. [DOI] [PubMed] [Google Scholar]
- 28.Halsted JA, Smith JC. Plasma-zinc in health and disease. Lancet. 1970;1(7642):322–324. doi: 10.1016/S0140-6736(70)90701-4. [DOI] [PubMed] [Google Scholar]
- 29.Hebbel RP. Beyond hemoglobin polymerization: the red blood cell membrane and sickle cell disease pathophysiology. Blood. 1991;77:214–237. [PubMed] [Google Scholar]
- 30.Joiner CH. Deoxygenation-induced cation fluxes in sickle cells: II. Inhibition by stilbene disulfonates. Blood. 1990;76:212–220. [PubMed] [Google Scholar]
- 31.Joiner CH. Cation transport and volume regulation in sickle red blood cells. Am J Physiol. 1993;264:C251–C270. doi: 10.1152/ajpcell.1993.264.2.C251. [DOI] [PubMed] [Google Scholar]
- 32.Joiner CH, Dew A, Ge DL. Deoxygenation-induced fluxes in sickle cells. I. Relationship between net potassium efflux and net sodium influx. Blood Cells. 1988;13:339–348. [PubMed] [Google Scholar]
- 33.Joiner CH, Jiang M, Claussen WJ, Roszell NJ, Yasin Z, Franco RS. Dipyridamole inhibits sickling-induced cation fluxes in sickle red blood cells. Blood. 2001;97:3976–3983. doi: 10.1182/blood.V97.12.3976. [DOI] [PubMed] [Google Scholar]
- 34.Joiner CH, Morris CL, Cooper ES. Deoxygenation-induced cation fluxes in sickle cells. III. Cation selectivity and response to pH and membrane potential. Am J Physiol. 1993;264:C734–C744. doi: 10.1152/ajpcell.1993.264.3.C734. [DOI] [PubMed] [Google Scholar]
- 35.Kaestner L. Cation channels in erythrocytes—historical and future perspective. Open Biol J. 2011;4:27–34. doi: 10.2174/1874196701104010027. [DOI] [Google Scholar]
- 36.Kaestner L, Tabellion W, Lipp P, Bernhardt I. Prostaglandin E2 activates channel-mediated calcium entry in human erythrocytes: an indication of a blood clot formation supporting process. Thromb Haemost. 2004;92:1269–1272. doi: 10.1160/TH04-06-0338. [DOI] [PubMed] [Google Scholar]
- 37.Kamp D, Sieberg T, Haest CWM. Inhibition and stimulation of phospholipid scrambling activity. Consequences for lipid asymmetry, echinocytosis, and microvesiculation of erythrocytes. Biochemistry. 2001;40:9438–9446. doi: 10.1021/bi0107492. [DOI] [PubMed] [Google Scholar]
- 38.Kiedaisch V, Akel A, Niemoeller OM, Wieder T, Lang F. Zinc-induced suicidal erythrocyte death. Am J Clin Nutr. 2008;87:1530–1534. doi: 10.1093/ajcn/87.5.1530. [DOI] [PubMed] [Google Scholar]
- 39.Kirk K. Membrane transport in the malaria-infected erythrocyte. Physiol Rev. 2001;81:495–537. doi: 10.1152/physrev.2001.81.2.495. [DOI] [PubMed] [Google Scholar]
- 40.Kuypers FA. Red cell membrane lipids in hemoglobinopathies. Curr Mol Med. 2008;8:633–638. doi: 10.2174/156652408786241429. [DOI] [PubMed] [Google Scholar]
- 41.Kuypers FA, Lewis RA, Hua M, Schott MA, Discher D, Ernst JD, Lubin BH. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood. 1996;87:1179–1187. [PubMed] [Google Scholar]
- 42.Lang F, Lang KS, Lang PA, Huber SM, Wieder T. Mechanisms and significance of eryptosis. Antioxid Redox Signal. 2006;8:1183–1192. doi: 10.1089/ars.2006.8.1183. [DOI] [PubMed] [Google Scholar]
- 43.Lang F, Lang KS, Wieder T, Myssina S, Birka C, Lang PA, Kaiser S, Kempe DS, Duranton C, Huber SM. Cation channels, cell volume and the death of an erythrocyte. Eur J Physiol. 2003;447:121–125. doi: 10.1007/s00424-003-1150-8. [DOI] [PubMed] [Google Scholar]
- 44.Lang KS, Myssina S, Tanneur V, Wieder T, Huber SM, Lang F, Duranton C. Inhibition of erythrocyte cation channels and apoptosis by ethylisopropylamiloride. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:391–396. doi: 10.1007/s00210-003-0701-z. [DOI] [PubMed] [Google Scholar]
- 45.Lang E, Qadri SM, Lang F. Killing me softly—suicidal erythrocyte death. Int J Biochem Cell Biol. 2012;44:1236–1243. doi: 10.1016/j.biocel.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Lew VL, Bookchin RM. Ion transport pathology in the mechanism of sickle cell dehydration. Physiol Rev. 2005;85:179–200. doi: 10.1152/physrev.00052.2003. [DOI] [PubMed] [Google Scholar]
- 47.Lew VL, Ortiz OE, Bookchin RM. Stochastic nature and red cell population distribution of the sickling-induced Ca2+ permeability. J Clin Investig. 1997;99:2727–2735. doi: 10.1172/JCI119462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindeman RD, Clark ML, Colmore JP. Influence of age and sex on plasma and red-cell zinc concentrations. J Gerontol. 1971;26:358–363. doi: 10.1093/geronj/26.3.358. [DOI] [PubMed] [Google Scholar]
- 49.Lubin B, Chiu D, Bastacky J, Roelofsen B, Van Deenen LLM. Abnormalities in membrane phospholipid organization in sickled erythrocytes. J Clin Investig. 1981;67:1643–1649. doi: 10.1172/JCI110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Y-L, Rees DC, Gibson JS, Ellory JC. The conductance of red blood cells from sickle cell patients: ion selectivity and inhibitors. J Physiol. 2012;590:2095–2105. doi: 10.1113/jphysiol.2012.229609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makhro A, Wang J, Vogel J, Boldyrev AA, Gassman M, Kaestner L, Bogdanova A. Functional NMDA receptors in rat erythrocytes. Am J Cell Physiol. 2010;298:C1315–C1325. doi: 10.1152/ajpcell.00407.2009. [DOI] [PubMed] [Google Scholar]
- 52.Middelkoop E, Lubin B, Bevers EM, Op den Kamp JA, Comfuriius B, Chiu DT, Zwaal RF, van Deenen LLM, Roelofsen B. Studies on sickle erythrocytes provide evidence that the asymmetric distribution of phosphatidylserine in the red cell membrane is maintained by both ATP-dependent translocation and interaction with membrane skeletal proteins. Biochim Biophys Acta. 1988;937:281–288. doi: 10.1016/0005-2736(88)90250-7. [DOI] [PubMed] [Google Scholar]
- 53.Mohandas N, Rossi ME, Clark MR. Association between morphologic distortion of sickle cells and deoxygenation-induced cation permeability increases. Blood. 1986;68:450–454. [PubMed] [Google Scholar]
- 54.Nguyen DB, Wagner-Britz L, Maia S, Steffen P, Wagner C, Kaestner L, Bernhardt I. Regulation of phosphatidylserine exposure in red blood cells. Cell Physiol Biochem. 2011;28:847–856. doi: 10.1159/000335798. [DOI] [PubMed] [Google Scholar]
- 55.Ortiz OE, Lew VL, Bookchin RM. Deoxygenation permeabilizes sickle cell anaemia red cells to magnesium and reverses its gradient in the dense cells. J Physiol. 1990;427:211–226. doi: 10.1113/jphysiol.1990.sp018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prasad AS. Zinc deficiency in patients with sickle cell disease. Am J Clin Nutr. 2002;75:181–182. doi: 10.1093/ajcn/75.2.181. [DOI] [PubMed] [Google Scholar]
- 57.Reed JD, Redding-Lallinger R, Orringer EP. Nutrition and sickle cell disease. Am J Hematol. 1987;24:441–455. doi: 10.1002/ajh.2830240416. [DOI] [PubMed] [Google Scholar]
- 58.Rhoda MD, Apovo M, Beuzard Y, Giraud F. Ca2+ permeability in deoxygenated sickle cells. Blood. 1990;75:2453–2458. [PubMed] [Google Scholar]
- 59.Setty BN, Kulkarini S, Stuart MJ. Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood. 2002;99:1564–1571. doi: 10.1182/blood.V99.5.1564. [DOI] [PubMed] [Google Scholar]
- 60.Shen M-R, Chou C-Y, Chiu WT. Streptomycin and its analogues are potent inhibitors of the hypotonicity-induced Ca2+ entry and Cl− channel activity. FEBS Lett. 2003;554:494–500. doi: 10.1016/S0014-5793(03)01231-6. [DOI] [PubMed] [Google Scholar]
- 61.Shi J, Gilbert GE. Lactadherin inhibits enzyme complexes of blood coagulation by competing for phospholipid-binding sites. Blood. 2003;101:2628–2636. doi: 10.1182/blood-2002-07-1951. [DOI] [PubMed] [Google Scholar]
- 62.Speake PF, Roberts CA, Gibson JS. Effect of changes in respiratory blood parameters on equine red blood cell K-Cl cotransporter. Am J Physiol. 1997;273:C1811–C1818. doi: 10.1152/ajpcell.1997.273.6.C1811. [DOI] [PubMed] [Google Scholar]
- 63.Steffen P, Jung A, Nguyen DB, Muller T, Bernhardt I, Kaestner L, Wagner C. Stimulation of human red blood cells leads to Ca2+-mediated intracellular adhesion. Cell Calcium. 2011;50:54–61. doi: 10.1016/j.ceca.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Stuart MJ, Setty BN. Hemostatic alterations in sickle cell disease: relationships to disease pathophysiology. Pediatr Pathol Mol Med. 2001;20:27–46. [PubMed] [Google Scholar]
- 65.Tosteson DC, Carlsen E, Dunham ET. The effects of sickling on ion transport. I. Effect of sickling on potassium transport. J Gen Physiol. 1955;39:31–53. doi: 10.1085/jgp.39.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss E, Cytlak UM, Rees DC, Osei A, Gibson JS. Deoxygenation-induced and Ca2+-dependent phosphatidylserine externalisation in red blood cells from normal individuals and sickle cell patients. Cell Calcium. 2012;51:51–56. doi: 10.1016/j.ceca.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Wood BL, Gibson DF, Tait JF. Increased erythrocyte phosphatidylserine exposure in sickle cell disease: flow-cytometric measurement and clinical associations. Blood. 1996;88:1873–1880. [PubMed] [Google Scholar]
- 68.Yang L, Andrews DA, Low PS. Lysophosphatidic acid opens a Ca2+ channel in human erythrocytes. Blood. 2000;95:2420–2425. [PubMed] [Google Scholar]