Abstract
Background
Re-coarctation after the Norwood procedure increases risk for mortality. The Single Ventricle Reconstruction (SVR) trial randomized subjects with a single right ventricle undergoing a Norwood procedure to a modified Blalock-Taussig shunt (MBTS) or right ventricle-pulmonary artery shunt (RVPAS). We sought to determine incidence of re-coarctation, risk factors and outcomes in the SVR trial.
Methods and Results
Re-coarctation was defined by intervention, either catheter-based or surgical. Univariate analysis and multivariable Cox proportional hazard models were performed adjusting for center. Of the 549 SVR subjects, 97 (18%) underwent 131 interventions (92 balloon aortoplasty; 39 surgical) for re-coarctation at median age 4.9 months (range: 1.1–10.5). Intervention typically occurred at pre-stage II catheterization (n=71, 54%) or at stage II surgery (n=38, 29%). In multivariable analysis, re-coarctation was associated with the shunt type in place at the end of the Norwood procedure (HR 2.0 for RVPAS vs. MBTS, p=0.02), and Norwood discharge peak echo-Doppler arch gradient (HR 1.07 per 1 mmHg, p<0.01). Subjects with re-coarctation demonstrated comorbidities at pre-stage II evaluation including higher pulmonary arterial pressures (15.4±3.0 vs. 14.5±3.5 mm Hg; p=0.05), higher pulmonary vascular resistance (2.6±1.6 vs. 2.0±1.0 WU × m2; p=0.04) and increased echocardiographic volumes (end-diastolic volume: 126±39 vs. 112±33 ml/BSA1.3; p=0.02). There was no difference in 12-month post-randomization transplant-free survival between those with and without re-coarctation (p=0.14).
Conclusions
Re-coarctation is common after Norwood and contributes to pre-stage II comorbidities. Although with intervention there is no associated increase in 1-year transplant/mortality, further evaluation is warranted to evaluate effects of associated morbidities.
Keywords: Coarctation, heart defects, congenital, angioplasty
Introduction
Children born with the hypoplastic left heart syndrome (HLHS) and other single right ventricle lesions have a high mortality risk.1, 2 Individual centers have improved outcomes by identifying modifiable risk factors including residual or recurrent anatomic lesions.3, 4 Re-coarctation is one such lesion and contributes to mortality and also morbidity after the Norwood procedure.5, 6
Although re-coarctation is recognized as an important comorbidity, its true impact remains unknown. Current understanding is based on single center retrospective analyses which have reported incidence of re-coarctation ranging from as low as 2% to as high as 40%.7–13 These reports have not accurately defined risk factors for re-coarctation and it is unclear whether intervention occurs sufficiently early in the post-Norwood course when risk of mortality and morbidity is most significant. Furthermore there are limited data on the impact of intervention on morbidities and mortality.
The NHLBI-sponsored Pediatric Heart Network (PHN) Single Ventricle Reconstruction (SVR) Trial includes the largest multicenter, prospective cohort of infants with HLHS or related single right ventricle anomalies with longitudinal follow-up after the Norwood procedure.1 The purpose of this analysis is: 1) to describe the incidence and timing of intervention for re-coarctation by 12-months post-randomization, 2) to assess factors that predict intervention, and 3) to determine the impact of re-coarctation with intervention on morbidity and mortality in the first year after the Norwood procedure.
Methods
Study Design and Sample
The PHN SVR trial was a prospective trial that compared outcomes between subjects randomized to either a right ventricle pulmonary artery shunt (RVPAS) or a modified Blalock-Taussig shunt (MBTS) at the time of the Norwood procedure. Details of the trial design and main results have been previously reported.1 We performed a retrospective cohort study using data collected from the SVR trial. Of the 555 subjects enrolled in the SVR trial, six were excluded from the primary endpoint; five infants did not undergo a Norwood procedure after randomization, and one patient’s family withdrew research consent following the Norwood procedure. Thus, 549 subjects formed the analytic cohort for the trial. Each participating center’s Institutional Review Board approved the trial, and written informed consent was obtained from one or both parents.
Study Measurements
Prior to the Norwood procedure, a detailed pre-operative medical history was recorded, including demographics, subject characteristics, and anatomic diagnosis. Operative variables included: shunt type, type of arch reconstruction (including juxtaductal coarctectomy, and use of patch material(s), origin of PTFE graft (innominate, subclavian, common carotid, aorta or right ventricle) and additional cardiac operations. All post-Norwood surgical or percutaneous interventions were recorded including additional cardiac surgeries such as repair of re-coarctation at the time of stage II (superior cavopulmonary anastomosis) palliation. Hemodynamic data were collected for all subjects undergoing cardiac catheterization prior to stage II surgery.
Catheterizations were performed at the discretion of the individual centers after the Norwood procedure. A total of 389 catheterizations were performed. Hemodynamic data were submitted to the data coordinating center. Angiograms were interpreted at a core laboratory. Interpretable aortic angiograms were available for 331/389 (85%) study subjects with catheterization data. Angiographic measurements were performed in the lateral projection during systole. The coarctation index was defined as the ratio between the narrowest isthmic diameter and the diameter of the descending aorta at the level of the diaphragm. Cases of re-coarctation were defined as those cases for which surgical or catheter-based intervention was performed by 12-months post randomization. All other variables were defined as per the SVR study.1
Echocardiograms were obtained post-Norwood (either at the time of discharge or at approximately 30 days of age if still hospitalized), pre-stage II surgery (during the pre-operative evaluation for the stage II procedure) and at 14-months of age (end of study visit). There was no clinically meaningful difference in age at echocardiography for those with and without coarctation intervention [age at Norwood echocardiogram: 18 ± 10 vs. 23 ± 14 days; age at stage II echocardiogram: 5.0 ± 1.5 vs. 4.8 ± 1.5 months and age at 14 month echocardiogram: 14.5 ± 1.4 vs. 14.5 ± 1.2 months for those with and without coarctation by 12-months respectively]. Echocardiograms were interpreted centrally at a core laboratory. Echocardiographic variables included: native ascending aorta diameter, distal transverse arch diameter, continuous wave Doppler of the descending aorta, right ventricular fractional area change, body surface area (BSA) -indexed end diastolic dimension, BSA-indexed end systolic dimension and degree of atrioventricular valve regurgitation (none, mild, moderate, severe).
Statistical Methods
The unit of observation for this analysis was an infant enrolled in the study. We used standard summary statistics to describe the study variables including means and standard deviations and frequency counts and percentages. We evaluated risk factors for residual or recurrent coarctation using a Cox proportional hazards model with a shared gamma frailty to account for clustering by site.14 Individual centers included in the SVR trial defined the groups over which the frailties were shared. We treated the frailties of the final multivariable model as gamma distributed with a mean of one and a variance estimated from the data θ=0.394 with p=0.02 from the likelihood ratio test of H0: θ=0, suggesting a significant frailty effect. The hazard ratios were treated conditionally on θ fixed at its optimal value that maximized the penalized log likelihood.14 For the purpose of the model, time was defined as days from randomization to first intervention for re-coarctation (trans-catheter or surgical). The outcome of interest was an intervention for re-coarctation defined as a dichotomous variable and infants were censored at the time of death or at 12-months post randomization. Variables significantly associated with re-coarctation in a univariate analysis as well as those associated with coarctation in previous studies were included in the multivariable model. We tested the proportional hazards assumption using observed (Kaplan Meier) versus expected (Cox model) plots and goodness of fit tests on the basis of Schoenfeld residuals. For all variables included in the model, the observed versus predicted curves were visually inspected and the p-values from the goodness of fit tests were all greater than 0.05. We compared catheterization, stage II operation, and 14-month echocardiographic variables between subjects with and without intervention using Chi square tests of association for categorical variables and Wilcoxon rank-sum tests for continuous variables. We used a Wilcoxon signed-rank test to compare matched paired measurements of the coarctation index before and after balloon angioplasty in the same subject. For mortality outcomes, we used the Kaplan Meier method and compared 12 month transplant-free mortality between the groups with and without coarctation using the Log Rank test. We conducted all analyses using Stata 12.0 (College Station, Tx), and considered a p-value<0.05 statistically significant.
Results
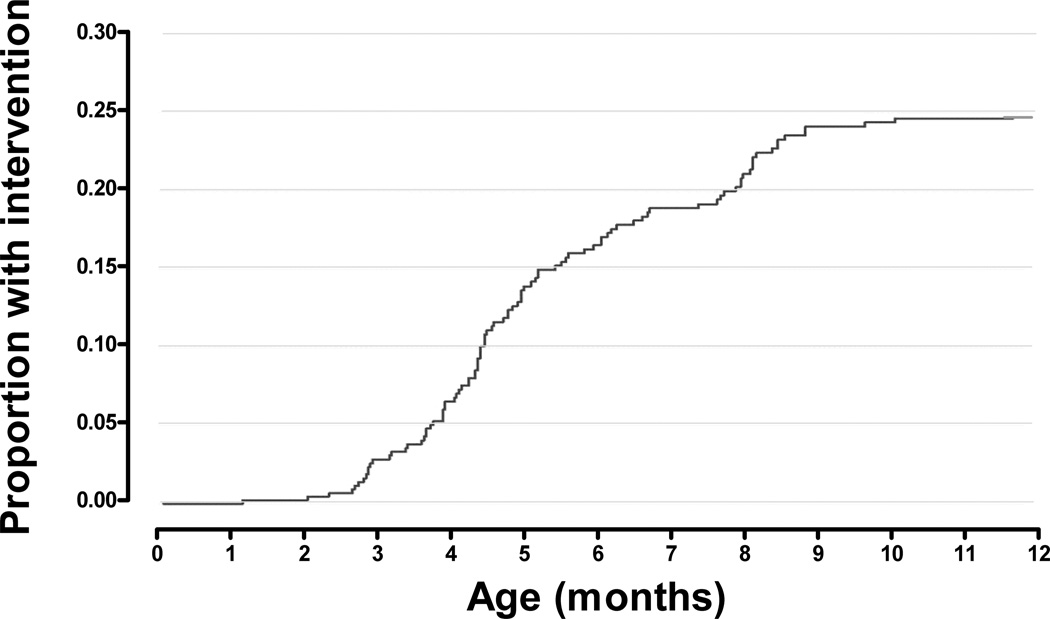
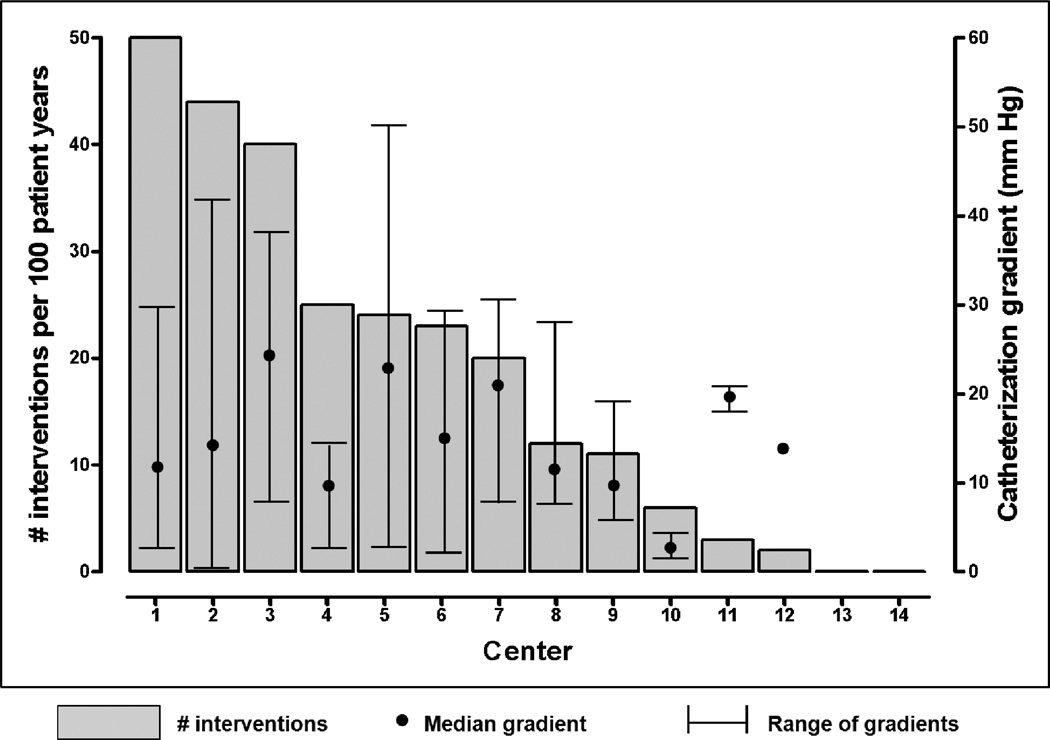
Of the 549 SVR subjects, 97 (18%) underwent a total of 131 interventions (92 balloon aortoplasty, 39 surgical) for re-coarctation within the first 12-months post randomization. Incidence of re-coarctation intervention at 1, 3, 6 and 12-months was 0%, 3%, 16% and 23% respectively (Figure 1). Intervention typically occurred at the time of pre-stage II cardiac catheterization (n=71, 54%), or concomitant with stage II surgery (n=38, 29%). Median age at initial intervention was 4.9 months (range: 1.1–10.5 months). There was wide center variation with intervention incidence rates ranging from 0 – 50 interventions per 100 patient years for the 14 centers enrolling ≥ 10 patients. Center variation did not appear to reflect differing indications for intervention, as centers with higher intervention incidence rates did not use lower catheterization peak re-coarctation gradients as an indication for intervention (Figure 2).
Figure 1. Proportion with re-coarctation intervention by 12 months post-randomization.
Kaplan Meier Curve for re-coarctation intervention by 12-months post-randomization.
Figure 2. Intervention rates for re-coarctation and catheterization re-coarctation gradients by center.
There was wide center variability in intervention rates (0–50%) for the 14 SVR centers that enrolled ≥ 10 subjects during the trial. Median and range of catheterization re-coarctation gradients are also presented by center.
By univariate Cox proportional hazards analysis (Table 1), intervention for re-coarctation by 12 months was not associated with any demographic or surgical variables but was associated with a smaller native ascending aorta (p=0.03) as well as echocardiographic indices assessed at Norwood hospital discharge, including smaller echocardiographic distal transverse arch diameter (p=0.04) and increased peak echo-Doppler arch gradient (p<0.01). Overall 46/355 (13%) subjects demonstrated a pre-Norwood discharge peak echo-Doppler gradient > 20 mm Hg. Of those, 22 ultimately received an intervention, 4 died in the interstage period and 20 survived without any intervention (sensitivity 31%, specificity 92%, positive predictive value 57%, negative predictive value 83%).
Table 1.
Univariate predictors of intervention for re-coarctation by 12 months
| n | Overall (N=449) |
Re- coarctation (N=97) |
No Re- coarctation (N=452) |
HR | p | |
|---|---|---|---|---|---|---|
| Demographic Features | ||||||
| Gestational age (weeks) | 549 | 38.2 ± 1.6 | 38.1 ± 1.5 | 38.2 ± 1.6 | 0.96 | 0.56 |
| Birth weight (grams) | 549 | 3103 ± 541 | 3058 ± 478 | 3113 ± 554 | 1.0 | 0.14 |
| Age at Norwood (days) | 549 | 5.8 ± 4.1 | 4.8 ± 3.3 | 6.0 ± 4.2 | 0.94 | 0.08 |
| Race | 544 | 95 | 439 | 0.16 | ||
| White | 436 (80.2) | 80 (84.2) | 356 (81.1) | 0.95 | ||
| Black | 86 (15.8) | 13 (13.7) | 73 (16.6) | 0.88 | ||
| Asian | 10 (1.8) | 2 (2.1) | 8 (2.0) | Ref | ||
| Hispanic | 539 | 95 | 444 | 0.94 | 0.84 | |
| Yes | 101 (18.7) | 20 (21.3) | 81 (18.2) | |||
| Morphologic Features | ||||||
| HLHS subtype | 480 | 87 | 393 | 0.47 | ||
| Mitral atresia/Aortic atresia | 209 (43.5) | 42 (48.3) | 167 (42.5) | 1.43 | ||
| Mitral stenosis/Aortic atresia | 138 (28.8) | 19 (21.8) | 119 (30.3) | 1.16 | ||
| Mitral atresia/Aortic stenosis | 23 (4.8) | 6 (6.9) | 17 (4.3) | 1.92 | ||
| Mitral stenosis/Aortic stenosis | 110 (22.9) | 20 (23.0) | 90 (22.9) | Ref | ||
| Aortic atresia | 480 | 347 (72.3) | 61 (70.1) | 286 (72.8) | 1.02 | 0.47 |
| Native ascending aorta diameter | 527 | 97 | 430 | 0.59 | 0.03 | |
| > 2mm | 98 (18.6) | 24 (24.7) | 74 (17.2) | |||
| Surgical Variables | ||||||
| Bypass time (minutes) | 549 | 144 ± 54 | 137 ± 50 | 145 ± 55 | 1.0 | 0.65 |
| Coarctectomy | 548 | 96 | 452 | 1.1 | 0.63 | |
| Yes | 184 (33.6) | 30 (31.2) | 154 (34.1) | |||
| Shunt type (Intention to treat) | 549 | 97 | 452 | |||
| RV-PA shunt | 274 (49.9) | 56 (42.2) | 218 (48.3) | 1.2 | 0.32 | |
| MBTS | 275 (50.1) | 41 (57.8) | 234 (51.7) | Ref | ||
| Shunt type (actual shunt received) | 549 | 97 | 452 | |||
| RV-PA shunt | 281 (51.2) | 60 (61.9) | 221 (48.9) | 1.3 | 0.19 | |
| MBTS | 268 (48.8) | 37 (38.1) | 231 (51.1) | Ref | ||
| Echo Variables (Stage I Discharge) | ||||||
| Peak arch gradient (mm Hg) | 355 | 11.2 ± 7.9 | 14.9 ± 1.1 | 10.2 ± 6.5 | 1.06 | <0.01 |
| Distal arch diameter (mm) | 442 | 6.3 ± 1.5 | 5.9 ± 1.6 | 6.4 ± 1.5 | 0.18 | 0.04 |
| RV end-diastolic volume (mL/BSA1.3) | 309 | 93 ± 50 | 93 ± 32 | 93 ± 24 | 1.0 | 0.61 |
| RV end-systolic volume (mL/BSA1.3) | 309 | 50 ± 17 | 49 ± 21 | 50 ± 16 | 1.0 | 0.89 |
| RV fractional area change (%) | 376 | 37 ± 7 | 37 ± 8 | 37 ± 7 | 1.5 | 0.61 |
Data represent n ± 1 SD or n (%), HLHS represents Hypoplastic Left Heart Syndrome, RV represents right ventricle. Hazard ratios are from univariate Cox proportional hazards models.
By multivariable Cox proportional hazard modeling adjusted for site, predictors of re-coarctation intervention included the peak echocardiographic arch gradient at the time of Norwood discharge (HR = 1.07 per 1 mm Hg, p<0.01) and receipt of an RVPAS (HR = 2.0 for RVPAS, p=0.02). However when the model was repeated using the assigned shunt type (intention to treat), there was no association with re-coarctation (HR = 1.4, p=0.24) (Table 2). The multivariable results were unchanged when excluding all Norwood hospital deaths and transplants.
Table 2.
Multivariable predictors of intervention for re-coarctation by 12 months
| Predictor | Hazard Ratio (95% CI) |
Model p value |
|---|---|---|
| Birth weight (grams) | 0.99 (0.99–1.0) | 0.87 |
| Bypass time (minutes) | 1.0 (0.99–1.0) | 0.44 |
| Age at Norwood (days) | 0.9 (0.9–1.0) | 0.18 |
| RVPAS (actual shunt received)* | 2.0 (1.1–3.6) | 0.02 |
| Coarctectomy | 0.99 (0.5–2.0) | 0.98 |
| Native ascending aorta > 2mm | 0.66 (0.3–1.3) | 0.22 |
| Norwood discharge echo gradient (mm Hg) | 1.07 (1.0–1.1) | <0.01 |
| Aortic atresia | 0.65 (0.4–1.2) | 0.19 |
Actual shunt received represents the shunt in place at the end of the Norwood procedure. When multivariable modeling was repeated using the intention to treat shunt type, use of an RVPAS was no longer associated with re-coarctation (HR = 1.4; 95% CI 0.8–2.4; p=0.24)
Re-coarctation intervention and pre-stage II comorbidities
By univariate analysis (Table 3), intervention for re-coarctation prior to stage II surgery was associated with hemodynamic variables measured at pre-stage II catheterization including a higher peak systolic catheterization gradient (p<0.01), higher mean pulmonary arterial pressures (p=0.05) and higher pulmonary vascular resistance (p=0.04). Subjects undergoing intervention also demonstrated increased echocardiographic BSA-indexed right ventricular end-systolic (p<0.04) and end-diastolic volumes (p<0.02) at the time of stage II surgery. However, right ventricular volumes were not different for those with and without re-coarctation at the 14-month follow up echocardiogram. This relationship remained when comparing only the 182 subjects who had both stage II and 14-month echocardiographic data. Importantly, subjects who underwent intervention for re-coarctation did not demonstrate decreased right ventricular fractional area change or increased atrioventricular valve regurgitation at the time of stage II surgery or at 14-month follow up compared to those who did not have a re-coarctation intervention by 12 months.
Table 3.
Hemodynamic and echocardiographic consequences
| N | Re- coarctation |
No Re- coarctation |
p | |
|---|---|---|---|---|
| Pre-Stage II Cath | ||||
| End diastolic pressure (mm Hg) | 69/311 | 8.6±3.3 | 8.2±3.3 | 0.30 |
| Wedge pressure (mm Hg) | 45/205 | 10.7±3.4 | 10.8±3.9 | 0.80 |
| PA pressure (mm Hg) | 48/204 | 15.4±3.0 | 14.5±3.5 | 0.05 |
| PVRI (WU × m2) | 40/230 | 2.6±1.6 | 2.0±1.0 | 0.04 |
| AVO2 difference | 64/306 | 25.4±7.6 | 24.8±7.4 | 0.80 |
| Coarctation index | 68/257 | 0.6±0.2 | 0.9±0.1 | <0.01 |
| Peak systolic gradient (mm Hg) | 79/280 | 19.8±14.3 | 3.3±7.1 | <0.01 |
| Stage II Echo | ||||
| RV end-diastolic volume (mL/BSA1.3) | 57/210 | 126±39 | 112±33 | 0.02 |
| RV end-systolic volume (mL/BSA1.3) | 57/210 | 74±32 | 63±23 | 0.04 |
| RV fractional area change (%) | 84/276 | 32±8 | 34±8 | 0.24 |
| Tricuspid insufficiency | 68/329 | 0.95 | ||
| None/mild | 50 (73.5) | 242 (73.6) | ||
| Mod/severe | 18 (26.5) | 87 (26.4) | ||
| 14 month Echo | ||||
| RV end-diastolic volume (mL/BSA1.3) | 48/181 | 94±32 | 89±30 | 0.51 |
| RV end-systolic volume (mL/BSA1.3) | 48/181 | 54±24 | 52±23 | 0.71 |
| RV fractional area change (%) | 66/225 | 33±7 | 32±7 | 0.56 |
| Tricuspid insufficiency | 73/247 | 0.48 | ||
| None/mild | 56 (76.7) | 191 (77.3) | ||
| Mod/severe | 17 (23.3) | 56 (22.7) | ||
Data represent mean ± 1SD or n (%), N = numbers of subjects with and without re-coarctation respectively, p values are from Pearson’s chi-square or Wilcoxon rank-sum tests where appropriate, PA represents pulmonary arterial, PVRI represents pulmonary vascular resistance index, WU represents Wood units, AVO2 difference represents the difference between the systemic and mixed venous saturation, RV represents right ventricle.
Intervention outcomes
Overall 33/97 (34%) subjects received a second (n=31) or third (n=2) intervention for re-coarctation within 12-months post randomization. Reintervention rate was 39% (n=33/83) for subjects undergoing balloon angioplasty as their initial intervention. While balloon angioplasty significantly improved the coarctation index (0.6 ± 0.1 vs. 0.8 ± 0.2 for pre- versus post-angioplasty index, p<0.01), there was no association between degree of improvement and need for subsequent reintervention. For those subjects undergoing surgical re-coarctation intervention, 5% (n=2/39) required subsequent reintervention for re-coarctation.
Outcomes
At 12-months post randomization there were 7 deaths and 2 heart transplants out of the 97 subjects with prior intervention for re-coarctation. Comparing all patients undergoing pre-stage II cardiac catheterization (n=380), 12-month post randomization transplant free survival did not differ for those with intervention (86% versus 91% for intervention versus no intervention respectively; p=0.14). There was also no difference in 1 year transplant free survival in the subset of subjects who survived to stage II surgery (88% versus 93%; for prior intervention versus no intervention respectively, p=0.07).
While mortality in the SVR cohort was more likely during the Norwood hospitalization or interstage period, very few subjects with early mortality had prior intervention for re-coarctation (n=1/97 and 1/51 for Norwood hospital and interstage mortality respectively). However, 29% (7/24) of subjects who died after stage II surgery through 12 months, had prior re-coarctation intervention. To assess whether this disproportionate contribution to late mortality might reflect a higher incidence of “unrecognized” re-coarctation in those with interstage mortality; pre-Norwood discharge echo-Doppler arch gradients were assessed. While peak gradients were significantly higher in those with interstage mortality, quantifiable differences were small (11 ± 8 vs. 13 ± 6 mm Hg for survivors vs. interstage deaths respectively, p=0.03) and only 4/51 subjects with interstage mortality demonstrated a pre-Norwood discharge gradient > 20 mm Hg.
Discussion
The major findings of the present multi-center analysis include an 18% cumulative incidence of intervention for re-coarctation with incidence by center ranging widely from 0 – 50%. Re-coarctation appears difficult to predict early in the clinical course and intervention typically occurred later in the post-Norwood course, most commonly at the time of pre-stage II catheterization. Subjects with intervention demonstrated pre-stage II comorbidities including higher PVR and right ventricular dilation; however, echocardiographic dimensions demonstrate relative normalization after intervention. Initial intervention for re-coarctation was most often balloon aortoplasty which was acutely effective at increasing the coarctation index but either inadequate or short-lived requiring subsequent reintervention in 39%. There was no difference in overall survival between those with and without re-coarctation intervention; however, re-coarctation may have been unrecognized in those with pre-stage II mortality who did not undergo cardiac catheterization.
Re-coarctation following single ventricle surgery has been shown to exacerbate atrioventricular valve regurgitation, ventricular dysfunction and to increase mortality risk.5, 7, 15 Despite these recognized consequences, the true scope of the problem has been difficult to define with reported incidence ranging from 2% to 40%.7–13 These prior reports have been retrospective, single center analyses. The present report is the first multi-center analysis with prospectively collected data. These data confirm that re-coarctation is a significant concern, with intervention in nearly 1 in 5 Norwood recipients.
In this analysis there was no difference in 12-month post randomization transplant free survival or post-stage II survival for subjects with intervention. Although encouraging that with intervention late mortality outcomes are unaffected, it is less clear whether earlier mortality in the pre-discharge or interstage period might be impacted by re-coarctation. Mortality risk is significantly higher during these earlier stages. However, in this cohort intervention for re-coarctation was performed rarely in subjects during Norwood hospitalization or in those children with interstage mortality. This suggests that re-coarctation either does not develop until late in the course or is under-diagnosed in those with earlier mortality. Under-diagnosis is a concern as Fraisse et al. demonstrated 40% mortality in patients with a missed diagnosis of re-coarctation that was subsequently identified by autopsy.6 To assess the likelihood of missed diagnoses, pre-Norwood discharge echocardiographic arch gradients were evaluated. Although gradients were significantly higher in those with interstage death, the measured differences were small and sensitivity of gradients commonly used to predict re-coarctation (i.e. > 20mm Hg) was poor. Previous studies have evaluated similar pre-stage I discharge echocardiographic indices including arch gradients, atrioventricular valve regurgitation and ventricular dysfunction and have also demonstrated variable sensitivity with differences typically only seen with more severe re-coarctation.6, 16 There were also no specific anatomic or demographic risk factors that predicted intervention. Our impression is that re-coarctation is not easy to predict early in the post-surgical course using echocardiographic or clinical variables. This conclusion is supported by the timing of intervention which occurred relatively late in the stage I course, typically close to stage II surgery. Some centers are now moving towards catheterization or alternative imaging prior to Norwood discharge. It remains to be seen if this will allow for earlier identification and intervention for re-coarctation or if earlier intervention would change outcomes.
Beyond mortality, several morbidity measures were associated with re-coarctation intervention including increasing echocardiographic ventricular dimensions, mean pulmonary arterial pressures and pulmonary vascular resistance. While echocardiographic ventricular end-systolic and end-diastolic dimensions were increased at the time of intervention, these dimensions demonstrated relative normalization at 1-year follow up. This is encouraging and suggests that some of the deleterious effects of re-coarctation can be reversed with intervention.
Surgical risk factors for re-coarctation
Several reports have suggested that re-coarctation is dynamic and may result from contraction of residual ductal tissue. In autopsy specimens ductal tissue has been demonstrated at the re-coarctation site and surgeons have reported decreased incidence when using techniques that eliminate ductal tissue.8, 9, 17, 18 These techniques include complete resection using coarctectomy or coarctectomy with an interdigitating technique to further prevent ductal constriction.9, 17 In this present analysis interdigitation was not reported but complete coarctectomy was used in one third of subjects and did not decrease incidence of re-coarctation intervention. Despite the absence of a clear cut surgical risk factor, the wide center variability suggests that perhaps surgical technique may be an important factor.
An interesting finding in this analysis was the association of assigned shunt type but not intention to treat shunt type with reintervention. One possibility is that re-coarctation is a greater risk for early mortality in those with a MBTS than in those with an RVPAS. Other SVR trial analysis has demonstrated increased interstage mortality with a MBTS and it is plausible that this circulation is more tenuous and incapable of tolerating the hemodynamic burden of re-coarctation.19 Another explanation is that factors leading to cross-over from one shunt type to the other might have impacted risk of re-coarctation. The intraoperative cross-over rate in the SVR trial was 9% with an approximately equal number in each direction.1 The most frequent reason for shunt cross-over at the time of Norwood was cardiac or arch anatomy that prevented use of the assigned shunt. Potentially those subjects with complex arch anatomy that prohibited use of the MBTS were at increased risk for re-coarctation.
Interventions for re-coarctation
Balloon aortoplasty has become the intervention of choice for re-coarctation at most centers. Our data confirm the findings of prior reports indicating that balloon aortoplasty is effective at acutely increasing isthmic diameter. 7, 11–13, 20–22However 39% of subjects required subsequent reintervention which is higher than previous reports. While surgical intervention is associated with a lower recurrence rate, many caretakers find it reasonable to use aortoplasty as the first line intervention as surgical intervention is more invasive. However aortoplasty is not risk free and several prior reports have demonstrated intra-procedural arrest or bradyarrhythmias with need for cardiopulmonary resuscitation in 20–30% of those undergoing aortoplasty.12, 13, 20, 22
Limitations of the current study include the noted use of intervention to define re-coarctation. The advantage of this definition is that it is a well-defined endpoint. However indications for intervention as well as timing of intervention likely vary by center which introduces some heterogeneity. This definition also limits ability to detect re-coarctation in subjects who did not undergo cardiac catheterization. There were no invasive hemodynamic or angiographic data available for the majority of subjects with early mortality. Other limitations include the absence of specific data on surgical technique or procedural details during catheterization, including specific details on balloon size, post intervention hemodynamics and procedural complications.
In conclusion, intervention for re-coarctation was performed in almost 1 in 5 patients following single ventricle surgical reconstruction. Intervention was typically performed at the time of pre-stage II catheterization and in this analysis very few patients with early mortality (pre-stage II) had intervention for re-coarctation. It is counterintuitive that re-coarctation is protective against early mortality and more likely that re-coarctation is under-diagnosed. It is enticing to speculate that earlier diagnosis may decrease interstage mortality. There was wide center variability and this finding suggests that either some centers have managed to significantly decrease re-coarctation incidence or alternatively, that some centers are simply more aggressive with intervention. The former explanation seems more likely as gradients at the time of intervention did not vary substantially by center. Regardless of the explanation, re-coarctation remains a common problem. Although associated intermediate term morbidities such as ventricular dilation appear to be reversible with intervention, it remains unclear whether long-term outcome is improved.
Supplementary Material
Acknowledgements
Funding Sources
This publication was made possible by Grant Numbers HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057, HL109737 and HL085057 from the National Heart Lung and Blood Institute.
Footnotes
Disclosures
None
See Appendix for a complete list of the Pediatric Heart Network Investigators
References
- 1.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fixler DE, Nembhard WN, Salemi JL, Ethen MK, Canfield MA. Mortality in first 5 years in infants with functional single ventricle born in Texas, 1996 to 2003. Circulation. 2010;121:644–650. doi: 10.1161/CIRCULATIONAHA.109.881904. [DOI] [PubMed] [Google Scholar]
- 3.Ghanayem NS, Cava JR, Jaquiss RD, Tweddell JS. Home monitoring of infants after stage one palliation for hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:32–38. doi: 10.1053/j.pcsu.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Ghanayem NS, Hoffman GM, Mussatto KA, Cava JR, Frommelt PC, Rudd NA, Steltzer MM, Bevandic SM, Frisbee SS, Jaquiss RD, Litwin SB, Tweddell JS. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg. 2003;126:1367–1377. doi: 10.1016/s0022-5223(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 5.Bartram U, Grunenfelder J, Van Praagh R. Causes of death after the modified Norwood procedure: a study of 122 postmortem cases. Ann Thorac Surg. 1997;64:1795–1802. doi: 10.1016/s0003-4975(97)01041-2. [DOI] [PubMed] [Google Scholar]
- 6.Fraisse A, Colan SD, Jonas RA, Gauvreau K, Geva T. Accuracy of echocardiography for detection of aortic arch obstruction after stage I Norwood procedure. Am Heart J. 1998;135:230–236. doi: 10.1016/s0002-8703(98)70086-9. [DOI] [PubMed] [Google Scholar]
- 7.Zeltser I, Menteer J, Gaynor JW, Spray TL, Clark BJ, Kreutzer J, Rome JJ. Impact of re-coarctation following the Norwood operation on survival in the balloon angioplasty era. J Am Coll Cardiol. 2005;45:1844–1848. doi: 10.1016/j.jacc.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 8.Lamers LJ, Frommelt PC, Mussatto KA, Jaquiss RD, Mitchell ME, Tweddell JS. Coarctectomy combined with an interdigitating arch reconstruction results in a lower incidence of recurrent arch obstruction after the Norwood procedure than coarctectomy alone. J Thorac Cardiovasc Surg. 2012;143:1098–1102. doi: 10.1016/j.jtcvs.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkhart HM, Ashburn DA, Konstantinov IE, De Oliveira NC, Benson L, Williams WG, Van Arsdell GS. Interdigitating arch reconstruction eliminates recurrent coarctation after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;130:61–65. doi: 10.1016/j.jtcvs.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 10.Ishino K, Stumper O, De Giovanni JJ, Silove ED, Wright JG, Sethia B, Brawn WJ, de Leval M. The modified Norwood procedure for hypoplastic left heart syndrome: early to intermediate results of 120 patients with particular reference to aortic arch repair. J Thorac Cardiovasc Surg. 1999;117:920–930. doi: 10.1016/s0022-5223(99)70373-9. [DOI] [PubMed] [Google Scholar]
- 11.Chessa M, Dindar A, Vettukattil JJ, Stumper O, Wright JG, Silove ED, De Giovanni J. Balloon angioplasty in infants with aortic obstruction after the modified stage I Norwood procedure. Am Heart J. 2000;140:227–231. doi: 10.1067/mhj.2000.108238. [DOI] [PubMed] [Google Scholar]
- 12.Tworetzky W, McElhinney DB, Burch GH, Teitel DF, Moore P. Balloon arterioplasty of recurrent coarctation after the modified Norwood procedure in infants. Catheter Cardiovasc Interv. 2000;50:54–58. doi: 10.1002/(sici)1522-726x(200005)50:1<54::aid-ccd11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Zellers TM. Balloon angioplasty for recurrent coarctation of the aorta in patients following staged palliation for hypoplastic left heart syndrome. Am J Cardiol. 1999;84:231–233. doi: 10.1016/s0002-9149(99)00242-8. A239. [DOI] [PubMed] [Google Scholar]
- 14.Glidden DV, Vittinghoff E. Modelling clustered survival data from multicentre clinical trials. Stat Med. 2004;23:369–388. doi: 10.1002/sim.1599. [DOI] [PubMed] [Google Scholar]
- 15.Larrazabal LA, Selamet Tierney ES, Brown DW, Gauvreau K, Vida VL, Bergersen L, Pigula FA, del Nido PJ, Bacha EA. Ventricular function deteriorates with recurrent coarctation in hypoplastic left heart syndrome. Ann Thorac Surg. 2008;86:869–874. doi: 10.1016/j.athoracsur.2008.04.074. discussion 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemler MS, Zellers TM, Harris KA, Ramaciotti C. Coarctation index: identification of recurrent coarctation in infants with hypoplastic left heart syndrome after the Norwood procedure. Am J Cardiol. 2000;86:697–699. doi: 10.1016/s0002-9149(00)01058-4. A699. [DOI] [PubMed] [Google Scholar]
- 17.Bautista-Hernandez V, Marx GR, Gauvreau K, Pigula FA, Bacha EA, Mayer JE, Jr, del Nido PJ. Coarctectomy reduces neoaortic arch obstruction in hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2007;133:1540–1546. doi: 10.1016/j.jtcvs.2006.12.067. [DOI] [PubMed] [Google Scholar]
- 18.Machii M, Becker AE. Nature of coarctation in hypoplastic left heart syndrome. Ann Thorac Surg. 1995;59:1491–1494. doi: 10.1016/0003-4975(95)00154-d. [DOI] [PubMed] [Google Scholar]
- 19.Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, Eghtesady P, Frommelt PC, Gruber PJ, Hill KD, Kaltman JR, Laussen PC, Lewis AB, Lurito KJ, Minich LL, Ohye RG, Schonbeck JV, Schwartz SM, Singh RK, Goldberg CS. Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore JW, Spicer RL, Mathewson JW, Kirby WC. High-risk angioplasty. Coarctation of the aorta after Norwood Stage 1. Tex Heart Inst J. 1993;20:48–50. [PMC free article] [PubMed] [Google Scholar]
- 21.Porras D, Brown DW, Marshall AC, Del Nido P, Bacha EA, McElhinney DB. Factors associated with subsequent arch reintervention after initial balloon aortoplasty in patients with Norwood procedure and arch obstruction. J Am Coll Cardiol. 2011;58:868–876. doi: 10.1016/j.jacc.2010.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soongswang J, McCrindle BW, Jones TK, Vincent RN, Hsu DT, Kuhn MA, Moskowitz WB, Cheatham JP, Kholwadwala DH, Benson LN, Nykanen DG. Outcomes of transcatheter balloon angioplasty of obstruction in the neo-aortic arch after the Norwood operation. Cardiol Young. 2001;11:54–61. doi: 10.1017/s1047951100012427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.