Abstract
Background
Locoregionally advanced stage head and neck cancer (HNC) is known for unfavorable outcome with only ~ 40–50 % 3-year overall survival (OS). Clinical T4 stage includes a wide range of tumor burden. The lack of further nonsurgical subgrouping of cT4 stage makes intercenter outcome of irradiated cohorts difficult. Aim of this analysis was to further stratify cT4 stage HNC using volumetric staging.
Material and methods
Between January 2002 and January 2013, a total of 201 cT4 stage squamous cell cancer (SCC) HNC patients referred to our center for curative definitive radiation were consecutively irradiated. Radiation was performed using modulated techniques. Total gross tumor volumes (tGTV: primary + nodal tumor volume) of all patients have retrospectively been stratified using a prospectively evaluated volumetric staging system which bases on 3 cut-offs (15/70/130 ml), translating into 4 prognostic subgroups [V1: 1–15 ml (n = 15), V2: 16–70 ml (108), V3: 71–130 ml (62), V4: > 130 ml (16)]. OS, disease-free survival (DFS), locoregional control (LRC), and distant metastasis-free survival (DMFS) rates were calculated.
Results
The mean/median follow-up was 31/23 months (range 1–116 months). The 3-year OS, DFS, LRC, and DMFS rates of the entire cohort were 63, 44, 48, and 77 %, respectively. Volumetric staging revealed its potential to prognostically statistically significantly divide the cT4 cohort into 4 volume subgroups (V1/2/3/4): OS: 90 %/72 %/58 %/18 %; DFS: 83 %/50 %/39 %/10 %; LRC: 81 %/53 %/47 %/15 %; DMFS: 93 %/90 %/70 %/41 %, all p < 0.0001.
Conclusion
Volumetric staging allowed a highly statistically significant stratification of cT4 HNC stages into prognostic subgroups, which offers the chance of better intercenter comparability of irradiated advanced stage HNC cohorts.
Keywords: Volumetric staging, cT4 stage tumors, Head and neck neoplasms, Neoplasm staging, Prognosis
Abstract
Hintergrund
Lokoregionär fortgeschrittene Kopf-Hals-Tumoren (KHT) haben eine schlechte Prognose mit nur ~ 40–50 % 3-Jahres-Gesamtüberleben (GÜ). cT4-Stadien beinhalten eine große Spanne von Tumorvolumina. Das Fehlen einer weiteren nichtchirurgischen Unterteilung von cT4-Stadien macht den Vergleich der Resultate bestrahlter Kohorten aus verschiedenen Zentren schwierig. Ziel unserer Arbeit war, cT4-Stadien bei definitiv bestrahlten KHT-Patienten mittels volumetrischem Staging zu stratifizieren.
Material und Methodik
Zwischen Januar 2002 und Januar 2013 wurden uns 201 KHT-Patienten mit einem Plattenepitelkarzinom im Stadium cT4 zur kurativen definitiven Radiotherapie zugewiesen. Alle Patienten wurden mit modulierten Techniken bestrahlt. Das Gesamttumorvolumen (tGTV: Primärtumor + Lymphknotenmetastasen) aller Patienten wurde retrospektiv mittels eines prospektiv getesteten volumetrischen Staging-Systems mit 3 Schnittwerten (15/70/130 ml) stratifiziert, was zu 4 prognostischen Subgruppen führt [V1: 1–15 ml (n = 15), V2: 16–70 ml (n = 108), V3: 71–130 ml (n = 62), V4: > 130 ml (n = 16)]. GÜ, krankheitsfreies Überleben (KFÜ), lokoregionäre Kontrolle (LRK) und metastasenfreies Überleben (MFÜ) wurden berechnet.
Ergebnisse
Die mittlere/mediane Bobachtungszeit betrug 31/23 Monate (Spanne 1–116 Monate). Das 3-Jahres-GÜ, -KFÜ, -LRK und -MFÜ der gesamten Kohorte betrug 63, 44, 48 und 77 %. Mittels volumetrischem Staging konnte die cT4-Kohorte in 4 statistisch hochsignifikant unterschiedliche prognostische Untergruppen stratifiziert werden (jeweils V1/2/3/4): GÜ: 90 %/72 %/58 %/18 %; KFÜ: 83 %/50 %/39 %/10 %; LRK: 81 %/53 %/47 %/15 %; MFÜ: 93 %/90 %/70 %/41 %, alle p < 0,0001.
Schlussfolgerung
Volumetrisches Staging erlaubte eine statistisch hochsignifikante Stratifizierung in prognostisch unterschiedliche Untergruppen, was eine bessere Vergleichbarkeit von Resultaten verschiedener Zentren nach primärer intensitätsmodulierter Strahlentherapie (IMRT) von cT4 KHT ermöglichte.
Schlüsselwörter: Volumetrisches Staging, cT4-Tumorstadium, Kopf-Hals-Tumore, Tumorstaging, Prognose
Advanced stage head and neck cancer (HNC) is known for generally unfavorable outcome with only ~ 40–50 % 3-year overall survival [1, 2, 3]. Clinical T4 stage includes a wide range of tumor volumes. The lack of further nonsurgical subgrouping of cT4 stage makes intercenter comparison of outcome results in irradiated cT4 patient cohorts difficult. The estimation of operability (cT4a versus cT4b) is sometimes quite dependent of a surgeon’s individual opinion and experience. In addition, the in- or exclusion of very advanced cT4 any NM0 into curatively aimed treatment regimens remains quite subjective.
The aim of this analysis was to further stratify cT4 stage squamous cell HNC disease using volumetric staging. This was performed with the help of a formerly prospectively tested and published volumetric scoring system [4, 5, 6, 7]. Using this scoring system, we previously demonstrated that volumetric staging was superior compared to the standard TN/AJCC systems regarding predictive power of disease control and survival of our irradiated cohorts.
Included in the presented analysis were all cT4 stage primary squamous cell cancer (SCC) HNC patients referred for definitive radiation.
Methods
Between January 2002 and January 2013, a total of 201 cT4 stage SCC HNC patients were referred to our department. All were treated with curative intent with modulated radiotherapy ± chemotherapy. All patients were retrospectively stratified using a prospectively evaluated volumetric staging system. T4 lymphoepithelial nasopharynx tumors (n = 13) and paranasal tumors (n = 8) were excluded. The used staging system is based on three cut-offs (15/70/130 ml, see also previous publications [4, 5, 6, 7]) to stratify the total gross tumor volumes (tGTV: primary and nodal tumor volume), allowing a subdivision of cT4 stages into 4 prognostic subgroups [1–15 ml (n = 15), 16–70 ml (n = 108), 71–130 ml (n = 62), > 130 ml (n = 16)]. Overall survival (OS), disease-free survival (DFS), locoregional control (LRC), and distant metastasis-free survival (DMFS) rates were calculated using Kaplan–Meier curves. Demographic data and tumor characteristics are listed in Tab. 1.
Tab. 1.
Patient and tumor characteristics
| Parameters | cT4 | ||
|---|---|---|---|
| Patients (n) | 201 | ||
| Gender (female:male) | 25 %:75 % | ||
| Mean age (range) | 62 (38–91) years | ||
| Mean/median folllow-up (range) | 31/23 (1–116) months | ||
| Histology | Squamous cell carcinoma | 201 | |
| Diagnosis | Mesopharynx | 116 (58 %) | |
| Hypopharynx | 42 (21 %) | ||
| Oral cavity | 24 (12 %) | ||
| Larynx | 19 (9 %) | ||
| N stage | N0 | 43 (21 %) | |
| N1–2b | 61 (30 %) | ||
| N2c | 88 (44 %) | ||
| N3 | 9 (5 %) | ||
| Total gross tumor volume (tGTV) | Mean | Range | 64 ml (7–216) |
| V1 | 1–15 ml | 15 (7 %) | |
| V2 | 16–70 ml | 108 (54 %) | |
| V3 | 71–130 ml | 62 (31 %) | |
| V4 | > 30 ml | 16 (8 %) | |
| Concomitant systemic therapy | None | 31 (15 %) | |
| Cisplatin only | 112 (56 %) | ||
| Cetuximab only | 25 (12 %) | ||
| Cisplatin switched to cetuximab | 33 (16 %) | ||
| Induction chemotherapy | 36 (17 %) | ||
All patients underwent modulated radiation therapy using simultaneously integrated boost techniques [SIB-IMRT/SIB-volumetric modulated arc therapy (SIB-VMAT)]. In 84 %, concomitant cisplatin chemotherapy (40 mg/m2/radiation week) or cetuximab (loading dose 400 mg/m2, followed by concomitant doses of 2250 mg/m2/radiation week) was administered. In 36 patients with very advanced disease of questionably curable stage, TPF (docetaxel, cisplatin, 5-fluorouracil)-based induction chemotherapy was given as a decision aid to add or not curatively intended radiation. The remaining 16 % of patients were treated with radiation only because of age or substantial comorbidity.
All GTVs were contoured or reviewed by at least one of the authors on all relevant axial computerized images without using interpolation; in most cases the contouring was also reviewed by a third staff physician. In addition, the wide volumetric ranges (cut-offs 15/70/130 ml) render the system quite robust with respect to interindividual contouring differences. Volumetric three-dimensional measurements (cm3) of contoured structures were calculated by the Varian Treatment Planning System volume algorithm (Eclipse® External Beam Planning System, Version 7.3.10 and PRO 8.9, AAA 8.9, Varian Medical Systems). A detailed description of the applied SIB modulated techniques and contouring of gross tumor volume (GTV) and planning target volumes (PTVs) has formerly been published [7]. In several patients with very large GTVs, dose compromises were performed delivering 66–68 Gy to the boost volume, while the 70 Gy dose volume was limited to the GTV.
Statistical analysis
Statistical calculations were performed using the statistics program implemented in StatView® (version 4.5; SAS Institute, Cary, NC, USA). Univariate analyses were performed with a Cox proportional hazards regression model in StatView®. Actuarial survival data were calculated using Kaplan–Meier curves and log-rank tests implemented in StatView®. P values < 0.05 were considered statistically significant.
Results
Outcome prediction by volumetric scoring
Between January 2002 and January 2013, a total of 201 cT4 stage SCC HNC patients were curatively treated at our department. The mean/median follow-up was 31/23 months (range 1–116 months). In all, 67 % of all patients were alive at last follow-up, and 49 % had no signs of disease. Of the 33 % of patients who had died, 24 % died due to disease-related reasons. The 3-year OS, DFS, LRC, and DMFS rates of the entire cohort were 63, 44, 48, and 77 %, respectively.
Volumetric staging revealed its potential to prognostically statistically significantly divide the cT4 cohort into 4 volume subgroups (V1/2/3/4): OS: 90 %/72 %/58 %/18 %; DFS: 83 %/50 %/39 %/10 %; LRC: 81 %/53 %/47 %/15 %; DMFS: 93 %/90 %/70 %/41 %, all p < 0.0001, (Tab. 2, Fig. 1).
Tab. 2.
Outcome according to volume subgroups (V1-4, using cut-off values of 15/70/130 ml)
| 3-year survival rates | ||||||
|---|---|---|---|---|---|---|
| LRC | DMFS | DFS | OAS | |||
| cT4 | n (%) | % | % | % | % | |
| V1 | 1–15 ml | 15 (7 %) | 81 | 93 | 83 | 90 |
| V2 | 16–70 ml | 108 (54 %) | 53 | 90 | 50 | 72 |
| V3 | 71–130 ml | 62 (31 %) | 47 | 70 | 39 | 58 |
| V4 | > 130 ml | 16 (8 %) | 15 | 41 | 10 | 18 |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||
LRC locoregional control rate, DMFS distant metastasis-free survival rate, DFS disease-free survival rate, OS overall survival rate.
Fig. 1.
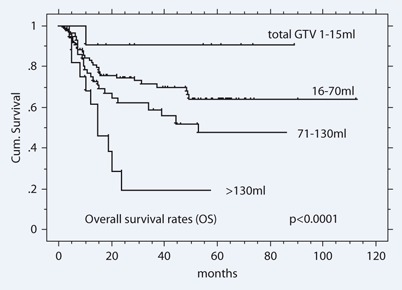
Overall survival rates of 201 cT4 patients staged by total tumor volume (ml)
Additional parameters with potential impact on disease control and OAS
The following parameters were tested in univariate analysis:
histopathological grading (grade 2 versus 3, no grade 1 cases), not significant,
age (>/< 70 years), not significant,
cT4a versus cT4b: in 63 % of the cases this differentiation was not indicated; most of the remaining cases were scored as cT4a (therefore statistically not evaluable),
nodal status (cN0 vs N1 vs N2a vs N2b vs N2c vs N3; cN0 vs N1–2b vs N2c vs N3; cN0 vs cN1–2 vs cN3), not significant,
systemic therapy: as the sample sizes of the subgroup with versus without systemic therapy was unbalanced (84 % vs 16 %—not the same patients with respect to substantial comorbidity and age), and systemic therapy was not homogeneous, no reliable information can be drawn from this analysis, which, however, showed a significant difference in favor of the combined modality subgroup (p = 0.2; OS 65 % vs 50 % at 3 years).
Treatment tolerance
With respect to treatment tolerance, the following findings in 117 locoregionally controlled patients were stated as based on the last clinical visit: 16/117 patients experienced any late term grade 3/4 side effects (LENT-SOMA, 14 %). Only 6/16 patients (38 %; 3 % of all patients) suffered from persistent late term sequelae (1 × xerostomia G3, 1 × loss of taste G3, 1 × chondronecrosis, 1 × dysphagia G3, 2 × feeding tube dependence).
Discussion
Aim of this work was to assess the potential of volumetric stratification of our cT4 SCC HNC cohort into different prognostic subgroups. We found volumetric stratification highly statistically significant in predicting outcome for different volume subgroups in the assessed cT4 HNC cohort. The volumetric system itself is considered robust with respect to interobserver GTV contouring, as its cut offs values differ markedly (15 ml/70 ml/130 ml) [4, 7]. The potential benefit of the assessed stratification lays in its more precise prediction of disease control in irradiated cT4 patient cohorts, and therefore more accurate characterization of cT4 cohorts for intercenter comparison purposes.
A weakness of this study is its retrospective stratification approach, which however applied a prospectively tested staging system [4, 5, 6, 7]. In addition, the assessed cohort includes different unbalanced tumor sites as well as unbalanced volume subgroups (Tab. 1).
To our knowledge there are no similar comparable volumetric staging analyses published. Most published volumetric focused outcome analyses were based on dichotomizing the GTV (i.e., using just one cut-off), (Tab. 3, [4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35]). Four [17, 18, 20, 25] of the 31 listed reports were based on two or three cut-off values, our own system included. All but two analyses showed significant difference in outcome between larger vs smaller tumor volumes. Been et al. [34] failed to demonstrate statistical significance between pGTV and locoregional outcome, perhaps due to not considering the nodal tumor volume which may significantly impact locoregional outcome. Mendenhall et al. [8] found no outcome difference in tumors of the hypopharynx/base of tongue/posterior tonsillar pillar when using a cut off value of 6 ml. This cut-off may have been too low.
Tab. 3.
Literature on head and neck cancer (HNC) outcome prediction based on volumetric classifications
| Author [ref] | Year | HNC entity | Number | T | Treatment | RT technique | Mean PGTV (ml) | Cut-off value (ml) | p value LC | p value OS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mendenhall et al. [8] | 2003 | Soft pal/supragl/glottic/tonsil ant pilar | 12/114/55/37 | T1-4 | RT(-CT) | 3DCRT | 5/12/8/3/12 | 6 | < 0.05 | Nl | |||
| Mendenhall et al. [8] | 2003 | Hypo/BoT/tonsil post pilar | 45/72/69 | T1-4 | RT(-CT) | 3DCRT | 6/24/18 | 6 | NS | Nl | |||
| Pameijer et al. [9] | 1998 | Pyrifrom sinus | 23 | T1/2 | RT | 3DCRT | Nl | 6.5 | 0.021 | Nl | |||
| Keberle et al. [10] | 2004 | Hypo | 45 | T1-4 | S(-RT) | 3DCRT | 8.1 | 8.1 | 0.004 | Nl | |||
| Tsou et al. [19] | 2006 | Hypo | 51 | III–IV | RT-CT | 3DCRT | Nl | 19 | < 0.001 | 0.036 | |||
| Chen et al. [21] | 2009 | Hypo | 76 | III–IV | RT-CT | 3DC + IMRT | 33.4 | 30 | < 0.0001 | Nl | |||
| Grabenbauer et al. [12] | 1998 | OC/Oro/hypo/larynx | 87 | III–IV | RT(-CT) | 3DCRT | Median 110 | 110 | Nl | 0.0001 | |||
| Rudat et al. [13] | 1999 | OC/Oro/hypo/larynx | 68 | T2-4 | RT-CT | 3DCRT | Median 112 TGTV | 112 | 0.0008 | Nl | |||
| Plataniotis et al. [11] | 2004 | OC/Oro/hypo/larynx | 101 | III–IV | RT(-CT) | 3DCRT | 17/13/22.6/14.8 median TGTV | 22.8 | Nl | 0.01 | |||
| Strongin et al. [20] | 2012 | Oro/hypo/larynx | 78 | T1-4 | RT-CT | 3DC + IMRT | 38.7 | 35 | Nl | < 0.001 | |||
| Freeman et al. [15] | 1990 | Supraglottic | 31 | T1-4 | RT | 3DCRT | Nl | 6 | 0.038 | Nl | |||
| Mukherji et al. [14] | 2000 | Supraglottic | 37 | T1-4 | S(-RT) | 3DCRT | 9.3 | 16 | 0.04 | Nl | |||
| Gilbert et al. [16] | 1987 | Larynx | 37 | T2-4 | RT | 3DCRT | 21.8* vs 8.9* | – | Nl | 0.02 | |||
| Lee et al. [23] | 1993 | Glottic | 29 | T3 | RT | 3DCRT | Nl | 3.5 | 0.02 | Nl | |||
| Pameijer et al. [24] | 1997 | Glottic | 42 | T3 | RT | 3DCRT | Nl | 3.5 | 0.0002 | Nl | |||
| Hamilton et al. [18] | 2004 | Larynx | 47 | T2-3 | RT | 3DCRT | 3.5 | 3 (glottic:1) | 0.003 | Nl | |||
| Chua et al. [25] | 1997 | NPC | 290 | T1-3 | RT(-CT) | 3DCRT | 6.9/18.8/52.4 in T1,2,3 | 20/> 60 | < 0.05 | Nl | |||
| Lee et al. [17] | 2008 | NPC | 66 | T1-4 | RT(-CT) | 3DCRT | 19.5 | 12.5/25/50 | Nl | 0.02 | |||
| Nathu et al. [26] | 2000 | Oro | 114 | T2-4 | RT(-CT) | 3DCRT | 6.8/14.8/42.6 in T2,3,4 | Nl | NS | Nl | |||
| Hermans et al. [28] | 2001 | Oro | 112 | T1-4 | RT | 3DCRT | 3.1/10.6/14.5/44.9 in T1-4 | 6/14.5/31 | 0.047 | NS | |||
| Keberle et al. [27] | 2003 | Oro | 80 | T1-4 | S(-RT) | 3DCRT | Median 4.7 | Nl | NS | Nl | |||
| Chao et al. [11] | 2004 | Oro | 31 | I–V | RT(-CT) | IMRT | 30.5 | Nl | 0.05 | Nl | |||
| Been et al. [34] | 2008 | Oro | 79 | T1-4 | RT(-CT) | 3DC + IMRT | 13.1 | 13.1 | 0.6 LRC | Nl | |||
| Chung et al. [35] | 2009 | Oro | 42 | T1-4 | RT±S | 3DCRT | NI | 35 | NI | 0.05 | |||
| Studer et al. [7] | 2012 | Oro | 277 | T1-4 | RT(-CT) | IMRT | 50.5 (totalGTV) | 15/70/130 | < 0.0001 LRC | < 0.0001 | |||
| Lok et al. [33] | 2012 | Oro | 340 | T1-4 | RT-CT | IMRT | 42.5 | 32.8 | 0.004 | < 0.0001 | |||
| Johnson et al. [31] | 1995 | All | 51 | Advanced | RT | 3DCRT | Median 35 TGTV | 35 | < 0.0001 | Nl | |||
| Doweck et al. [30] | 2002 | All | 64 | III–IV | RT-CT | 3DCRT | 35.4 | 19.6 | Nl | 0.0018 | |||
| Kurek et al. [32] | 2003 | All | 107 | T1-4 | RT(-CT) | 3DCRT | Median 32.5 and 44.4 | Nl | Nl | 0.02 | |||
| Studer et al. [4] | 2007 | All but larynx | 172 | T1-4 | RT(-CT) | IMRT | 37.7 | 15/70 | < 0.02 | Nl | |||
| Hoebers et al. [22] | 2008 | All but NPC | 46 | T3-4 (92 %) | RT-CT | 3DCRT | 28 | 23 | 0.036 LRC | 0.045 | |||
| Present work | 2013 | All but LE NPC | 201 | T4 | RT(-CT) | IMRT | 64 (total GTV) | 15/70/130 | < 0.0001 LRC | < 0.0001 | |||
Soft pal soft palate, ant/post tons pil anterior/posterior tonsillar pillar, hypo hypopharyngeal tumor, OC oral cavity tumor, oro oropharyngeal tumor, LE NPC lymphoepithelial nasopharyngeal tumor, RT radiotherapy, CT chemotherapy, 3DCRT three-dimensional conventional radiotherapy, IMRT intensity-modulated radiation therapy, PGTV primary gross tumor volumes, NI not indicated, TGTV total gross tumor volume, LC local control, OS overall survival.
The data presented here are derived from a cohort treated with IMRT techniques, with previous careful staging (in most cases using PET-CT) [36, 37].
Conclusion
Volumetric staging was shown to allow for highly statistically significantly stratification of cT4 stage SCC HNC into different prognostic subgroups, offering the option of better comparability of irradiated advanced stage HNC cohorts.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Compliance with ethical guidelines
Conflict of interest. G. Studer and C. Glanzmann state that there are no conflicts of interest.
The accompanying manuscript does not include studies on humans or animals.
Footnotes
©The Authors (2013) This article is published with open access at Springerlink.com.
References
- 1.Garden Int J Radiat Oncol Biol Phys. 2008;71:1351. doi: 10.1016/j.ijrobp.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez-Millan Clin Transl Oncol. 2013;15:321. doi: 10.1007/s12094-012-0925-9. [DOI] [PubMed] [Google Scholar]
- 3.Hauswald Radiat Oncol. 2011;6:70. doi: 10.1186/1748-717X-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Studer Acta Oncol. 2007;46:386. doi: 10.1080/02841860600815407. [DOI] [PubMed] [Google Scholar]
- 5.Studer Strahlenther Onkol. 2008;184:580. doi: 10.1007/s00066-008-1951-y. [DOI] [PubMed] [Google Scholar]
- 6.Studer Radiat Oncol. 2011;6:120. doi: 10.1186/1748-717X-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studer Oral Oncol. 2013;49:269. doi: 10.1016/j.oraloncology.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Mendenhall Head Neck. 2003;25:535. doi: 10.1002/hed.10253. [DOI] [PubMed] [Google Scholar]
- 9.Pameijer Head Neck. 1998;20:159. doi: 10.1002/(SICI)1097-0347(199803)20:2<159::AID-HED10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Keberle Eur Radiol. 2004;14:286. doi: 10.1007/s00330-003-1994-5. [DOI] [PubMed] [Google Scholar]
- 11.Plataniotis Int J Radiat Oncol Biol Phys. 2004;59:1018. doi: 10.1016/j.ijrobp.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Grabenbauer Radiother Oncol. 1998;47:175. doi: 10.1016/S0167-8140(98)00016-4. [DOI] [PubMed] [Google Scholar]
- 13.Rudat Radiother Oncol. 1999;53:119. doi: 10.1016/S0167-8140(99)00119-X. [DOI] [PubMed] [Google Scholar]
- 14.Mukherji Head Neck. 2000;22:282. doi: 10.1002/(SICI)1097-0347(200005)22:3<282::AID-HED11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Freeman Int J Radiat Oncol Biol Phys. 1990;19:485. doi: 10.1016/0360-3016(90)90562-X. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert Ann Otol Rhinol Laryngol. 1987;96:514. [Google Scholar]
- 17.Lee Acta Otolaryngol. 2008;128:93. doi: 10.1080/00016480701361921. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton J Otolaryngol. 2004;33:289. doi: 10.2310/7070.2004.03088. [DOI] [PubMed] [Google Scholar]
- 19.Tsou ORL J Otorhinolaryngol Relat Spec. 2006;68:206. doi: 10.1159/000091803. [DOI] [PubMed] [Google Scholar]
- 20.Strongin Int J Radiat Oncol Biol Phys. 2012;82:1823. doi: 10.1016/j.ijrobp.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 21.Chen Head Neck. 2009;31:709. doi: 10.1002/hed.21011. [DOI] [PubMed] [Google Scholar]
- 22.Hoebers Head Neck. 2008;30:1216. doi: 10.1002/hed.20865. [DOI] [PubMed] [Google Scholar]
- 23.Lee Int J Radiat Oncol Biol Phys. 1993;25:683. doi: 10.1016/0360-3016(93)90016-O. [DOI] [PubMed] [Google Scholar]
- 24.Pameijer Int J Radiat Oncol Biol Phys. 1997;37:1011. doi: 10.1016/S0360-3016(96)00626-8. [DOI] [PubMed] [Google Scholar]
- 25.Chua Int J Radiat Oncol Biol Phys. 1997;39:711. doi: 10.1016/S0360-3016(97)00374-X. [DOI] [PubMed] [Google Scholar]
- 26.Nathu Head Neck. 2000;22:1. doi: 10.1002/(SICI)1097-0347(200001)22:1<1::AID-HED1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Keberle Rofo. 2003;175:61. doi: 10.1055/s-2003-36610. [DOI] [PubMed] [Google Scholar]
- 28.Hermans Int J Radiat Oncol Biol Phys. 2001;50:37. doi: 10.1016/S0360-3016(00)01559-5. [DOI] [PubMed] [Google Scholar]
- 29.Chao Int J Radiat Oncol Biol Phys. 2004;59:43. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Doweck Laryngoscope. 2002;112:1742. doi: 10.1097/00005537-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Johnson Int J Radiat Oncol Biol Phys. 1995;33:281. doi: 10.1016/0360-3016(95)00119-J. [DOI] [PubMed] [Google Scholar]
- 32.Kurek Strahlenther Onkol. 2003;179:292. doi: 10.1007/s00066-003-1017-0. [DOI] [PubMed] [Google Scholar]
- 33.Lok Int J Radiat Oncol Biol Phys. 2012;82:1851. doi: 10.1016/j.ijrobp.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Been Laryngoscope. 2008;118:1377. doi: 10.1097/MLG.0b013e318172c82c. [DOI] [PubMed] [Google Scholar]
- 35.Chung Clin Exp Otorhinolaryngol. 2009;2:78. doi: 10.3342/ceo.2009.2.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abramyuk Strahlenther Onkol. 2013;189:197. doi: 10.1007/s00066-012-0283-0. [DOI] [PubMed] [Google Scholar]
- 37.Fakhrian Strahlenther Onkol. 2013;189:293. doi: 10.1007/s00066-012-0297-7. [DOI] [PubMed] [Google Scholar]


