Abstract
Tailoring the interface interactions between a biomaterial and the surrounding tissue is a capital aspect to consider for the design of medical devices. Poly(vinyl alcohol) (PVA) hydrogels present suitable mechanical properties for various biological substitutes, however the lack of cell adhesion on their surface is often a problem. The common approach is to incorporate biomolecules, either by blending or coupling. But these modifications disrupt PVA intra- and intermolecular interactions leading therefore to a loss of its original mechanical properties. In this work, surface modification by glow discharge plasma, technique known to modify only the surface without altering the bulk properties, has been investigated to promote cell attachment on PVA substrates. N2/H2 microwave plasma treatment has been performed, and the chemical composition of PVA surface has been investigated. X-ray photoelectron and Fourier transform infrared analyses on the plasma-treated films revealed the presence of carbonyl and nitrogen species, including amine and amide groups, while the main structure of PVA was unchanged. Plasma modification induced an increase in the PVA surface wettability with no significant change in surface roughness. In contrast to untreated PVA, plasma-modified films allowed successful culture of mouse fibroblasts and human endothelial cells. These results evidenced that the grafting was stable after rehydration and that it displayed cell adhesive properties. Thus plasma amination of PVA is a promising approach to improve cell behavior on contact with synthetic hydrogels for tissue engineering.
Keywords: hydrogel, poly(vinyl alcohol), surface analysis, nitrogen plasma, amine grafting
Introduction
Tissue engineering aims at producing artificial constructs with the suitable mechanical and biological properties to replace or regenerate damaged tissue in vivo. In this context, a large spectrum of bulk materials has been investigated, including natural polymers (collagen, fibrin, polysaccharides), synthetic polymers (polycaprolactone, polylactic acid, polyglycolic acid), metals and inorganic compounds. Among all these synthetic polymers, poly(vinyl alcohol) (PVA) stands out due to its excellent biocompatibility and safety,1,2 further it is already approved by FDA and Conformité Européenne (CE) for clinical uses in humans.3,4 It was found as a high-potential material for use in vascular,7 ophptalmic,8 cartilage, and skin applications.5,6 PVA-based materials have been processed by casting and electrospinning. They possess high stability under a large range of temperatures and pH conditions. They also show excellent mechanical properties, closely reaching those of human soft tissue,3 mainly attributable to the numerous interchain hydrogen bonds between hydroxyl groups and Van der Waals interactions in hydrocarbon polymer backbones.7 These interactions are recognized to be strongly dependent on the processing parameters such as molecular weight of the polymer, PVA concentration used during gelation process and preparation method.7,8
Previous works from Chaouat et al. have also demonstrated the feasibility of preparing PVA tubular grafts with internal diameter as small as 2 mm. Implanted in rats, these scaffolds presented no rupture or rip or aneurysm.9 In order to limit the thrombosis process, the endothelialization of the internal wall of the prostheses would increase its hemocompatibility and viability at long-term. However, PVA is recognized for its hydrophilic nature rendering thus difficult to support cell adhesion and spreading.5,10
Two approaches can be considered to increase wettability and cell adhesion: macromolecules blending and chemical modification. Blending PVA with biomacromolecules such as fibronectin,11 chitosan,12 or heparin,13 is very attractive because these components have intrinsic biological properties which should be benefit for cell adhesion and proliferation. Unhappily, despite an improvement of the PVA scaffolds behavior toward cells, the resulted mechanical properties are strongly affected.14 In fact, PVA intramolecular interactions are strongly compromised by the presence of another macromolecule. Similarly, functionalization of the PVA polymer chains by chemical modification led to changes in intramolecular interactions.7,15
Surface modification of PVA-based materials appears preferable in order to maintain bulk properties.3,16 Thus in the present study, we propose to improve cell attachment on PVA scaffolds using plasma-induced functionalization. Indeed, it is a well-recognized technique in the biomedical field for modifying the surfaces of biomaterials without affecting the bulk properties.17 Furthermore, it is a solvent-free process and leads to sterilized surfaces.17,18 Thus, plasma surface modification might represent a valid alternative to conventional PVA chemical modifications, which require multi-step reactions and subdue PVA to harsh conditions.19,20 In addition, the surface chemistry can be modulated with plasma modification by changing plasma parameters (gas, pressure, power, and treatment time).17,21,22
Despite its high-potential for changing surface properties and in particular wetabbility, only one study on the effects of surface modifications by plasma on PVA was found.23 This work investigated PVA modification by plasma using argon and ammonia gas to obtain optimal epithelialization on artificial corneas. Results showed that only argon plasma modified surfaces supported cell proliferation after 7 d. However, it has already been proved that amination by plasma on various polymer substrates (polyethylene, Teflon and Dacron) had a beneficial effect on cells in terms of adhesion, spreading and growth.24-26
Therefore, the main objective of this work was to demonstrate that amination of PVA scaffolds can be successfully obtained using a H2/N2 plasma treatment. This gas mixture was privileged to simple ammonia mainly because H2/N2 blends are less toxic, and the gas composition can be also modulated affecting thus the wettability of the surface.27 In this study, PVA hydrogels chemically crosslinked by sodium trimetaphosphate (STMP) with appropriate mechanical properties for vascular replacement, as described previously, were used. X-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FTIR) analyses, contact angle measurements and atomic force microscopy (AFM) were used to investigate surface modifications. Finally, cell adhesion and morphology of fibroblasts and endothelial cells were investigated on virgin and plasma-modified PVA membranes by cell quantification and fluorescence microscopy.
Results and Discussion
Plasma functionalization and chemical characterization of PVA substrates
The PVA membranes used in this study were obtained by casting method with STMP acting as a crosslinking agent between hydroxyl groups.9 The film is mainly composed of carbon and oxygen as expected, which is confirmed by XPS. However, the untreated PVA film had a lower ratio of oxygen to carbon (O/C) than the theoretical value i.e ~0.4 and 0.5 respectively (Table 1). This could be explained by the crosslinking process where phosphorous and sodium atoms, elements coming from the crosslinker agent STMP, were also detecting. After plasma treatment, ~6% of N was incorporated on the PVA film, leading to a slight decrease of O/C ratio which is due to oxygen substitution by nitrogen components (Table 1).
Table 1. Surface chemical composition obtained from XPS survey spectra and water contact angle of PVA surface before and after plasma treatment (n = 3) .
| Material | C (%) | O (%) | N (%) | N/C ratio | O/C ratio | % NH2 | Contact angle (°) |
|---|---|---|---|---|---|---|---|
| PVAa |
67.6 ± 1.7 |
27.0 ± 1.9 |
1.0 ± 0.8 |
0.01 ± 0.01 |
0.40 ± 0.03 |
N.D |
40.0 ± 2.4 |
| PVA plasmaa | 65.9 ± 1.3 | 24.8 ± 1.2 | 6.0 ± 1.8 | 0.09 ± 0.03 | 0.38 ± 0.03 | 3.4 ± 0.6 | 18.1 ± 2.1 |
a The balance in XPS for untreated and plasma-treated PVA films were Si, Na, P, Cl. N.D, non detectable
The N1s high resolution spectra did not permit to differentiate the nitrogen species (amine, amide, imine, nitrile) grafted on the surface because they do not have a significant difference in their chemical shifts (Fig. 1). Thus, amine grafting was assessed by chemical derivatization. As chlorobenzaldehyde reacts specifically with amines in derivatization reaction, the amount of chlorine detected by XPS on the modified surface allows to quantify the grafted amines.28 No amine groups on native PVA films were detected, whereas on the plasma-treated films 3.4% of amines were estimated. This value corresponds to an amino group relative surface concentration of 0.5–2 amine/nm2.29 Keeping in mind that initially 6% of nitrogen was grafted, the amine specificity was efficient with ~55% of NH2/N and similar to the highest values described in literature ~66% (66.7% Ruelle et al.,30 66% Sarra-Bournet et al.,27 65% Vallières et al.31).
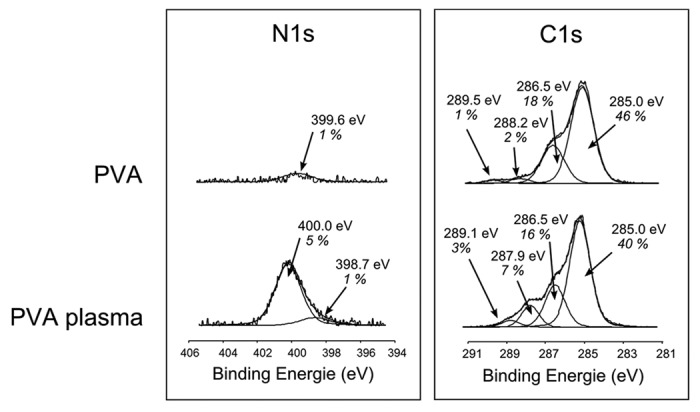
Figure 1. XPS high resolution spectra of N1s and C1s before and after plasma treatment.
High resolution spectra of C1s confirmed that the main chemical structure of PVA was unchanged (Fig. 1). Indeed, the two characteristic bands of initial PVA at 285.0 eV and 286.5 eV from CH2 and CH-OH respectively, were not affected by the plasma treatment. It should also be noticed that there was an incorporation of amide and carboxylic acid groups during the plasma treatment as shown by an increase of their characteristic bands at 287.9 eV and 289.5 eV, respectively. These observations were also noticed in FTIR spectra with the detection of shoulders in the carbonyl area at bands 1743 cm−1, 1705 cm−1, and 1664 cm−1 from ester carboxylic acid and amide groups, respectively (Fig. 2). The main peak of PVA (ν C-O at 1087 cm−1) was unchanged meaning that the PVA main composition and chain interactions are unchanged in the bulk. Indeed, FTIR depth analysis is deeper compared with XPS one: 1 μm vs. ~5 nm, respectively.
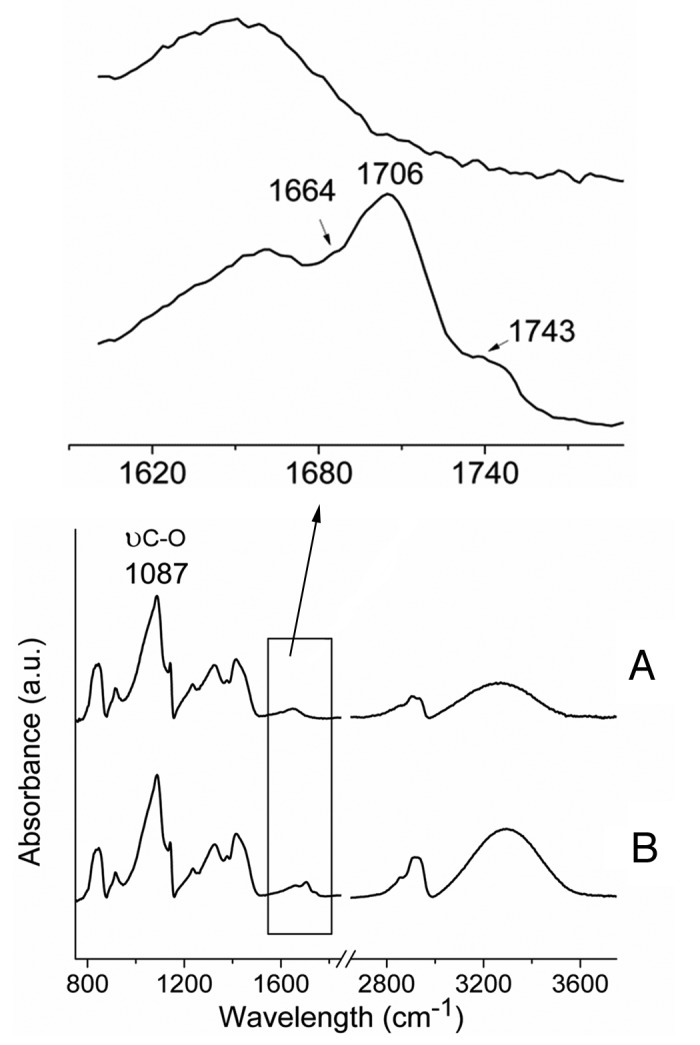
Figure 2. FTIR spectra of PVA films before (A) and after plasma treatment (B). New peaks and bands appeared at 1654 cm−1, 1705 cm−1 and 3200–3400 cm−1, associated to amide, carboxylic acid and OH/NH functionalities, respectively. A.u., arbitrary units.
Both XPS and FTIR analyses prooved that plasma surface modification is an efficient procedure to graft amino groups on a PVA hydrogel, without affecting its main chemical structure and chain interactions. Furthermore, the FTIR spectrum of the plasma-modified substrate (Fig. 2) compared with the untreated one showed a higher intensity of the vibration band of OH/NH functionalities (3200–3400 cm−1) which indicate an increase in hydrophilic moieties on the PVA film.
Physical surface properties
The extent of hydrophilicity for plasma-treated PVA substrates was investigated by contact angle (CA) measurements (Table 1). A significant increase in wettability resulted by plasma modification was observed: untreated PVA substrates displayed a contact angle of 40.0° ± 2.4 which decreased to 18.1° ± 2.1 after surface modification. Contact angle values can be directly correlated with the species created on the surface. However, during plasma treatment two main processes can occurred: the addition of new polar groups which leads to lower contact angles, and the etching process inducing the opposite effect (higher CA values). Thus, the important decrease in contact angle value observed on PVA films after plasma treatment can be explained by the presence of new polar groups such as amide and amine groups on the polymer surface, as shown in XPS and FTIR analyses.
Furthermore, as illustrated in Figure 3 with atomic force microscopy (AFM) images, plasma treatment did not seem to induce drastic changes in the surface topography. Despite numerous small round nodules on PVA treated film, membranes displayed similar roughness before and after plasma modification over an area of 20 × 20 µm2 and 5 × 5 µm2. These nodules are due to chain scission and reorganization of these highly hydrophilic fragments compared with the underlying material as previously reported. Despite chain scission, the fragments are long enough as they stay on the surface whereas for etching process, the polymer scission leads to small fragments which are removed from the surface. Therefore, it can be assumed that the polymer surface was not etched by the plasma conditions, and that the hydrophilic character of the PVA treated film is mainly due to surface functionalization.32 In contrast, Lee et al. did not notice any change in contact angle values after their plasma treatment with ammonia on PVA substrates because their process mainly induced etching.20 In their study, the samples were directly treated in the discharge with high energy species (radicals, ions, or electrons) known to induce etching, whereas our treatment was performed in post-discharge (7 cm), meaning that these species lose energy and are less able to reach the surface, therefore reducing the etching.
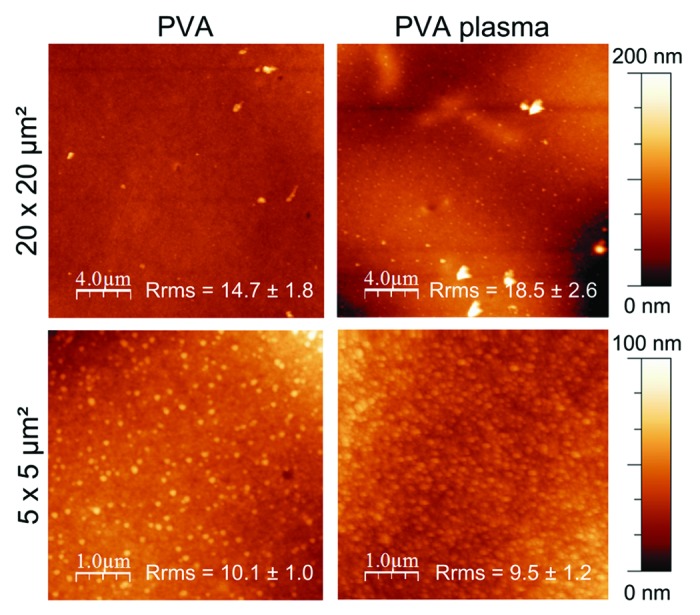
Figure 3. Surface morphology of untreated and plasma-treated PVA films observed by AFM. Roughness was calculated by the root mean square roughness (Rrms).
The efficiency of the plasma treatment on PVA substrates has been clearly evidenced in term of chemical modification by attaching 3.4% of amino groups to the surface without altering the bulk composition. Furthermore, the film hydrophilicity has been increased without affecting the surface topography as evidenced by AFM images. Thus, the influence of the modified surface on cell behavior can be considered.
Cell culture
Cell adhesion and proliferation on native and plasma-treated PVA substrates were investigated using two cell types, mouse fibroblasts (NIH-3T3) and human endothelial cells (EAhy.926), which were cultured separately. Substrates were precoated with serum, in order to test the surfaces in more realistic conditions, as there are numerous proteins in human blood. Furthermore, it is known that surface modifications affect the protein adhesion and configuration as well as cell attachment, so precoating with serum appeared the more realistic approach.
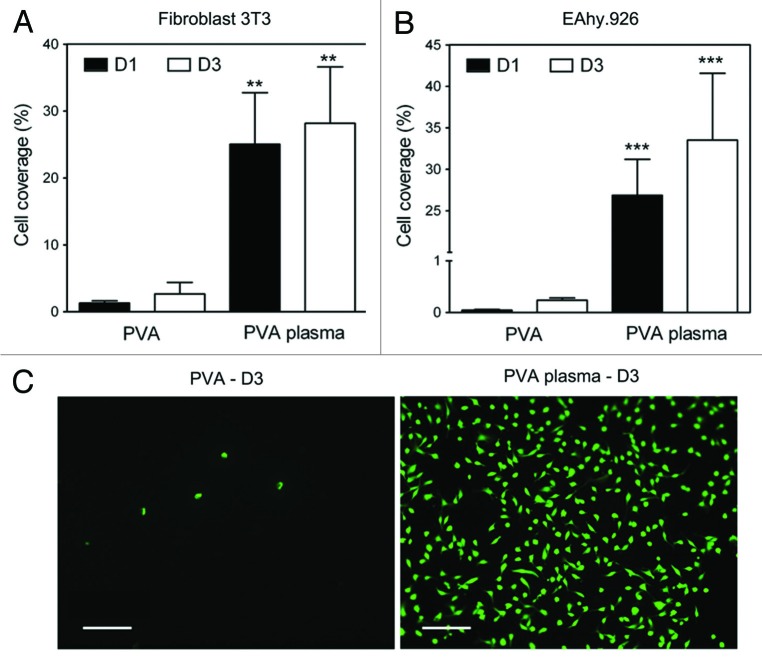
After seeding the cells on the surfaces, cell behaviors at day 1 and day 3 were assessed by fluorescence microscopy images with green-fluorescent labeling live cells (calcein-AM). In addition, cells on plasma-modified PVA substrates and untreated control substrates were quantified and compared at day 1 and day 3. Cell coverage results are presented in Figure 4. For both cell types, very few cells were observed on the unmodified PVA films at day 1. However, higher cell densities were observed on plasma-treated PVA surfaces at day 1, and cell coverage increased overall from day 1 to day 3 (25% to 28% and 27% to 34% for fibroblasts and endothelial cells, respectively).
Figure 4. (A and B) Cell coverage for NIH-3T3 and EAhy.926 cultured on untreated and plasma-treated PVA substrates was quantified using a Calcein assay on day 1 and 3 (n = 4). At all times, cell density on PVA plasma films was significantly higher than on untreated PVA films: **p < 0.01, ***p < 0.001. (C) Fluorescence images of EAhy.926 cells at day 3. Scale bar: 100 µm.
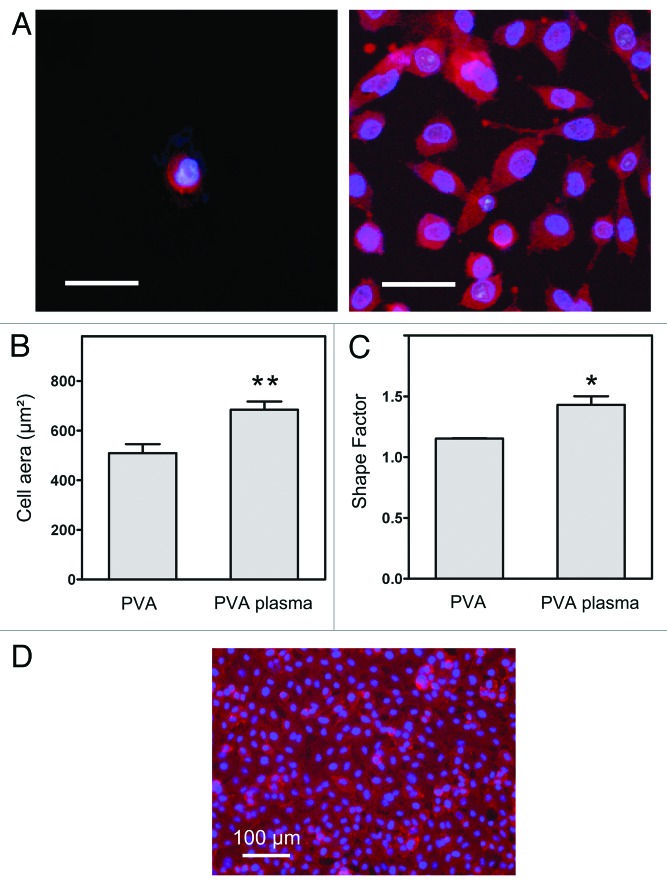
In order to further investigate these changes in cell-contacting properties, we evaluated the cell spreading by measuring cell area and cell roundness. After three days of culture, the overall endothelial cell morphology was visualized by the fluorescent staining of nuclei and cytoplasmic F-actins, as shown in Figure 5. Cells on the untreated PVA remained round, while a large number of cells cultured on the plasma-treated PVA were flat and well spread, with a polygonal shape, suggesting that the presence of amines was beneficial for the initial cell attachment. Moreover, area and shape of cells on plasma-treated surfaces were significantly different from that of the control i.e corresponding mean cell area was 509 ± 108 µm2 and 684 ± 130 µm2 respectively. Additionally, the shape factor, which is related to the cell roundness, was 1.15 ± 0.01 for untreated PVA (almost discoid cells) and 1.43 ± 0.28 for plasma-treated PVA substrates. Cell culture results also evidenced that a homogenous monolayer of endothelial cells could be formed onto the surface of plasma-treated substrates (Fig. 5D). Thus based on prior works from our laboratories, amine-grafted PVA hydrogels appear as promising scaffolds for vascular biomaterials.9 Future studies will investigate hemocompatibility of this material.
Figure 5. (A) Endothelial cells were stained with Phalloidin/DAPI and observed on (left) untreated PVA and (right) plasma-treated PVA substrates with fluorescence microscopy at day 3. Scale bar: 50 µm. Cell area (B) and shape factor (C) were calculated from digital images (n = 15). Significant difference from untreated PVA: *p < 0.05 and **p < 0.01. (D) Cells eventually reached confluence on a treated PVA surface, forming an endothelium-like monolayer.
Surface characterization and cytocompatibility results demonstrated that cell-contacting properties of PVA substrates significantly improve due to the presence of surface amine groups. Recent works on various synthetic substrates also demonstrated that nitrogen-containing groups, especially amine and amide functionalities, were optimal for adhesion proteins adsorption and cell adhesion.33,34 Most authors ascribe the observed enhancements to a combination of increased hydrophilicity and surface charge. Cell-substrate adhesion is a process that involves several steps, including adsorption of extracellular matrix (ECM) proteins on the surface, recognition of ECM components by cells through integrins, cytoskeletal reorganization and overall cell spreading. A large portion of cells, serum and ECM proteins (fibronectin, vitronectin and collagen) are recognized as being negatively charged, so they tend to be more adsorbed through electrostatic interactions on positively charged surfaces.35 Amine groups are protonated at a physiological pH (pKa~10) and can interact with negatively charged proteins and/or with proteoglycans of the cellular membrane. Therefore in the early stage of cellular attachment, amines are likely to enhance proteins adsorption from the culture medium and subsequent cellular interactions of adherent cells with the adsorbed protein layer.
A drawback generally observed with plasma techniques is the limited stability of the treated surfaces toward aging and washing.36 This is particularly important when designing a biomaterial that will eventually have to be stored, sterilized and implanted. The aging behavior of amine-grafted surfaces prepared by plasma has been extensively described, and is usually due to post-plasma oxidation and surface rearrangements.27,37 Latkany et al. also noticed that nitrogen species grafting on PVA substrates by ammonia plasma treatment, was not effective on enhancing cell adhesion.23 Indeed, they stated that the grafted amine groups were unstable. Here, we demonstrated that our nitrogen plasma grafting on PVA surfaces was stable enough to display cell adhesive properties after UV sterilization, rehydration and subsequent cell culture. Moreover, immobilization of highly chemically reactive groups like amines would allow the subsequent grafting of bioactive molecules that are tailored to specific need, such as adhesion peptides.38
Materials and Methods
PVA film preparation
Chemical reagents commonly used as hydrogel crosslinker (glutaraldehyde, formaldehyde, epichlorhydrine) could be further leached in vivo and induce secondary effects (inflammatory response, toxic effect).5,10 In order to avoid these complications, we have used sodium trimetaphosphate (STMP), as chemical crosslinker, because it is already accepted for food applications.9
Poly(vinyl alcohol) (PVA) films were prepared with a casting method as previously described.9 Briefly, PVA (Sigma-Aldrich; Mw 84–124 kDa and saponification degree > 99.9%) (400 mg) was dissolved without further purification in deionized water (4 mL), while stirring at 90°C until complete dissolution. The solution was cooled down to room temperature and STMP (Sigma-Aldrich) (300 µL, 15% w/v) was added under stirring. Sodium hydroxide (120 µL, 30% w/v) was then added dropwise. Four grams of the mixture were poured into a 90 mm diameter polystyrene Petri dish and kept at room temperature until completely evaporated. Resulting films were washed with deionized water to remove residual sodium hydroxide and STMP.
Plasma treatment
Dried PVA films were placed in a commercial microwave plasma reactor from Plaminionique Inc. (Varennes). The surface activation was performed in the downstream region of the plasma (7 cm) consisting of high purity nitrogen and hydrogen (80 sccm, 20 sccm) at 300 W, 2.45 GHz, and 500 mTorr for 5 min.
Chemical surface analyses
The surface chemical composition was investigated by means of XPS using a PHI 5600-ci spectrometer (Physical Electronics). A standard aluminum X-ray source (1486.6 eV) was used at 300 W with a neutralizer to record the survey spectra, and the high resolution spectra were obtained using a standard magnesium X-ray source (1253.6 eV) at 300 W with no charge neutralization. Photoelectron detection was generally performed at 45° with respect to the surface plane.
Amine surface concentration on the PVA samples after plasma treatment was quantified through a vapor phase chemical derivatization technique using chlorobenzaldehyde and followed by XPS analyses. This derivatization technique is described with more details elsewhere.28 Briefly, it consists in performing a chemical reaction between a molecule containing an atom that is not already present in the composition of the treated surface (in this case, chlorine) and the surface chemical functionality of interest (amines). The concentration of this added atom is surveyed by XPS and can be directly linked to the relative concentration of the reacted functionality.
Film compositions were determined by FTIR using a Nicolet Magna 550 (Thermo-Nicolet, Madison) equipped with a deuterated triglycinesulphate (DTGS) detector. The attenuated total reflection (ATR) mode was used with a split pea attachment (Harrick-Scientific Corp.) equipped with a silicon hemispherical internal reflection element. The maximum depth of analysis was estimated to be 1µm, and 150 scans were acquired at a spectral resolution of 4 cm−1.
Surface characterization
Film surface topography was investigated using a DimensionTM 3100 atomic force microscope (Digital Instruments) in tapping mode with an etched silicon tip (OTESPATM, tip radius < 10 nm, aspect ratio ≈1.6/1). The surface roughness for areas of 20 × 20 µm2 and 5 × 5 µm2 was calculated using the Root Mean Square Roughness parameter (Rrms). Visualization and analysis of the morphology were performed using the WSxM software.39
Static contact angle measurements of the samples were recorded using a VCA 2500 XE system (AST). Three µL of deionized water were deposited on the surface of dried films and pictures were taken within 5 sec. Contact angles were measured on three drops randomly deposited on different parts of each sample, followed by triplicate analyses.
Cell culture
PVA and plasma-treated PVA films were punched into disks and placed in 24 well plates. After 15 min of UV exposure, films were rehydrated in PBS for 2 h. A Teflon O-ring was positioned on top of each film to prevent floating. The fibroblast cell line (NIH-3T3, ATCC) was cultured with Dulbecco’s Modified Eagle Medium (DMEM) containing 10% calf serum (CS, ATCC) and 1% penicillin/streptomycin/amphotericin B at 37 °C in 5% CO2. Cells were seeded on films (105 cell/cm2) and maintained in culture for 3 d. Endothelial cell culture was investigated according to the same protocol, using an EAhy.926 cell line with DMEM, supplemented with 10% fetal bovine serum (FBS) and hypoxanthine/aminopterin/thymidine. Films were coated with CS (NIH-3T3) or FBS (EAhy.926) for 1 h at 37 °C prior to seeding.
At day 1 and day 3, seeded films were incubated with calcein-AM (Calbiochem) for 1 h at 37°C. Viable cells were observed with a Nikon Eclipse E400 fluorescence microscope (4×) and cell viability was quantified on digital images. Then, samples were fixed with paraformaldehyde (4% in PBS). Cells were permeabilized with Triton X-100 (0.1% in PBS) and stained for nuclei with DAPI (Sigma D8417, 1:10 000) and for cytoplasmic F-actin with Phalloidin-TRITC (Sigma P1951, 1:200). Cell area and perimeter were measured manually on digital images using Archimed and Adobe Photoshop software. A cell shape factor was defined as the ratio of the real perimeter to the apparent perimeter if the cell was considered a circle with a similar area: S = P/[2 × (Π × A)]1/2, with S: Shape factor, A: Cell area (µm2) and P: Cell perimeter (µm).
Statistical analysis
Data are presented as mean ± standard errors. Cell culture results were analyzed using a non-parametric Kruskal-Wallis one-way analysis of variance with Dunns’s post hoc test. Morphological data were evaluated by an unpaired Student’s t-test. A value of p < 0.05 was accepted as statistically significant.
Conclusion
In the present study, we demonstrated that H2/N2 plasma treatment can successfully modify a chemically crosslinked PVA hydrogel leading to an improvement in cell adhesion and growth. FTIR and XPS spectroscopies confirmed the presence of nitrogen groups on the functionalized surfaces. Derivatization technique also indicated that the plasma condition used for amination was efficient and that the plasma selectively was good with 3.4% of amines out of 6% of nitrogen initially grafted. Furthermore, the plasma treatment led to an increase in substrate wettability mainly induced by surface functionalization. No evidence of surface etching was noticed as exhibited by AFM images with no change in the surface morphology as well as roughness. Mouse NIH-3T3 fibroblasts and human endothelial cells EAhy.926 were used to evaluate cell affinity on PVA substrates. Contrary to native PVA, plasma-modified surfaces allowed cell attachment and viability for both cell types. This procedure appears promising in terms of hydrogel application as scaffold for tissue engineering. The next step of this work is to go further by transferring this surface modification to the aforementioned cylindrical tube in order to perform in vivo tests.
Acknowledgments
This study was supported by Inserm, Universities Paris 7 and Paris 13. JMI is a recipient of the French Ministry of Higher Education and Research Scholarship (ED Galilée, University Paris 13). The authors would like to thank E Michel and J Lagueux for technical help. They also acknowledge the Franco-Québécois program between Inserm and FRSQ (Fond de Recherche Santé Québec), the Canadian Institute of Health Research (CIHR), the Natural Sciences and Engineering Research Council (NSERC) and the Research Center of the University Hospital Research Center (CR-CHUQ) at Saint-François d’Assise Hospital in Quebec City, Canada, and funding from Agence Nationale pour la Recherche (ANR-10 INTB-1502-01).
Submitted
03/04/2013
Revised
05/21/2013
Accepted
06/14/2013
Note
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/25414
References
- 1.Chiellini E, Corti A, D'Antone S, Solaro R. Biodegradation of poly (vinyl alcohol) based materials. Prog Polym Sci. 2003;28:963–1014. doi: 10.1016/S0079-6700(02)00149-1. [DOI] [Google Scholar]
- 2.DeMerlis CC, Schoneker DR. Review of the oral toxicity of polyvinyl alcohol (PVA) Food Chem Toxicol. 2003;41:319–26. doi: 10.1016/S0278-6915(02)00258-2. [DOI] [PubMed] [Google Scholar]
- 3.Chong S-F, Smith AAA, Zelikin AN. Microstructured, Functional PVA Hydrogels through Bioconjugation with Oligopeptides under Physiological Conditions. Small 2012:n/a-n/a. [DOI] [PubMed]
- 4.Weaver JD, Ku DN. Mechanical Evaluation of Polyvinyl Alcohol Cryogels for Covered Stents. J Med Device. 2010;4:031002–6. doi: 10.1115/1.4001863. [DOI] [Google Scholar]
- 5.Gupta S. T G, Basu B, Goswami S, Sinha A. Stiffness- and wettability-dependent myoblast cell compatibility of transparent poly(vinyl alcohol) hydrogels. J Biomed Mater Res B Appl Biomater. 2013;101B:346–54. doi: 10.1002/jbm.b.32845. [DOI] [PubMed] [Google Scholar]
- 6.Sailaja GS, Sreenivasan K, Yokogawa Y, Kumary TV, Varma HK. Bioinspired mineralization and cell adhesion on surface functionalized poly(vinyl alcohol) films. Acta Biomater. 2009;5:1647–55. doi: 10.1016/j.actbio.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Alves M-H, Jensen BEB, Smith AAA, Zelikin AN. Poly(vinyl alcohol) physical hydrogels: new vista on a long serving biomaterial. Macromol Biosci. 2011;11:1293–313. doi: 10.1002/mabi.201100145. [DOI] [PubMed] [Google Scholar]
- 8.Bolto B, Tran T, Hoang M, Xie Z. Crosslinked poly(vinyl alcohol) membranes. Prog Polym Sci. 2009;34:969–81. doi: 10.1016/j.progpolymsci.2009.05.003. [DOI] [Google Scholar]
- 9.Chaouat M, Le Visage C, Baille WE, Escoubet B, Chaubet F, Mateescu MA, et al. A Novel Cross-linked Poly(vinyl alcohol) (PVA) for Vascular Grafts. Adv Funct Mater. 2008;18:2855–61. doi: 10.1002/adfm.200701261. [DOI] [Google Scholar]
- 10.Padavan DT, Hamilton AM, Millon LE, Boughner DR, Wan W. Synthesis, characterization and in vitro cell compatibility study of a poly(amic acid) graft/cross-linked poly(vinyl alcohol) hydrogel. Acta Biomater. 2011;7:258–67. doi: 10.1016/j.actbio.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 11.Millon LE, Padavan DT, Hamilton AM, Boughner DR, Wan W. Exploring cell compatibility of a fibronectin-functionalized physically crosslinked poly(vinyl alcohol) hydrogel. J Biomed Mater Res B Appl Biomater. 2012;100:1–10. doi: 10.1002/jbm.b.31860. [DOI] [PubMed] [Google Scholar]
- 12.Yan E, Fan S, Li X, Wang C, Sun Z, Ni L, et al. Electrospun polyvinyl alcohol/chitosan composite nanofibers involving Au nanoparticles and their in vitro release properties. Mater Sci Eng C. 2013;33:461–5. doi: 10.1016/j.msec.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Negishi J, Nam K, Kimura T, Fujisato T, Kishida A. High-hydrostatic pressure technique is an effective method for the preparation of PVA-heparin hybrid gel. Eur J Pharm Sci. 2010;41:617–22. doi: 10.1016/j.ejps.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Liu Q, Chen X, Yu F, Zhu Z. Investigation of PVA/ws-chitosan hydrogels prepared by combined γ-irradiation and freeze-thawing. Carbohydr Polym. 2008;73:401–8. doi: 10.1016/j.carbpol.2007.12.008. [DOI] [Google Scholar]
- 15.Hamidi M, Ashrafi H, Azadi A. Surface Functionalized Hydrogel Nanoparticles. Biomedical Materials and Diagnostic Devices: John Wiley & Sons, Inc., 2012:191-213. [Google Scholar]
- 16.Rafat M, Rotenstein LS, You J-O, Auguste DT. Dual functionalized PVA hydrogels that adhere endothelial cells synergistically. Biomaterials. 2012;33:3880–6. doi: 10.1016/j.biomaterials.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Gomathi N, Suresthkumar A, Neogi S. RF plasma-treated polymers for biomedical applications. Curr Sci. 2008;94:9. [Google Scholar]
- 18.Akitsu T, Ohkawa H, Tsuji M, Kimura H, Kogoma M. Plasma sterilization using glow discharge at atmospheric pressure. Surf Coat Tech. 2005;193:29–34. doi: 10.1016/j.surfcoat.2004.07.042. [DOI] [Google Scholar]
- 19.Fundueanu G, Constantin M, Bortolotti F, Cortesi R, Ascenzi P, Menegatti E. Cellulose acetate butyrate-pH/thermosensitive polymer microcapsules containing aminated poly(vinyl alcohol) microspheres for oral administration of DNA. Eur J Pharm Biopharm. 2007;66:11–20. doi: 10.1016/j.ejpb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Mahanta N, Valiyaveettil S. Surface modified electrospun poly(vinyl alcohol) membranes for extracting nanoparticles from water. Nanoscale. 2011;3:4625–31. doi: 10.1039/c1nr10739a. [DOI] [PubMed] [Google Scholar]
- 21.Calderon JG, Harsch A, Gross GW, Timmons RB. Stability of plasma-polymerized allylamine films with sterilization by autoclaving. J Biomed Mater Res. 1998;42:597–603. doi: 10.1002/(SICI)1097-4636(19981215)42:4<597::AID-JBM16>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Gancarz I, Bryjak J, Pozniak G, Tylus W. Plasma modified polymers as a support for enzyme immobilization - II. Amines plasma. Eur Polym J. 2003;39:2217–24. doi: 10.1016/S0014-3057(03)00160-5. [DOI] [Google Scholar]
- 23.Latkany R, Tsuk A, Sheu MS, Loh IH, Trinkaus-Randall V. Plasma surface modification of artificial corneas for optimal epithelialization. J Biomed Mater Res. 1997;36:29–37. doi: 10.1002/(SICI)1097-4636(199707)36:1<29::AID-JBM4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Hamerli P, Weigel T, Groth T, Paul D. Surface properties of and cell adhesion onto allylamine-plasma-coated polyethylenterephtalat membranes. Biomaterials. 2003;24:3989–99. doi: 10.1016/S0142-9612(03)00312-0. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Jung HW, Kang IK, Lee HB. Cell behaviour on polymer surfaces with different functional groups. Biomaterials. 1994;15:705–11. doi: 10.1016/0142-9612(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 26.Tseng DY, Edelman ER. Effects of amide and amine plasma-treated ePTFE vascular grafts on endothelial cell lining in an artificial circulatory system. J Biomed Mater Res. 1998;42:188–98. doi: 10.1002/(SICI)1097-4636(199811)42:2<188::AID-JBM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Sarra-Bournet C, Ayotte G, Turgeon S, Massines F, Laroche G. Effects of chemical composition and the addition of H2 in a N2 atmospheric pressure dielectric barrier discharge on polymer surface functionalization. Langmuir. 2009;25:9432–40. doi: 10.1021/la900652y. [DOI] [PubMed] [Google Scholar]
- 28.Chevallier P, Castonguay M, Turgeon S, Dubrulle N, Mantovani D, McBreen PH, et al. Ammonia RF−Plasma on PTFE Surfaces: Chemical Characterization of the Species Created on the Surface by Vapor−Phase Chemical Derivatization. J Phys Chem B. 2001;105:12490–7. doi: 10.1021/jp011607k. [DOI] [Google Scholar]
- 29.Gauvreau V, Chevallier P, Vallières K, Petitclerc E, Gaudreault RC, Laroche G. Engineering surfaces for bioconjugation: developing strategies and quantifying the extent of the reactions. Bioconjug Chem. 2004;15:1146–56. doi: 10.1021/bc049858u. [DOI] [PubMed] [Google Scholar]
- 30.Ruelle B, Peeterbroeck S, Godfroid T, Bittencourt C, Hecq M, Snyders R, et al. Selective Grafting of Primary Amines onto Carbon Nanotubes via Free-Radical Treatment in Microwave Plasma Post-Discharge. Polymers. 2012;4:296–315. doi: 10.3390/polym4010296. [DOI] [Google Scholar]
- 31.Vallières K, Chevallier P, Sarra-Bournet C, Turgeon S, Laroche G. AFM imaging of immobilized fibronectin: does the surface conjugation scheme affect the protein orientation/conformation? Langmuir. 2007;23:9745–51. doi: 10.1021/la701323q. [DOI] [PubMed] [Google Scholar]
- 32.Oteyaka MO, Chevallier P, Robitaille L, Laroche G. Effect of Surface Modification by Ammonia Plasma on Vascular Graft: PET Film and PET Scaffold. Acta Phys Pol A. 2012;121:125–7. [Google Scholar]
- 33.Rimmer S, Johnson C, Zhao B, Collier J, Gilmore L, Sabnis S, et al. Epithelialization of hydrogels achieved by amine functionalization and co-culture with stromal cells. Biomaterials. 2007;28:5319–31. doi: 10.1016/j.biomaterials.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Sosnik A, Sefton MV. Methylation of poloxamine for enhanced cell adhesion. Biomacromolecules. 2006;7:331–8. doi: 10.1021/bm050693h. [DOI] [PubMed] [Google Scholar]
- 35.Lavigne D, Guerrier L, Gueguen V, Michel JB, Boschetti E, Meilhac O, et al. Culture of human cells and synthesis of extracellular matrix on materials compatible with direct analysis by mass spectrometry. Analyst. 2010;135:503–11. doi: 10.1039/b914539g. [DOI] [PubMed] [Google Scholar]
- 36.Siow KS, Britcher L, Kumar S, Griesser HJ. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization - A Review. Plasma Process Polym. 2006;3:392–418. doi: 10.1002/ppap.200600021. [DOI] [Google Scholar]
- 37.Gengenbach TR, Ximing X, Chatelier RC, Griesser HJ. Evolution of the surface composition and topography of perfluorinated polymers following ammonia-plasma treatment. J Adhes Sci Technol. 1994;8:305–28. doi: 10.1163/156856194X00267. [DOI] [Google Scholar]
- 38.Boucher C, Ruiz J-C, Thibault M, Buschmann MD, Wertheimer MR, Jolicoeur M, et al. Human corneal epithelial cell response to epidermal growth factor tethered via coiled-coil interactions. Biomaterials. 2010;31:7021–31. doi: 10.1016/j.biomaterials.2010.05.072. [DOI] [PubMed] [Google Scholar]
- 39.Horcas I, Fernández R, Gómez-Rodríguez JM, Colchero J, Gómez-Herrero J, Baro AM. WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev Sci Instrum. 2007;78:013705. doi: 10.1063/1.2432410. [DOI] [PubMed] [Google Scholar]