Abstract
Human African trypanosomiasis (HAT) is a parasitic neglected tropical disease that affects 10,000 patients each year. Current treatments are sub-optimal, and the disease is fatal if not treated. Herein, we report our continuing efforts to repurpose the human phosphodiesterase 4 (hPDE4) inhibitor piclamilast to target trypanosomal phosphodiesterase TbrPDEB1. We prepared a range of substituted heterocyclic replacements for the 4-amino-3,5-dichloro-pyridine head group of piclamilast, and found that these compounds exhibited weak inhibitory activity of TbrPDEB1.
Human African trypanosomiasis (HAT) is a neglected tropical disease caused by the parasites Trypanosoma brucei gambiense and T. b. rhodesiense. Together, over 60 million people in 36 countries in sub-Saharan Africa are at risk, with approximately 10,000 infections annually.1 HAT is fatal unless treated and the four drugs approved for this indication: pentamidine, suramin, eflornithine, and melarsoprol, are inadequate for a variety of reasons, including cost, toxicity, and lack of oral bioavailability. For instance, melarsoprol is especially toxic as it induces reactive encepalophathy in 5–10% of patients, killing approximately half of them.2 As such, new medicines are desperately needed but pharmaceutical companies tend to deprioritize diseases such as HAT due to an inability to recover research costs from the extremely poor who are the most affected by the disease.
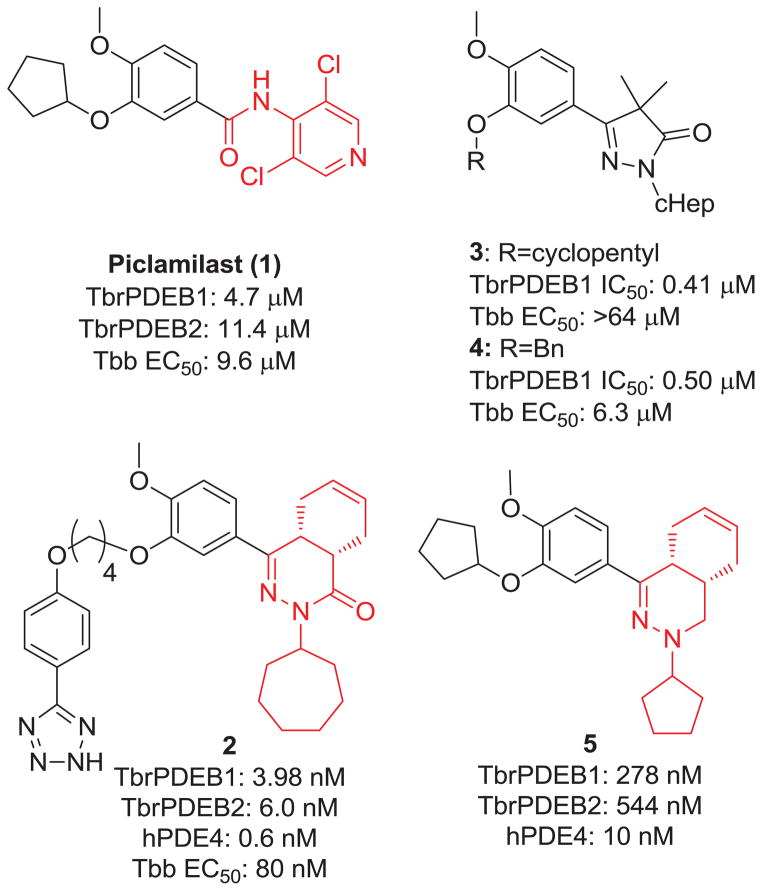
In order to speed up the drug discovery process, a drug repurposing approach3 has been taken against two T. brucei phosphodiesterases (PDEs), TbrPDEB1 and TbrPDEB2.4–6 Simultaneous RNAi knockdown of both is fatal,7 suggesting that small molecule inhibitors of these enzymes could be useful interventions.4, 8 Humans have 11 PDEs that have been well explored, producing numerous clinical drug candidates.9 The catalytic domains of human PDEs are 30–35% homologous to those of the parasite enzymes TbrPDEB1 and TbrPDEB2. Recent crystallographic evidence confirms that there are key regions of the trypanosomal protein that may allow for selective inhibition over human PDEs.10 We previously reported that human PDE4 inhibitor piclamilast (1) represents a promising lead series for optimization towards selective TbrPDEB inhibitors,4 and others have shown analogous catechol-derived inhibitors to have high potency against the trypanosomal enzyme.8, 11 In this report, we describe our efforts to explore replacements for the 2,6-dichloro-4-pyridylamide headgroup of 1 by assessing their potency against TbrPDEB1.
Our rationale for focusing first on the headgroup region of 1 is shown in Figure 1. The optimization of phthalazinones as human PDE4 (hPDE4) inhibitors has been described, leading to the discovery of 2 with an IC50 of 0.6 nM against hPDE4.12 This compound was later discovered in a screening campaign to be a potent inhibitor of TbrPDEB1 (3.98 nM),8 and we found that this compound comparably inhibits TbrPDEB2 (6 nM). A related set of compounds (3 and 4, Figure 1) has recently been disclosed as TbrPDEB1 inhibitors.11 There is obvious structural similarity between compounds 1–4, though 1 inhibits TbrPDEB1 with an IC50=4.7 μM, whereas 2–4 inhibit TbrPDEB1 with sub-micromolar potency. We prepared 5,12–15 an analog of 1 and 2, to more directly compare the effect of the headgroup region of the inhibitor, and confirmed its activity against TbrPDEB1 and B2 (IC50=278 and 544 nM, respectively). Thus, in observing the 15-fold increase in potency with 5 versus 1, we hypothesized that the headgroup region must be a major driver of potency against TbrPDEB1. This became our rationale in future compound design and the resulting structure-activity-relationship (SAR) exploration.
Figure 1.
Headgroup replacement rationale based upon related TbrPDEB1 inhibitor chemotypes.
We prepared putative heterocyclic aromatic headgroup replacements for the phthalizinone moiety as shown in Scheme 1. Electrophilic bromination of guaiacol by a previously published method afforded 5-bromo-2-methoxyphenol, intermediate 6.16 This intermediate reacted with cyclopentanol under Mitsunobu conditions to produce the dialkoxy catechol intermediate 7a. A small library of boronic acids was used in Suzuki reactions with intermediate 7a to yield analogs 8a–c, which were found to be weak inhibitors of TbrPDEB1 (Table 1).
Scheme 1.
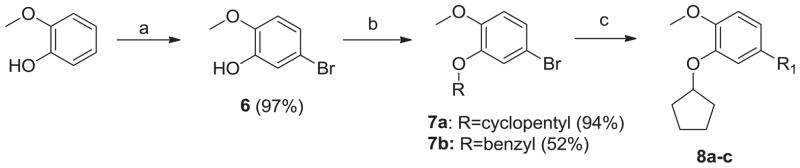
Synthesis of 8a–c. Reagents and conditions: (a) (i) TFAA, t-BuOK, MeCN, rt, 45 min; (ii) NBS, MeCN, rt, o/n; (b) ROH, PPh3, DEAD, toluene, rt, 2h; (c) R-B(OH)2, Na2CO3, Pd(PPh3)4, toluene/EtOH/H2O (4:1:1),105°C, o/n.
Table 1.
Aryl analogs tested against TbrPDEB1
Data shown are average of 3 replicate independent experiments.
Replicate of 5 independent experiments.
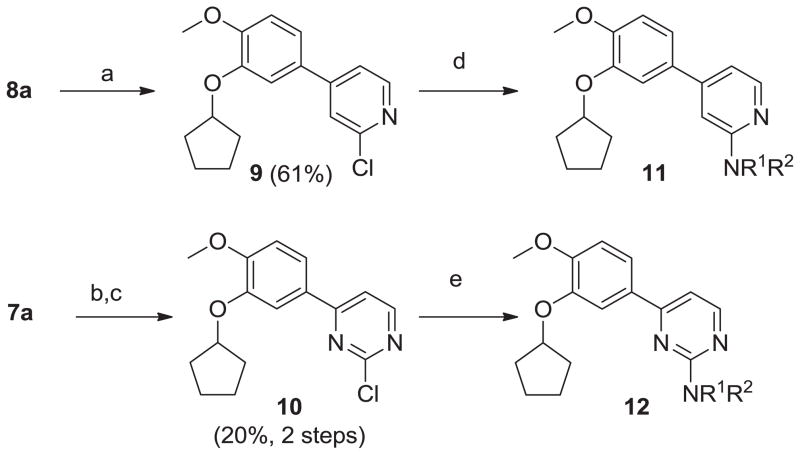
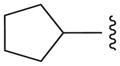
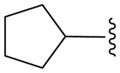
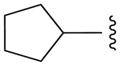
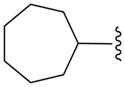
We also prepared a series of pyrimidine and pyridine-derived inhibitors, as shown in Scheme 2. Compound 8a could be converted to the chloropyridine intermediate 9 by a sequence of N-oxide formation and chlorination with POCl3. The analogous pyrimidine intermediate 10 was made via borylation of intermediate 7a, followed by a Suzuki reaction with 2,4-dichloropyrimidine. Chloride displacement with small aliphatic and benzylic amines was achieved to provide the pyrimidine and pyridine-derived analogs 11 and 12 (Table 2).
Scheme 2.
Synthesis of 11 and 12. Reagents and conditions: (a) (i) m-CPBA, CHCl3, 0°C to RT, o/n; (ii) POCl3, TEA, CHCl3, MW, 100°C, 1 h; (b) n-BuLi, then bis-(pinacolato)diborane, −78°C to rt, o/n; (c) 2,4-dichloropyrimidine, Na2CO3, PPh3, Pd2(dba)3, tol/EtOH/H2O (4:1:1), 105°C, o/n; (d) R1R2NH, DIEA, NMP or DMF, MW, 250°C, 1 h; (e) R1R2NH, DIEA, NMP or DMF, 80°C, o/n.
Table 2.
Pyridine and Pyrimidine analogs tested against TbrPDEB1
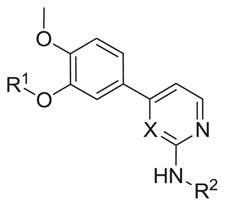
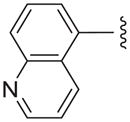
| ||||
|---|---|---|---|---|
| Compound | R1 | R2 | X | TbrPDEB1 (% inh)a |
| 11a |
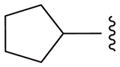
|
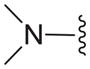
|
C | 34.0± 22.8 |
| 11b |
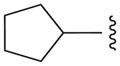
|
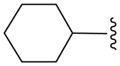
|
C | 27.1 ± 6.1 |
| 12a |
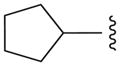
|
|
N | 15.8 ±21.2 |
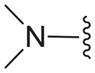
| 12b |
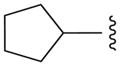
|
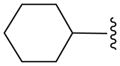
|
N | 29.8 ± 0.3 |
| 12c |
|
|
N | 12.7 ± 17.9 |
| 12d |
|
|
N | 20.6 ± 8.8 |
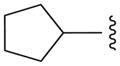
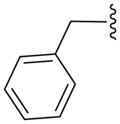
| 12e |
|
|
N | 32.9 ± 6.0 |
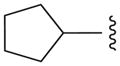
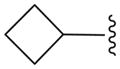
| 12f |
|
|
N | 3.9 ± 0.5 |
| 15a |
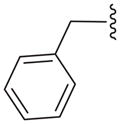
|
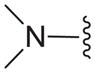
|
C | 48.0 ± 3.1 |
| 15b |
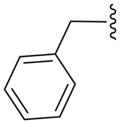
|
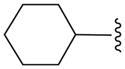
|
C | 28.4 ± 2.8 |
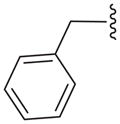
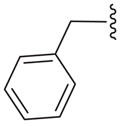
| 15c |
|
|
C | 7.2 ± 33 |
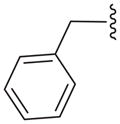
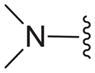
| 16a |
|
|
N | 14.5 ±20.5 |
| 16b |
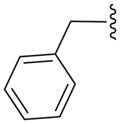
|
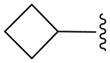
|
N | 16.1 ± 22.8 |
| 16c |
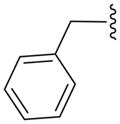
|
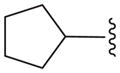
|
N | 21.8 ± 4.6b |
| 16d |
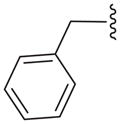
|
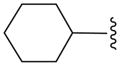
|
N | 23.2 ± 10.9 |
| 16e |
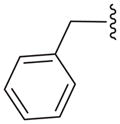
|
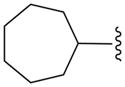
|
N | 22.3 ± 11.2 |
| 16f |
|
|
N | 18.5 ± 5.14 |
Data shown are average of 2 replicate independent experiments.
Replicate of 4 independent experiments.
Figure 1 shows a related TbrPDEB1 inhibitor chemotype (4) that showed equal enzymatic potency (though improved cellular potency) where the cyclopentyl catechol ether was replaced with a benzyl ether.11 In light of this, we opted to also make benzyl-substituted analogs of the compounds in Scheme 2, which was achieved by the route shown in Scheme 3. The brominated intermediate 7b was converted to the 2-chloropyridyl intermediate 14a under Suzuki conditions in modest yields. We prepared the pyrimidine template 16 via the boronate; we elected to utilize palladium catalysis for the preparation of 13, as the lithium-halogen exchange route applied in Scheme 2 led to undesirable side reactions. The crude boronate 13 was reacted with 2,4-dichloropyrimidine under Suzuki conditions to obtain intermediate 14b. As before, the pyrimidine and pyridine libraries were produced from halide displacement with various amines. We note that the dimethyl-substituted analogs (11a, 12a, 15a, 16a) were obtained as side-products when performing the displacement reactions in DMF, the chloride having reacted with dimethyl amine generated by thermal decomposition of the solvent.
Scheme 3.
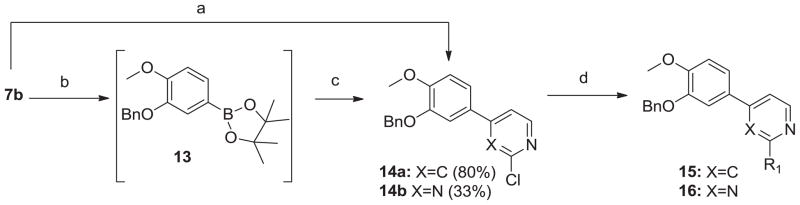
Synthesis of 15 and 16. Reagents and conditions: (a) 2-chloro-4-pyridylboronic acid, Na2CO3, PPh3, Pd2(dba)3, tol/EtOH/H2O (4:1:1), 105°C, o/n; (b) bis-(pinacolato)diborane, KOAc, PdCl2(dppf), dioxane, MW, 45 min, 145°C; (c) 2,4-dichloropyrimidine, Na2CO3, Pd2(dba)3, tol/EtOH/H2O (4:1:1), 105°C, o/n; (d) R1R2NH, DMF or NMP, heat.
All analogs generated were tested for inhibition of TbrPDEB1 at a concentration of 10 μM (Table 2). Regrettably, we observed that all analogs showed weak (< 50%) inhibition of TbrPDEB1. We conclude that, though the headgroup of 5 is responsible for significant improvement of potency over 1, the substituted aromatic headgroup replacements we prepared do not appropriately occupy the space that the headgroup of 5 does. We hypothesize that larger, nonplanar headgroups may be needed.
Figure 2 shows an overlay of 5 (green) against the cyclopentyl substituted analogs in 8c (black) and 11a (purple). This figure was generated by creating a conformer database of the analogs (using OMEGA version 2.4.3 OpenEye Scientific Software, Santa Fe, NM.),17 and generating optimal shape and electrostatic overlays against a minimized conformation of 5 (using ROCS version 3.0.0 and EON version 2.1.0). It is clear from this analysis that there is poor overlap between these new analogs and the fused ring of the N-alkylphthalizinone core, which most likely attributes to the observed differences in potency. Further, though the pyridyl, pyrimidinyl, or quinolinyl nitrogen is positioned close to the carbonyl of 5, these lone pairs may not reach far enough into the metal binding pocket to create good binding interactions suggested by previous crystallography results.10, 18–21 With this in mind, our optimization efforts continue in this class of compounds, with an eye towards a better recapitulation of the headgroup-metal interactions.
Figure 2.
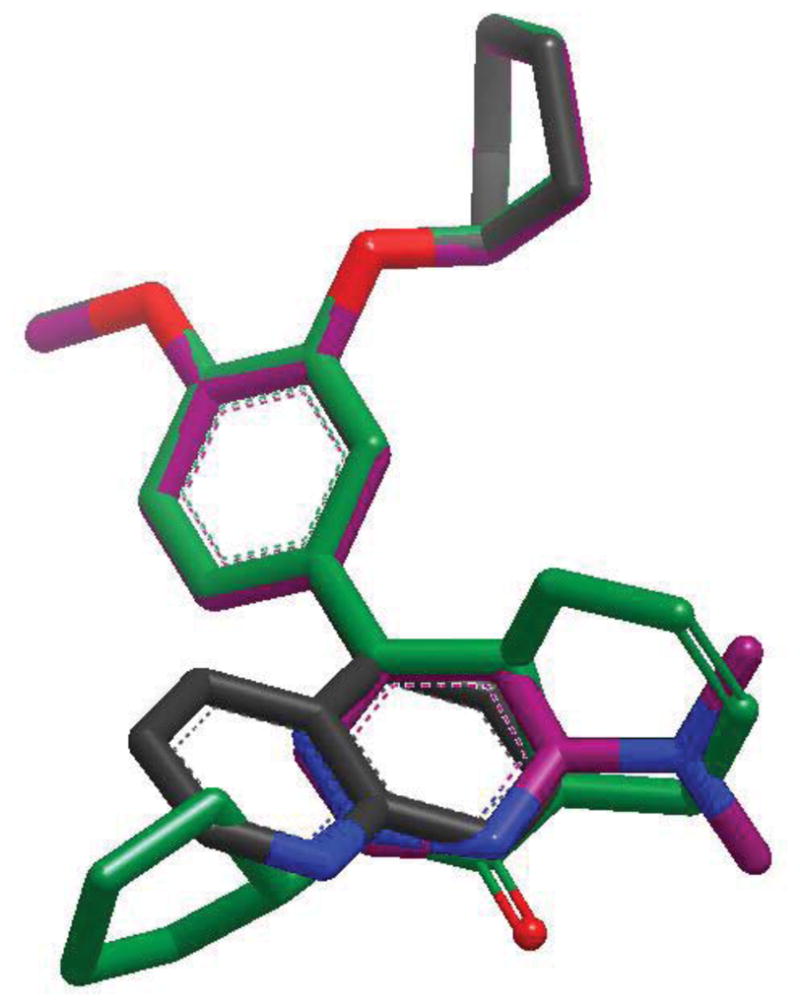
Overlay of 5(green) with compounds 8c (black)and 11a (purple).
Supplementary Material
Acknowledgments
We thank Dr. Geert-Jan Sterk (MercaChem) for helpful discussions. We acknowledge funding from the National Institutes of Health (R01AI082577), and are grateful for a free academic license from OpenEye Scientific Software for ROCS and EON.
Footnotes
Supporting information. Supporting information includes synthetic preparations, compound characterization, biological assay conditions, and a recapitulation of the tables from the manuscript that include compound registry numbers. All data is freely available on the Collaborative Drug Discovery database (www.collaborativedrug.com).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Savioli L, Daumerle D. World Health Organization. 2013 http://apps.who.int/iris/bitstream/10665/77950/1/9789241564540_eng.pdf.
- 2.Chappuis F, Udayraj N, Stietenroth K, Meussen A, Bovier PA. Clin Infect Dis. 2005;41:748. doi: 10.1086/432576. [DOI] [PubMed] [Google Scholar]
- 3.Pollastri MP, Campbell RK. Future Med Chem. 2011;3:1307. doi: 10.4155/fmc.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bland ND, Wang C, Tallman C, Gustafson AE, Wang Z, Ashton TD, Ochiana SO, McAllister G, Cotter K, Fang AP, Gechijian L, Garceau N, Gangurde R, Ortenberg R, Ondrechen MJ, Campbell RK, Pollastri MP. J Med Chem. 2011;54:8188. doi: 10.1021/jm201148s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochiana SO, Gustafson A, Bland ND, Wang C, Russo MJ, Campbell RK, Pollastri MP. Bioorg Med Chem Lett. 2012;22:2582. doi: 10.1016/j.bmcl.2012.01.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Ashton TD, Gustafson A, Bland ND, Ochiana SO, Campbell RK, Pollastri MP. Bioorg Med Chem Lett. 2012;22:2579. doi: 10.1016/j.bmcl.2012.01.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberholzer M, Marti G, Baresic M, Kunz S, Hemphill A, Seebeck T. FASEB J. 2007;21:720. doi: 10.1096/fj.06-6818com. [DOI] [PubMed] [Google Scholar]
- 8.de Koning HP, Gould MK, Sterk GJ, Tenor H, Kunz S, Luginbuehl E, Seebeck T. J Infect Dis. 2012;206:229. doi: 10.1093/infdis/jir857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender AT, Beavo JA. Pharmacol Rev. 2006;58:488. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 10.Jansen C, Wang H, Kooistra AJ, de Graaf C, Orrling KM, Tenor H, Seebeck T, Bailey D, de Esch IJ, Ke H, Leurs R. J Med Chem. 2013;56:2087. doi: 10.1021/jm3017877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orrling KM, Jansen C, Vu XL, Balmer V, Bregy P, Shanmugham A, England P, Bailey D, Cos P, Maes L, Adams E, van den Bogaart E, Chatelain E, Ioset JR, van de Stolpe A, Zorg S, Veerman J, Seebeck T, Sterk GJ, de Esch IJ, Leurs R. J Med Chem. 2012;55:8745. doi: 10.1021/jm301059b. [DOI] [PubMed] [Google Scholar]
- 12.Van der Mey M, Hatzelmann A, Van Klink GP, Van der Laan IJ, Sterk GJ, Thibaut U, Ulrich WR, Timmerman H. J Med Chem. 2001;44:2523. doi: 10.1021/jm010838c. [DOI] [PubMed] [Google Scholar]
- 13.Sterk G-J. personal communication.
- 14.Hatzelmann A, Boss H, Hafner D, Beume R, Kley H-P, Van DMM, Van DLIJ, Timmerman H, Sterk GJ. 9931071A1. WO. 1999:40.
- 15.Van DMM, Van DLIJ, Timmerman H, Hatzelmann A, Boss H, Hafner D, Beume R, Kley H-P, Sterk GJ. 9831674A1. WO. 1998:59.
- 16.Conde JJ. 2001019785A1. WO. 2001:16.
- 17.Hawkins PCD, Skillman AG, Warren GL, Ellingson BA, Stahl MT. J Chem Inf Model. 2010;50:572. doi: 10.1021/ci100031x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Card GL, England BP, Suzuki Y, Fong D, Powell B, Lee B, Luu C, Tabrizizad M, Gillette S, Ibrahim PN, Artis DR, Bollag G, Milburn MV, Kim SH, Schlessinger J, Zhang KY. Structure. 2004;12:2233. doi: 10.1016/j.str.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Yan Z, Geng J, Kunz S, Seebeck T, Ke H. Mol Microbiol. 2007;66:1029. doi: 10.1111/j.1365-2958.2007.05976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu RX, Hassell AM, Vanderwall D, Lambert MH, Holmes WD, Luther MA, Rocque WJ, Milburn MV, Zhao Y, Ke H, Nolte RT. Science. 2000;288:1822. doi: 10.1126/science.288.5472.1822. [DOI] [PubMed] [Google Scholar]
- 21.Huai Q, Liu Y, Francis SH, Corbin JD, Ke H. J Biol Chem. 2004;279:13095. doi: 10.1074/jbc.M311556200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.