Abstract
The order Botryosphaeriales represents several ecologically diverse fungal families that are commonly isolated as endophytes or pathogens from various woody hosts. The taxonomy of members of this order has been strongly influenced by sequence-based phylogenetics, and the abandonment of dual nomenclature. In this study, the phylogenetic relationships of the genera known from culture are evaluated based on DNA sequence data for six loci (SSU, LSU, ITS, EF1, BT, mtSSU). The results make it possible to recognise a total of six families. Other than the Botryosphaeriaceae (17 genera), Phyllostictaceae (Phyllosticta) and Planistromellaceae (Kellermania), newly introduced families include Aplosporellaceae (Aplosporella and Bagnisiella), Melanopsaceae (Melanops), and Saccharataceae (Saccharata). Furthermore, the evolution of morphological characters in the Botryosphaeriaceae were investigated via analysis of phylogeny-trait association. None of the traits presented a significant phylogenetic signal, suggesting that conidial and ascospore pigmentation, septation and appendages evolved more than once in the family. Molecular clock dating on radiations within the Botryosphaeriales based on estimated mutation rates of the rDNA SSU locus, suggests that the order originated in the Cretaceous period around 103 (45-188) mya, with most of the diversification in the Tertiary period. This coincides with important periods of radiation and spread of the main group of plants that these fungi infect, namely woody Angiosperms. The resulting host-associations and distribution could have influenced the diversification of these fungi.
Taxonomic novelties:
New families - Aplosporellaceae Slippers, Boissin & Crous, Melanopsaceae Phillips, Slippers, Boissin & Crous, Saccharataceae Slippers, Boissin & Crous.
Key words: Aplosporellaceae, Melanopsaceae, molecular dating, Phyllostictaceae, Planistromellaceae, Saccharataceae, systematics
INTRODUCTION
DNA sequence-based phylogenetics has dramatically influenced both the taxonomy and systematics of the Botryosphaeriaceae during the course of the past decade (Crous et al. 2006), as it has done in most other groups of Fungi (James et al. 2006, Hibbett et al. 2007). At a higher taxonomic level, DNA sequence data have led to the recognition that the Botryosphaeriaceae represents a distinct order within the Dothideomycetes, leading Schoch et al. (2006) to introduce the Botryosphaeriales. The circumscription of the Botryosphaeriales has suffered from insufficient sampling and it was only recently that Minnis et al. (2012) provided molecular evidence to show that the Planistromellaceae resides in this order. In a subsequent study, Liu et al. (2012) provided a comprehensive phylogenetic analysis of genera in the Botryosphaeriales and they also concluded that, other than the Botryosphaeriaceae and Planistromellaceae, a number of clearly defined evolutionary lineages exist.
Apart from the Planistromellaceae, the genera traditionally associated with Botryosphaeria and Phyllosticta have sexual morphs that are clearly distinct phylogenetically, morphologically and ecologically. However, both are still grouped within the Botryosphaeriaceae. Members of the Botryosphaeria group are common endophytes of leaf and woody tissue of many woody plant species, have hyaline to dark ascospores, multilocular ascomata, and a wide range of asexual morphs that typically lack a mucoid sheath and apical appendage. Species in the Guignardia group (= Phyllosticta) typically infect leaves and fruit, less commonly wood, have unilocular ascomata with smaller ascospores that typically have mucoid appendages, and Phyllosticta asexual morphs. The Phyllostictaceae has been resurrected to accommodate this group of taxa (see Wikee et al. 2013b, this volume).
Substantial changes to the definition of sexual and asexual genera linked to the Botryosphaeriaceae have been made during the past decade (e.g. Crous et al. 2006, Phillips et al. 2008, Liu et al. 2012). Only a selection of the most common examples is discussed here. The first DNA sequence data for the Botryosphaeriaceae appeared to reveal a distinction between asexual morphs with hyaline fusicoccum-like conidia and those with pigmented diplodia-like conidia, termed sections Hyala and Brunnea (Jacobs & Rehner 1998, Denman et al. 2000, Zhou & Stanosz 2001). This distinction became increasingly less obvious as sampling increased and it was evident that conidial pigmentation is a feature that evolved more than once. It was, for example, shown that dark, septate and even muriformly septate dichomera-like conidia could be synasexual morphs of well-known genera such as Fusicoccum and Neofusicoccum (Barber et al. 2005, Phillips et al. 2005). Furthermore, dark, septate ascospores were shown to be a polyphyletic character of several genera and more common than previously believed (Phillips et al. 2008). As the true phylogenetic diversity within the group emerged, a number of new genera were described (e.g. Botryobambusa, Cophinforma, Neofusicoccum, Neoscytalydium, Pseudofusicoccum, etc.) or older genera re-defined (e.g. Auerswaldia, Barriopsis, Dothiorella, etc.) (e.g. Crous et al. 2006, Phillips et al. 2008, Liu et al. 2012). The most recent work by Liu et al. (2012) reviewed these genera, and this study reflects a growing consensus regarding the circumscription of the majority of the genera (29 in total, of which sequence data are available for 20).
DNA-based sequence analyses have also resulted in significant changes to the nomenclature, identification and circumscription of species in the Botryosphaeriaceae. These changes have resulted in the implementation of a single nomenclature for all morphs of a species (Crous et al. 2006, Hawksworth et al. 2011, Wingfield et al. 2012). For the Botryosphaeriaceae, this has included the description of cryptic species based on DNA sequence data, where morphological characters were not variable enough for this purpose (Pavlic et al. 2009a, Sakalidis et al. 2011).
Insights gained from contemporary studies on the Botryosphaeriaceae have led to uncertainty regarding the application of names published in the older literature. The analyses show for example that morphological characters typically used for species identification (chiefly conidia or ascospore dimensions, shape, septation and pigmentation) are frequently unreliable. Even ecological and geographical data are difficult to interpret, with some species occurring on numerous hosts, and single locations or hosts often yielding numerous co-occurring species (Slippers & Wingfield 2007, Slippers et al. 2009). For this reason (together with the significant changes in generic descriptions mentioned above) many, if not most, of the taxa dealt with before the introduction of DNA sequence-based phylogenetic inference will need to be redefined (possibly neo- or epitypified), to allow meaningful comparisons with currently applied names (also see the discussion in Phillips et al. 2013, this volume). Where it is not possible to follow this approach, older names may have to be ignored and new species introduced that are supported by DNA data (see Slippers et al. 2014).
The Botryosphaeriales is an important group of fungi due to the ecological and economic significance of many of its species. All species are plant-associated, and many are classified as pathogens, known to cause disease on a wide range of ecologically and economically important plants (Mehl et al. 2012). Some species are also known to cause opportunistic infections in humans (de Hoog et al. 2000). Most species exist as endophytes living in healthy plant tissues for extended periods of time (Slippers & Wingfield 2007). Their roles as endophytes or pathogens often overlap, as is for example found in the case of Diplodia sapinea. This well-known pathogen of Pinus (Swart et al. 1991) is also a common endophyte in branches, the trunks and seed cones of these trees. In an extreme example, D. sapinea has been isolated from the wood of Pinus in South Africa, where it must have existed without causing disease subsequent to the tree being infected as long as a decade previously (Bihon et al. 2011).
Unlike the case for D. sapinea, the ecological roles for the majority of species of Botryosphaeriaceae are unknown. The changes to the taxonomy of the group are already strongly promoting an ability to characterise the diversity in this group. In turn, this is providing an evolutionary framework making it possible to study the ecological role that remains obscure for the majority of these fungi.
In this paper, the phylogenetic relationships of all the genera known from culture and considered to reside in the Botryosphaeriales and Botryosphaeriaceae are determined based on DNA sequence data for six loci. The Planistromellaceae is well defined within the Botryosphaeriales. As expected, Phyllosticta (= Guignardia) also forms a strongly supported monophyletic lineage, recognised as the Phyllostictaceae (see Wikee et al. 2013b, this volume). Saccharata, however, groups separately with respect to all other genera in the Botryosphaeriales, as does Aplosporella, Bagnisiella and Melanops. The nomenclatural changes necessary to reflect these distinctions are considered in this study. With the well-supported phylogeny provided by these analyses, we also test hypotheses regarding the evolution of major morphological features typically used in taxonomy of the Botryosphaeriaceae. Finally, we use the nuclear ribosomal subunit data to date the divergence in the major groups of the Botryosphaeriales.
MATERIALS AND METHODS
Isolates and DNA extractions
A total of 96 strains corresponding to 85 species were grown on 2 % potato dextrose agar (PDA) plates incubated at 25 °C. Genomic DNA was extracted from mycelium using the PrepMan™ Ultra protocol (Applied Biosystems). Sequences from additional species were retrieved from GenBank. A total of 140 taxa were included in the ingroup and six taxa in the outgroup (see Table 1 for details).
Table 1.
Isolates subjected to DNA analysis in this study.
CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CMW: Tree Pathology Co-operative Program, Forestry and Agricultural Biotechnology Institute, University of Pretoria, South Africa; IRAN: Iranian Fungal Culture Collection, Iranian Research Institute of Plant Protection, Iran; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Mai, Thailand; MUCC: Culture Collection, Laboratory of Plant Pathology, Mie University, Tsu, Mie prefecture, Japan.
PCR and sequencing
A total of six partial gene portions were used in this study: the nuclear ribosomal small subunit (SSU), the nuclear ribosomal large subunit (LSU), the intergenic spacer (ITS), the translation elongation factor 1-alpha (EF1), the β-tubulin gene (BT) and the mitochondrial ribosomal small subunit (mtSSU).
The primers used were NS1 and NS4 (White et al. 1990) for SSU, LROR and LR5 (Vilgalys Laboratory, Duke university, www.biology.duke.edu/fungi/mycolab/primers.htm) for LSU, ITS-1 and ITS-4 (White et al. 1990) for ITS, EF-AF and EF-BR (Sakalidis et al. 2011) for EF1, BT2A and BT2B (Glass & Donaldson 1995) for BT and mrSSU1 and mrSSU3R (Zoller et al. 1999) for mtSSU. All PCR reactions were conducted in 15 μL containing 1.5 mM of MgCl2, 0.5 mM of dNTP, 1 × final concentration of buffer, 1 μM of each primer, 0.25 U of FastStart Taq Polymerase (Roche), 1.5 μL of DNA template and Sabax sterilised water (Adcock Ingram) to complete up to 15 μL. The cycling parameters were as follows: a first step of denaturation at 95 °C for 5 min followed by 35 cycles of (i) denaturation at 95 °C for 60 s, (ii) annealing at optimal temperature (55 °C for ITS, EF1, LSU and 45 °C for SSU, mtSSU, BT) for 80 s, (iii) elongation at 72 °C for 90 s, and a final elongation step of 5 min was applied.
Sephadex columns (Sigma-Aldrich) were used to clean the samples both before and after the sequencing reactions. The sequencing PCRs were performed in 10 μL containing 1 μL of PCR product, 0.7 μL of Big Dye Terminator v. 3.1 (Applied Biosystems), 2.5 μL of sequencing buffer (provided with Big Dye), 1 μL of primer (10 μM) and 4.8 μL of Sabax sterilised water. Cycling parameters consisted of 25 cycles with three steps each: 15 s at 95 °C, 15 s at 55 °C (for ITS, EF1, LSU) or 45 °C (for SSU, mtSSU, BT) and 4 min at 60 °C. The sequencing PCR products were sent to a partner laboratory for sequencing of both strands (Sequencing Facility, University of Pretoria).
Data analyses
Sequences were aligned using MAFFT v. 6 online (Katoh & Toh 2008) and refined visually. In order to retrieve the genetic information from indels, GapCoder (Young & Healy 2003) was used to code the gaps contained in the EF1 and the mSSU alignments. Analyses were run both with gaps coded and not coded.
The datasets were combined because this is believed to increase phylogenetic accuracy (Bull et al. 1993, Cunningham 1997). The six phylogenies resulting from the six data sets were first inspected visually to check whether there were conflicts between the histories of the genes that would preclude the combination of data. Additionally, the Incongruence Length Difference (ILD) test (Farris et al. 1995a, b) also known as the partition homogeneity test was run using PAUP v. 4.0b10 (Swofford 2003).
MrAIC (Nylander 2004) was used to determine the best fit model of nucleotide substitutions for each gene. Phylogenetic relationships of the samples were investigated using both Maximum Likelihood (ML) and Bayesian Inference (BI). The ML analysis was conducted using PhyML online (Guindon et al. 2005). The reliability of each node was assessed using the bootstrap (Felsenstein 1985) resampling procedure (100 replicates).
The BI was conducted using the software Beast v. 1.7.4 (Drummond et al. 2012). The phylogenetic relationships were estimated by running 10 000 000 generations and sampling every 100th generation. Bayes Factors were computed to choose between the different options available in Beast (four clock models: strict clock, exponential or lognormal uncorrelated relaxed clocks, and random local clock and two tree priors: Yule process or Birth-Death speciation model). The Birth-Death speciation model and a relaxed uncorrelated exponential clock were selected as best fitting our data. Six independent runs were performed and outputs were combined using LogCombiner (in the Beast package). The programme Tracer v. 1.5 (available on the Beast website) was used to check that the effective sampling sizes (ESS) were above 200 (as advised by the programmers to ensure an accurate estimation of phylogeny and parameters of interest). The programme Tree Annotator (available in the Beast package) was used to summarize the resulting trees using the maximum clade credibility option. The final tree was visualised in FigTree v. 1.4.
Molecular clock dating
Using a mean molecular rate of 1-1.25 % per lineage per 100 million years, commonly accepted in fungi for the SSU gene (Berbee & Taylor 2010), a rough estimation of the times to the most recent common ancestors of groups of interest was assessed using Beast. A normal distribution was used as prior and this was centred on a 95 % interval spanning 1.0-1.25 % (mean = 0.000113; standard deviation = 0.000006). The dating of the distinct groups of interest and their 95 % highest posterior density (HPD) were retrieved using Tracer v. 1.5.
Analysis of phylogeny-trait association
In order to investigate the evolution of morphological characters in the Botryosphaeriaceae, ancestral trait reconstructions and tests for phylogenetic signal were conducted in Mesquite v. 2.74 (Maddison & Maddison 2010). The characters considered were 1) ascospore colour; 2) presence or absence of ascospore septa; 3) conidial colour; 4) presence or absence of conidial septa; and 5) presence or absence of a mucus sheath. Both parsimony and ML reconstructions were used in Mesquite to test for phylogenetic signal. The observed distribution of character states at the tips of the phylogeny was compared to null distributions obtained when reshuffling the tip characters on the tree topology (10 000 times). Where the number of steps (or likelihood value) in the observed trait reconstruction fell outside the 95 % range of the null distribution, this was seen to indicate that character states are not distributed randomly on the phylogeny (i.e. there is a phylogenetic component).
RESULTS
Phylogenetic relationships
The alignment included a total of 4498 bp from six gene portions. The results from the ILD test were not significant and supported a decision to combine the 6 gene datasets. The number of polymorphic and parsimony informative sites ranged from 108 for the SSU, 165 for the mtSSU, 170 for the LSU, 222 for BT, 335 for the EF1 to 361 for the ITS.
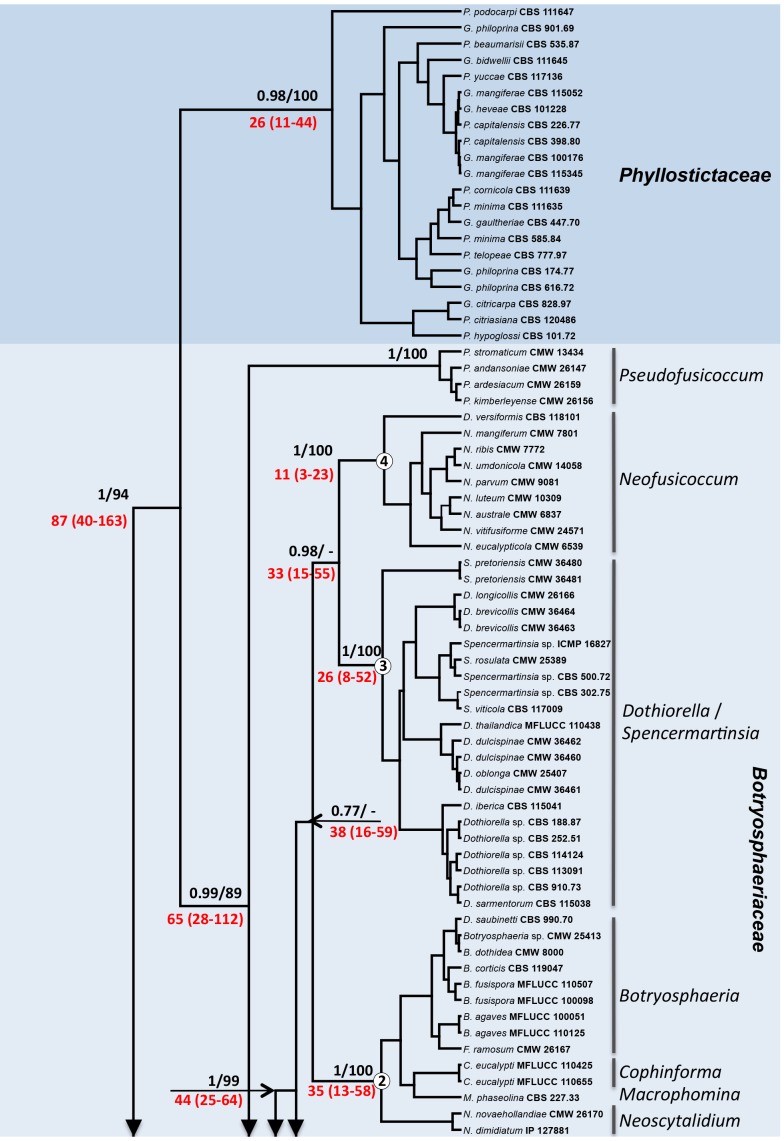
The Maximum Likelihood (ML) and Bayesian Inference (BI) phylogenetic reconstructions were similar, and the BI tree is shown on Fig. 1. Species residing in the genera Aplosporella and Bagnisiella (in one clade), Melanops, Saccharata and Kellermania had a basal position on the tree with respect to other genera in the Botryosphaeriales. The remaining genera in the Botryosphaeriales clustered together with a bootstrap support value of 94 % and Posterior Probability (PP) value of 1. The first cluster to split from the rest of the main group was formed by species of Phyllosticta, hereafter treated as Phyllostictaceae (see Wikee et al. 2013b, this volume). The main clade below Phyllostictaceae was defined as Botryosphaeriaceae s. str. (0.99 PP and 89 % bootstrap).
Fig. 1.
Phylogenetic relationships of the Botryosphaeriales using Bayesian reconstruction and six gene portions (LSU, SSU, ITS, EF1, BT and mtSSU). Numbers above branches indicate bootstrap values/posterior probabilities. Numbers highlighted in red below branches indicate estimated dates in million years with the 95 % Highest Posterior Density interval given in brackets. Clades 1-4 in the Botryosphaeriaceae are indicated by a circled number on the corresponding node.
Pseudofusiccocum was basal within the Botryosphaeriaceae, followed by Endomelanconiopsis. The remaining species formed a clade having strong bootstrap support v (99 %) and could be further subdivided into four sub-clades. Sub-clade 1 encompassed species of the genera Diplodia, Neodeightonia, Lasiodiplodia, Macrovalsaria, Phaeobotryosphaeria, Phaeobotryon, Barriopsis, Botryobambusa and Tiarosporella. The recently described Auerswaldia lignicola clustered in sub-clade 1, together with Lasiodiplodia. Sub-clade 2 encompassed species of Neoscytalidium, Cophinforma, Botryosphaeria (= Fusicoccum) and Macrophomina together with Dichomera saubinetti. Sub-clade 3 accommodated species in the genera Dothiorella, Spencermartinsia and the recently described Auerswaldia dothiorella. Sub-clade 4 included species of Neofusicoccum and Dichomera.
Molecular dating
Based on the models used, families split between 57 (28-100) - 103 (45-188) mya. Divergence within some families was also very ancient, such as the split between Pseudofusicoccum [65 (28-112) mya] and Endomelanconiopsis [52 (27-78) mya] and the rest of the Botryosphaeriaceae. The split between sub-clades 1 to 4 within the Botryosphaeriaceae was estimated to be between 33 (15-55) and 44 (25-64) mya.
Analysis of phylogeny-trait association
None of the five morphological traits that were investigated had a significant phylogenetic signal (Table 2; Fig. 2A-E).
Table 2.
Test of phylogenetic signal for each of the 5 traits investigated using Parsimony and Maximum Likelihood reconstructions.
| Phylogenetic Signal |
Parsimony (number of steps) |
Maximum Likelihood (-log likelihood values) |
||||
|---|---|---|---|---|---|---|
| Observed | Expected Mean (Range) | P-value | Observed | Expected Mean (Range) | P-value | |
| Ascospore colour | 3 | 5,89 (3-7) | ns | 11,35 | 12,87 (10,6-13,7) | ns |
| Ascospore septation | 4 | 5,29 (3-6) | ns | 11,7 | 12,79 (8,9-13,7) | ns |
| Conidial colour | 6 | 8,99 (5-13) | ns | 15,51 | 17,76 (15,1-18,4) | ns |
| Conidial septation | 8 | 8,90 (5-12) | ns | 18,02 | 17,77 (15,8-18,6) | ns |
| Mucus | 4 | 3,98 (2-4) | ns | 13,59 | 14,09 (8,0-18,1) | ns |
Fig. 2.
Ancestral state reconstruction using parsimony and mapping the five traits onto the phylogenetic tree. Traits are coded as presence (in black) or absence (in white).
Taxonomy
Based on the phylogenetic distinctions found in this study, as well as the morphological and in some cases ecological distinction between the major groups in the Botryosphaeriales, six families are recognised. Of these, the Planistromellaceae (accommodating Kellermania) and Phyllostictaceae (accommodating Phyllosticta) are accepted as previously described (Minnis et al. 2012, Wikee et al. 2013b, this volume). The Botryosphaeriaceae is redefined, while the Aplosporellaceae, Saccharataceae and Melanopsaceae are newly described.
Botryosphaeriaceae Theis. & P. Syd., Ann. Mycol. 16: 16. 1918.
Type genus: Botryosphaeria Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 211. 1863.
Type species: B. dothidea (Moug.: Fr.) Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 212. 1863.
Genera included based on support by DNA sequence data: Barriopsis, Botryobambusa, Botryosphaeria (= Fusicocccum, incl. Dichomera pro parte), Cophinforma, Dothiorella, Diplodia, Endomelanconiopsis, Lasiodiplodia (incl. Auerswaldia, Macrovalsaria), Macrophomina, Neodeightonia, Neofusicoccum (incl. Dichomera pro parte), Neoscytalidium, Phaeobotryon, Phaeobotryosphaeria, Pseudofusicoccum, Spencermartinsia, Tiarosporella.
Genera lacking DNA sequence data: Auerswaldiella, Leptoguignardia, Microdiplodia, Phyllachorella, Pyrenostigma, Septorioides, Sivanesania, Thyrostroma, Vestergrenia (Liu et al. 2012).
Ascostromata uni- to multilocular, solitary or in clusters, fully or partially erumpent at maturity, with multi-layered, dark brown walls, infrequently embedded in stromatic tissue. Asci bitunicate, fissitunicate, chiefly 8-spored, with a thick endotunica and well-developed apical chamber, short stipitate, clavate. Pseudoparaphyses intermixed with asci, hyaline, septate, frequently constricted at septa, hyphae-like, branched or not, frequently deliquescing at maturity. Ascospores 2-3 seriate, hyaline to pigmented, smooth to verruculose, septate or not, fusoid to ellipsoid or ovoid, with or without a mucoid sheath or rarely with appendages. Asexual morphs mostly have uni-, rarely multilocular pycnidial conidiomata, infrequently embedded in stromatic tissue. Conidiophores mostly reduced to conidiogenous cells. Conidiogenous cells hyaline, phialidic, proliferating percurrently or via periclinal thickening, with or without collarettes. Conidia hyaline to pigmented, aseptate, one or multi-septate, sometimes muriform, smooth or striate, thin to thick-walled, and sometimes with mucoid sheaths or appendages. Synasexual morphs coelomycetous or hyphomycetous (see Crous et al. 2006). Spermatogonia similar to conidiomata in anatomy. Spermatogenous cells ampulliform to lageniform or subcylindrical, hyaline smooth, phialidic. Spermatia developing in conidiomata or spermatogonia, hyaline, smooth, granular, subcylindrical or dumbbell-shaped, with rounded ends.
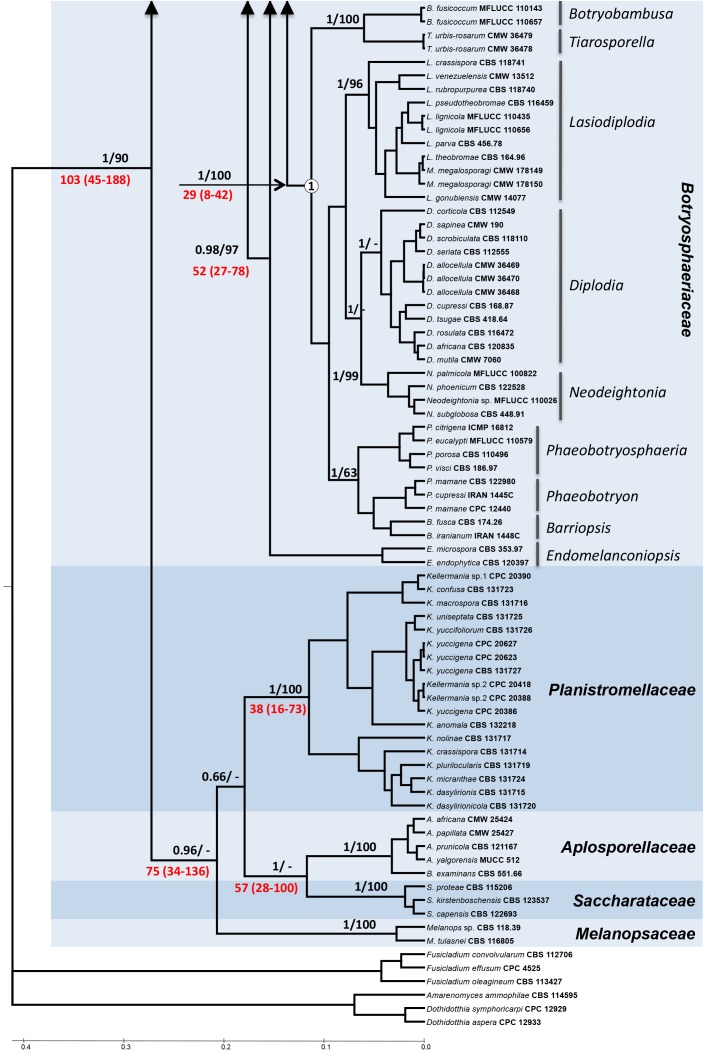
Saccharataceae Slippers, Boissin & Crous, fam. nov. MycoBank MB805794. Fig. 3.
Fig. 3.
Saccharataceae (Saccharata proteae, CBS 121406). A. Symptomatic leaves with tip die-back. B. Superficial view of immersed ascomata, showing clypeus-like structure. C, D. Asci and ascospores. E-G. Conidiogenous cells and paraphyses. H, I. Conidia and spermatia. Scale bars = 10 μm.
Type genus: Saccharata Denman & Crous, In: Crous et al., CBS Biodiversity Ser. (Utrecht) 2: 104. 2004.
Type species: S. proteae (Wakef.) Denman & Crous, In: Crous et al., CBS Biodiversity Ser. (Utrecht) 2: 104. 2004.
Genus supported by DNA sequence data: Saccharata.
Ascomata pseudothecial, unilocular, solitary or in clusters, with multilayered dark brown walls, infrequently embedded in stromatic tissue, with upper ascomatal layer darkened and thickened. Asci bitunicate, fissitunicate, 8-spored, with a thick endotunica, stalked or sessile, clavate, with a well-developed apical chamber. Pseudoparaphyses intermixed with asci, hyaline, septate, hyphae-like, branched or not. Ascospores hyaline to pigmented, granular, septate or not, ellipsoid to ovoid, without mucoid appendages or sheath. Asexual morph has unilocular pycnidial conidiomata, infrequently embedded in stromatic tissue with thickened, darkened upper layer. Conidiophores sparingly branched, hyaline, subcylindrical, or reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth, phialidic, proliferating via periclinal thickening or percurrent proliferation, with or without collarettes. Conidia hyaline, thin-walled, granular, fusoid, aseptate. Synasexual morph formed in separate conidiomata, or in same conidiomata with asexual morph. Synasexual conidia pigmented, thick-walled, finely verruculose, ellipsoid or oval, aseptate. Spermatogonia similar to conidiomata in anatomy. Spermatogenous cells ampulliform to lageniform or subcylindrical, hyaline smooth, phialidic. Spermatia developing in conidiomata or spermatogonia, hyaline, smooth, granular, subcylindrical or dumbbell-shaped, with rounded ends.
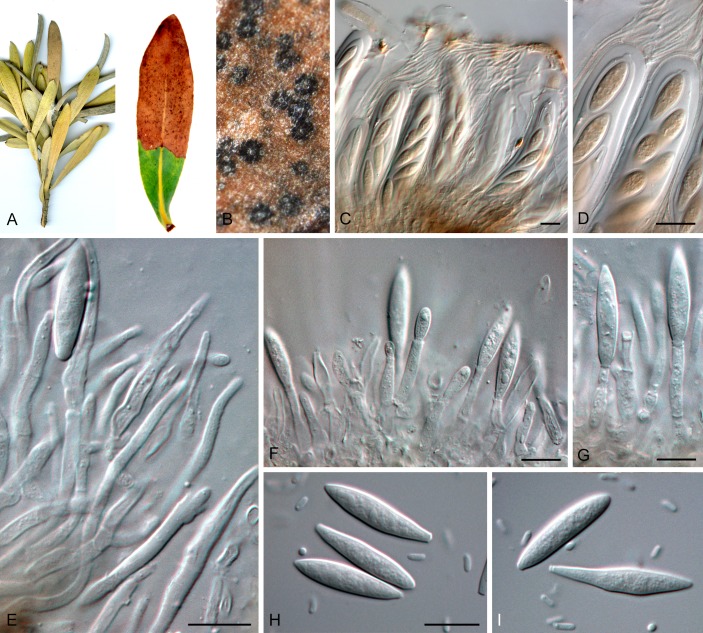
Aplosporellaceae Slippers, Boissin & Crous, fam. nov. MycoBank MB805795. Fig. 4.
Fig. 4.
Aplosporellaceae (Aplosporella prunicola, CBS 121167). A, B. Oozing spore masses from submerged conidiomata. C. Transverse section through multilocular conidioma. D, E. Conidiogenous cells. F. Paraphyses. H. Conidia and branched paraphyses. G-J. Conidia. Scale bars: A-C = 250 μm, F = 20 μm, D, E, G-J = 10 μm (adapted from Damm et al. 2007).
Type genus: Aplosporella Speg., Anal. Soc. cient. argent. 10(5-6): 158. 1880.
Type species: A. chlorostroma Speg., Anal. Soc. cient. argent. 10(5-6): 158. 1880.
Genera supported by DNA sequence data: Aplosporella, Bagnisiella.
Ascomata pseudothecial, mostly multilocular with multilayered dark brown walls, embedded in stromatic tissue. Asci bitunicate, with a thick endotunica, stalked or sessile, clavate, with a well-developed apical chamber, intermixed with hyaline, septate, hyphal-like pseudoparaphyses, branched or not. Ascospores hyaline to pigmented, septate or not, ellipsoid to ovoid, without mucoid appendages or sheath. Asexual morphs with uni- to multilocular pycnidial conidiomata, embedded in stromatic tissue. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, phialidic, proliferating percurrently or with periclinal thickening at apex. Paraphyses present or absent, hyaline, smooth-walled, septate, branched or not, hyphae-like. Conidia ellipsoid to subcylindrical, initially hyaline becoming pigmented, aseptate, thin-walled and smooth, becoming thick-walled and spinulose.
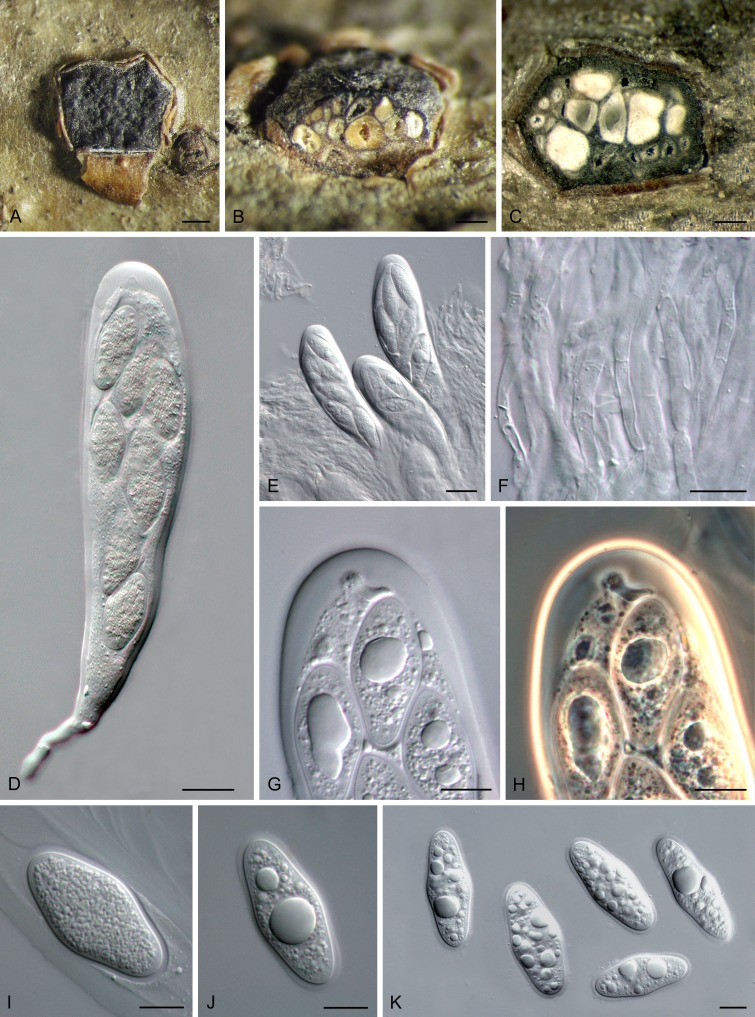
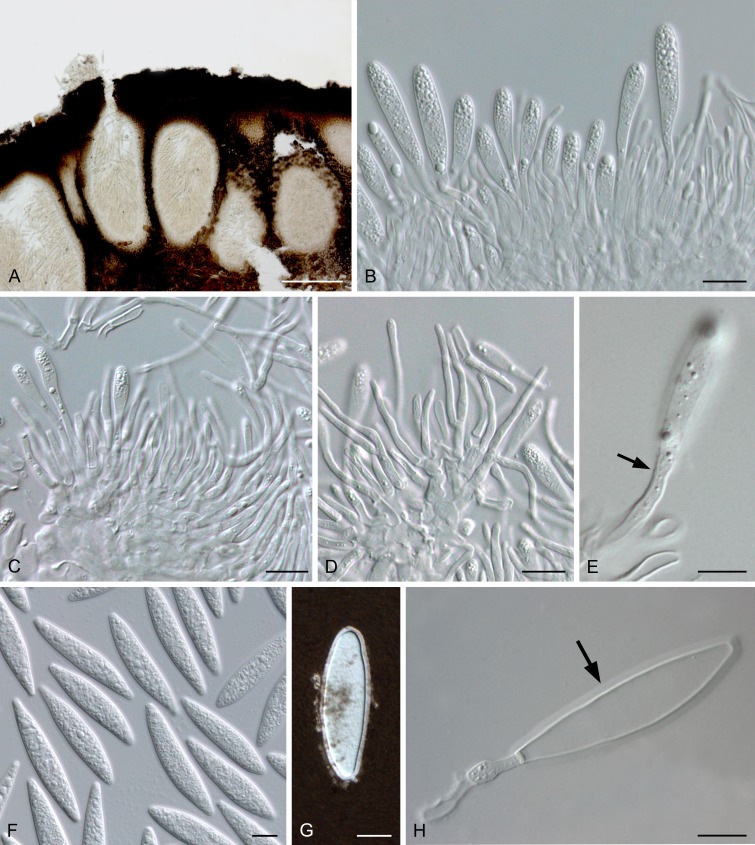
Melanopsaceae Phillips, Slippers, Boissin & Crous, fam. nov. MycoBank MB805796. Figs 5, 6.
Fig. 5.
Melanopsaceae (Melanops tulasnei, LISE 95179). A. Stroma erumpent through bark. B, C. Sections through stromata revealing ascomata and conidiomata. D. Ascus. E. Asci and pseudoparaphyses. F. Pseudoparaphyses. G, H. Ascus tips viewed under differential interference contrast (G) and phase contrast (H). I-K. Ascospores. Scale bars: A-C = 250 μm, D, E = 20 μm, F-K = 10 μm (adapted from Phillips & Alves 2009).
Fig. 6.
Melanopsaceae (Melanops tulasnei, LISE 95179). A. Section through conidiomata. B. Conidiogenous layers with developing conidia among paraphyses. C. Immature conidiogenous cells. D. Paraphyses. E. Conidiogenous cell with percurrent proliferations (arrowed). F. Conidia. G. Conidium in indian ink, revealing sheath. H. Conidium attached to conidiogenous cell with mucus sheath (arrowed). Scale bars: A = 200 μm, B-D, F, G = 10 μm, E, H = 5 μm (adapted from Phillips & Alves 2009).
Type genus: Melanops Nitschke ex Fuckel, In: Fuckel, Jahrb. Nassau. Ver. Naturk. 23-24: 225. 1870.
Type species: Melanops tulasnei Nitschke, In: Fuckel, Jahrb. Nassau. Ver. Naturk. 23-24: 225. 1870.
Genus supported by DNA sequence data: Melanops (see Phillips & Alves 2009).
Ascomata pseudothecial, multiloculate, immersed, partially erumpent at maturity, black, subglobose, thick-walled; wall composed of thick-walled textura angularis. Asci 8-spored, bitunicate, fissitunicate, stipitate, clavate. Pseudoparaphyses hyaline, thin-walled, hyphal-like, septate. Ascospores hyaline, aseptate, thin-walled, ellipsoid to rhomboid, with a persistent mucus sheath. Conidiomata indistinguishable from ascomata and often formed in the same stroma. Paraphyses hyaline, septate, branched or not, filiform, arising from between the conidiogenous cells. Conidiophores hyaline, smooth, 1-2-septate, branched or not, or reduced to conidiogenous cells. Conidiogenous cells subcylindrical, hyaline, branched or unbranched, discrete, formed from the inner wall of the conidioma, proliferating percurrently at apex, or with periclinal thickening. Conidia hyaline, aseptate, fusoid, with a persistent mucus sheath, rarely with minute marginal frill.
Phyllostictaceae Fr. (as “Phyllostictei”), Summa veg. Scand., Section Post. (Stockholm): 420. 1849.
Type genus: Phyllosticta Pers., Traité sur les Champignons Comestibles (Paris): 55. 147. 1818.
Type species: P. convallariae Pers., Traité sur les Champignons Comestibles (Paris): 148. 1818.
Genus supported by DNA sequence data: Phyllosticta (see Wikee et al. 2013b, this volume).
Planistromellaceae M.E. Barr, Mycotaxon 60: 433. 1996. Fig. 7.
Fig. 7.
Planistromellaceae (Kellermania yuccigena, CPC 20627). A. Conidiomata sporulating on OA. B, C. Conidiogenous cells showing percurrent proliferation. D. Spermatia. E-G. One-septate macroconidia with apical appendages. Scale bars = 10 μm.
Type genus: Planistromella A.W. Ramaley, Mycotaxon 47: 260. 1993.
Type species: P. yuccifoliorum A.W. Ramaley, Mycotaxon 47: 261. 1993 (= Kellermania yuccifoliorum)
Genus supported by DNA sequence data: Kellermania (= Alpakesa, Piptarthron, Planistroma, Planistromella, Septoplaca (possibly), see Minnis et al. 2012).
Ascomata pseudothecial, multi- or uniloculate, immersed to erumpent, solitary to gregarious, with papillate, periphysate ostiole; walls of several layers of dark brown textura angularis. Hamathecium mostly lacking pseudoparaphyses at maturity. Asci 8-spored, bitunicate, fissitunicate, thick-walled, oblong to clavate or subcylindrical, stipitate, with well-developed ocular chamber. Ascospores 1-3-seriate, hyaline or pale brown, guttulate, ellipsoid to broadly obovoid, aseptate or with 1-2 transverse septa, thin-walled, with or without a gelatinous sheath. Conidiomata pycnidial to acervular, subepidermal, dark brown, immersed to semi-erumpent, solitary to gregarious; wall comprising several layers with cells of dark brown textura angularis, becoming hyaline towards the inner region. Conidiophores reduced to conidiogenous cells. Conidiogenous cells ampulliform to sub-cylindrical, hyaline, smooth, phialidic, proliferating via percurrent proliferation or periclinal thickening. Conidia obclavate to ellipsoid-cylindrical, aseptate or transversely multiseptate, hyaline to brown, smooth to verruculose, with or without one or more apical appendages, a persistent mucoid sheath, and a basal marginal frill. Spermatogonia similar to conidiomata in anatomy. Spermatogenous cells ampulliform to lageniform or subcylindrical, hyaline smooth, phialidic. Spermatia developing in conidiomata or spermatogonia, hyaline, smooth, granular, sub-cylindrical or dumbbell-shaped, with rounded ends.
DISCUSSION
Using the DNA sequence data for the six loci analysed in this study, together with unique morphological and ecological characteristics (as discussed below), we have distinguished six families in the Botryosphaeriales. The Planistromellaceae that has recently been defined based on DNA sequence data (Minnis et al. 2012) has been retained. Furthermore, the Aplosporellaceae, Melanopsaceae, and Saccharataceae are distinguished from the Botryosphaeriaceae and introduced as novel families. These families are also phylogenetically distinct from the Phyllostictaceae, which has been defined in a separate study (Wikee et al. 2013b, this volume).
Botryosphaeriaceae
The Botryosphaeriaceae as it has been defined in this study includes the type genus Botryosphaeria (asexual morph Fusicoccum), as well as 16 other genera. This group corresponds to a group traditionally referred to as botryosphaeria-like. This term is, however, now understood to be much more restricted, including B. dothidea and a few closely related species (as defined in Slippers et al. 2004, Crous et al. 2006, Phillips et al. 2013, this volume). Using Botryosphaeria to refer to the assemblage of genera including Diplodia, Lasiodiplodia, Neofusicoccum and others, of which the sexual morphs were formerly described in Botryosphaeria, is thus taxonomically incorrect. These groups are now referred to using a single genus name, which is typically the asexual morph, irrespective of whether a sexual morph is known or not. This convention has been applied subsequent to the taxonomic changes introduced by Crous et al. (2006), and is also consistent with the recent decisions to abolish the dual nomenclatural system for fungal taxonomy (Hawksworth et al. 2011, Wingfield et al. 2012).
The data presented in this study, together with those emerging from more focused earlier studies, as well as the recent changes resulting in the abandonment of a dual nomenclature, necessitates reducing a number of genera in the Botryosphaeriaceae to synonymy with others. Most importantly, there is no longer just cause to maintain Botryosphaeria and Fusicoccum as distinct genera. In the interests of maintaining taxonomic stability, and the fact that B. dothidea is the type species of the order and family, it is recommended that Botryosphaeria be retained (Slippers et al. 2004, Schoch et al. 2006, Phillips et al. 2013, this volume). Botryosphaeria must thus be redefined to include species where only the asexual (Fusicoccum and dichomera-like) morphs are known. Species such as F. ramosum and Dichomera saubinetti must then be redefined in Botryosphaeria. For F. ramosum, an ex-type isolate was available and it has thus been redescribed in Botryosphaeria in a companion paper (Phillips et al. 2013, this volume).
Dichomera is polyphyletic and most likely also includes synasexual morphs of other genera. Two of the species included in this analysis, D. eucalypti and D. versiformis, clearly group in Neofusicoccum and we consider them as synonyms of species in this genus. Dichomera saubinetti grouped with Botryosphaeria and should be redescribed in this genus. Unfortunately no ex-type isolates of these species are presently available.
A number of genera grouped in Lasiodiplodia s. lat. in our analyses and are possibly synonyms of this genus. Macrovalsaria (see Sivanesan 1975) clearly grouped amongst species of Lasiodiplodia. This was also pointed out by Liu et al. (2012), but they did not find the available LSU and SSU data sufficiently convincing to make taxonomic changes. Isolates of Macrovalsaria however, grouped extremely closely with L. theobromae and we view them as representing a synonym of Lasiodiplodia, rather than Lasiodiplodia being polyphyletic. Lasiodiplodia and Macrovalsaria are both tropical fungi. Our analyses differ from those of Liu et al. (2012), indicating that Auerswaldia is a synonym of Lasiodiplodia. This was also confirmed in Phillips et al. (2013, this volume), who redescribed A. lignicola as L. lignicola. These findings suggest that the taxonomy of Lasiodiplodia needs to be re-evaluated, but for the present we do not recognize Auerswaldia as a genus in the Botryosphaeriaceae.
Diplodia juglandis and D. corylii both grouped in Dothiorella, which is consistent with the results of a previous study (Phillips et al. 2008). Type specimens were not available for these species and they were, therefore, not re-described. These species require epitypification. It is likely that a number of other Diplodia species will similarly reside in Dothiorella or Spencermartinsia, or vice versa, given the confusion of these names in the past (Phillips et al. 2005, 2008). The conidia of these genera remain difficult to distinguish, which can create problems when interpreting older descriptions or poorly preserved herbarium specimens.
There was no statistically significant pattern that could be discerned in the Botryosphaeriaceae with respect to the evolution of hyaline or pigmented conidia or ascospores. These characters appear to be more or less randomly spread amongst the clades of this section of the phylogenetic tree for the family. This would suggest that these characters predate the divergence of the genera in this family, and that they have been independently lost or suppressed (character not expressed under all conditions) in different groups. This would also explain the “appearance” of darker or even dark muriform conidia in genera such as Botryosphaeria and Neofusicoccum that were traditionally considered not to have such synasexual morphs (Barber et al. 2005, Phillips et al. 2005b, Crous et al. 2006). Clearly these characters, which have traditionally been commonly used for phylogenetic and taxonomic purposes, have very little phylogenetic and taxonomic value above the genus level.
The distinction and more narrow definition of the Botryosphaeriaceae is important when considering the economic and ecological importance of this group. Many of the Botryosphaeriaceae species share a common ecology in being endophytic and latent pathogens in virtually all parts of woody plants (Slippers & Wingfield 2007). While not all species have been isolated as endophytes, or from all plant parts, most of those that have been carefully studied have conformed to this pattern, and it is thus expected for the group as a whole. Many of the genera in the family are also very widespread, with wide host ranges and broad levels of environmental tolerance (e.g. N. parvum, N. australe, B. dothidea, L. theobromae, L. pseudotheobromae). Sakalidis et al. (2013) for example reported N. parvum from 90 hosts in 29 countries on six continents. This broad ecological range, together with their cryptic nature as endophytes, makes these fungi important to consider as a group prone to being spread with living plant material. Ample evidence exists that these fungi can infect both native and non-native trees, once they have been introduced into a region. The observed (Dakin et al. 2010, Piskur et al. 2011) and expected (Desprez-Loustau et al. 2006) increase of the importance of this group due to pressure on plant communities as a result of climate change provides another reason to focus future efforts on characterising the diversity, distribution and pathogenicity of this group of fungi.
Aplosporellaceae
An unexpected outcome of this study was the consistent connection between Aplosporella and Bagnisiella and their distinction from other members of the Botryosphaeriales. While it has been suggested that some Aplosporella spp. might be asexual morphs of Bagnisiella, this connection has never been proven. Neither of these genera were treated in molecular phylogenetic re-evaluations of the Botryosphaeriaceae until very recently. The first analyses to include DNA sequence data for Aplosporella (Damm et al. 2007, Liu et al. 2012) and Bagnisiella (Schoch et al. 2009) hinted at a distant relationship with other Botryosphaeriales. However, none of these studies included both genera. The phylogenetic relationship between these genera revealed in this study is further supported by their remarkably similar multiloculate sporocarps, expressed in both the asexual morphs and sexual morphs, which is thus not the product of parallel evolution in two distinct groups. There are, however, undoubtedly many species of Aplosporella and Bagnisiella that would not be connected to the phylogenetic clade identified here, because both genera are heterogeneous and likely contain unrelated species.
The data presented in this study suggest that Aplosporella and Bagnisiella are not only related, but that they should be synonymized. Both genera were described in 1880, and historical precedence can thus not be used to choose an appropriate genus. Aplosporella includes many more species (352) than Bagnisiella (65) (www.MycoBank.org, accessed August 2013). More species have also recently been described in the former genus, possibly because the asexual structures are more common than the sexual structures (as is found in other Botryosphaeriales). An argument based on taxonomic stability, relevance and frequency of occurrence is thus favoured and has led us to decide that Bagnisiella should be reduced to synonymy with Aplosporella.
Examination of the distribution of species of Aplosporella and Bagnisiella is complicated by the fact that the literature is old (which means the taxonomic accuracy is difficult to judge) and commonly lacking relevant information. However, most of the well-known and recently characterised species, and all species included here, are from the Southern Hemisphere (Damn et al. 2007, Taylor et al. 2009). This result suggests the possibility of a Southern Hemisphere and Gondwanan origin and divergence pattern, which should be considered in future studies based on more robust sampling.
Melanopsaceae
Melanops tulasnei and an undescribed Melanops sp. grouped most basal in the Botryosphaeriales, together with the Aplosporellaceae, Planistromellaceae and Saccharataceae. This group is unique amongst these families in having persistent mucous sheath around its ascospores and conidia (Phillips & Alves 2009). Conidia in M. tulasnei are typically hyaline and fusoid and it resembles the Aplosporellaceae in having multiloculate ascomata and conidiomata, often with locules at different levels. Little is known regarding the ecology and distribution of this group, given the paucity of recent reports that could be verified using DNA sequence data. It appears similar, however, to other Botryosphaeriales that infect woody tissue of plants, and sporulates on the dead tissue. Whether it is pathogenic or endophytic is not known.
Saccharataceae
Saccharata (the only genus in the Saccharataceae) grouped separately from all other families that were basal in the phylogenetic tree, suggesting a long, separate evolutionary history. The genus was first described by Crous et al. (2004) from Proteaceae in the South Western Cape region of South Africa. Subsequently, three additional species were added to the genus, two also from Proteaceae and one from Encephalartos (Marincowitz et al. 2008, Crous et al. 2008, 2009). All known species are thus from the same region on indigenous flora. These species are typically associated with leaf spots and stem cankers and they appear to be pathogens. Separate studies have also shown that they are endophytes (Swart et al. 2000, Taylor et al. 2001), similar to members of the Botryosphaeriaceae.
Apart from its restricted distribution and host range, Saccharata is also unique in its asexual morphology, which includes a hyaline, fusicoccum-like and a pigmented diplodia-like asexual morph. These characters are shared with its related families; fusicoccum-like conidia in Melanopsaceae and pigmented diplodia-like conidia in Aplosporellaceae. It is clear that these variations in conidial morphology are very old (tens of millions of years) ancestral characters that must have existed prior to the divergence of this group from other Botryosphaeriales. It is thus remarkable how similar, especially in the fusicoccum-like conidia and ascospore morphology, the spores of these fungi have remained over time. The diplodia-like state is somewhat different from other Botryosphaeriales in that the conidia are typically almost half the size of other Diplodia conidia. We do not currently have enough data to address the selective pressure that could have played a role in the development these interesting morphological changes.
Phyllostictaceae and Planistromellaceae
Phyllosticta clearly warrants a separate family to accommodate this morphologically and ecologically unique, widespread and economically important genus in the Phyllostictaceae. These fungi typically infect leaves and fruit, rather than woody tissue, and they can cause serious damage (Glienke et al. 2011, Wong et al. 2012). Species of Phyllosticta are also known to have an endophytic phase (Wikee et al. 2013a), as is true for most other Botryosphaeriales. The Phyllostictaceae is also morphologically unique in terms of the ascospores and conidia (Van der Aa & Vanev 2002). The species included in this study are only representative of a small extent of the diversity in this group and a more complete analysis of the Phyllostictaceae is presented in Wikee et al. (2013b, this volume).
The Planistromellaceae has previously been recognised as distinct within the Botryosphaeriales (Minnis et al. 2012) and this is supported by the analyses in the present study. The family is currently considered to include only species of Kellermania. Species in the Planistromellaceae have unique conidia with fairly long appendages that are quite distinct from other genera in the Botryosphaeriales. Kellermania spp. are mostly leaf infecting, and one species has been associated with Yucca Leaf Blight in California and Florida in the USA (Horst 2008). Species are commonly collected sporulating on dead leaves of Agavaceae, and appear to be endophytic, as they have been isolated from healthy leaves in many countries where Yucca spp. are grown as ornamentals (P.W. Crous, unpubl data). Minnis et al. (2012) have also shown apparent patterns of host specificity and co-evolution with major plant lineages. As is true for many other families in the Botryosphaeriales, additional sampling is needed for a better understanding of species diversity, host range and geographic distribution.
Molecular dating
The molecular dating on the radiations within the Botryosphaeriales in this study, based on general estimated mutation rates of the rDNA SSU locus by Taylor & Berbee (2010), must be viewed as preliminary. From the dating that was conducted, the group appears to have originated in the Cretaceous period around 103 (45-188) mya, with most of the subsequent diversification within the families occurring in the Tertiary period. This date is well within the estimated date of the emergence of the Dothideomycetes (Berbee & Taylor 2010, Gueidan et al. 2011). This coincides with important periods of Angiosperm radiation and spread, which is the main group of plants on which these fungi are found and that might be expected to have influenced the diversification of these fungi. Of particular relevance is the rapid diversification of the Eurosid and other dominant woody Angiosperm groups and their prominence in Angiosperm dominated forests from around 110 mya onwards (Soltis et al. 2008, Fawcett et al. 2009, Wang et al. 2009, Bell et al. 2010). De Wet et al. (2008) pointed out that the members of the Botryosphaeriaceae are most diverse on Angiosperms, and showed that ancestral state reconstruction suggests that this is the main group of plants on which the Botryosphaeriaceae co-evolved. A much smaller number of species, especially those in Diplodia, occur commonly on coniferous hosts and appear to have emerged and diversified more recently. They also tend to be more host-specific than some of the other genera discussed above that are more common on Angiosperms (De Wet et al. 2008, Sakalidis et al. 2013), suggesting a specific acquired trait that allowed them to infect coniferous hosts.
The major changes in dominant plant hosts in forests globally during the Cretaceous period would be expected to have influenced fungal evolution beyond the Botryosphaeriales, and this indeed appears to be the case. For example, studies on another Dothideomycetes order, the sooty molds in the Capnodiales, also appear to have been influenced by the rise of Angiosperm forests in the Cretaceous period (Schmidt et al. 2013). Furthermore, the divergence of a prominent fungal complex, Fusarium, was estimated to be later at around 93 mya, and is also thought to have been influence by this Angiosperm divergence (O’Donnell et al. 2013).
Molecular dating, together with the geographic distribution (and restriction) of different groups in the Botryosphaeriales should provide rich information to explore in future to understand the patterns that have shaped the diversity of this important group of plant-associated fungi. For example, the Saccharataceae has previously been known only from southern Africa, and is most diverse on the Proteaceae. Recent research has shown, however, that it has also been introduced as endophyte into other countries where South African Proteaceae are now being cultivated (Marincowitz et al. 2008). It is known that this plant family, which has a high endemic richness in southern Africa, has evolved in the region for more than 100 million years (Barker et al. 2007). This date allows for the estimated 57 (28-100) mya (based on rDNA SSU) of separation of the Saccharataceae to have evolved with these endemic plant hosts in the region.
Diversification within the most diverse family, the Botryosphaeriaceae, occurred between 52-65 (27-112) mya for the two earliest diverging (and least diverse) genera, Pseudofusicoccum and Endomelanconiopsis. These dates correspond to the diversification between some other families in the order [e.g. the Aplosporellaceae, Melanopsaceae, Planistromellaceae and Saccharataceae that split between 57-75 (28-136) mya]. At present, however, there does not appear sufficiently robust morphological or ecological validation for a further split of the Botryosphaeriaceae to accommodate Pseudofusicoccum and Endomelanconiopsis in distinct families. If these genera were to be considered as residing in distinct families, the question would arise as to whether further family level distinction in the Botryosphaeriaceae is necessary. The other major lineages within the Botryosphaeriaceae (clades 1-4) appeared around 11-35 (3-58) mya. The most recent diversification for which there was support was within Neofusicoccum clade, dated at around 11 (3-23) mya.
This study has provided a systematic framework for future taxonomic and ecological studies of the Botryosphaeriales. It has also highlighted a number of interesting host association and geographic patterns amongst the genera that are worthy of further investigation. It is hoped that this framework, in conjunction with the growing body of DNA-based sequence data reflecting the species diversity and their distribution will ultimately lead to a model supporting an improved understanding of the co-evolution of woody plants and their fungal endophytes/latent pathogens.
Acknowledgments
We thank members of the Tree Protection Co-operative Programme (TPCP), the DST/NRF Centre of Excellence in Tree Health Biotechnology (CTHB), THRIP (project no. TP2009061100011) and the University of Pretoria, South Africa for financial support. We also thank Ms Katrin Fitza and Ms Fahimeh Jami for assistance with submissions of sequences.
REFERENCES
- Aa HA van der, Vanev S. (2002). A revision of the species described in Phyllosticta. (Aptroot A, Summerbell RC, Verkley GJ, eds) CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands: [Google Scholar]
- Abdollahzadeh J, Mohammadi Goltapeh E, Javadi A, Shams-bakhsh M, Zare R, Phillips AJL. (2009). Barriopsis iraniana and Phaeobotryon cupressi: two new species of the Botryosphaeriaceae from trees in Iran. Persoonia 23: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves A, Crous PW, Correia A, Phillips AJL. (2008). Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Diversity 28: 1–13 [Google Scholar]
- Barber P, Burgess T, Hardy G, Slippers B, Keane P, Wingfield MJ. (2005). Botryosphaeria species from Eucalyptus in Australia are pleoanamorphic, producing Dichomera synanamorphs in culture. Mycological Research 109: 1347–1363 [DOI] [PubMed] [Google Scholar]
- Barker NP, Weston PH, Rutschmann F, Sauquet H. (2007). Molecular dating of the ‘Gondwanan’ plant family Proteaceae is only partially congruent with the timing of the break-up of Gondwana. Journal of Biogeography 34: 2012–2027 [Google Scholar]
- Bell CD, Soltis DE, Soltis PS. (2010). The age and diversification of the Angiosperms re-revisited. American Journal of Botany 97: 1296–1303 [DOI] [PubMed] [Google Scholar]
- Berbee ML, Taylor JW. (2010). Dating the molecular clock in fungi - how close are we? Fungal Biology Reviews 24: 1–16 [Google Scholar]
- Bihon W, Burgess T, Slippers B, Wingfield MJ, Wingfield BD. (2011). Distribution of Diplodia pinea and its genotypic diversity within asymptomatic Pinus patula trees. Australasian Plant Pathology 40: 540–548 [Google Scholar]
- Bull JJ, Huelsenbeck JP, Cunningham CW, Swofford DL, Waddell PJ. (1993). Partitioning and combining data in phylogenetic analysis. Systematic Biology 42: 384–397 [Google Scholar]
- Crous PW, Denman S, Taylor JE, Swart L, Palm ME. (2004). Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea. CBS Biodiversity Series 2: 1–228 CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands: [Google Scholar]
- Crous PW, Groenewald JZ, Taylor JE. (2009). Fungal Planet 43 - Saccharata intermedia. Persoonia 23: 198–199 [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ. (2006). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wood AR, Okada G, Groenewald JZ. (2008). Foliicolous microfungi occurring on Encephalartos. Persoonia 21: 135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CW. (1997). Can three incongruence tests predict when data should be combined? Molecular Biology and Evolution 14: 733–740 [DOI] [PubMed] [Google Scholar]
- Dakin N, White D, Hardy GStJ, Burgess TI. (2010). The opportunistic pathogen, Neofusicoccum australe, is responsible for crown dieback of peppermint (Agonis flexuosa) in Western Australia. Australasian Plant Pathology 39: 202–206 [Google Scholar]
- Damm U, Fourie PH, Crous PW. (2007). Aplosporella prunicola, a novel species of anamorphic Botryosphaeriaceae. Fungal Diversity 27: 3543 [Google Scholar]
- Denman S, Crous PW, Groenewald JZ, Slippers B, Wingfield BD, Wingfield MJ. (2003). Circumscription of Botryosphaeria species associated with Proteaceae based on morphology and DNA sequence data. Mycologia 95: 294–307 [PubMed] [Google Scholar]
- Desprez-Loustau ML, Marçais B, Nageleisen LM, Piou D, Vannini A. (2006). Interactive effects of drought and pathogens in forest trees. Annals of Forest Science 63: 597–612 [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, Bult C. (1995a). Constructing a significance test for incongruence. Systematic Biology 44: 570–572 [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, Bult C. (1995b). Testing significance of incongruence. Cladistics 10: 315–319 [Google Scholar]
- Fawcett JA, Maere S, Peer Y van de. (2009). Plants with double genomes might have had a better chance to survive the Cretaceous-Tertiary extinction event. Proceedings of the National Academy of Sciences of the United States of America 106: 5737–5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Applied and Environmental Microbiology 61: 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glienke C, Pereira OL, Stringari D, Fabris J, Kava-Cordeiro V, et al. (2011). Endophytic and pathogenic Phyllosticta species, with reference to those associated with Citrus Black Spot. Persoonia 26: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueidan C, Ruibal C, Hoog GS de, Schneider H. (2011) Rock-inhabiting fungi originated during periods of dry climate in the late Devonian and middle Triassic. Fungal Biology 115: 987–996 [DOI] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, Gascuel O. (2005). PHYML Online - a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Research 33: W557–W559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, et al. (2011). The Amsterdam declaration on fungal nomenclature. IMA Fungus 2: 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, et al. (2007). A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509–547 [DOI] [PubMed] [Google Scholar]
- Horst RK. (2008). Westcott’s Plant Disease Handbook. 7th Edn. Springer-Verlag, Dordrecht, Netherlands: [Google Scholar]
- Huelsenbeck JP, Bull JJ, Cunningham CW. (1996). Combining data in phylogenetic analysis. Trends in Ecology and Evolution 11: 152–158 [DOI] [PubMed] [Google Scholar]
- James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, et al. (2006). Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443: 818–822 [DOI] [PubMed] [Google Scholar]
- Katoh K, Toh H. (2008). Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9: 286–298 [DOI] [PubMed] [Google Scholar]
- Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, et al. (2012). Towards a natural classification of Botryosphaeriales. Fungal Diversity 57: 149–210 [Google Scholar]
- Maddison WP, Maddison DR. (2010). Mesquite: a modular system for evolutionary analysis. Version 2.74. Available from: http://mesquiteproject.org
- Marincowitz S, Groenewald JZ, Wingfield MJ, Crous PW. (2008). Species of Botryosphaeriaceae occurring on Proteaceae. Persoonia 21: 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnis AM, Kennedy AH, Grenier DB, Palm ME, Rossman AY. (2012). Phylogeny and taxonomic revision of the Planistromellaceae including its coelomycetous anamorphs: contributions towards a monograph of the genus Kellermania. Persoonia 29: 11–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander JAA. (2004). MrAIC.pl. Program distributed by the author, Evolutionary Biology Centre, Uppsala University, Sweden: [Google Scholar]
- O’Donnell K, Rooney AP, Proctor RH, Brown DW, McCormick SP, et al. (2013). Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genetics and Biology 52: 20–31 [DOI] [PubMed] [Google Scholar]
- Pavlic D, Slippers B, Coutinho TA, Wingfield MJ. (2009a). Molecular and phenotypic characterization of three phylogenetic species discovered within the Neofusicoccum parvum/N. ribis complex. Mycologia 101: 636–647 [DOI] [PubMed] [Google Scholar]
- Pavlic D, Slippers B, Coutinho TA, Wingfield MJ. (2009b). Multiple gene genealogies and phenotypic data reveal cryptic species of the Botryosphaeriaceae: A case study on the Neofusicoccum parvum/N. ribis complex. Molecular Phylogenetics and Evolution 51: 259–268 [DOI] [PubMed] [Google Scholar]
- Phillips A, Alves A, Correia A, Luque J. (2005). Two new species of Botryosphaeria with brown, 1-septate ascospores and Dothiorella anamorphs. Mycologia 97: 513–529 [DOI] [PubMed] [Google Scholar]
- Phillips AJL, Alves A. (2009). Taxonomy, phylogeny, and epitypification of Melanops tulasnei, the type species of Melanops. Fungal Diversity 38: 155–166 [Google Scholar]
- Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, et al. (2013). The Botryosphaeriaceae: genera and species known from culture. Studies in Mycology 76: 51–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, et al. (2008). Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21: 29–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJL, Rumbos JC, Alves A, Correia A. (2005b). Morphology and phylogeny of Botryosphaeria dothidea causing fruit rot of olives. Mycopathologia 159: 433–439 [DOI] [PubMed] [Google Scholar]
- Piskur B, Pavlic D, Slippers B, Ogris N, Maresi G, et al. (2011). Diversity and pathogenicity of Botryosphaeriaceae on declining Ostrya carpinifolia in Slovenia and Italy following extreme weather conditions. European Journal of Forest Research 130: 235–249 [Google Scholar]
- Sakalidis ML. (2004). Resolving the Botryosphaeria ribis-B. parva species complex; a molecular and phenotypic investigation. School of Biological Sciences and Biotechnology, Murdoch University, Western Australia: [Google Scholar]
- Sakalidis ML, Hardy GEStJ, Burgess TI. (2011). Use of the Genealogical Sorting Index (GSI) to delineate species boundaries in the Neofusicoccum parvum-Neofusicoccum ribis species complex. Molecular Phylogenetics and Evolution 60: 333–344 [DOI] [PubMed] [Google Scholar]
- Sakalidis ML, Slippers B, Wingfield BD, Hardy GEStJ, Burgess TI. (2013). The challenge of understanding the origin, pathways and extent of fungal invasions: global populations of the Neofusicoccum parvum-N. ribis species complex. Diversity and Distributions 19: 873–883 [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, et al. (2009). A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Shoemaker R, Seifert K, Hambleton S, Spatafora JW, Crous PW. (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–52 [DOI] [PubMed] [Google Scholar]
- Schmidt AR, Beimforde C, Seyfullah LJ, Wege S, Dörfelt H, et al. (2013). Review of Palaeobotany and Palynology Amber fossils of sooty moulds. Review of Palaeobotany and Palynology 200: 53–64 [Google Scholar]
- Sivanesan A. (1975). Redisposition and descriptions of some Amphisphaeria species and a note on Macrovalsaria. Transactions of the British Mycological Society 65: 395–402 [Google Scholar]
- Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ. (2004). Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96: 83–101 [PubMed] [Google Scholar]
- Slippers B, Roux J, Wingfield MJ, Walt FJJ van der, Jami F, Mehl JWM, Marais GJ. (2014). Confronting the constraints of morphological taxonomy in the fungi: A Botryosphaeriaceae case study. Persoonia: in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis DE, Bell CD, Kim S, Soltis PS. (2008). Origin and early evolution of angiosperms. Annals of the New York Academy of Sciences 1133: 3–25 [DOI] [PubMed] [Google Scholar]
- Swart L, Crous PW, Petrini O, Taylor JE. (2000). Fungal endophytes of Proteaceae, with particular emphasis on Botryosphaeria proteae. Mycoscience 41: 123–127 [Google Scholar]
- Swart WJ, Wingfield MJ. (1991). The biology and control of Sphaeropsis sapinea on Pinus species in South Africa. Plant Disease 75: 761–766 [Google Scholar]
- Swofford DL. (2003). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts: [Google Scholar]
- Taylor JE, Denman S, Crous PW. (2001). Endophytes isolated from three species of Protea in a nature reserve in the Western Cape, South Africa. Sydowia 53: 247–260 [Google Scholar]
- Taylor JW, Berbee ML. (2006). Dating divergences in the Fungal Tree of Life: review and new analyses. Mycologia 98: 838–849 [DOI] [PubMed] [Google Scholar]
- Taylor K, Barber PA, Hardy GESJ, Burgess TI. (2009). Botryosphaeriaceae from tuart (Eucalyptus gomphocephala) woodland, including the description of four new species. Mycological Research 113: 337–353 [DOI] [PubMed] [Google Scholar]
- Wang H, Moore MJ, Soltis PS, Bell CD, Brockington SF, et al. (2009). Rosid radiation and the rapid rise of angiosperm-dominated forests. Proceedings of the National Academy of Sciences of the United States of America 106: 3853–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, California: 315–322 [Google Scholar]
- Wikee S, Lombard L, Crous PW, Nakashima C, Motohashi K, et al. (2013a). Phyllosticta capitalensis, a widespread endophyte of plants. Fungal Diversity 60: 91–105 [Google Scholar]
- Wikee S, Lombard L, Nakashima C, Motohashi K, Chukeatirote E, et al. (2013b). A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Studies in Mycology 76: 1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield MJ, De Beer ZW, Slippers B, Wingfield BD, Groenewald JZ, et al. (2012). One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology 13: 604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M-H, Crous PW, Henderson J, Groenewald JZ, Drenth A. (2012). Phyllosticta species associated with freckle disease of banana. Fungal Diversity 56: 173–187 [Google Scholar]
- Young ND, Healy J. (2003). GapCoder automates the use of indel characters in phylogenetic analysis. BMC Bioinformatics 4: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Stanosz GR. (2001). Relationships among Botryosphaeria species and associated anamorphic fungi inferred from the analyses of ITS and 5.8S rDNA sequences. Mycologia 93: 516–527 [Google Scholar]
- Zoller S, Scheidegger C, Sperisen C. (1999). PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. The Lichenologist 31: 511–516 [Google Scholar]