Abstract
Phyllosticta is a geographically widespread genus of plant pathogenic fungi with a diverse host range. This study redefines Phyllosticta, and shows that it clusters sister to the Botryosphaeriaceae (Botryosphaeriales, Dothideomycetes), for which the older family name Phyllostictaceae is resurrected. In moving to a unit nomenclature for fungi, the generic name Phyllosticta was chosen over Guignardia in previous studies, an approach that we support here. We use a multigene DNA dataset of the ITS, LSU, ACT, TEF and GPDH gene regions to investigate 129 isolates of Phyllosticta, representing about 170 species names, many of which are shown to be synonyms of the ubiquitous endophyte P. capitalensis. Based on the data generated here, 12 new species are introduced, while epitype and neotype specimens are designated for a further seven species. One species of interest is P. citrimaxima associated with tan spot of Citrus maxima fruit in Thailand, which adds a fifth species to the citrus black spot complex. Previous morphological studies lumped many taxa under single names that represent complexes. In spite of this Phyllosticta is a species-rich genus, and many of these taxa need to be recollected in order to resolve their phylogeny and taxonomy.
Taxonomic novelties:
New species - Phyllosticta abieticola Wikee & Crous, P. aloeicola Wikee & Crous, P. citrimaxima Wikee, Crous, K.D. Hyde & McKenzie, P. leucothoicola Wikee, Motohashi & Crous, P. mangifera-indica Wikee, Crous, K.D. Hyde & McKenzie, P. neopyrolae Wikee, Motohashi, Crous, K.D. Hyde & McKenzie, P. pachysandricola Wikee, Motohashi & Crous, P. paxistimae Wikee & Crous, P. podocarpicola Wikee, Crous, K.D. Hyde & McKenzie, P. rhaphiolepidis Wikee, C. Nakash. & Crous, P. rubra Wikee & Crous, P. vacciniicola Wikee, Crous, K.D. Hyde & McKenzie; New combinations - P. foliorum (Sacc.) Wikee & Crous, P. philoprina (Berk. & M.A. Curtis) Wikee & Crous; Epitypifications (basionyms) - P. concentrica Sacc., P. cussoniae Cejp, P. owaniana G. Winter; Neotypifications (basionyms) - Phyllosticta cordylinophila P.A. Young, Physalospora gregaria var. foliorum Sacc., Sphaeropsis hypoglossi Mont., Sphaeropsis minima Berk. & M.A. Curtis.
Key words: Botryosphaeriales, foliar pathogens, Guignardia, Phyllosticta, Phyllostictaceae, Multi-Locus Sequence Typing (MLST), systematics
INTRODUCTION
The genus Phyllosticta was introduced by Persoon (1818) with P. convallariae (nom. inval., lacking description) designated as the type species (Donk 1968), which is a synonym of P. cruenta (van der Aa 1973), which van der Aa & Vanev (2002) cited as type of the genus. Species of Phyllosticta are mostly plant pathogens of a broad range of hosts, and responsible for numerous diseases, including leaf and fruit spots. Some of these pathogens cause diseases of significant economic importance, e.g., P. citricarpa, the cause of citrus black spot, which is regarded as a quarantine pest in Europe and the USA (Baayen et al. 2002, Glienke et al. 2011). Other economically important plant pathogenic species include the P. ampelicida species complex that causes black rot disease on grapevines (Wicht et al. 2012), and the P. musarum species complex that causes banana freckle disease (Pu et al. 2008, Wong et al. 2012). Some species of Phyllosticta have also been isolated as endophytes from a wide range of hosts, e.g., P. capitalensis. Other species are regarded as saprobes, e.g., P. carpogena and P. ericae (van der Aa 1973, Baayen et al. 2002, van der Aa & Vanev 2002, Glienke et al. 2011, Wikee et al. 2011). Presently there are approximately 3 340 epithets known for Phyllosticta (www.MycoBank.org; accessed August 2013), but many of these reflect old concepts of the genus, and have since been accommodated elsewhere (van der Aa & Vanev 2002). Many species also produce spermatial or sexual states, which in some cases have been named in Leptodothiorella and Guignardia, respectively (van der Aa 1973).
For many years researchers have confused the generic circumscription of Phoma and Phyllosticta. Both genera were recognised as pycnidial fungi forming unicellular, hyaline conidia. Allescher (1898) separated the two genera based on the infected part of the plant part, with Phyllosticta as foliar pathogens, and Phoma on other plant parts. This concept was further refined by Grove (1935) who regarded Phyllosticta as a parasite and Phoma as saprobe or wound parasite. Seaver (1922) and Grove (1935) separated “Phyllosticta” species based on host preference, as was common taxonomic practice in the 20th century. Seaver (1922) described 300 species, and Grove (1935) approximately 150. In both cases the host plant was the main criterion on which species were separated. Indeed, Seaver’s classification was largely characterised on spore size on host plants, while Grove arranged species under the alphabetically arranged host genera. Many Phyllosticta species were given specific epithets based on the host family, genus or species. For example, P. iridis on Iris versicolor (Iridaceae), P. eugeniae on Eugenia buxifolia (Myrtaceae), P. minor on Vinca minor (Apocynaceae), etc. (Seaver 1922). For the plant pathogenic Phyllosticta species, separation based on host species (or sometimes genus) has proven to be a good method to distinguish species, but this does not hold true for the endophytic or saprobic species.
Viala & Ravaz (1892) introduced Guignardia as a replacement name for Laestadia Auersw. (1869), which was a later homonym of Laestadia Kunth ex Lessing (1832). Viala & Ravaz applied the name only to Sphaeria bidwellii (≡ G. bidwellii), a species that is different from L. alnea, the type species of Laestadia Auersw. (Bissett 1986). Petrak (1957) concluded that G. bidwellii and related species could be accommodated in Botryosphaeria, and Barr (1970, 1972) agreed with Petrak and placed Guignardia and Phyllosticta in Botryosphaeria, and other related species in Discosphaerina.
Punithalingam (1974) suggested that the genus Guignardia must be confined to only those taxa with Phyllosticta morphs as typified by G. bidwellii (= P. ampelicida, see Zhang et al. 2013). He stated that Botryosphaeria usually has larger ascomata and ascospores, and also a multilocular stroma, features that distinguish it from Guignardia. Van der Aa (1973) also pointed out that these two genera had different growth characteristics in culture. Following molecular studies, Schoch et al. (2006) placed Phyllosticta in the Botryosphaeriales. Since Botryosphaeria has been shown to be poly- and paraphyletic, numerous genera have been distinguished in the Botryosphaeriaceae (Crous et al. 2006, Phillips et al. 2008, Liu et al. 2012). With the increasing use of molecular data to link asexual and sexual morphs, and the end of dual nomenclature for fungi (Hawksworth et al. 2011, Wingfield et al. 2012), the oldest, more important and commonly used name, Phyllosticta, was chosen over that of Guignardia (Glienke et al. 2011, Sultan et al. 2011, Wikee et al. 2011, 2013, Wong et al. 2012).
The principal character by which a fungus is recognised as a species of Phyllosticta is by the production of pycnidia containing aseptate, hyaline conidia that are usually covered by a mucoid layer and bearing a single apical appendage (van der Aa 1973). However, the mucoid layer and appendage is not necessarily a universal feature, and some species such as P. colocasiicola, P. minima, and P. sphaeropsoidea lack these features. Furthermore, mucoid appendages formed on agar media may disappear with age, or vary in size and shape when the same isolate is compared on different media, e.g., pine needle agar, oatmeal agar, or potato dextrose agar. Presently Phyllosticta is circumscribed by pycnidia that are usually globose to subglobose, flattened above, and closely connected with the subepidermal pseudostroma. They are mostly unilocular but may be multilocular. The conidia are commonly hyaline, aseptate, ovoid, obovoid to ellipsoid, or short cylindrical, seldomly pyriform, globose or subglobose, and usually covered by a mucoid layer and bearing a single apical appendage (van der Aa 1973). The sexual morph is characterised by erumpent ascomata that are globose to pyriform in section, often irregularly shaped, unilocular, and with a central ostiole. The peridium is thin, comprising a few layers of angular cells. Asci are 8-spored, bitunicate, clavate to broadly ellipsoid, with a wide, obtusely rounded or slightly square apex, tapering gradually to a small pedicel, and with a well-developed ocular chamber. Ascospores are ellipsoid to limoniform, sometimes slightly elongated, aseptate, hyaline, often multiguttulate or with a large central guttule, and may have mucilaginous polar appendages or a sheath. A spermatial state may form in culture. Spermatia are hyaline, aseptate, cylindrical to dumbbell-shaped with guttules at each end (van der Aa 1973).
Phyllosticta s. str. was first monographed by van der Aa (1973), who described and illustrated 46 species, and listed the sexual morphs for 12 species, and the spermatial morphs for 17 based mostly on material collected in Europe and North America. More recently van der Aa & Vanev (2002) revised all species names described in Phyllosticta, and provided a list of 190 accepted epithets, as well as a second list of excluded names that indicated their current disposition if known.
In recent years DNA sequencing of conserved loci has vastly improved our knowledge of fungal phylogeny. Several studies have shown that phylogenetic analysis can resolve the taxonomy and identification of Phyllosticta spp. (Baayen et al. 2002, Wulandari et al. 2009, Glienke et al. 2011, Wikee et al. 2011). Indeed, new species of Phyllosticta are increasingly described based on molecular results (Crous et al. 2012, Wang et al. 2012, Su & Cai 2012, Wong et al. 2012, Zhang et al. 2012).
Phyllosticta was placed in the order Botryosphaeriales by Schoch et al. (2006), who proposed that the Botryosphaeriaceae contained both Botryosphaeria and Phyllosticta, although no support was obtained for this relationship. Crous et al. (2006) and Liu et al. (2012) also classified Phyllosticta in the Botryosphaeriaceae. In both studies it was noted that Phyllosticta was distinct from other genera in the Botryosphaeriaceae, and that these authors eventually expected it to be placed elsewhere. Seaver (1922) used the order Phyllostictales and family Phyllostictaceae for the genus Phyllosticta. The family name Phyllostictaceae (as Phyllostictei) was first proposed by Fries (1849) and accepted by Hawksworth & David (1989). This family name is still available for use, and we suggest that Phyllosticta again be placed in this family, which is sister to the Botryosphaeriaceae (Botryosphaeriales).
Although phylogenetic analysis has become a standard approach in fungal identification, phylogenetic studies should combine both molecular and morphological data to help discriminate taxa (Crous & Groenewald 2005, Hyde et al. 2010). Suitable type material that can be sequenced is not available for many species of fungi, and thus neo- or epitypification is required in order to create a stable and workable taxonomy. The objectives of this study are: (1) to clarify relationships among species of Phyllosticta using multi-gene sequence data [internal transcribed spacer region (ITS), translation elongation factor 1-α gene (TEF1), actin gene (ACT), 28S rRNA gene (LSU) and glyceraldehyde-3-phosphate dehydrogenase gene (GPDH)] combined with morphological characteristics; (2) to provide a phylogenetic backbone for the genus Phyllosticta, and (3) to designate neo- or epitype specimens for fungal isolates that correlate well with original type material, thereby fixing the genetic application of these names.
MATERIAL AND METHODS
Isolates
A global collection of 160 strains of Phyllosticta associated with both leaf spot diseases and healthy leaves of various host plants were studied (Table 1). All isolates were sequenced and analysed together with sequences downloaded from GenBank. If fruit bodies were present on diseased tissue then a single spore isolation procedure as described by Chomnunti et al. (2011) was used to obtain cultures. To obtain isolates of Phyllosticta from diseased leaves of host plants when fruit bodies were not present, the leaf surface was cleaned by wiping with 70 % ethanol. Small pieces of leaf were then cut from the interface between healthy and diseased tissue. These were surface sterilised in 70 % ethanol, washed and plated onto ½ strength potato dextrose agar (½PDA). For isolation of endophytic species, healthy leaves were washed in tap water and wiped with 70 % ethanol. They were then cut into small pieces (about 1 × 1 cm), suspended in 70 % ethanol for 15 min (three times) and washed in distilled water (three times) before placing on ½PDA. All plates were incubated at 27 °C for 1 wk and observed daily. The growing tips of hyphae of Phyllosticta colonies that developed were cut out and transferred to fresh PDA plates. Isolates are deposited in Mae Fah Luang University Culture Collection (MFLUCC) and in the working collection of Pedro Crous housed at the CBS-KNAW Fungal Biodiversity Centre (CBS), Utrecht, The Netherlands (CPC). Other fungal isolates of representative Phyllosticta spp. were obtained from the CBS and added to this study (Table 1).
Table 1.
Phyllosticta isolates investigated in this study.
| Species | Culture no.1 | Host | Country |
GenBank no.2 |
||||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | TEF1 | ACT | GPDH | ||||
| Botryosphaeria obtusa | CMW 8232 | Conifers | South Africa | AY972105 | - | DQ280419 | AY972111 | - |
| Guignardia mangiferae | CPC 17469 | Cymbidium sp. | India | KF206189 | - | - | KF289285 | - |
| IMI 260576 | Mangifera indica | India | JF261459 | KF206222 | JF261501 | JF343641 | JF343748 | |
| CPC 20260 | Arecaceae | Thailand | KF206193 | KF206243 | KF289187 | KF289294 | KF289114 | |
| G. rhodorae | CBS 901.69 | Rhododendron sp. | Netherlands | KF206174 | KF206292 | KF289230 | KF289256 | KF289166 |
| Phyllosticta abieticola | CBS 112067 | Abies concolor | Canada | KF170306 | EU754193 | - | KF289238 | - |
| P. aloeicola | CPC 21020 | Aloe ferox | South Africa | KF154280 | KF206214 | KF289193 | KF289311 | KF289124 |
| CPC 21021 | Aloe ferox | South Africa | KF154281 | KF206213 | KF289194 | KF289312 | KF289125 | |
| CPC 21022 | Aloe ferox | South Africa | KF154282 | KF206212 | KF289195 | KF289313 | KF289126 | |
| CPC 21023 | Aloe ferox | South Africa | KF154283 | KF206211 | KF289196 | KF289314 | KF289127 | |
| CPC 21024 | Aloe ferox | South Africa | KF154284 | KF206210 | KF289197 | KF289315 | KF289128 | |
| P. beaumarisii | CBS 535.87 = IMI 298910 | Muehlenbekia adpressa | Australia | AY042927 | KF306229 | KF289170 | KF306232 | KF289074 |
| P. bifrenariae | CBS 128855 = VIC30556 | Bifrenaria harrisoniae | Brazil | JF343565 | KF206209 | JF343586 | JF343649 | JF343744 |
| CPC 17467 | Bifrenaria harrisoniae | Brazil | KF170299 | KF206260 | KF289207 | KF289283 | KF289138 | |
| P. brazillianiae | CBS 126270 = LGMF330 | Mangifera indica | Brazil | JF343572 | KF206217 | JF343593 | JF343656 | JF343758 |
| LGMF 333 | Mangifera indica | Brazil | JF343574 | KF206216 | JF343595 | JF343658 | JF343760 | |
| LGMF 334 | Mangifera indica | Brazil | JF343566 | KF206215 | JF343587 | JF343650 | JF343752 | |
| P. capitalensis | CBS 173.77 | Citrus aurantiifolia | New Zealand | KF206179 | KF306231 | FJ538393 | KF289244 | KF289100 |
| CBS 226.77 | Paphiopedilum callosum | Germany | FJ538336 | KF206289 | FJ538394 | FJ538452 | JF343718 | |
| CBS 356.52 | Ilex sp. | Unknown | FJ538342 | KF206300 | FJ538400 | FJ538458 | KF289087 | |
| CBS 100175 | Citrus sp. | Brazil | FJ538320 | KF206327 | FJ538378 | FJ538436 | JF343699 | |
| CBS 101228 | Nephelium lappaceum | Hawaii | FJ538319 | KF206325 | FJ538377 | FJ538435 | KF289086 | |
| CBS 114751 | Vaccinium sp. | New Zealand | EU167584 | EU167584 | FJ538407 | FJ538465 | KF289088 | |
| CBS 115046 | Myracrodruon urundeuva | Brazil | FJ538322 | KF206319 | FJ538380 | FJ538438 | KF289082 | |
| CBS 115047 | Aspidosperma polyneuron | Brazil | FJ538323 | KF206318 | FJ538381 | FJ538439 | KF289077 | |
| CBS 115049 | Bowdichia nitida | Brazil | FJ538324 | KF206317 | FJ538382 | FJ538440 | KF289084 | |
| CBS 117118 | Musa acuminata | Indonesia | FJ538339 | JQ743603 | FJ538397 | FJ538455 | KF289090 | |
| CBS 119720 | Musa acuminata | Hawaii | KF206178 | KF206316 | FJ538398 | KF289240 | KF289098 | |
| CBS 120428 | Sansevieria sp. | Netherlands | JN692544 | KF206315 | JN692532 | JN692520 | JN692509 | |
| CBS 123373 | Musa paradisiaca | Thailand | FJ538341 | JQ743604 | FJ538399 | FJ538457 | JF343703 | |
| CBS 123404 | Musa paradisiaca | Thailand | FJ538333 | JQ743601 | FJ538391 | FJ538449 | KF289095 | |
| CBS 128856 | Stanhopea sp. | Brazil | JF261465 | KF206304 | JF261507 | JF343647 | JF343776 | |
| CPC 11337 | Eucalyptus grandis | Brazil | KF206180 | - | - | KF289259 | - | |
| CPC 11867 | Acacia crassicarpa | Thailand | KF206181 | KF206283 | KF289184 | KF289260 | KF289108 | |
| CPC 12157 | Acacia crassicarpa | Thailand | KF206182 | - | - | KF289261 | - | |
| CPC 13987 | Protea repens | Portugal | KF206183 | KF206281 | KF289176 | KF289263 | KF289083 | |
| CPC 14609 | Zyzygium sp. | Madagascar | KF206184 | KF206280 | KF289175 | KF289264 | KF289081 | |
| CPC 16590 | Citrus limon | Argentina | KF206185 | KF206272 | KF289177 | KF289271 | KF289091 | |
| CPC 16591 | Citrus limon | Argentina | KF206186 | KF206271 | KF289179 | KF289272 | KF289093 | |
| CPC 16592 | Citrus limon | Argentina | KF206187 | KF206270 | KF289178 | KF289273 | KF289092 | |
| CPC 17468 | Cymbidium sp. | Brazil | KF206188 | KF206259 | KF289189 | KF289284 | KF289120 | |
| CPC 17748 | Heliconia sp. | Thailand | KF206190 | KF206258 | KF289180 | KF289286 | KF289096 | |
| CPC 18848 | Stanhopea graveolens | Brazil | JF261465 | KF206255 | JF261507 | KF289289 | JF343776 | |
| CPC 20251 | Wild plant | Thailand | KC291333 | KF206252 | KC342553 | KC342530 | KF289101 | |
| CPC 20252 | Punica granatum | Thailand | KC291334 | KF206251 | KC342554 | KC342531 | KF289097 | |
| CPC 20253 | Scheffera venulosa | Thailand | KF206192 | KF206250 | KF289181 | KF289293 | KF289102 | |
| CPC 20254 | Saccharum officinarum | Thailand | KC291335 | KF206249 | KC342555 | KC342532 | KF289103 | |
| CPC 20255 | Arecaceae | Thailand | KC291336 | KF206248 | KC342556 | KC342533 | KF289115 | |
| CPC 20256 | Ophiopogon japonicus | Thailand | KC291337 | KF206247 | KC342557 | KC342534 | KF289089 | |
| CPC 20257 | Ficus benjamina | Thailand | KC291338 | KF206246 | KC342558 | KC342535 | KF289099 | |
| CPC 20258 | Ophiopogon japonicus | Thailand | KC291339 | KF206245 | KC342559 | KC342536 | KF289094 | |
| CPC 20259 | Orchidaceae | Thailand | KC291340 | KF206244 | KC342560 | KC342537 | KF289104 | |
| CPC 20263 | Magnoliaceae | Thailand | KC291341 | KF206241 | KC342561 | KC342538 | KF289085 | |
| CPC 20265 | Euphobiaceae | Thailand | KF206194 | KF206239 | KF289182 | KF289297 | KF289105 | |
| CPC 20266 | Polyscias sp. | Thailand | KC291342 | KF206238 | KC342562 | KC342539 | KF289109 | |
| CPC 20267 | Baccaurea ramiflora | Thailand | KF206195 | KF206237 | KF289173 | KF306233 | KF289078 | |
| CPC 20268 | Hibiscus syriacus | Thailand | KC291343 | KF206236 | KC342563 | KC342540 | KF289117 | |
| CPC 20269 | Ophiopogon japonicus | Thailand | KC291344 | KF206235 | KC342564 | KC342541 | KF289118 | |
| CPC 20270 | Tectona grandis | Thailand | KC291345 | KF206234 | KC342565 | KC342542 | KF289110 | |
| CPC 20271 | Crinum asiaticum | Thailand | KF206196 | KF206233 | KF289183 | KF289298 | KF289106 | |
| CPC 20272 | Orchidaceae | Thailand | KC291346 | KF206232 | KC342566 | KC342543 | KF289079 | |
| CPC 20274 | Mangifera indica | Thailand | KF206197 | KF206231 | KF289188 | KF289299 | KF289119 | |
| CPC 20275 | Polyalthia longifolia | Thailand | KC291347 | KF206230 | KC342567 | KC342544 | KF289107 | |
| CPC 20278 | Euphorbia milii | Thailand | KC291348 | KF206227 | KC342568 | KC342545 | KF289113 | |
| CPC 20423 | Philodendron sp. | Thailand | KC291349 | KF206226 | KC342569 | KC342546 | KF289116 | |
| CPC 20508 | Ixora chinensis | Thailand | KF206198 | KF206225 | KF289185 | KF289302 | KF289111 | |
| CPC 20509 | Cordyline fruticosa | Thailand | KF206199 | KF206224 | KF289186 | KF289303 | KF289112 | |
| CPC 20510 | Pyrrosia adnascens | Thailand | KF206200 | KF206223 | KF289174 | KF289304 | KF289080 | |
| CPC 21035 | Citrus sp. | KF206201 | - | - | KF289305 | - | ||
| LGMF 219 | Citrus sinensis | Brazil | KF206202 | KF206220 | JF261490 | KF289306 | JF343737 | |
| LGMF 220 | Citrus sinensis | Brazil | KF206203 | KF206219 | JF261488 | KF289307 | JF343735 | |
| LGMF 222 | Citrus sinensis | Brazil | KF206204 | KF206218 | JF261492 | KF289308 | JF343739 | |
| P. citriasiana | CBS 120486 | Citrus maxima | Thailand | FJ538360 | KF206314 | FJ538418 | FJ538476 | JF343686 |
| CBS 120487 | Citrus maxima | China | FJ538361 | KF206313 | FJ538419 | FJ538477 | JF343687 | |
| CBS 120488 | Citrus maxima | Thailand | JN692545 | KF206312 | JN692533 | JN692521 | KF289144 | |
| CBS 123370 | Citrus maxima | Vietnam | FJ538355 | KF206310 | FJ538413 | FJ538471 | JF343689 | |
| CBS 123371 | Citrus maxima | Vietnam | FJ538356 | KF206309 | FJ538414 | FJ538472 | JF343690 | |
| CBS 123372 | Citrus maxima | Vietnam | FJ538357 | KF206308 | FJ538415 | FJ538473 | KF289145 | |
| P. citribraziliensis | CBS 100098 | Citrus limon | Brazil | FJ538352 | KF206221 | FJ538410 | FJ538468 | JF343691 |
| CPC 17464 | Citrus sp. | Brazil | KF170300 | KF206263 | KF289224 | KF289280 | KF289159 | |
| CPC 17465 | Citrus sp. | Brazil | KF170301 | KF206262 | KF289225 | KF289281 | KF289160 | |
| CPC 17466 | Citrus sp. | Brazil | KF170302 | KF206261 | KF289226 | KF289282 | KF289161 | |
| P. citricarpa | CBS 102374 | Citrus aurantium | Brazil | FJ538313 | KF206324 | GU349053 | FJ538429 | JF343679 |
| CBS 120489 | Citrus sinensis | Brazil | FJ538315 | KF206311 | FJ538373 | FJ538431 | KF289150 | |
| CBS 127454 | Citrus limon | Australia | JF343583 | KF206306 | JF343604 | JF343667 | JF343771 | |
| CBS 127452 | Citrus reticulata | Australia | JF343581 | KF206307 | JF343602 | KF289241 | JF343769 | |
| CBS 127455 | Citrus sinensis | Australia | JF343584 | KF206305 | JF343605 | JF343668 | JF343772 | |
| CBS 122482 | Citrus sinensis | Zimbabwe | FJ538317 | KF306230 | FJ538375 | KF289265 | KF289146 | |
| CPC 16586 | Citrus limon | Argentina | KF170293 | KF206274 | KF289220 | KF289269 | KF289155 | |
| CPC 16587 | Citrus limon | Argentina | KF170294 | KF206273 | KF289219 | KF289270 | KF289154 | |
| CPC 16603 | Citrus limon | Uruguay | KF170295 | KF206269 | KF289213 | KF289274 | KF289147 | |
| CPC 16604 | Citrus limon | Uruguay | KF206191 | - | - | KF289292 | - | |
| CPC 16605 | Citrus limon | Uruguay | KF170296 | KF206268 | KF289214 | KF289275 | KF289148 | |
| CPC 16606 | Citrus limon | Uruguay | KF170297 | KF206267 | KF289215 | KF289276 | KF289149 | |
| CPC 16609 | Citrus sp. | Argentina | KF170298 | KF206266 | KF289217 | KF289277 | KF289152 | |
| CPC 16149 | Citrus sp. | Argentina | KF170290 | KF206277 | KF289216 | KF289266 | KF289151 | |
| CPC 16151 | Citrus sp. | South Africa | KF170291 | KF206276 | KF289221 | KF289267 | KF289156 | |
| CPC 16152 | Citrus sp. | South Africa | KF170292 | KF206275 | KF289218 | KF289268 | KF289153 | |
| P. citrichinaensis | ZJUCC 200956 | Citrus reticulata | China | JN791620 | - | JN791459 | JN791533 | - |
| ZJUCC 200964 | Citrus maxima | China | JN791611 | - | JN791461 | JN791535 | - | |
| ZJUCC 2010150 | Citrus maxima | China | JN791662 | - | JN791514 | JN791582 | - | |
| ZJUCC 2010152 | Citrus sinensis | China | JN791664 | - | JN791515 | JN791589 | - | |
| P. citrimaxima | CPC 20276 = MFLUCC10-0137 = CBS 136059 | Citrus maxima | Thailand | KF170304 | KF206229 | KF289222 | KF289300 | KF289157 |
| P. concentrica | CBS 937.70 | Hedera helix | Italy | FJ538350 | KF206291 | FJ538408 | KF289257 | JF411745 |
| CBS 134749 = CPC 18842 | Hedera sp. | Spain | KF170310 | KF206256 | KF289228 | KF289288 | KF289163 | |
| P. cordylinophila | CPC 21880 = MUCCJ 521 | Cordyline fruticosa | Japan | AB454357 | AB454357 | - | AB704244 | - |
| CPC 20261 = MFLUCC10-0166 = WK024 | Cordyline fruticosa | Thailand | KF170287 | KF206242 | KF289172 | KF289295 | KF289076 | |
| CPC 20277 = MFLUCC12-0014 = WK048 | Cordyline fruticosa | Thailand | KF170288 | KF206228 | KF289171 | KF289301 | KF289075 | |
| P. cornicola | CBS 111639 | Cornus florida | USA | KF170307 | - | - | KF289234 | - |
| P. cussonia | CPC 13812 | Cussonia sp. | South Africa | KF170311 | KF206282 | KF289223 | KF289262 | KF289158 |
| CPC 14873 | Cussonia sp. | South Africa | JF343578 | KF206279 | JF343599 | JF343662 | JF343764 | |
| CPC 14875 | Cussonia sp. | South Africa | JF343579 | KF206278 | JF343600 | JF343663 | JF343765 | |
| P. elongata | CBS 126.22 | Oxycoccus macrocarpos | USA | FJ538353 | AB095508 | FJ538411 | FJ538469 | KF289164 |
| P. ericarum | CBS 132534 = CPC 19744 | Erica gracilis | South Africa | KF206170 | KF206253 | KF289227 | KF28291 | KF289162 |
| P. eugeniae | CBS 445.82 | Eugenia aromatica | Indonesia | AY042926 | KF206288 | KF289208 | KF289246 | KF289139 |
| P. foliorum | CBS 174.77 | Cryptomeria japonica | USA | KF170308 | KF206290 | KF289200 | KF289245 | KF289131 |
| CBS 447.68 | Taxus baccata | Netherlands | KF170309 | KF206287 | KF289201 | KF289247 | KF289132 | |
| P. gaultheriae | CBS 447.70 | Gaultheria humifusa | USA | JN692543 | KF206298 | JN692531 | KF289248 | JN692508 |
| P. hamamelidis | MUCC 149 | Hamamelis japonica | Japan | KF170289 | - | - | KF289309 | - |
| P. hostae | CGMCC 3.14355 | Hosta plantaginea | China | JN692535 | - | JN692523 | JN692511 | JN692503 |
| CGMCC 3.14356 | Hosta plantaginea | China | JN692536 | - | JN692524 | JN692512 | JN692504 | |
| CGMCC 3.14357 | Hosta plantaginea | China | JN692537 | - | JN692525 | JN692513 | JN692505 | |
| P. hubeiensis | CGMCC 3.14986 | Viburnum odoratissimim | China | JX025037 | - | JX025042 | JX025032 | JX025027 |
| CGMCC 3.14987 | Viburnum odoratissimim | China | JX025038 | - | JX025043 | JX025033 | JX025028 | |
| CGMCC 3.14988 | Viburnum odoratissimim | China | JX025039 | - | JX025044 | JX025034 | JX025029 | |
| P. hymenocallidicola | CBS 131309 | Hymenocallis littoralis | Australia | JQ044423 | JQ044443 | KF289211 | KF289242 | KF289142 |
| CPC 19331 | Hymenocallis littoralis | Australia | KF170303 | KF206254 | KF289212 | KF289290 | KF289143 | |
| P. hypoglossi | CBS 101.72 | Ruscus aculeatus | Italy | FJ538365 | KF206326 | FJ538423 | FJ538481 | JF343694 |
| CBS 167.85 | Ruscus hypoglossum | Italy | FJ538366 | KF206302 | FJ538424 | FJ538482 | JF343696 | |
| CBS 434.92 | Ruscus aculeatus | Italy | FJ538367 | KF206299 | FJ538425 | FJ538483 | JF343695 | |
| P. ilicis-aquifolii | CGMCC 3.14358 | Ilex aquifolium | China | JN692538 | - | JN692526 | JN692514 | - |
| CGMCC 3.14359 | Ilex aquifolium | China | JN692539 | - | JN692527 | JN692515 | - | |
| CGMCC 3.14360 | Ilex aquifolium | China | JN692540 | - | JN692528 | JN692516 | - | |
| P. leucothoicola | MUCC 553 = CBS 136073 | Leucothoe catesbaei | Japan | AB454370 | AB454370 | - | KF289310 | - |
| P. mangifera-indica | CPC 20274 = MFLUCC10-0029 | Mangifera indica | Thailand | KF170305 | KF206240 | KF289190 | KF289296 | KF289121 |
| P. minima | CBS 585.84 = IFO 32917 | Acer rubrum | USA | KF206176 | KF206286 | KF289204 | KF289249 | KF289135 |
| P. neopyrolae | CPC 21879 = MUCC 125 | Pyrola asarifolia | Japan | AB454318 | AB454318 | - | AB704233 | - |
| P. owaniana | CBS 776.97 = CPC 1009 | Brabejum stellatifolium | South Africa | FJ538368 | KF206293 | FJ538426 | KF289254 | JF343767 |
| CPC 14901 | Brabejum stellatifolium | South Africa | JF261462 | KF206303 | JF261504 | KF289243 | JF343766 | |
| P. pachysandricola | MUCC 124 = NBRC 102276 | Pachysandra terminalis | Japan | AB454317 | AB454317 | - | AB704232 | - |
| P. paxistimae | CBS 112527 | Paxistima mysinites | USA | KF206172 | KF206320 | KF289209 | KF289239 | KF289140 |
| P. philoprina | CBS 587.69 | Ilex aquifolium | Spain | KF154278 | KF206297 | KF289206 | KF289250 | KF289137 |
| CBS 616.72 | Ilex aquifolium | Germany | KF154279 | KF206296 | KF289205 | KF289251 | KF289136 | |
| P. podocarpicola | CBS 728.79 | Podocarpus maki | USA | KF206173 | KF206295 | KF289203 | KF289252 | KF289134 |
| P. podocarpi | CBS 111646 | Podocarpus falcatus | South Africa | AF312013 | KF206323 | KC357671 | KC357670 | KF289169 |
| CBS 111647 | Podocarpus lanceolata | South Africa | KF154276 | KF206322 | KF289232 | KF289235 | KF268168 | |
| P. pseudotsugae | CBS 111649 | Pseudotsuga menziesii | USA | KF154277 | KF206321 | KF289231 | KF289236 | KF289167 |
| P. rhaphiolepidis | MUCC 432 | Rhaphiolepis indica | Japan | DQ632660 | - | DQ632724 | AB704242 | - |
| P. rubra | CBS 111635 | Acer rubrum | USA | KF206171 | EU754194 | KF289198 | KF289233 | KF289129 |
| P. sphaeropsoidea | CBS 756.70 = IFO 32905 | Aesculus hippocastanum | Germany | AY042934 | KF206294 | KF289202 | KF289253 | KF289133 |
| P. spinarum | CBS 292.90 | Chamaecyparis pisifera | France | JF343585 | KF206301 | JF343606 | JF343669 | JF343773 |
| P. styracicola | CGMCC 3.14985 | Styrax gradiflorus | China | JX052040 | - | JX025045 | JX025035 | JX025030 |
| CGMCC 3.14989 | Styrax gradiflorus | China | JX052041 | - | JX025046 | JX025036 | JX025031 | |
| P. telopeae | CBS 777.97 | Telopea speciosissima | Tasmania | KF206205 | KF206285 | KF289210 | KF289255 | KF289141 |
| P. vacciniicola | CPC 18590 | Vaccinium macrocarpum | USA | KF170312 | KF206257 | KF289229 | KF289287 | KF289165 |
| P. yuccae | CBS 112065 | Yucca elephantipes | USA | KF206175 | - | - | KF289237 | - |
| Phyllosticta sp. | CPC 11336 | Eucalyptus globulus | Spain | KF206177 | KF206284 | KF289199 | KF289258 | KF289130 |
| MUCC 147 | Rhododendron keiskei | Japan | AB454319 | AB454319 | - | AB704234 | - | |
| CPC 17454 | Mangifera indica | Brazil | KF206206 | KF206265 | KF289192 | KF289278 | KF289123 | |
| CPC 17455 | Mangifera indica | Brazil | KF206207 | KF206264 | KF289191 | KF289279 | KF289122 | |
CPC: Culture collection of P.W. Crous, housed at CBS; IFO: Institute For Fermentation, Osaka, Japan; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; LGMF: Culture collection of Laboratory of Genetics of Microorganisms, Federal University of Parana, Curitiba, Brazil; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands; ZJUCC: Zhejiang University Culture Collection, China; MFLUCC: Mae Fah Luang University Culture Collection; CGMCC: China, General Microbiological Culture Collection, Beijing, China; MUCC: Culture Collection, Laboratory of Plant Pathology, Mie University, Tsu, Mie prefecture, Japan. Type and ex-type cultures are in bold.
ITS: Internal transcribed spacers 1 and 2 together with 5.8S nrDNA; LSU: large subunit 28S nrDNA; TEF1: partial translation elongation factor 1-α gene; ACT: partial actin gene; GPDH: partial glyceraldehyde-3-phosphate dehydrogenase gene.
Morphology
Growth rates, culture characteristics, and morphology of the isolates were determined at 27 °C. Sporulation was induced by growth on pine needle agar (PNA) (Smith et al. 1996) and synthetic nutrient-poor agar (SNA) under near UV-light. Colony colour and growth rate were established on PDA, malt extract agar (MEA) and oatmeal agar (OA) according to Crous et al. (2009). Culture characteristics were assessed, and the colour of upper and lower sides of cultures was determined after 14 d in the dark at 27 °C. Colony colour on MEA, OA and PDA was determined with the colour charts of Rayner (1970). Nomenclatural novelties and descriptions were deposited in MycoBank (www.mycobank.org; Crous et al. 2004).
DNA extraction, amplification, and sequencing
Total genomic DNA was extracted from cultures grown on MEA for 2-3 d using the UltraClean™ Microbial DNA isolation kits (Mo Bio Laboratories, Inc., California, USA) according to the manufacturer’s protocol. Partial regions of five loci were amplified including actin (ACT) using primers ACT-512F and ACT-783R (Carbone & Kohn 1999); the internal transcribed spacer region (ITS) of the nuclear rDNA using primers V9G (de Hoog & Gerrits van den Ende 1998) and ITS4 (White et al. 1990), the 28S large subunit nrDNA (LSU) using primers LROR (Moncalvo et al. 1995) and LR5 (Vilgalys & Hester 1990); translation elongation factor 1-α using primers EF1-728F (Carbone & Kohn 1999) and EF2 (O’Donnell et al. 1998); and glyceraldehyde-3-phosphate dehydrogenase (GPDH) using primers Gpd1-LM and Gpd2-LM (Myllys et al. 2002). For Phyllosticta citricarpa isolates, GPDH was amplified using primers Gpd1 (Guerber et al. 2003) and GPDHR2 (Glienke et al. 2011). The PCR reaction mixtures and cycling conditions were followed as described by Glienke et al. (2011).
Amplified fragments were sequenced in both directions using the same primers pairs used for amplification. For this purpose, the BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems™, Foster City, CA, USA) containing AmpliTaq DNA Polymerase was used. The amplified products were analysed on an automated 3730xl DNA analyzer (Life Technologies Europe BV, Applied Biosystems™, Bleiswijk, The Netherlands). Sequences generated were assembled and aligned using MEGA v. 5.05 (Tamura et al. 2011) and MAFFT v. 6 (http://mafft.cbrc.jp/alignment/server/), respectively. The sequences were manually aligned as necessary.
Molecular phylogeny
Phylogenetic analyses were based on both Maximum Parsimony (MP) and Bayesian inference (BI). The MP analyses were done using PAUP (Phylogenetic Analysis Using Parsimony, v. 4.0b10; Swofford 2003). Phylogenetic relationships were estimated by heuristic searches with 1 000 random addition sequences. Tree bisection-reconnection was used, with the branch swapping option set on “best trees” only with all characters weighted equally and alignment gaps treated as fifth state. Tree length (TL), consistency index (CI), retention index (RI) and rescaled consistence index (RC) were calculated for parsimony and bootstrap analysis (Hillis & Bull 1993) was based on 1 000 replications.
For BI, the best evolutionary models for each partition were determined using MrModeltest (Nylander 2004) and incorporated into the analyses. MrBayes v. 3.2.1. (Ronquist & Huelsenbeck 2003) was used to generate phylogenetic trees under optimal criteria per partition. A Markov Chain Monte Carlo (MCMC) algorithm of four chains was started in parallel from a random tree topology with the heating parameter set at 0.3. The MCMC analysis lasted until the average standard deviation of split frequencies came below 0.01 with trees saved every 1 000 generations. The first 25 % of saved trees were discarded as the “burn-in” phase and posterior probabilities (PP) determined from the remaining trees.
RESULTS
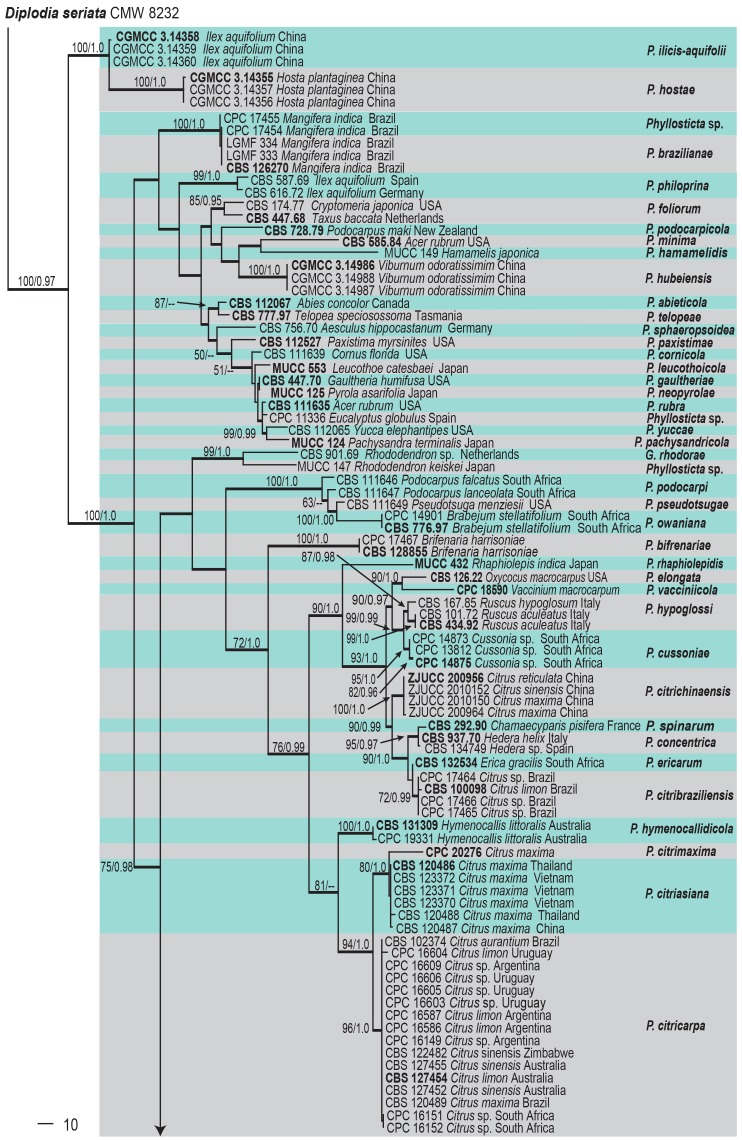
Phylogenetic relationships were determined for the ITS and ACT gene sequences of 160 Phyllosticta strains (including one outgroup). The combined partial dataset of Phyllosticta comprised 883 characters (including gaps), of which 341 characters are constant, and 150 characters are variable and parsimony-uninformative. Parsimony analysis generated 1 000 trees, one of which is presented as shown in Fig. 1 (TL = 2099, CI = 0.481, RI = 0.898, RC = 0.432). The phylogenetic tree of the ITS and ACT region resolved 46 clades (see Table 1 for details). The Bayesian consensus tree confirmed the tree topology and bootstrap support of the strict consensus tree obtained with MP.
Fig. 1.
One of 1 000 equally most parsimonious trees obtained from a heuristic search with 1 000 random taxon additions of the combined ACT and ITS sequence alignments. Bootstrap support values and Bayesian posterior probability values are indicated at the nodes. Branches present in both the consensus trees of the MP and BI are thickened. Substrate and country of origin, where known, are indicated next to the strain numbers. The tree was rooted to Diplodia seriata (CMW 8232)
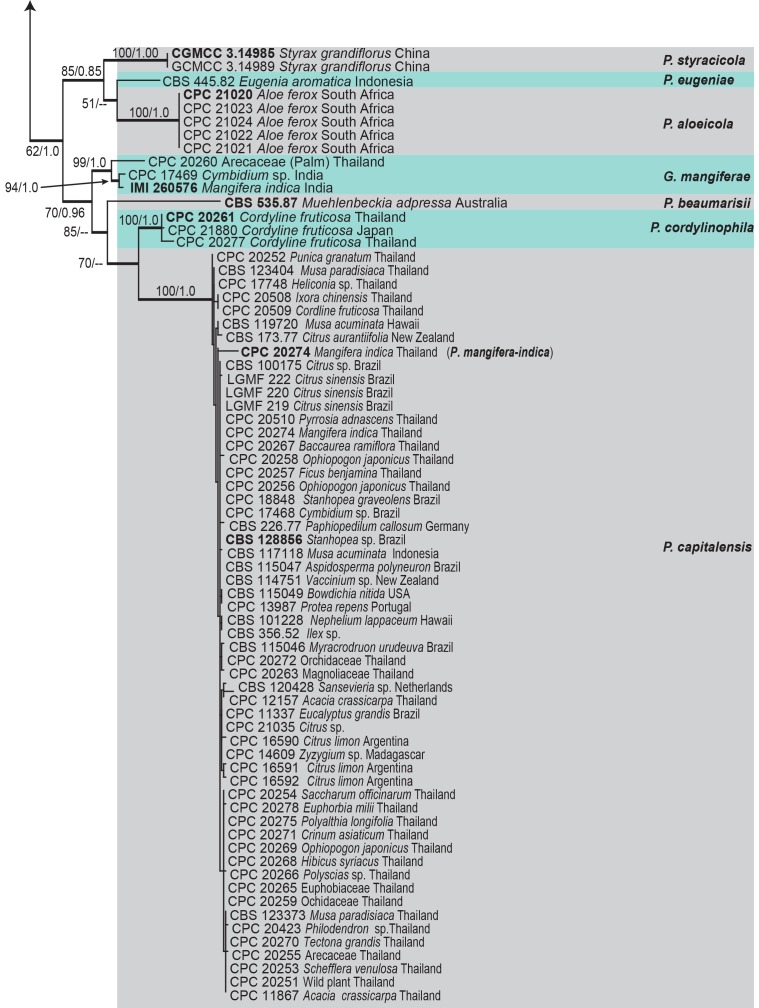
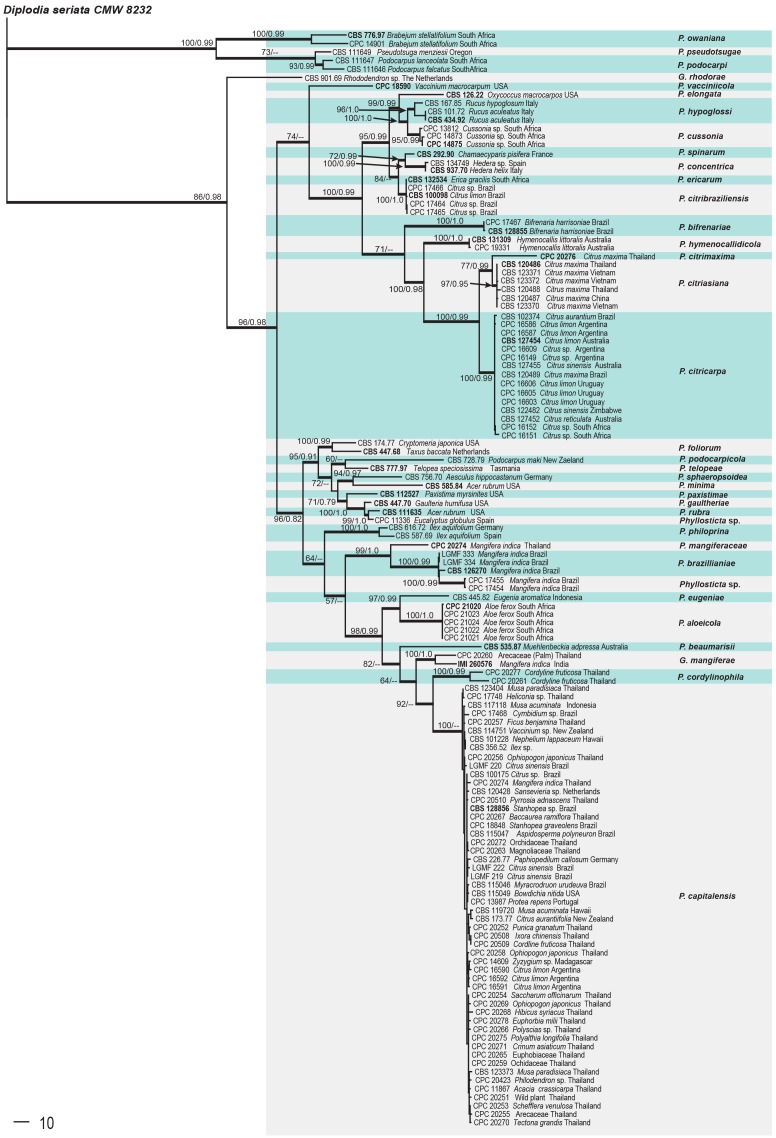
A second analysis including all isolates for which a complete dataset were available (129 strains including the outgroup) was run based on ITS, LSU, ACT, TEF1 and GPDH (Table 1). The combined partial dataset of Phyllosticta comprised 2 577 characters (including gaps), of which 1 547 characters are constant, 296 characters are variable and parsimony-uninformative. Parsimony analysis generated 1 000 trees, of which one is shown in Fig. 2 (TL = 3173, CI = 0.517, RI = 0.906, RC = 0.468). The phylogenetic tree using combined multi-gene data resolved 33 clades (see Table 1 for details). The Bayesian consensus tree confirmed the tree topology and bootstrap support of the strict consensus tree obtained with MP.
Fig. 2.
One of 1 000 equally most parsimonious trees obtained from a heuristic search with 1 000 random taxon additions of the combined ACT, GPDH, ITS, LSU and TEF1 sequence alignments. Bootstrap support values and Bayesian posterior probability values are indicated at the nodes. Branches present in both the consensus trees of the MP and BI are thickened. Substrate and country of origin, where known, are indicated next to the strain numbers. The tree was rooted to Diplodia seriata (CMW 8232).
Taxonomy
Phyllosticta is distinct from members of the Botryosphaeriaceae in cultural characteristics (slow growing, black erumpent colonies vs. grey, fluffy, fast-growing colonies). Morphologically it is also distinct, having conidia encased in a mucoid sheath and often with an apical appendage. The sexual morph has ascomata unilocular, ascospores frequently with mucoid caps, and hamathecial tissue disintegrating at maturity, which collectively differs from those in the Botryosphaeriaceae. Phyllosticta is also phylogenetically supported as distinct from members of the Botryosphaeriaceae (see Slippers et al. 2013, this volume), we choose to place it in the Phyllostictaceae that was originally erected to accommodate this genus.
Phyllostictaceae Fr. (as “Phyllostictei”), Summa veg. Scand., Section Post. (Stockholm): 420. 1849.
Foliicolous, plant pathogenic, endophytic or saprobic. Ascomata pseudothecial, separate to gregarious, globose, brown to black, with a central ostiole. Asci bitunicate, fissitunicate, clavate to subcylindrical, 8-spored, fasciculate, stipitate, with an ocular chamber. Pseudoparaphyses mostly absent at maturity (see Sultan et al. 2013), filamentous, branched, septate when present. Ascospores bi- to triseriate, hyaline, aseptate, ellipsoid-fusoid to limoniform, smooth-walled, usually with mucilaginous caps at ends, or surrounded by a mucilaginous sheath. Asexual morph: Conidiomata pycnidial globose, dark brown, separate to aggregated, with a central ostiole; wall of 3-6 layers of brown textura angularis. Conidiogenous cells lining the inner wall, hyaline, smooth, subcylindrical to ampulliform or doliiform, proliferating percurrently near apex, frequently covered in mucilaginous sheath. Conidia hyaline, smooth, ellipsoid-fusoid to obovoid or ovoid, aseptate, smooth-walled, guttulate or granular, frequently surrounded by a mucilaginous sheath, and bearing a single mucilaginous apical appendage.
Type genus: Phyllosticta Pers.
Phyllosticta Pers., Traité sur les Champignons Comestibles (Paris): 55. 147. 1818.
Conidiomata and spermatogonia pycnidial, immersed, subepidermal to erumpent, unilocular, rarely multilocular, glabrous, ostiolate, dark brown to black; ostiole circular to oval; pycnidial wall of thick-walled, dark brown textura angularis, with inner layers of hyaline to pale brown, thin-walled textura prismatica to angularis. Conidiophores lining the cavity of the conidioma, reduced to conidiogenous cells, invested in mucus. Conidiogenous cells discrete, producing macroconidia and spermatia (also produced in separate spermatogonia), ampulliform, lageniform, doliiform to subcylindrical, hyaline, smooth, proliferating several times percurrently near the apex, invested in a mucoid layer. Spermatogenous cells ampulliform to lageniform or subcylindrical, hyaline smooth, phialidic. Conidia ellipsoid-fusoid to obovoid or ovoid, rarely subcylindrical, aseptate, broadly rounded at the apex, often tapering strongly toward the base, unicellular, hyaline, smooth-walled, guttulate to granular, often enclosed in a persistent mucilaginous sheath, and bearing an unbranched, tapering, straight to curved, mucoid apical appendage. Spermatia hyaline, smooth, granular, subcylindrical or dumbbell-shaped, with rounded or blunt ends. Ascomata pseudothecial, separate to gregarious, globose to subglobose, brown to black, unilocular with a central ostiole. Asci bitunicate, fissitunicate, clavate to subcylindrical, 8-spored, fasciculate, stipitate, with an ocular chamber. Pseudoparaphyses mostly absent at maturity, filamentous, branched, septate when present. Ascospores bi- to triseriate, hyaline, guttulate to granular, aseptate, ellipsoid, ellipsoid-fusoid to limoniform, smooth-walled, usually with mucilaginous caps at ends, or surrounded by a mucilaginous sheath.
Type species: P. convallariae Pers., nom. inval. (= P. cruenta (Fr.) J. Kickx f.)
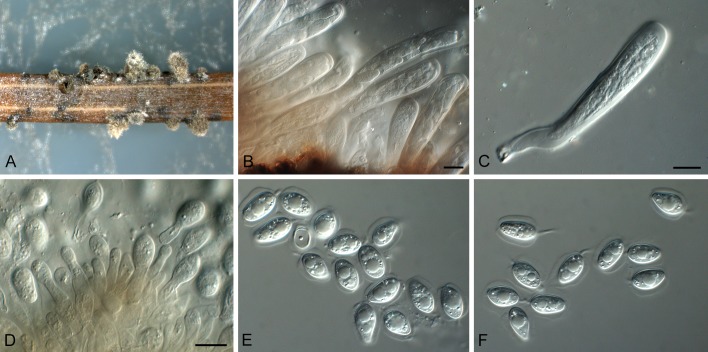
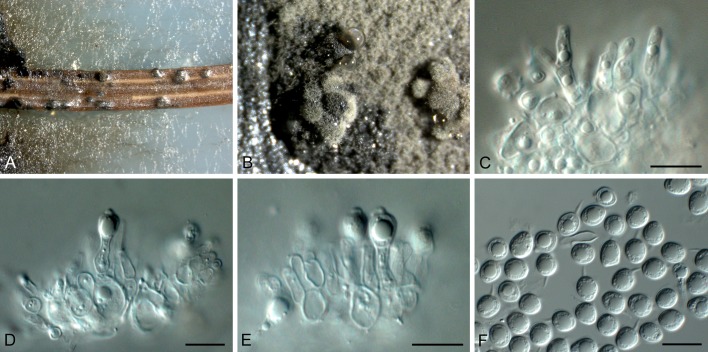
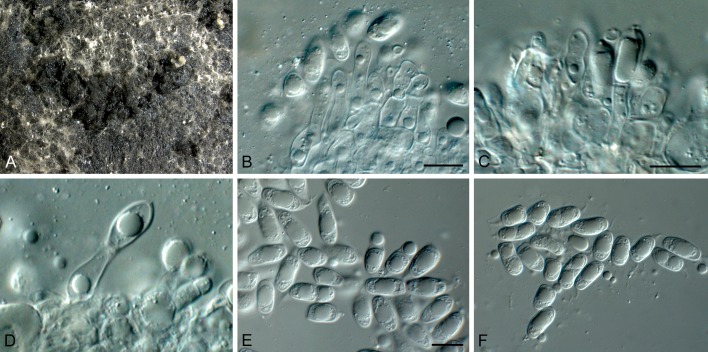
Phyllosticta abieticola Wikee & Crous, sp. nov. MycoBank MB805654. Fig. 3.
Fig. 3.
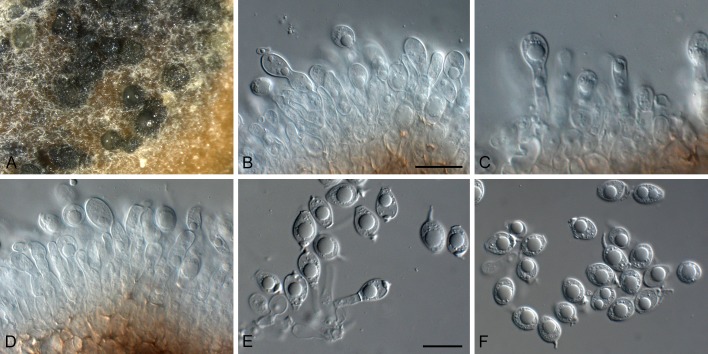
Phyllosticta abieticola (CBS 112067). A. Conidiomata and ascomata forming on PNA. B, C. Asci with ascospores. D. Conidiogenous cells giving rise to conidia. E, F. Conidia with mucoid sheaths and apical appendages. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Abies.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 250 μm diam, elongated in culture on PNA; pycnidial wall of several layers of textura angularis, up to 30 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 15 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1 supporting cell, that can be branched at the base, 10-25 × 4-6 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 7-15 × 3-5 μm; proliferating several times percurrently near apex. Conidia (11-)13-16(-18) × (7-)8 μm, solitary, hyaline, aseptate, thin and smooth-walled, granular, or with a single large central guttule, fusoid-ellipsoid, tapering towards a narrow truncate base, 2-3 μm diam, enclosed in a thin persistent mucoid sheath, 3-4 μm thick, and bearing a hyaline, apical mucoid appendage, (15-)20-25(-30) × 1.5(-2) μm, flexible, unbranched, tapering towards an acutely rounded tip. Ascomata similar to conidiomata in general anatomy. Asci bitunicate, hyaline, clavate to broadly fusoid-ellipsoid, with visible apical chamber, 2 μm diam, 65-120 × 12-15 μm. Ascospores bi- to multiseriate, hyaline, smooth, granular to guttulate, aseptate, straight, rarely curved, widest in the middle, limoniform with obtuse ends, (15-)16-18(-20) × (6-)7 μm.
Culture characteristics: Colonies erumpent, spreading with moderate aerial mycelium, covering dish after 1 mo at 25 °C. On OA surface iron-grey. On PDA and MEA surface grey-olivaceous, reverse iron-grey.
Specimen examined. Canada, on living leaf of Abies concolor, Jan. 2001, M. Forve (holotype CBS H-21389, ex-type culture CBS 112067).
Notes: The present isolate of P. abieticola was originally identified as P. abietis, which is distinguished by having smaller conidia (7-12 × 6.5-9 μm), and a sheath up to 1.5 μm wide, with apical appendages up to 2.5 μm long when present (Bissett & Palm 1989).
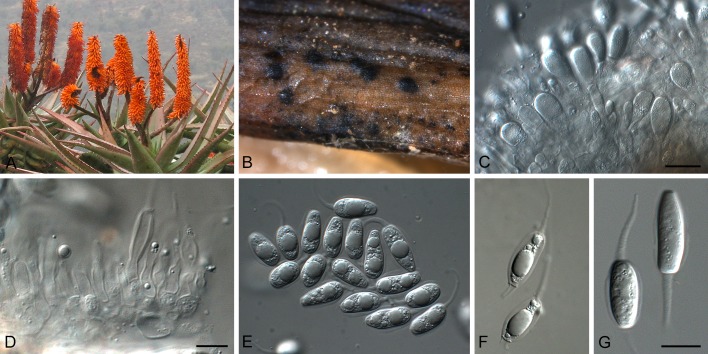
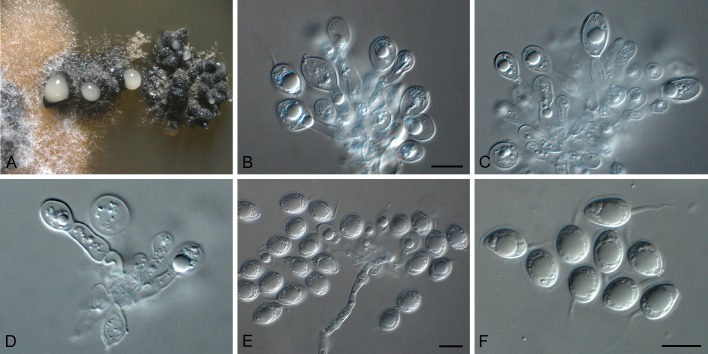
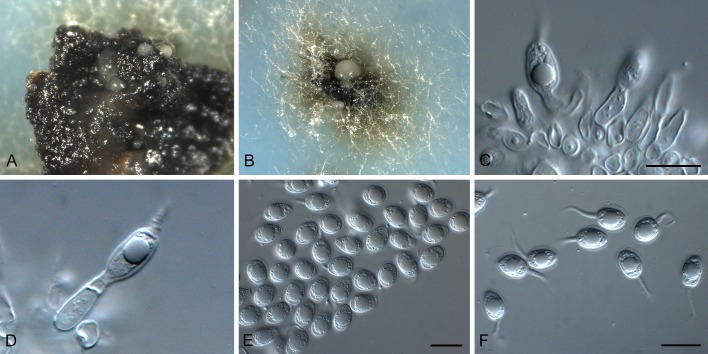
Phyllosticta aloeicola Wikee & Crous, sp. nov. MycoBank MB805655. Fig. 4.
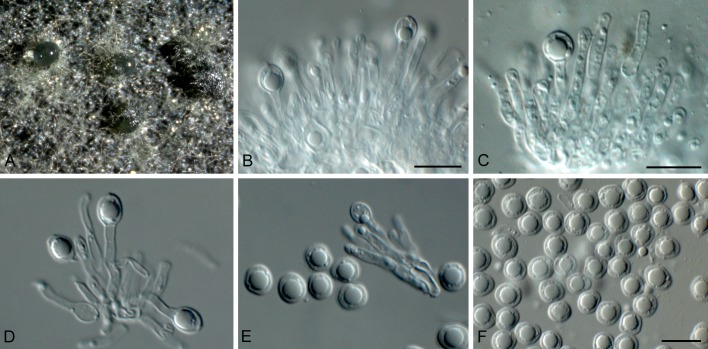
Fig. 4.
Phyllosticta aloeicola (CPC 20677). A. Aloe with dead leaf tips that harbour P. aloeicola. B. Immersed conidiomata on leaf tissue. C, D. Conidiogenous cells giving rise to conidia. E-G. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Aloe.
Associated with leaf tip blight. Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 250 μm diam; pycnidial wall of several layers of textura angularis, up to 40 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 20 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 5-13 × 3-4 μm; proliferating several times percurrently near apex. Conidia (8-)14-18(-27) × (6.5-) 7-8(-9) μm, solitary, hyaline, aseptate, thin and smooth walled, granular, or with a single large central guttule, ellipsoid to obovoid or subcylindrical, tapering towards a narrow truncate base, 3-5 μm diam, enclosed in a thin, persistent mucoid sheath, 1-2 μm thick, and bearing a hyaline, apical mucoid appendage, (7-)15-20(-23) × 2-3(-3.5) μm, flexible, unbranched, tapering towards an acutely rounded tip.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium and feathery margins, covering the dish in 1 mo. On MEA surface olivaceous-grey, reverse iron-grey; on OA and PDA iron-grey on surface and reverse.
Specimen examined: South Africa, Free State Province, Bloemfontein Botanical Garden, Bloemfontein, on living leaf of Aloe ferox, 7 May 2012, P.W. Crous & W.J. Swart (holotype CBS H-21390, culture ex-type CPC 21020 = CBS 136058).
Notes: Phyllosticta aloeicola and P. aloës were both isolated from Aloe latifolia in South Africa. Van der Aa & Vanev (2002) examined the type specimen of P. aloës (deposited in B), and concluded that it was either a Phoma or Asteromella sp.
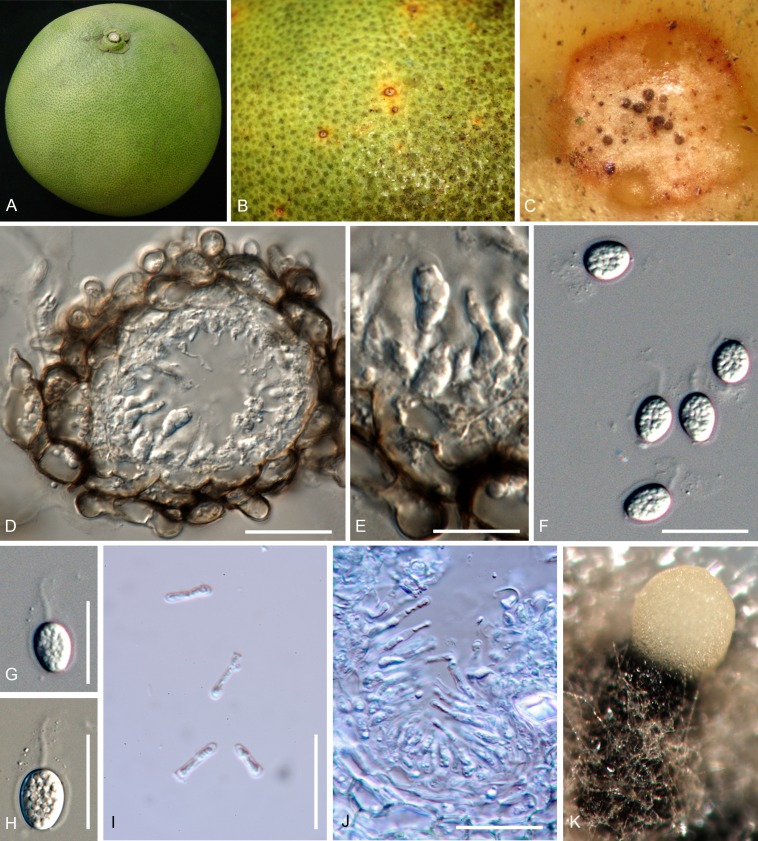
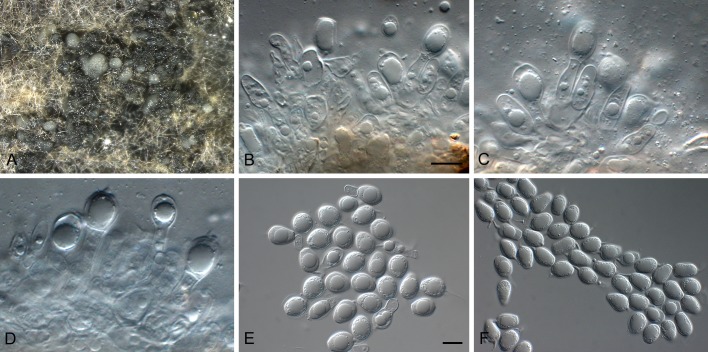
Phyllosticta citrimaxima Wikee, Crous, K.D. Hyde & McKenzie, sp. nov. MycoBank MB803675. Fig. 5.
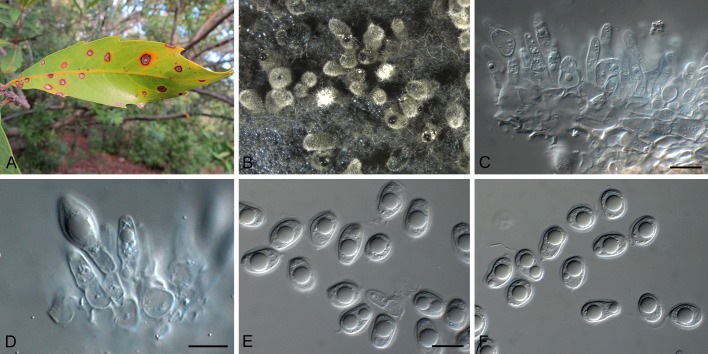
Fig. 5.
Phyllosticta citrimaxima (CPC 20276). A-C. Symtoms on host. D, E. Vertical section through conidioma showing developing conidia. F-H.Conidia. I, J. Spermatial state, spermogonium. K. Conidia produced on OA. Scale bars: D = 30 μm; E-J = 10 μm.
Etymology: Named after this host on which it occurs, Citrus maxima.
Conidiomata pycnidial (on PNA), forming after 4 d of incubation, black, superficial, globose, 150-160 × 120-130 μm; wall 1-3 layers, 20-30 μm thick. Conidiogenous cells developing after 5 d, lining wall of pycnidium, phialidic, cylindrical, hyaline, 3-5 × 1-2 μm. Conidia ellipsoidal, hyaline, 1-celled, smooth, 5(-8) × (3-)4(-7) μm, surrounded by mucilaginous sheath, 1 μm thick, bearing a single, apical appendage, 2-16 μm long.
Culture characteristics: On OA, colonies flat, with irregular margin, initially hyaline with abundant mycelium, gradually becoming greenish after 2-3 d. On MEA colonies woolly, irregular, initially white with abundant mycelium, gradually becoming greenish to dark green after 2-3 d with white hyphae on the undulate margin, eventually turning black; reverse dark green to black. After 25 d in the dark at 27 °C the colony covered the whole plate. On PDA, colonies were flat, rather fast growing, initially white with abundant mycelium, gradually becoming greenish to dark green after 2-3 d, with white hyphae at the margin, eventually turning black; reverse black and after 14 d in the dark at 27 °C colony covered the whole plate.
Specimen examined: Thailand, Chiangrai, Weing Khaen, on fruit peel of Citrus maxima, Jun. 2011, S. Wikee (holotype MFLU 13-0001, ex-type culture CPC 20276 = MFLUCC10-0137 = CBS 136059).
Notes: Phyllosticta citrimaxima was isolated from tan spots on the fruit surface of Citrus maxima, which is grown as an economically important crop in Thailand and Asia. Recently, P. citriasiana, and P. citrichinaensis were described from Citrus maxima in Vietnam and China (Wulandari et al. 2009, Wang et al. 2012), and P. citribraziliensis from Brazil (Glienke et al. 2011). Phyllosticta citrimaxima is well supported phylogenetically (Fig. 1). Wang et al. (2012) provided a table in which they compared the morphology of five Phyllosticta species associated with citrus: P. citricarpa, P. citriasiana, P. capitalensis, P. citribraziliensis, and P. citrichinaensis. Phyllosticta citrimaxima produces smaller conidia (5-8 × 3-7 μm) than P. citricarpa (11-12 × 6-8 μm), P. citriasiana (12-14 × 6-7 μm), P. capitalensis (11-12 × 6-7 μm), P. citribraziliensis (10-12 × 6-7 μm) and P. citrichinaensis (8-12 × 6-9 μm), and has longer apical appendages (2-16 μm) than any of these four species, except P. citrichinaensis (14-26 μm).
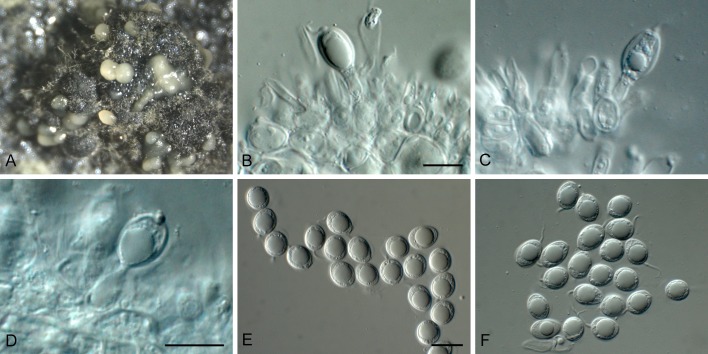
Phyllosticta concentrica Sacc., Nuovo Giorn. Bot. Ital. 8: 203. 1876. Fig. 6.
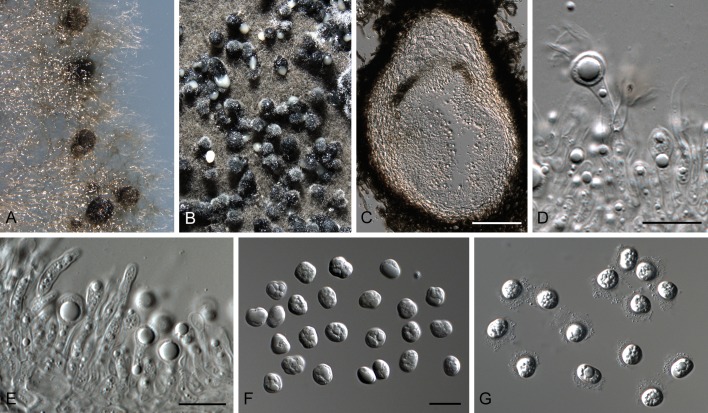
Fig. 6.
Phyllosticta concentrica (CBS 937.70). A. Conidiomata sporulating on OA. B-D. Conidiogenous cells giving rise to conidia. E, F. Conidia. Scale bars = 10 μm.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 400 μm diam, elongated in culture on PNA; pycnidial wall of several layers of textura angularis, up to 30 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 25 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1 supporting cell, that gives rise to 1-2 conidiogenous cells, 12-20 × 4-6 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 7-10 × 3-6 μm; proliferating several times percurrently near apex. Conidia (10-)11-13(-14) × (6-)8(-9) μm, solitary, hyaline, aseptate, thin and smooth-walled, granular, or with a single large central guttule, ellipsoid, tapering towards a narrow truncate base, 2-3 μm diam, enclosed in a thin persistent mucoid sheath, 1-2 μm thick, and bearing a hyaline, apical mucoid appendage, (5-)8-12(-15) × (1-)1.5 μm, flexible, unbranched, tapering towards an acutely rounded tip.
Culture characteristics: Colonies flat, spreading with sparse aerial mycelium, and feathery, lobate margins, reaching 30 mm after 2 wk at 25 °C. On PDA surface greenish black, reverse iron-grey; on OA surface iron-grey; on MEA surface olivaceous-grey in centre, pale olivaceous-grey in outer region, olivaceous-grey underneath.
Specimens examined. Italy, Padua, on withering leaves of Hedera helix, Jul. 1875, syntype (L); Sardegna, Cologne near Oleina, leaf litter of Hedera helix, 31 Aug. 1970, W. Gams (epitype designated here CBS H-16992, culture ex-epitype CBS 937.70; MBT176244). Spain, on living leaf of Hedera sp., 10 Jul. 2010, U. Damm, culture CPC 18842 = CBS 134749.
Notes: Phyllosticta concentrica, and its purported sexual state, Guignardia philoprina, represent different taxa, with each name representing a species complex for which numerous old names are available. Phyllosticta concentrica was originally introduced by Saccardo for a species occurring on Hedera helix in Italy, but which appears to be common in Europe on this host. The present collection closely matches the original description of P. concentrica in morphology, for which an epitype is designated.
Phyllosticta cordylinophila P.A. Young, Bulletin of the Bernice P. Bishop Museum, Honolulu, Hawaii 19: 133. 1925. Fig. 7.
Fig. 7.
Phyllosticta cordylinophila (CPC 20261). A. Conidiomata sporulating on PNA. B. Conidiomata sporulating on OA. C. Conidioma with ostiole (arrowed). D, E. Conidiogenous cells giving rise to conidia. F. Conidia. Scale bars = 10 μm.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless to opaque conidial masses; pycnidia up to 200 μm diam; pycnidial wall of 3-6 layers of textura angularis, up to 40 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 20 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1 supporting cell, at times branched at base, 10-20 × 4-6 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 10-17 × 3-6 μm; proliferating several times percurrently near apex. Conidia (10-)11-13(-15) × 7-8(-11) μm, solitary, hyaline, aseptate, thin and smooth walled, coarsely guttulate, or with a single large central guttule, ellipsoid to obovoid, tapering towards a narrow truncate base, 2-3 μm diam, enclosed in a thin, persistent mucoid sheath, 1-2 μm thick, and bearing a hyaline, apical mucoid appendage, (10-)20-35(-40) × 2(-3) μm, flexible, unbranched, tapering towards an acutely rounded tip.
Culture characteristics: Colonies spreading, erumpent, with sparse aerial mycelium and even, smooth margins. On MEA surface pale olivaceous-grey in centre, dirty white in outer region, reverse iron-grey; on OA olivaceous-grey; on PDA olivaceous-grey on surface and reverse.
Specimens examined: Thailand, Chiangrai, Nang lae, Pasang, on leaf spot of Cordyline fruticosa, Nov. 2011, S. Wikee (neotype designated here CBS H-21391, ex-neotype culture CPC 20261 = WK024 = CBS 136244; MBT176245). Japan, Kagoshima, Amami-Ohshima, Amagi, on C. fruticosa, 22 Oct. 2003, Y. Ono & T. Kobayashi, culture ex-type MUCCJ 521 = CPC 21880 = CBS 136072.
Notes: Van der Aa (1973) did not locate type material, and the material studied by Petrak & Sydow (1927) was depauperate. As the present collections match the morphology of the original species description [conidia ellipsoid to ovoid, 7-12(-15) × 5-7.5(-8) μm], we herewith designate one specimen as neotype.
Phyllosticta cornicola (DC.) Rabenh., Klotzschii Herb. Viv. Mycol., Edn 2: no. 454. 1857. Fig. 8.
Fig. 8.
Phyllosticta cornicola (CBS 111639). A. Conidiomata sporulating on PNA. B. Conidiomata forming on OA. C-E. Conidiophores giving rise to conidia. F. Conidia. Scale bars = 10 μm.
Basionym: Sphaeria lichenoides var. cornicola DC., in de Candolle & Lamarck, Fl. franç., Edn 3 (Paris) 6: 148. 1815.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 200 μm diam; pycnidial wall of several layers of textura angularis, up to 30 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 10 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1-2 supporting cells, that can be branched at the base, 10-20 × 4-5 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 7-12 × 2.5-4 μm; proliferating several times percurrently near apex. Conidia (6-)7-8 × (5.5-)6(-7) μm, solitary, hyaline, aseptate, thin and smooth walled, granular, or with a single large central guttule, ellipsoid to obovoid, tapering towards a narrow truncate base, 2-3 μm diam, enclosed in a thin persistent mucoid sheath, 1 μm thick, and bearing a hyaline, apical mucoid appendage, (3-) 4-5(-7) × 1(-1.5) μm, flexible, unbranched, tapering towards an acutely rounded tip.
Culture characteristics: Colonies erumpent, spreading with moderate aerial mycelium and feathery, lobate margins, covering dish after 1 mo at 25 °C. On OA, MEA and PDA surface olivaceous-grey, reverse iron-grey.
Specimen examined. USA, on living leaf of Cornus florida, Jul. 1999, G. Carroll, CBS H-21392, culture CBS 111639.
Notes: The name P. cornicola is based on European collections (Cornus sanguinea, Czech Republic), and until fresh European material has been collected, we cannot be sure that the name is authentic for this taxon.
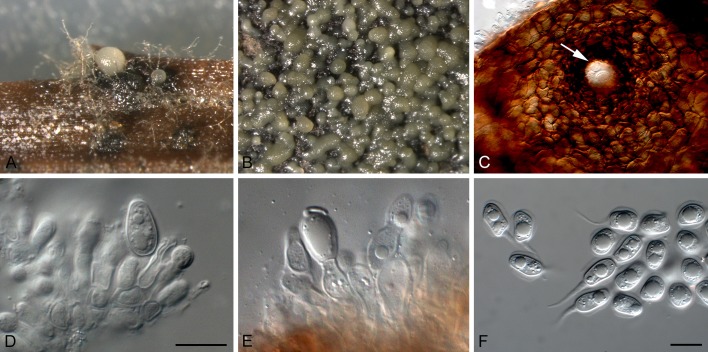
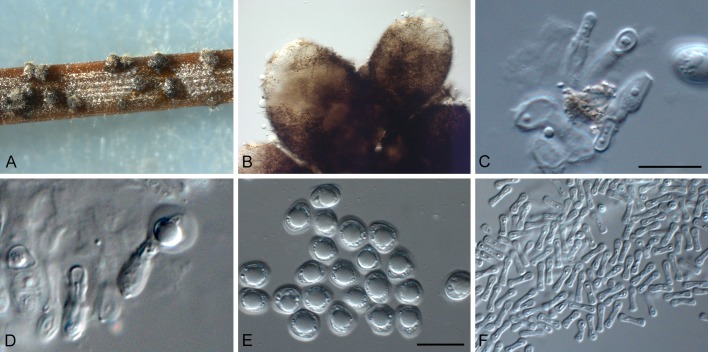
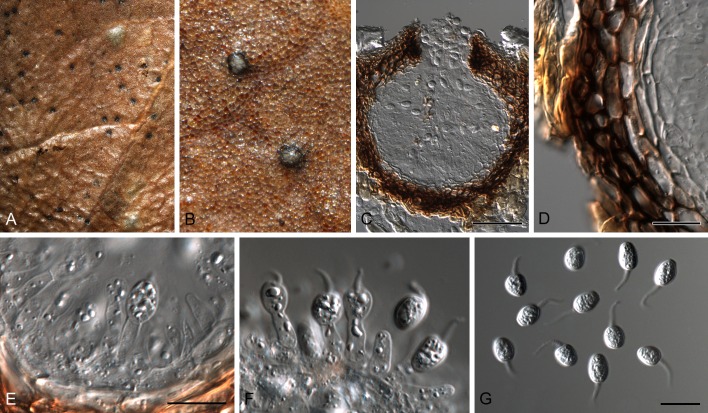
Phyllosticta cussoniae Cejp, Bothalia 10: 341. 1971. Fig. 9.
Fig. 9.
Phyllosticta cussoniae (CPC 14873). A. Symptomatic leaf of Cussonia sp. B. Conidiomata forming on PNA. C. Conidiomata sporulating on OA. D, E. Conidiogenous cells giving rise to conidia. F. Conidia. G. Spermatia. Scale bars = 10 μm.
Leaf spots amphigenous, subcircular, pale to medium brown, 0.5-1 cm diam, frequently surrounded by a red-purple margin. Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless to opaque conidial masses; pycnidia up to 200 μm diam; pycnidial wall of several layers of textura angularis; inner wall of hyaline textura angularis. Ostiole central, up to 20 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1-2 supporting cells, branched at base, 10-25 × 3-5 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 5-10 × 3-4 μm; proliferating several times percurrently near apex. Conidia (10-)12-15(-17) × (6-)7(-8) μm, solitary, hyaline, aseptate, thin and smooth walled, coarsely guttulate, or with a single large central guttule, ellipsoid to obovoid, tapering towards a narrow truncate base, 3-4 μm diam, enclosed in a thin, persistent mucoid sheath, 2-4 μm thick, and bearing a hyaline, apical mucoid appendage, (8-)10-12(-13) × 2(-3) μm, flexible, unbranched, tapering towards an acutely rounded tip. Spermatia occurring in same conidioma with conidia, hyaline, smooth, guttulate to granular, bacilliform, 7-10 × 2-3 μm.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium and feathery margins, covering the dish in 1 mo. On MEA surface olivaceous-grey, reverse iron-grey; on OA iron-grey; on PDA iron-grey on surface and reverse.
Specimens examined. South Africa, Mpumalanga, Schagen, Nelspruit, on Cussonia umbellifera, 25 Dec. 1933, L.C.C. Liebenberg, holotype PREM 32871; Eastern Cape, Graaff Reinet, Valley of Desolation, on leaf spot of Cussonia sp., 9 Jan. 2008, P.W. Crous (epitype designated here CBS H-21393, cultures ex-epitype CPC 14874, 14873 = CBS 136060; MBT176246); Gauteng, Walter Sisulu National Botanical Garden, on leaves of Cussonia sp., 2 Mar. 2007, P.W. Crous, cultures CPC 13812-13813.
Notes: Phyllosticta cussoniae occurs commonly on various Cussonia spp. throughout South Africa, where it causes a prominent leaf spot disease. All isolates collected from the various provinces where this host occurs, appear to have the same species (based on DNA sequence data) associated with the disease.
Phyllosticta foliorum (Sacc.) Wikee & Crous, comb. nov. MycoBank MB805656. Fig. 10.
Fig. 10.
Phyllosticta foliorum (CBS 447.68). A. Colony sporulating on MEA. B-D. Conidiogenous cells giving rise to conidia. E, F. Conidia. Scale bars = 10 μm.
Basionym: Physalospora gregaria var. foliorum Sacc., Syll. fung. (Abellini) 1: 435. 1882.
≡ Pyreniella foliorum (Sacc.) Theiss., Annls mycol. 14(6): 411. 1917 (1916).
≡ Melanops foliorum (Sacc.) Petr. (as “foliicola”), Kryptogamenflora Forsch. Bayer. Bot. Ges. Erforsch Leim. Flora 2(2): 165. 1931.
≡ Botryosphaeria foliorum (Sacc.) Arx & E. Müll., Beitr. Kryptfl. Schweiz 11(no. 1): 42. 1954.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose or with elongated body, exuding colourless to opaque conidial masses; pycnidia up to 400 μm diam; pycnidial wall of several layers of textura angularis; inner wall of hyaline textura angularis. Ostiole central, up to 40 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1-2 supporting cells, branched at base or not, 10-25 × 4-5 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 8-20 × 3-4 μm; proliferating several times percurrently near apex. Conidia (12-)13-14(-15) × (9-)10(-11) μm, solitary, hyaline, aseptate, thin and smooth walled, coarsely guttulate, or with a single large central guttule, broadly ellipsoid, tapering towards a narrow truncate base, 2-3 μm diam, enclosed in a thin, persistent mucoid sheath, 2-3 μm thick, and bearing a hyaline, apical mucoid appendage, (10-)12-15(-20) × 1.5(-2) μm, flexible, unbranched, tapering towards an acutely rounded tip.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium and feathery margins, covering the dish in 1 mo. On MEA surface olivaceous-grey, reverse iron-grey; on OA iron-grey; on PDA iron-grey on surface and reverse.
Specimens examined. Italy, on fallen leaves of Taxus baccata, holotype of Physalospora gregaria var. foliorum, Herb. P.A. Saccardo, PAD. Netherlands, Baarn, Maarschalksbos, on dead twigs and needles of Taxus baccata, Sep. 1969, H.A. van der Aa (neotype designated here CBS H-9495, culture ex-neotype CBS 447.68). USA, from bonsai tree of Cryptomeria japonica, 25 Feb. 1977, G.H. Boerema, specimens CBS H-13111, CBS H-619, culture CBS 174.77.
Notes: Guignardia philoprina (from Ilex) is a species complex with numerous old names. The oldest name linked to European specimens from Taxus appears to be Physalospora gregaria var. foliorum, which we recombine in Phyllosticta. As the holotype specimen in PAD only contains immature ascomata and spermatia, a neotype is herewith designated.
Phyllosticta hypoglossi (Mont.) Allesch., Rabenh. Krypt.-Fl., Edn 2 (Leipzig) 1(6): 163. 1898. Fig. 11.
Fig. 11.
Phyllosticta hypoglossi (CBS 434.92). A. Colony sporulating on OA. B-D. Conidiogenous cells giving rise to conidia. E, F. Conidia. Scale bars = 10 μm.
Basionym: Sphaeropsis hypoglossi Mont., Annls Sci. Nat., Bot., sér. 3 12: 307. 1849.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 200 μm diam; pycnidial wall of several layers of textura angularis, up to 30 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 15 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1-2 supporting cells, that can be branched at the base, 15-25 × 4-5 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 10-15 × 3-5 μm; proliferating several times percurrently near apex. Conidia (10-)11-12(-14) × (9-)10(-11) μm, solitary, hyaline, aseptate, thin and smooth walled, granular, or with a single large central guttule, broadly ellipsoid to obovoid or globose, tapering towards a narrow truncate base, 3-4 μm diam, enclosed in a thin, mucoid sheath, 1-3 μm thick, mostly not persistent, and bearing a hyaline, apical mucoid appendage, (8-)10-12(-15) × 1.5(-2) μm, flexible, unbranched, tapering towards an acute tip.
Culture characteristics: Colonies flat, spreading with sparse aerial mycelium and feathery, lobate margins, reaching 25 mm diam on MEA, 30 mm diam on PDA and 35 mm diam on OA after 2 wk at 25 °C. On OA centre olivaceous-grey, outer zone with diffuse pale yellow pigment in agar. On PDA surface olivaceous-grey, reverse iron-grey. On MEA surface iron-grey in centre, pale grey-olivaceous in outer region, iron-grey in reverse.
Specimens examined: France, near Marseille, on cladodes of Ruscus hypoglossum, 1845, J.L.M. Castagne, (type not found, presumably missing). Italy, Prov. Napoli, Cratere degli Astroni, on dead cladodes of Ruscus aculeatus, May 1992, W. Gams (neotype designated here CBS H-5331; ex-neotype culture CBS 434.92; MBT176248).
Notes: Judging from the number of specimens and cultures in the CBS collection, P. hypoglossi is a common European species on cladodes of Ruscus hypoglossum. The morphology of the neotype closely matches that described in the original description.
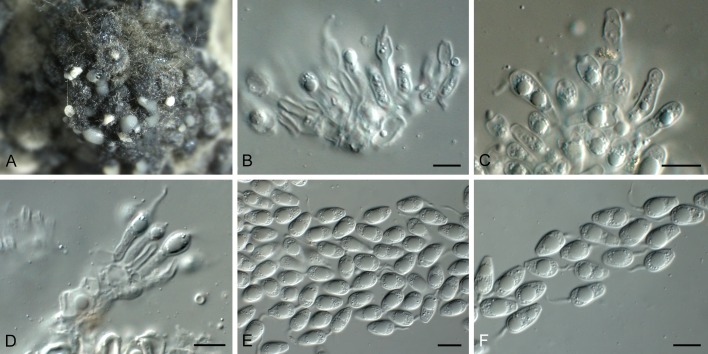
Phyllosticta leucothoicola Wikee, Motohashi & Crous, sp. nov. MycoBank MB805657. Fig. 12.
Fig. 12.
Phyllosticta leucothoicola (MUCC 553). A. Conidiomata forming on PNA. B. Conidiomata. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. F. Spermatia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Leucothoe.
Leaf spots purple-brown, scattered, enlarged and becoming confluent, subcircular to oblong, with brown to dark brown border (Takeuchi & Horie 1998). Conidiomata (on PNA) pycnidial, mostly aggregated in clusters, black, erumpent, globose to clavate or elongated with necks up to 500 μm long, exuding colourless to opaque conidial masses; pycnidia up to 300 μm diam; pycnidial wall of several layers of textura angularis, up to 40 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 15 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1 supporting cell, 6-20 × 3-4 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 6-15 × 3-4 μm; proliferating several times percurrently near apex. Conidia (6-)7-8(-9) × 6(-7) μm, solitary, hyaline, aseptate, thin and smooth walled, coarsely guttulate, or with a single large central guttule, ovoid to irregularly ellipsoid, at times enclosed in a thin mucoid sheath, up to 1.5 μm thick; apical mucoid appendage not seen. Spermatia developing in same conidioma as conidia, bacilliform, smooth, hyaline, guttulate, 5-7 × 2-3 μm.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium and feathery margins, covering the dish in 1 mo. On MEA surface olivaceous-grey, reverse iron-grey; on OA iron-grey; on PDA iron-grey on surface and reverse.
Specimen examined: Japan, Tokyo, on living leaf of Leucothoe catesbaei, May 1996, J. Takeuchi (holotype CBS H-21394, ex-type culture MUCC 553 = CPC 21881 = CBS 136073).
Notes: Phyllosticta leucothoës has been described from Leucothoe acuminata, although van der Aa & Vanev (2002) transferred this to Fusicoccum based on an examination of type material. Phyllosticta leucothoicola represents a distinct taxon on L. catesbaei, corroborating the morphological differences noted by Motohashi et al. (2009).
Phyllosticta mangifera-indica Wikee, Crous, K.D. Hyde & McKenzie, sp. nov. MycoBank MB805657. Fig. 13.
Fig. 13.
Phyllosticta mangifera-indica (CPC 20274). A. Healthy leaf of Mangifera indica. B. Isolation on WA. C. Culture sporulating on OA. D. Culture sporulating on SNA. E. Vertical section through a conidioma showing developing conidia. F-I. Appressoria. J, K. Conidia. L. Culture on MEA. Scale bars: E = 100 μm, F-K = 10 μm.
Etymology: Named after the host genus on which it occurs, Mangifera indica.
Conidiomata pycnidial (on PNA), initially forming after 4 d of incubation, black, superficial, subglobose or ellipsoidal, 220-300 × 160-180 μm; wall of 1-3 layers of brown textura angularis, 20-30 μm thick. Conidiogenous cells lining the inner wall, phialidic, cylindrical, hyaline, 3-5 × 3-4 μm. Conidia ellipsoidal, hyaline, aseptate, smooth, (6-)9(-13) × (4-)5(-6) μm, surrounded by mucilaginous sheath, 0.5-2 μm thick, bearing single apical appendage, 3-14 μm long.
Culture characteristics: On OA colonies appeared flat, with irregular margins, initially hyaline with abundant mycelium, gradually becoming greenish after 2-3 d. On MEA, colonies woolly, irregular, initially white with abundant mycelium, gradually becoming greenish to dark green after 2-3 d, with white hyphae at the undulate margin, eventually turning black; reverse dark green to black. After 25 d in the dark at 27 °C colony covering the whole plate. On PDA colonies flat, rather fast growing, initially white with abundant mycelium, gradually becoming greenish to dark green after 2-3 d with white hyphae at the margin, eventually turning black; reverse black and after 14 d in the dark at 27 °C colony covering the whole plate.
Specimen examined: Thailand, Chiangrai, Nanglae, on healthy leaf of Mangifera indica, July 2011, S. Wikee (holotype MFU13-0108; ex-type culture CPC 20274 = MFLUCC10-0029 = CBS 136061).
Notes: Phyllosticta mangifera-indica was isolated as an endophyte from a healthy leaf of Mangifera indica. Several species have been reported as pathogens on M. indica including G. mangiferae and P. brazilianiae (Glienke et al. 2011). Phyllosticta mangifera-indica produced abundant conidia on OA and formed appressoria within 2 d. Morphologically, it is distinct from P. capitalensis (conidia 8-11 × 5-6 μm) in having longer conidia (conidia 6-13 × 4-6), and represents a distinct lineage with 99 % bootstrap support with the inclusion of TEF1 and GPDH sequence data. It is phylogenetically distinct from P. mangiferae, and most closely related to P. brazilianiae, which occurs on the same host.
Phyllosticta minima (Berk. & M.A. Curtis) Underw. & Earle, Bull. Alabama Agric. Exp. Stn. 80: 168. 1897. Fig. 14.
Fig. 14.
Phyllosticta minima (CBS 585.84). A. Colony sporulating on MEA. B-E. Conidiogenous cells giving rise to conidia. F. Conidia. Scale bars = 10 μm.
Basionym: Sphaeropsis minima Berk. & M.A. Curtis, N. Amer. Fungi: no. 418. 1874.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 180 μm diam; pycnidial wall of several layers of textura angularis, up to 30 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 15 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1-2 supporting cells, that can be branched at the base, 15-50 × 5-6 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 8-20 × 3-4 μm; proliferating several times percurrently near apex. Conidia (9-)10-11(-12) × (6-)7(-8) μm, solitary, hyaline, aseptate, thin and smooth walled, granular, or with a single large central guttule, broadly ellipsoid to obovoid or globose, tapering towards a narrow truncate base, 2-3 μm diam, enclosed in a thin mucoid sheath, absent at maturity or 1 μm thick, and bearing a hyaline, apical mucoid appendage, 6-7(-10) × 1.5(-2) μm, flexible, unbranched, tapering towards an acute tip.
Culture characteristics: Colonies flat, spreading with sparse aerial mycelium and feathery, lobate margins, reaching 15 mm diam on MEA, 40 mm diam on PDA and 8 mm diam on OA after 2 wk at 25 °C. On OA surface olivaceous-grey. On PDA surface and reverse iron-grey. On MEA surface olivaceous-grey with patches of pale luteus.
Specimens examined: USA, North Dakota, New England, on Acer rubrum, R. Sprague 5314 (holotype not found); Tennessee, Gatlinburg, Great Smoky Mountains National Park, on leaf spot of Acer rubrum, June 1984, D.H. Defoe (neotype designated here CBS H-17023; ex-neotype culture CBS 585.84 = IFO 32917; MBT176250).
Note: This taxon appears to be common on Acer spp. in the USA, where it is associated with leaf spots (Bissett & Darbyshire 1984). The holotype could not be located in NY, LCR, IMI, S, K or BPI, and thus a neotype (from the original host in the USA) is designated.
Phyllosticta neopyrolae Wikee, Motohashi, Crous, K.D. Hyde & McKenzie, sp. nov. MycoBank MB803676. Fig. 15.
Fig. 15.
Phyllosticta neopyrolae (CBS 134750). A. Colony sporulating on OA. B-E. Conidiogenous cells giving rise to conidia. F. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus on which it occurs, Pyrola.
Leaf spots orbicular to ellipsoid, black. Conidiomata (on PNA) pycnidial, epiphyllous, sparse, solitary or aggregated, immersed at first, then erumpent breaking through the epidermis, brown to dark brown, subglobose, 60-100 × 60-113 μm; pycnidial wall composed of the depressed or irregular cells in 1-4 layers, brown to dark brown, hyaline or paler toward the inside, with a central ostiole, up to 10 μm diam. Conidiophores subcylindrical, reduced to conidiogenous cells, or with 1-2 supporting cells, branched at the base, 15-20 × 2-3 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 8-15 × 2-3 μm; proliferating several times percurrently near apex. Conidia (6-)7(-8) × (5-)6(-7) μm, solitary, hyaline, aseptate, thin and smooth walled, granular, or with a single large central guttule, broadly ellipsoid to globose, mucoid sheath and appendage lacking.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium and feathery margins, covering the dish in 1 mo. On MEA surface olivaceous-grey, reverse iron-grey; on OA iron-grey; on PDA iron-grey on surface and reverse.
Specimen examined. Japan, Nagano, Sugadaira, on living leaf of Pyrola asarifolia subsp. incarnata, 17 June 2006, T. Hosoya (holotype TFM: FPH 7887, isotype CBS H-21395, ex-type culture MUCC 125 = CPC 21879 = CBS 134750).
Notes: Two species of Phyllosticta are known from Pyrola spp., namely P. pyrolae Ellis & Everh. and P. pyrolae (Ehrenb.: Fr) Allesch. Of these, the latter species is an illegitimate homonym, with morphological characteristics (conidia 3-4 μm long) that indicate that it should be excluded from Phyllosticta s. str. (van der Aa & Vanev 2002). The other species, P. pyrolae Ellis & Everh. (conidia ovoid to globose, 4.5-7.5 × 4-9 μm, with mucoid layer and an apical appendage) resembles P. neopyrolae. Phyllosticta neopyrolae differs from these two species by having conidia that lack a mucoid sheath and apical appendage.
Phyllosticta owaniana G. Winter, Hedwigia 24: 31. 1885. Fig. 16.
Fig. 16.
Phyllosticta owaniana (CBS 776.97). A. Symptomatic leaf of Brabejum stellatifolium. B. Colony sporulating on OA. C, D. Conidiogenous cells giving rise to conidia. E, F. Conidia. Scale bars = 10 μm.
Leaf spots amphigenous, irregular to subcircular, pale to medium brown, turning greyish with age, surrounded by a broad purplish border, and chlorotic margin. Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 300 μm diam, frequently with elongated neck on OA and MEA; pycnidial wall of several layers of textura angularis, up to 30 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 10 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1-2 supporting cells, that can be branched at the base, 10-30 × 4-5 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 10-25 × 3-4.5 μm; proliferating several times percurrently near apex. Conidia (10-)11-12(-13) × (7-)8(-9) μm, solitary, hyaline, aseptate, thin and smooth walled, granular, or with a single large central guttule, ellipsoid to obovoid, tapering towards a bluntly obtuse or narrow truncate base, 2-3 μm diam, enclosed in a thin persistent mucoid sheath, 1-2 μm thick, and bearing a hyaline, apical mucoid appendage, (5-)8-12(-15) × (1-)1.5 μm, flexible, unbranched, tapering towards an acutely rounded tip.
Culture characteristics: Colonies flat, spreading with sparse aerial mycelium and feathery, lobate margins, reaching 30 mm diam on MEA, 40 mm diam on PDA and 25 mm diam on OA after 2 wk at 25 °C. On OA surface iron-grey. On PDA surface and reverse iron-grey. On MEA surface and reverse iron-grey.
Specimens examined: South Africa, Western Cape Province, Cape Town, Table Mountain, on leaves of Brabejum stellatifolium, 1884, P. McOwan, holotype in B; Western Cape Province, Jonkershoek Nature Reserve, on leaf spot of Brabejum stellatifolium, 3 Jan. 1995, A. den Breeÿen, (epitype designated here CBS H-21396, ex-epitype culture CPC 1009 = CBS 776.97; MBT176251).
Notes: Phyllosticta owaniana causes a serious leaf spot disease on Brabejum stellatifolium, and is generally found wherever this host occurs in South Africa. All isolates collected thus far (Crous, unpubl. data) are similar based on DNA sequence data, suggesting that it’s a common species on this host.
Phyllosticta pachysandricola Wikee, Motohashi & Crous, sp. nov. MycoBank MB805658. Fig. 17.
Fig. 17.
Phyllosticta pachysandricola (NBRC 102276). A. Colony sporulating on SNA. B. Colony sporulating on PDA. C. Vertical section through conidioma. D, E. Conidiogenous cells. F. Conidia mounted in lactic acid. G. Conidia mounted in water. Scale bars: C = 35 μm, all others = 10 μm.
Etymology: Named after the host genus from which it was collected, Pachysandra.
Leaf spots circular to ellipsoid, pale brown to brown, often extend with concentric rings, 6-16 mm diam, surrounded by a dark brown border. Conidiomata (on PNA) pycnidial, amphiphyllous, sparse, solitary or aggregated, immersed at first, then erumpent breaking through the epidermis, brown to dark brown, subglobular, 90-140 × 25-80 μm diam, with central ostiole; pycnidial wall composed of depressed or irregular cells with 1-4 layers, brown to dark brown, hyaline or paler toward the inside. Conidiogenous cells integrated, lining the innermost layer of the pycnidial wall, cylindrical, straight or slightly curved, hyaline, proliferating percurrently at least once, with minute periclinal thickenings, 5-12 × 2-2.5 μm. Conidia sporulating holoblastically, solitary, unicellular, spherical, ellipsoid to obovoid, 5.5-8.5 × 4.5-7.5 μm, truncate at the base or rounded at both ends, containing numerous greenish guttulae, surrounded by a mucous sheath, rarely with a short apical appendage.
Specimen examined: Japan, Hokkaido, Asahikawa, on Pachysandra terminalis, K. Motohashi, C. Nakashima & T. Akashi, 7 June 2006 (holotype TFM: FPH7877, isotype MUMH 10488, ex-type culture MUCC 124 = NBRC 102276).
Notes: One other species has been recorded from Pachysandra, P. pachysandrae, which van der Aa & Vanev (2002) excluded from Phyllosticta s. str. based on its conidia (unicellular, oblong, 4.5-6 × 1 μm) that indicate placement in Asteromella. The Japanese collection on Pachysandra is thus described as a new species, P. pachysandricola, in accordance to the morphological differences noted by Motohashi et al. (2009).
Phyllosticta paxistimae Wikee & Crous, sp. nov. MycoBank MB805659. Fig. 18.
Fig. 18.
Phyllosticta paxistimae (CBS 112527). A. Colony sporulating on OA. B-D. Conidiogenous cells giving rise to conidia. E, F. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Paxistima.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 200 μm diam; pycnidial wall of several layers of textura angularis, up to 30 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 10 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1 supporting cell, 15-30 × 4-6 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 10-20 × 4-5 μm; proliferating several times percurrently near apex. Conidia (10-)12-14(-16) × 6-7(-8) μm, solitary, hyaline, aseptate, thin and smooth walled, granular, or with a single large central guttule, broadly ellipsoid, tapering towards a narrow truncate base, 2-3 μm diam, enclosed in a thin persistent mucoid sheath, 1 μm thick, and bearing a hyaline, apical mucoid appendage, (5-)9-11(-13) × 1.5(-2) μm, flexible, unbranched, tapering towards an acutely rounded tip.
Culture characteristics: Colonies erumpent, spreading with moderate aerial mycelium and feathery, lobate margins, reaching 60 mm diam after 1 mo at 25 °C. On OA surface iron-grey with patches of olivaceous-grey. On PDA surface and reverse iron-grey. On MEA surface dirty white with patches of iron-grey, reverse iron-grey.
Specimen examined. USA, Oregon, on living leaf of Paxistima myrsinites, 1994, G. Carroll (holotype CBS H-21397, ex-type culture CBS 112527).
Notes: We have been unable to trace the holotype of P. pachystimae (USA, Wyoming, Hoback Canyon, near Granite Creek, on Paxistima myrsinites, 1 Aug. 1940, L.E. Wehmeyer No 1198). The conidia of P. pachystimae (9-14 × 4-5 μm; Wehmeyer 1946) are much narrower than those of P. paxistimae (10-16 × 6-8 μm).
Phyllosticta philoprina (Berk. & M.A. Curtis) Wikee & Crous, comb. nov. MycoBank MB805660.
Basionym: Sphaeria philoprina Berk. & M.A. Curtis, Grevillea 4 (32): 154. 1876.
≡ Guignardia philoprina (Berk. & M.A. Curtis) Aa, Stud. Mycol. 5: 44. 1973.
For additional synonyms see van der Aa (1973).
Specimens examined: Spain, on living leaf of Ilex aquifolium, July 1970, H.A. van der Aa, specimen CBS H-13113, culture CBS 587.69. Germany, on Ilex aquifolium, Aug. 1972, R. Schneider, CBS 616.72.
Notes: The oldest name for a Phyllosticta sp. occurring on Ilex is Sphaeria philoprina. However, this name was based on material collected in the USA, and the present isolates were derived from European collections.
Phyllosticta podocarpicola Wikee, Crous, K.D. Hyde & McKenzie, sp. nov. MycoBank MB805661. Fig. 19.
Fig. 19.
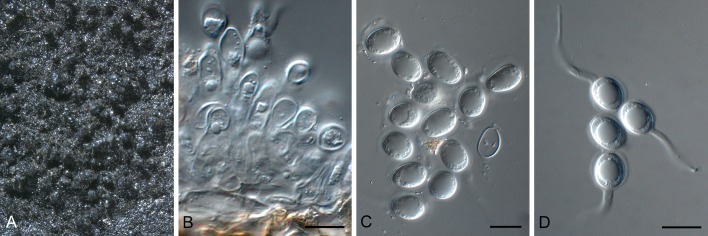
Phyllosticta podocarpicola (CBS 728.79). A. Colony sporulating on OA. B. Conidiogenous cells giving rise to conidia. C, D. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Podocarpus.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless to opaque conidial masses; pycnidia up to 200 μm diam; pycnidial wall of 3-6 layers of brown textura angularis; inner wall of hyaline textura angularis. Ostiole central, up to 20 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1-2 supporting cells, at times branched at base, 10-25 × 4-6 μm. Conidiogenous cells terminal, subcylindrical to doliiform, hyaline, smooth, coated in a mucoid layer, 10-17 × 4-6 μm; proliferating several times percurrently near apex. Conidia 12-13(-16) × 8-9(-9.5) μm, solitary, hyaline, aseptate, thin and smooth walled, coarsely guttulate, or with a single large central guttule, broadly ellipsoid, tapering towards a narrow truncate base, 2-5 μm diam, enclosed in a thin, persistent mucoid sheath, 3-4 μm thick, and bearing a hyaline, apical mucoid appendage, (25-)30-45(-55) × 3-4(-5) μm, flexible, unbranched, tapering towards an acutely rounded tip.
Culture characteristics: Colonies after 3 wk reaching 30 mm diam on MEA, 60 mm on PDA and OA. Colonies flattened, spreading, with sparse aerial mycelium and feathery margins. On MEA surface and reverse olivaceous-grey; on OA olivaceous-grey; on PDA iron-grey on surface and reverse.
Specimen examined: USA, Florida, on seed of Podocarpus maki (intercepted in New Zealand), Sep. 1979, G. Laundon (holotype CBS H-13109; ex-type culture CBS 728.79).
Notes: The isolate described here as Phyllosticta podocarpicola (CBS 728.79) was originally treated as part of the G. philoprina species complex, from which it is phylogenetically distinct (Figs 1, 2). It is also distinct from Phyllosticta podocarpi, which was originally described from Podocarpus elongatus leaf litter collected in South Africa [conidia (10-)14(-17) × (8-)9(-10) μm, appendages 10-40 × 1.5-2 μm; Crous et al. 1996].
Phyllosticta rhaphiolepidis Wikee, C. Nakash. & Crous, sp. nov. MycoBank MB805662. Fig. 20.
Fig. 20.
Phyllosticta rhaphiolepidis (MUCC 432). A, B. Close-up of immersed conidiomata on leaf tissue. C. Vertical section through conidioma. D. Conidiomatal wall of textura angularis. E, F. Conidiogenous cells. G. Conidia. Scale bars: C = 25 μm, all others = 10 μm.
Etymology: Named after the host genus from which it was collected, Rhaphiolepis.
Leaf spots irregular, pale brown. Conidiomata (on PDA) pycnidial, amphiphyllous, immersed, subglobose to globose, composed of depressed or irregular cells in 2-3 layers, dark brown to black, hyaline or paler toward the inside, 85-175 × 100-110 μm diam, with central ostiole, 10-13 μm diam. Conidiogenous cells integrated, lining the inner layer of pycnidia, hyaline, lageniform, cylindrical or conical, 3-10 × 3-4 μm, proliferating percurrently near apex. Conidia unicellular, spherical, ellipsoid to obovoid, truncate at base, later rounded at both ends, surrounded by a mucoid layer, containing numerous minute guttules, 7.5-10 × 4.6-6 μm, with a slender and short apical appendage 4-6 × 1-2 μm.
Specimen examined: Japan, Kagoshima, Tokunoshima Is., on living leaf of Rhaphiolepis indica var. umbellata, T. Kobayashi & Y. Ono, 22 Oct. 2003 (holotype CBS H-21408, culture ex-type MUCC 432).
Notes: Phyllosticta rhaphiolepicola, which occurs on Rhaphiolepis japonica in Germany, has somewhat wider conidia (7-9 × 6-8 μm; van der Aa & Vanev 2002) than the Japanese collection. Phyllosticta rhaphiolepidis is also phylogenetically distinct from other species of Phyllosticta that have been deposited in GenBank (Figs 1, 2).
Phyllosticta rubra Wikee & Crous, sp. nov. MycoBank MB805663. Fig. 21.
Fig. 21.
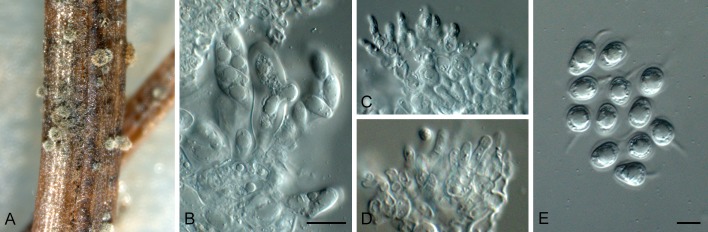
Phyllosticta rubra (CBS 111635). A. Conidiomata forming on PNA. B. Asci with ascospores. C, D. Conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Etymology. Named after the host species from which it was collected, Acer rubrum.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 200 μm diam; pycnidial wall of several layers of textura angularis, up to 30 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 15 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1 supporting cell, that can be branched at the base, 7-10 × 2-3 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 3-8 × 2-3 μm; proliferating several times percurrently near apex. Conidia (6-)6.5-7(-8) × (4-)5(-5.5) μm, solitary, hyaline, aseptate, thin and smooth walled, granular, or with a single large central guttule, ellipsoid to obovoid, tapering towards a narrow truncate base, 1.5-2 μm diam, enclosed in a thin persistent mucoid sheath, 1-1.5 μm thick, and bearing a hyaline, apical mucoid appendage, (5-)6-7(-9) × (1-)1.5 μm, flexible, unbranched, tapering towards an acutely rounded tip. Ascomata similar to conidiomata in general anatomy. Asci bitunicate, hyaline, clavate to broadly fusoid-ellipsoid, with visible apical chamber, 1 μm diam, 30-50 × 10-12 μm. Ascospores bi- to triseriate, hyaline, smooth, granular to guttulate, aseptate, straight, rarely curved, widest in the upper third, limoniform, (8-)9-10(-12) × (4-)5 μm.
Culture characteristics: Colonies erumpent, spreading with moderate aerial mycelium, covering dish after 1 mo at 25 °C. On OA surface iron-grey. On PDA and MEA surface olivaceous-grey, to iron-grey, reverse iron-grey.
Specimen examined: USA, Missouri, on Acer rubrum, July 1999, G. Carroll, (holotype CBS H-21398, culture ex-type CBS 111635).
Notes: Phyllosticta rubra is part of the P. minima species complex. Phyllosticta rubra has larger conidia (10 μm long), than two proposed synonyms, namely P. arida (on Acer negundo, conidia 8-10 × 6-7 μm), and P. acericola (on Acer rubrum, conidia 5-8 × 3-3.5 μm) (see van der Aa 1973).
Phyllosticta spinarum (Died.) Nag Raj & M. Morelet, Bull. Soc. Sci. nat. Arch. Toulon et du Var 34 (219): 12. 1978. Fig. 22.
Fig. 22.
Phyllosticta spinarum (CBS 292.90). A, B. Colony sporulating on SNA. C, D. Conidiogenous cells giving rise to conidia. E, F. Conidia. Scale bars = 10 μm.
Basionym: Phoma spinarum Died., Krypt.-Fl. Brandenburg (Leipzig) 9: 148. 1912.
Conidiomata (on PNA) pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 200 μm diam; pycnidial wall of several layers of textura angularis, up to 30 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 15 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1 supporting cell, that can be branched at the base, 10-15 × 4-5 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 5-12 × 3-5 μm; proliferating several times percurrently near apex. Conidia (10-)12-14(-15) × (7-)7.5(-8) μm, solitary, hyaline, aseptate, thin and smooth walled, granular, or with a single large central guttule, ellipsoid to obovoid, tapering towards a narrow truncate base, 3-4 μm diam, enclosed in a thin persistent mucoid sheath, 1-2 μm thick, and bearing a hyaline, apical mucoid appendage, (7-)8-12(-20) × (2-)2.5(-3) μm, flexible, unbranched, tapering towards an acutely rounded tip.
Culture characteristics: Colonies flat, spreading with sparse aerial mycelium and feathery, lobate margins, reaching 70 mm diam after 1 mo at 25 °C. On OA surface olivaceous-grey. On PDA surface olivaceous-grey, reverse iron-grey. On MEA surface pale olivaceous-grey in outer region, olivaceous-grey in centre; in reverse iron-grey in centre, smoke-grey in outer region.
Specimens examined: France, St. Denis en Val, on living leaf of Chamaecyparis pisifera, 1970, M. Morelet (CBS H-17034, culture CBS 292.90). Germany, Nieder Lauslitz: Colbus, on Juniperus sp., 4 Jul. 1910, Diedicke, holotype in B.
Notes: Nag Raj & Morelet (1979) provide a detailed description of the type specimen, which closely corresponds with isolate CBS 292.90 studied here.
Phyllosticta vacciniicola Wikee, Crous, K.D. Hyde & McKenzie, sp. nov. MycoBank MB805664. Fig. 23.
Fig. 23.
Phyllosticta vacciniicola (CPC 18590). A. Colony sporulating on OA. B-D. Conidiogenous cells giving rise to conidia. E, F. Conidia. Scale bars = 10 μm.
Conidiomata pycnidial, solitary, black, erumpent, globose, exuding colourless conidial masses; pycnidia up to 200 μm diam; pycnidial wall of several layers of textura angularis, up to 25 μm thick; inner wall of hyaline textura angularis. Ostiole central, up to 20 μm diam. Conidiophores subcylindrical to ampulliform, reduced to conidiogenous cells, or with 1 supporting cell, that can be branched at the base, 10-20 × 3-5 μm. Conidiogenous cells terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer, 8-15 × 3-4 μm; proliferating several times percurrently near apex. Conidia (9-)10-12(-13) × (6-)7(-8) μm, solitary, hyaline, aseptate, thin and smooth walled, granular, with a single large central guttule, ellipsoid to obovoid, tapering towards a narrow truncate base, 3-4 μm diam, enclosed in a thin persistent mucoid sheath, 0.5-1 μm thick, and bearing a hyaline, apical mucoid appendage, 7-25 × (1.5-)2 μm, flexible, unbranched, tapering towards an acutely rounded tip.
Culture characteristics: Colonies flat, spreading with sparse aerial mycelium and feathery, lobate margins, reaching 15 mm diam after 2 wk at 25 °C. On OA surface iron-grey. On PDA surface and reverse iron-grey. On MEA surface pale olivaceous-grey to olivaceous-grey, reverse olivaceous-grey.
Specimen examined: USA, on living leaf of Vaccinium macrocarpum, Mariusz Tadych, (holotype CBS H-21399, ex-type culture CPC 18590 = CBS 136062).
Notes: A recent study published by Zhang et al. (2013) revealed P. vaccinii (ex-epitype ATCC 46255) to be distinct from Guignardia vaccinii (ex-holotype CBS 126.22), and also revealed that several undescribed Phyllosticta spp. are associated with Vaccinium, one of which is described here as P. vacciniicola. The correct name for G. vaccinii should thus be that of its asexual morph P. elongata, in accordance with Weidemann et al. (1982).
Phyllosticta sp.
Specimen examined: Spain, on living leaf of Eucalyptus globulus, 4 Jan. 2004, M.J. Wingfield, culture CPC 11336.
Notes: Two species of Phyllosticta are known from Eucalyptus. Van der Aa & Vanev (2002) treated P. eucalyptorum (on E. grandis from Brazil, conidia (7.5-)11-20 × (5-6(-6.5) μm; Crous et al. 1993) as synonymous with P. eucalyptina (on E. globulus, Tunisia, conidia 18-20 × 5-6 μm). No cultures of P. eucalyptina are available, and P. eucalyptorum was considered a synonym of P. capitalensis (Fig. 1). Although the present collection appears to represent a novel species, it is not treated further as the cultures proved to be sterile.
Phyllosticta sp.
Specimens examined: Brazil, São Paulo, Pompeia, on living leaf of Mangifera indica, 14 May 2007, C. Glienke & D. Stringari CPC 17454; ibid., CPC 17455.
Notes: Although phylogenetically distinct (Fig. 2), both cultures of this species proved to be sterile, and thus are not treated further.
DISCUSSION
The resurrection of the Phyllostictaceae, and its separation from the Botryosphaeriaceae is justified based on morphology and DNA phylogeny (Crous et al. 2006, Liu et al. 2012, Slippers et al. 2013, this volume). Phyllosticta is a well-established genus, distinct from genera in the Phoma complex (Aveskamp et al. 2010, de Gruyter et al. 2009, 2012, 2013), while the Botryosphaeria complex has also been shown to represent numerous genera (Crous et al. 2006, Phillips et al. 2008, Liu et al. 2012), and even families (Slippers et al. 2013, this volume).
Traditionally species of Phyllosticta have been chiefly identified by their host association. Several recent papers have shown that many traditional morphological species represent complexes of species, e.g. P. citricarpa on citrus, P. musarum on banana (Glienke et al. 2011, Wang et al. 2012), and P. elongata on Vaccinium (Zhang et al. 2013).
Freckle disease of banana was usually referred to in literature under its sexual name, Guignardia musae, or that of its purported asexual morph, Phyllosticta musarum. By employing multigene DNA analysis combined with a morphological comparison, Wong et al. (2012) demonstrated that these two names were not conspecific, and that the the common species occurring on banana cultivar Cavendish was in fact a novel taxon, which they described as P. cavendishii. The commonly occurring species in Southeast Asia and Oceania on non-Cavendish bananas was in fact another taxon, P. maculata. A third species on bananas, P. musarum was confirmed from India and Thailand. The most recent studies focusing on the taxonomy of Phyllosticta species associated with citrus black spot is that of Glienke et al. (2011) and Wang et al. (2012). Surprisingly, several species of Phyllosticta were shown to cause these symptoms on Citrus, although there was a difference in their host range and preference. The citrus black spot pathogen which is presently subjected to phytosanitary legislation in the EU and United States, P. citricarpa, was isolated from lemons, mandarins and oranges in China, although Wang et al. (2012) did define two subclades, one from mandarins, and another from oranges and lemons. Phyllosticta citriasiana was newly described on Citrus maxima in Asia by Wulandari et al. (2009), while Glienke et al. (2011) described P. citribraziliensis on Citrus limon from Brazil. Wang et al. (2012) also described P. citrichinaensis on C. maxima and C. reticulata from China. The present study adds yet a fifth species to this complex, namely P. citrimaxima, which is associated with tan spots on the fruit rind of Citrus maxima in Thailand. When considering that P. capitalensis can still co-occur as an endophyte in fruit or leaf lesions caused by these five species (Wikee et al. 2013), it is clear that these taxa are best distinguished by DNA sequence data. This has important biosecurity implications for the Citrus industries in many countries.
Guignardia philoprina (asexual morph P. concentrica) has been known as the taxon occurring on hosts such as Rhododendron, Hedera, Ilex, Magnolia, and Taxus (von Arx & Müller 1954). Not surprisingly, this turned out to represent a species complex, with numerous names available for consideration under the sexual and asexual morph. Although some of these names have been resurrected and applied in the present study, e.g. P. concentrica on Hedera helix, P. foliorum on Taxus, and P. philoprina on Ilex, many taxa still need to be recollected to resolve their phylogeny and correct taxonomy.
One aim of the present paper was to employ multigene DNA sequence analysis to discriminate among all species of Phyllosticta that were available to us from the CBS culture collection, supplemented by our own working collections, which resulted in a total of 160 strains. In addition to dealing with old synonymies that represented names that had to be resurrected, a further challenge has been to also merge Phyllosticta and Guignardia epithets, to obtain the best possible unit nomenclature for these species (Wingfield et al. 2012). In the present study we described 12 novel species, and designated a further eight epitype or neotype specimens. From the results obtained here, it is clear that in the case of epitypification, epitypes need to be designated based on the same host, recollected in the same geographic region (see Cannon et al. 2012). This is extremely difficult, as American names are commonly used for European of Asian taxa, and also vice versa (see the same situation in Cercospora and Pseudocercospora; Crous et al. 2013, Groenewald et al. 2013). In these cases the application of names to collections from other countries that appear morphologically similar, can at best be regarded as tentative, pending further collections.
Results obtained here clearly show that a multi-gene approach works well for distinguishing these taxa. In this study the intron dominated genes (ITS, ACT, TEF), and highly conserved gene coding regions (LSU, GPDH) were used. The result from the five gene analysis compared with the two gene analysis were similar (Figs 1, 2), indicating that the phylogeny of Phyllosticta derived from the ITS and ACT gene loci is sufficiently robust to distinguish most taxa, except those closely related to P. capitalensis. The biggest challenge ahead is to recollect specimens representative of the more than 3 000 names that exist in this complex.
Acknowledgments
We thank the technical staff, Arien van Iperen (cultures), and Marjan Vermaas (photographic plates) for their invaluable assistance. This study was financially supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0198/2552) to S. Wikee and Kevin D. Hyde. The National Research Council of Thailand is thanked for the award of grant No 55201020002 to study the genus Phyllosticta in Thailand. The Japanese studies were supported by JSPS KAKENHI (24780149 & 23780168).
REFERENCES
- Aa HA van der. (1973). Studies in Phyllosticta I. Studies in Mycology 5: 1–110 [Google Scholar]
- Aa HA van der, Vanev S. (2002). A revision of the species described in Phyllosticta. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands: [Google Scholar]
- Allescher A. (1898). Rabenhorst’s Kryptogamen-flora, Pilze - Fungi imperfecti. E. Kummer, Leipzig, Germany: [Google Scholar]
- Arx JA von, Müller E. (1954). Die Gattungen der amerosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz XI(1): 1–434 [Google Scholar]
- Aveskamp M, Gruyter J de, Woudenberg J, Verkley GJM, Crous PW. (2010). Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology 65: 1–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baayen R, Bonants P, Verkley GJM, Carroll G, Aa H van der, et al. (2002). Nonpathogenic isolates of the citrus black spot fungus, Guignardia citricarpa, identified as a cosmopolitan endophyte of woody plants, G. mangiferae (Phyllosticta capitalensis). Phytopathology 92: 464–477 [DOI] [PubMed] [Google Scholar]
- Barr ME. (1970). Some amerosporous ascomycetes on Ericaceae and Empetraceae. Mycologia 62: 377–394 [Google Scholar]
- Barr ME. (1972). Preliminary studies on the Dothideales in temperate North America. Contributions from the University of Michigan Herbarium 9: 523–638 [Google Scholar]
- Bissett J. (1986). Discochora yuccae sp. nov. with Phyllosticta and Leptodothiorella synanamorphs. Canadian Journal of Botany 64: 1720–1726 [Google Scholar]
- Bissett J, Darbyshire SJ. (1984). Phyllosticta minima. Fungi Canadenses: 277 National Mycological Herbarium, Biosystematics Research Institute, Agriculture Canada, Ottawa, Canada: [Google Scholar]
- Bissett J, Palm ME. (1989). Species of Phyllosticta on conifers. Canadian Journal of Botany 67: 2278–2285 [Google Scholar]
- Cannon PF, Damm U, Johnston PR, Weir BS. (2012). Colletotrichum - current status and future directions. Studies in Mycology 73: 181–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Chomnunti P, Schoch CL, Aguirre-Hudson B, Ko-Ko TW, Hongsanan S, et al. (2011). Capnodiaceae. Fungal Diversity 51: 103–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJM, et al. (2013). Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Groenewald JZ. (2005). Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463–470 [Google Scholar]
- Crous PW, Seifert KA, Castañeda-Ruiz RF. (1996). Microfungi associated with Podocarpus leaf litter in South Africa. South African Journal of Botany 62: 89–98 [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, et al. (2006). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, Burgess TI, Decock CA, et al. (2012). Fungal planet description sheets: 107-127. Persoonia 28: 138–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds) (2009). Fungal Biodiversity. CBS Laboratory Manual Series 1: 1–269 Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Crous PW, Wingfield MJ, Ferreira FA, Alfenas A. (1993). Mycosphaerella parkii and Phyllosticta eucalyptorum, two new species from Eucalyptus leaves in Brazil. Mycological Research 97: 582–584 [Google Scholar]
- Donk MA. (1968). Report of the committee for Fungi and Lichen 1964-1968. Taxon 17: 578–581 [Google Scholar]
- Fries EM. (1849). Summa vegetabilium Scandinaviae. Typographis Academica, Uppsala: [Google Scholar]
- Glienke C, Pereira O, Stringari D, Fabris J, Kava-Cordeiro V, et al. (2011). Endophytic and pathogenic Phyllosticta species, with reference to those associated with Citrus Black Spot. Persoonia 26: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewald JZ, Nakashima C, Nishikawa J, Shin H-D, Park J-H, et al. (2013). Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology 75: 115–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove WB. (1935). British stem-and leaf-fungi (Coelomycetes) 1 University Press, Cambridge, England: [Google Scholar]
- Gruyter J de, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW. (2009). Molecular phylogeny of Phoma and allied anamorph genera: Towards a reclassification of the Phoma complex. Mycological Research 113: 508–519 [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JH, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. (2010). Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102: 1066–1081 [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JH, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. (2013). Redisposition of phoma-like anamorphs in Pleosporales. Studies in Mycology 75: 1–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerber JC, Liu B, Correll JC, Johnston PR. (2003). Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 95: 872–895 [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, et al. (2011). The Amsterdam declaration on fungal nomenclature. IMA Fungus 2: 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, David JC. (1989). Family Names: Index of Fungi Supplement. Wallingford: CAB International; [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. (1998). Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41:183–189 [DOI] [PubMed] [Google Scholar]
- Hillis DM, Bull JJ. (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192 [Google Scholar]
- Hyde KD, Chomnunti P, Crous PW, Groenewald JZ, Damm U, et al. (2010). A case for re-inventory of Australia’s plant pathogens. Persoonia 25: 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, Ariyawansha H, et al. (2012). Towards a natural classification of Botryosphaeriales. Fungal Diversity 57: 149–210 [Google Scholar]
- Moncalvo JM, Wang HH, Hseu RS. (1995). Phylogenetic relationships in Ganoderma inferred from the internal transcribed spacer and 25S ribosomal DNA sequences. Mycologia 87: 223–238 [Google Scholar]
- Motohashi K, Inaba S, Anzai K, Takamatsu S, Nakashima C. (2009). Phylogenetic analyses of Japanese species of Phyllosticta sensu stricto. Mycoscience 50: 291–302 [Google Scholar]
- Myllys L, Stenroos S, Thell A. (2002). New genes for phylogenetic studies of lichenized fungi: glyceraldehyde-3-phosphate dehydrogenase and beta-tubulin genes. Lichenologist 34: 237–246 [Google Scholar]
- Nag Raj TR, Morelet M. (1979). Observations on Mucosetospora (Coelomycetes). Canadian Journal of Botany 57: 1295–1297 [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the USA 98: 2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persoon CH. (1818). Traité sur les champignons comestibles, contenant l’indication des espèces nuisibles; a l’histoire des champignons. Belin-Leprieur, Paris, France: [Google Scholar]
- Petrak F. (1957). Über die Gattungen Guignardia Viala & Ravaz und Discosphaerina v. Höhnel. Sydowia 11: 435–445 [Google Scholar]
- Petrak F, Sydow H. (1927). Die Gattungen der Pyrenomyzeten, Sphaeropsideen und Melanconieen. I. Der phaeosporen Sphaeropsideen und die Gattung Macrophoma. Feddes Repertorium Speciarum Novarum Regni Vegetabilum, Beihefte 42: 1–551 [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, et al. (2008). Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21: 29–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu J, Xie Y, Zhang X, Qi Y, Zhang C, Liu X. (2008). Preinfection behaviour of Phyllosticta musarum on banana leaves. Australasian Plant Pathology 37: 60–64 [Google Scholar]
- Punithalingam E. (1974). Studies on Spheropsidales in culture II. Mycological Papers 136: 1–63 [Google Scholar]
- Rayner RW. (1970). A mycological colour chart. CMI and British Mycological Society, Kew, Surrey, England: [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052 [DOI] [PubMed] [Google Scholar]
- Seaver FJ. (1922). Phyllostictaceae. North American Flora 6: 3–84 [Google Scholar]
- Slippers B, Boissin E, Phillips AJ, Groenewald JZ, Wingfield MJ, et al. (2013). Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Studies in Mycology 76: 31–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Wingfield MJ, Crous PW, Coutinho TA. (1996). Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African Journal of Botany 62: 86–88 [Google Scholar]
- Su YY, Cai L. (2012). Polyphasic characterisation of three new Phyllosticta spp. Persoonia 28: 76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan A, Johnston PR, Park D, Robertson AW. (2011). Two new pathogenic ascomycetes in Guignardia and Rosenscheldiella on New Zealand’s pygmy mistletoes (Korthalsella: Viscaceae). Studies in Mycology 68: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. (2003). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts: [Google Scholar]
- Takeuchi J, Horie H. (1998). First occurrence of leaf spot of Nandina and Japanese spurge caused by Phyllosticta sp. in Japan (in Japanese). Proceedings of the Kanto-Tosan Plant Protection Society 45: 143–145 [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viala P, Ravaz L. (1892). Sur la dénomination botanique (Guignardia bidwellii) du black-rot. Bulletin de la Société Mycologique de France 8: 63 [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen G, Huang F, Zhang J, Hyde KD, Li H. (2012). Phyllosticta species associated with citrus diseases in China. Fungal Diversity 52: 209–224 [Google Scholar]
- Wehmeyer LE. (1946). Studies on some fungi from north-western Wyoming. II. Fungi Imperfecti. Mycologia 38: 206–330 [PubMed] [Google Scholar]
- Weidemann G, Boone D, Burdsall H., Jr (1982). Taxonomy of Phyllosticta vaccinii (Coelomycetes) and a new name for the true anamorph of Botryosphaeria vaccinii (Dothideales, Dothioraceae). Mycologia 74: 59–65 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, California: 315–322 [Google Scholar]
- Wicht B, Petrini O, Jermini M, Gessler C, Broggini GAL. (2012). Molecular, proteomic and morphological characterization of the ascomycete Guignardia bidwellii, agent of grape black rot: a polyphasic approach to fungal identification. Mycologia 104: 1036–1045 [DOI] [PubMed] [Google Scholar]
- Wikee S, Lombard L, Crous PW, Nakashima C, Motohashi K, et al. (2013). Phyllosticta capitalensis, a widespread endophyte of plants. Fungal Diversity 60: 91–105 [Google Scholar]
- Wikee S, Udayanga D, Crous PW, Chukeatirote E, McKenzie EHC, et al. (2011). Phyllosticta - an overview of current status of species recognition. Fungal Diversity 51: 43–61 [Google Scholar]
- Wingfield MJ, De Beer ZW, Slippers B, Wingfield BD, Groenewald JZ, et al. (2012). One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology 13: 604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MH, Crous PW, Henderson J, Groenewald JZ, Drenth A. (2012). Phyllosticta species associated with freckle disease of banana. Fungal Diversity 56: 173–187 [Google Scholar]
- Wulandari N, To-Anun C, Hyde KD, Duong L, Gruyter J de, et al. (2009). Phyllosticta citriasiana sp. nov., the cause of Citrus tan spot of Citrus maxima in Asia. Fungal Diversity 34: 23–39 [Google Scholar]
- Zhang K, Zhang N, Cai L. (2013). Typification and phylogenetic study of Phyllosticta ampelicida and P. vaccinii. Mycologia 105: 1030–1042 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. (2012). Pleosporales. Fungal Diversity 53: 1–221 [DOI] [PMC free article] [PubMed] [Google Scholar]