For women with human epidermal growth factor receptor-2 (HER-2)–positive breast cancer, HER-2-directed therapy prolongs both time to disease progression and overall survival when combined with chemotherapy. This article reviews recent phase III trial data and discusses a practical approach to sequencing of HER-2-directed therapy in patients with HER-2-positive advanced breast cancer.
Keywords: Advanced breast cancer, HER-2-directed therapy, HER-2-positive, Trastuzumab, Lapatinib, T-DM1, Pertuzumab
Abstract
Untreated human epidermal growth factor receptor-2 (HER-2)-positive advanced breast cancer (ABC) is an aggressive disease, associated with a poor prognosis and short overall survival. HER-2-directed therapy prolongs both time to disease progression and overall survival when combined with chemotherapy and has become the standard of care for those with HER-2-positive breast cancer in the early and advanced settings. Despite the remarkable therapeutic impact HER-2-directed therapy has had on disease outcomes, some patients with HER-2-positive disease will have primary resistant disease and others will respond initially but will eventually have progression, underscoring the need for other novel therapeutic options. This article reviews recent phase III trial data and discusses a practical approach to sequencing of HER-2-directed therapy in patients with HER-2-positive ABC. The significant cumulative survival gains seen in these trials are slowly reshaping the landscape of HER-2-positive ABC outcomes.
Implications for Practice:
HER-2-directed therapy remains one of most promising areas of development in the management of advanced breast cancer (ABC). Results of eight trials investigating new HER-2-directed therapy regimens have been published or presented since 2012. Multiple HER-2-directed therapies have demonstrated significant and clinically meaningful survival benefits in the treatment of HER-2-positive ABC, including pertuzumab-combinations, trastuzumab-lapatinib combinations, and the antibody-cytotoxic conjugate, trastuzumab-emtansine. With the increased number of HER-2-directed therapy options, optimal sequencing of these regimens across multiple lines of therapy is integral to providing the best possible care for our patients. Consideration should also be given to management of central nervous system metastases and ensuring cardiac safety.
Introduction
Breast cancer (BC) is the leading cause of cancer-related death among women worldwide, with an estimated 1,383,500 new cases and 458,400 deaths in 2008 [1]. Approximately 6%–10% of patients have metastatic disease at diagnosis and systemic recurrence develops in approximately 25%–30% of patients treated with curative intent [2, 3]. Amplification of the human epidermal growth factor receptor (HER) 2 gene (HER-2) is observed in 15%–20% of all BCs [4, 5], and in the era prior to the availability of HER-2-directed therapy, patients with HER-2-positive tumors were observed to have more aggressive disease, poorer prognosis, and shorter overall survival (OS) compared with other subgroups [6, 7].
In 1998, trastuzumab was approved for use, either alone or in combination with paclitaxel, in the treatment of metastatic HER-2-positive disease [8] based on results of the pivotal trials by Slamon et al. [9, 10]. Outcomes of the M77001 trial subsequently confirmed these findings [11], leading to the establishment of trastuzumab-taxane therapies in the first-line metastatic disease setting [12, 13]. In 2007, lapatinib, a small-molecule dual (HER-1 and HER-2) tyrosine kinase inhibitor, plus capecitabine was approved for use in trastuzumab-resistant patients [14, 15]. Trial outcomes from the GBG26 trial established the use of trastuzumab therapy beyond progression on trastuzumab-based treatment [16–18].
HER-2-directed therapeutic approaches continue to evolve. Early data have shown activity with the following: HER-2-directed and endocrine therapy for patients who have both HER-2-positive and hormone receptor-positive (co-positive) disease [19–25], dual HER-2-blockade in the neoadjuvant and metastatic settings [26–32], the addition of other biologics to HER-2-directed therapy [33–35], and antibody–drug conjugates [36, 37]. Findings from phase III trials evaluating these and other concepts are expanding available treatment options for patients with HER-2-positive advanced BC (ABC). Trial data will be reviewed and provide context for discussion of practical clinical issues and guidance on the management of HER-2-positive ABC.
Methods
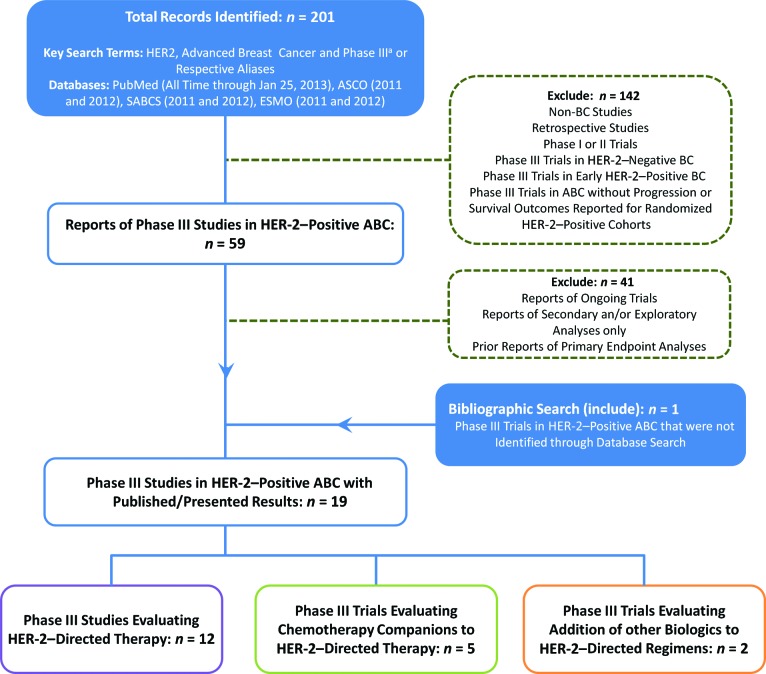
PubMed articles (to January 25, 2013) and the proceedings of the annual meeting of the American Society of Clinical Oncology, the San Antonio Breast Cancer Symposium, and the Annual Congress of the European Society for Medical Oncology (2011–2012) were searched for phase III clinical trials involving HER-2-directed therapies in HER-2-positive ABC, using the key search terms or aliases “HER-2,” “advanced breast cancer,” and “phase III clinical trials” (n = 19) (Fig. 1). Data were collected from the most up-to-date published or conference-presented source(s).
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses diagram of eligible HER-2–directed therapy trials.
aPubMed “Phase III” filter used for MEDLINE-indexed records.
Abbreviations: ABC, advanced breast cancer; ASCO, American Society of Clinical Oncology; BC, breast cancer, ESMO, European Society for Medical Oncology; HER-2, human epidermal growth factor receptor 2; SABCS, San Antonio Breast Cancer Symposium.
Findings
First-Line Trials of HER-2-Directed and Endocrine Therapy for HER-2/Hormone Receptor Co-Positive ABC
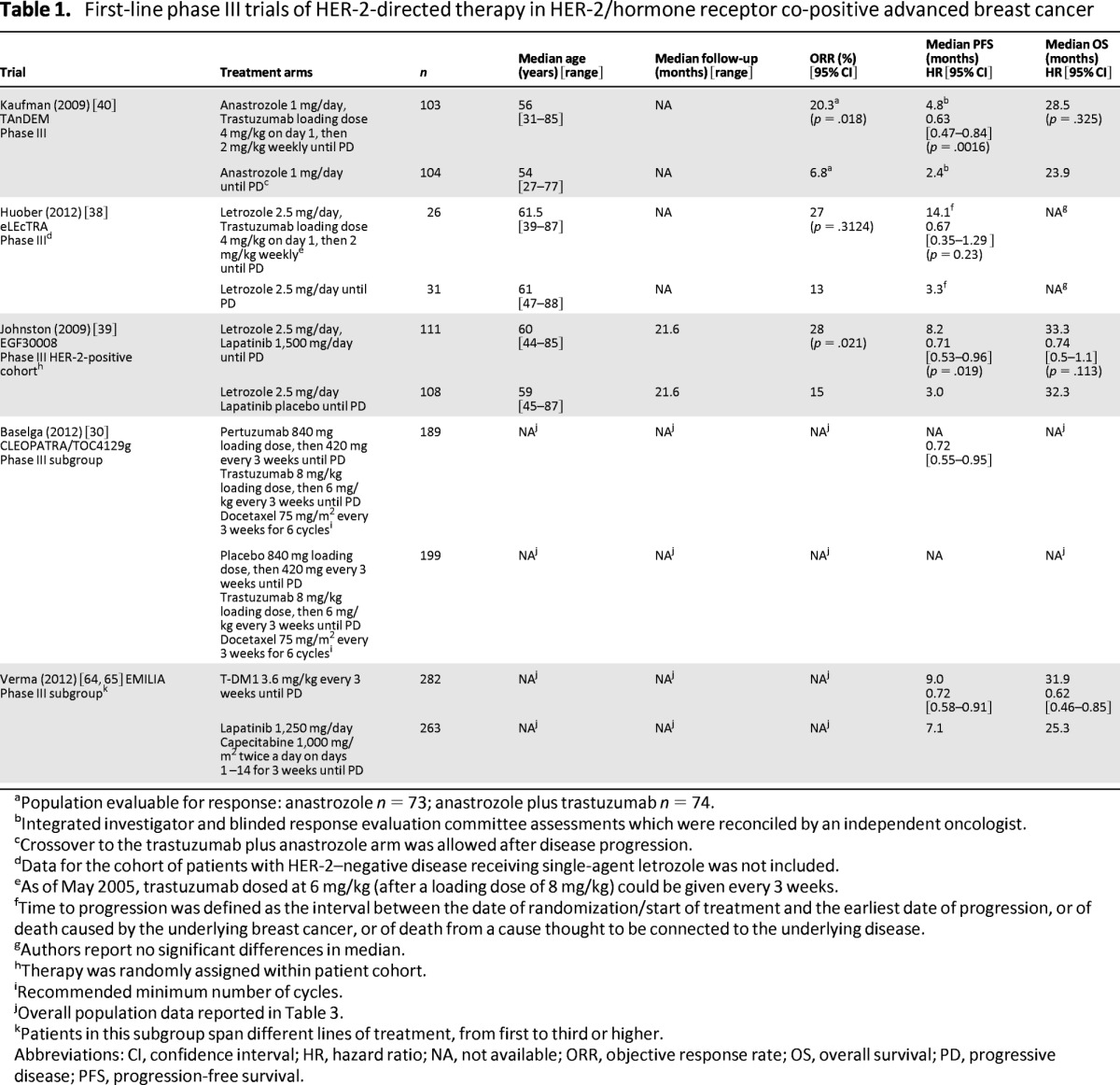
Three phase III trials have investigated the addition of HER-2-directed agents to standard endocrine therapy [38–41]. The larger TAnDEM trial (n = 208) [40] and the smaller eLEcTRA [38] trial (n = 57), which was halted because of low accrual, evaluated the addition of trastuzumab to nonsteroidal aromatase inhibitors (NSAIs) as first-line treatment for patients with HER-2/hormone receptor co-positive ABC (Table 1). At the time of final analysis, patients on the trastuzumab arm of the TAnDEM trial experienced a significant improvement in median progression-free survival (PFS) (4.8 months vs. 2.4 months; hazard ratio [HR] = 0.63; 95% confidence interval [CI] = 0.47–0.84; p = .0016) (Table 1) and those on the eLEcTRA trial experienced a trend toward an improved time to progression (14.1 months vs. 3.3 months; HR = 0.67; 95% CI = 0.35–1.29; p = .23) (Table 1). No significant differences in OS were reported when trastuzumab was added to NSAI therapy in either study (TAnDEM: 28.5 months vs. 23.9 months, p = .325; eLEcTRA: data not reported). Overall, the regimens were well tolerated, with an increase in adverse events (AEs) in the combination arm of each trial (TAnDEM and eLEcTRA, all-grade AEs: 87% vs. 65% and 96% vs. 71%, respectively; TAnDEM, grade 3 AEs: 23% vs. 15%, grade 4 AEs: 5% vs. 1%) compared with NSAIs alone.
Table 1.
First-line phase III trials of HER-2-directed therapy in HER-2/hormone receptor co-positive advanced breast cancer
aPopulation evaluable for response: anastrozole n = 73; anastrozole plus trastuzumab n = 74.
bIntegrated investigator and blinded response evaluation committee assessments which were reconciled by an independent oncologist.
cCrossover to the trastuzumab plus anastrozole arm was allowed after disease progression.
dData for the cohort of patients with HER-2–negative disease receiving single-agent letrozole was not included.
eAs of May 2005, trastuzumab dosed at 6 mg/kg (after a loading dose of 8 mg/kg) could be given every 3 weeks.
fTime to progression was defined as the interval between the date of randomization/start of treatment and the earliest date of progression, or of death caused by the underlying breast cancer, or of death from a cause thought to be connected to the underlying disease.
gAuthors report no significant differences in median.
hTherapy was randomly assigned within patient cohort.
iRecommended minimum number of cycles.
jOverall population data reported in Table 3.
kPatients in this subgroup span different lines of treatment, from first to third or higher.
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not available; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
The EGF30008 trial [39, 41], a multicenter, double-blind, placebo-controlled phase III study, evaluated the addition of lapatinib to NSAI therapy for first-line HER-2/hormone receptor co-positive ABC. Patients (n = 219) were randomly assigned to letrozole (2.5 mg) with or without lapatinib (1,500 mg), which was continued until disease progression. The primary endpoint was investigator-assessed PFS. After a median follow-up of 1.8 years, patients on the lapatinib–letrozole arm experienced a significant prolongation in median PFS (8.2 months vs. 3.0 months; HR = 0.71; 95% CI = 0.53–0.96; p = .019) (Table 1) but no improvement in median OS (33.3 months vs. 32.3 months; HR = 0.74; 95% CI = 0.5–1.1; p = .113) compared with the placebo–letrozole arm. The lapatinib–letrozole combination resulted in more serious AEs overall (8% vs. 4%) and increases in all-grade diarrhea and rash compared with placebo–letrozole (p < .05).
The overall benefit of adding pertuzumab to trastuzumab plus docetaxel in the CLEOPATRA trial extended to the co-positive cohort [30]. These trials support HER-2-directed therapy in combination with NSAIs as an appropriate chemotherapy-free treatment option for select patients with co-positive disease.
First-Line Trials of HER-2-Directed Therapy for HER-2-Positive ABC
Chemotherapy Companions to HER-2-Directed Therapy
Four other first-line trials have evaluated alternate chemotherapy companions for first-line disease; two investigated the addition of carboplatin to taxanes [42, 43] and two studied the use of vinorelbine compared with taxanes [44, 45]. Adding carboplatin did not improve survival compared with controls in either trial (median OS: HR = 0.9; 95% CI = 0.88–0.92; p = .76 [42]; BCIRG 007, HR = 1.015; 95% CI = 0.759–1.358; p = .99) (Table 2) and was associated with increased rates of neutropenia and grade 3 thrombocytopenia in both trials. Neither the HERNATA nor the TRAVIOTA phase III trials, evaluating the substitution of vinorelbine for docetaxel in combination with trastuzumab, showed significant improvement in survival (HERNATA, median OS: 38.8 months vs. 35.7 months; HR = 1.01; 95% CI = 0.71–1.42; p = .98). OS data were not reported for the TRAVIOTA trial (Table 2) [44, 45]. The combination of vinorelbine plus trastuzumab, however, was better tolerated than docetaxel plus trastuzumab in the HERNATA trial. Findings from these trials demonstrated that taxane–carboplatin plus trastuzumab combinations had limited clinical utility except in patients with good performance status requiring increased initial disease response. These trials also showed that vinorelbine plus trastuzumab was effective and better tolerated than docetaxel plus trastuzumab regimens, although the relative benefit of this regimen compared with weekly paclitaxel plus trastuzumab remains unclear.
Table 2.
Phase III trials of chemotherapy companions to HER-2-directed therapy in HER-2-positive advanced breast cancer
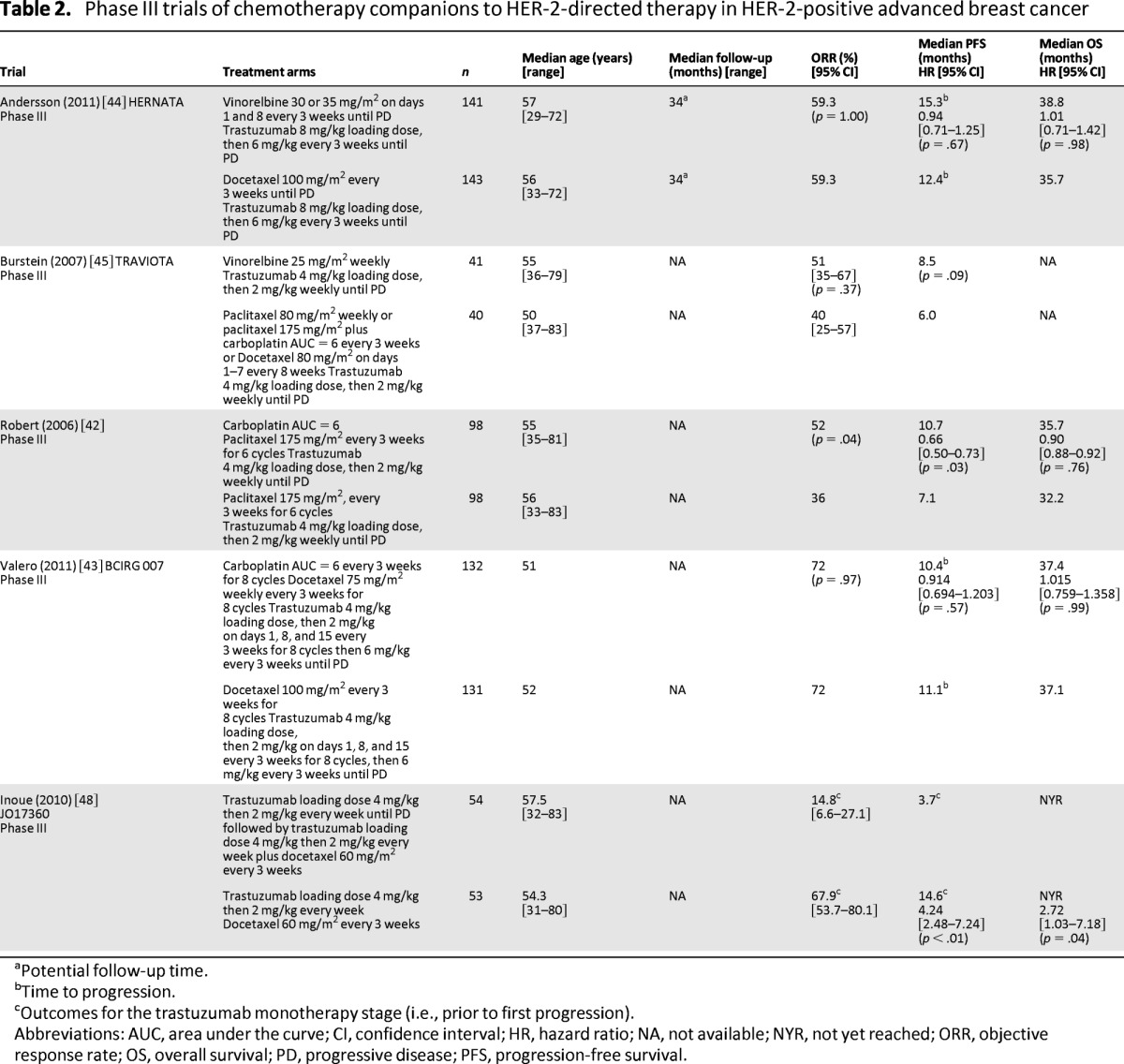
aPotential follow-up time.
bTime to progression.
cOutcomes for the trastuzumab monotherapy stage (i.e., prior to first progression).
Abbreviations: AUC, area under the curve; CI, confidence interval; HR, hazard ratio; NA, not available; NYR, not yet reached; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
In some centers, trastuzumab monotherapy is approved for use as first-line treatment [46, 47]. The open label, multicenter, phase III JO17360 trial evaluated delayed chemotherapy until first progression on trastuzumab and compared trastuzumab monotherapy followed by docetaxel plus trastuzumab with upfront docetaxel plus trastuzumab in HER-2-positive ABC [48]. The trial was stopped early because of decreased PFS (median: 14.6 months vs. 3.7 months; HR = 4.24; 95% CI = 2.48–7.24; p < .01) (Table 2) and OS (HR = 2.72; 95% CI = 1.03–7.18; p = .04) in the delayed chemotherapy companion arm, confirming the benefit of upfront chemotherapy companion use in first-line disease.
Second Generation HER-2-Directed Therapy
In the EGF104535 study, 444 Asian women with HER-2-positive ABC were randomly assigned to weekly paclitaxel (six cycles of paclitaxel 80 mg/m2 weekly for three out of four weeks) plus either lapatinib (1,500 mg daily) or placebo. The trial demonstrated that the addition of lapatinib to paclitaxel resulted in a significant improvement in the primary endpoint of OS (median, 27.8 months vs. 20.5 months; HR = 0.74; 95% CI = 0.58–0.94; p = .0124) (Table 3) as well as PFS (median, 9.7 months vs. 6.5 months; HR = 0.52; 95% CI = 0.42–0.64; p < .001) [49]. The combination arm was associated with higher rates of grade 3/4 diarrhea (20% vs. 1%), despite aggressive management, and neutropenia.
Table 3.
Recent phase III trials of HER-2-directed therapy in HER-2-positive advanced breast cancer
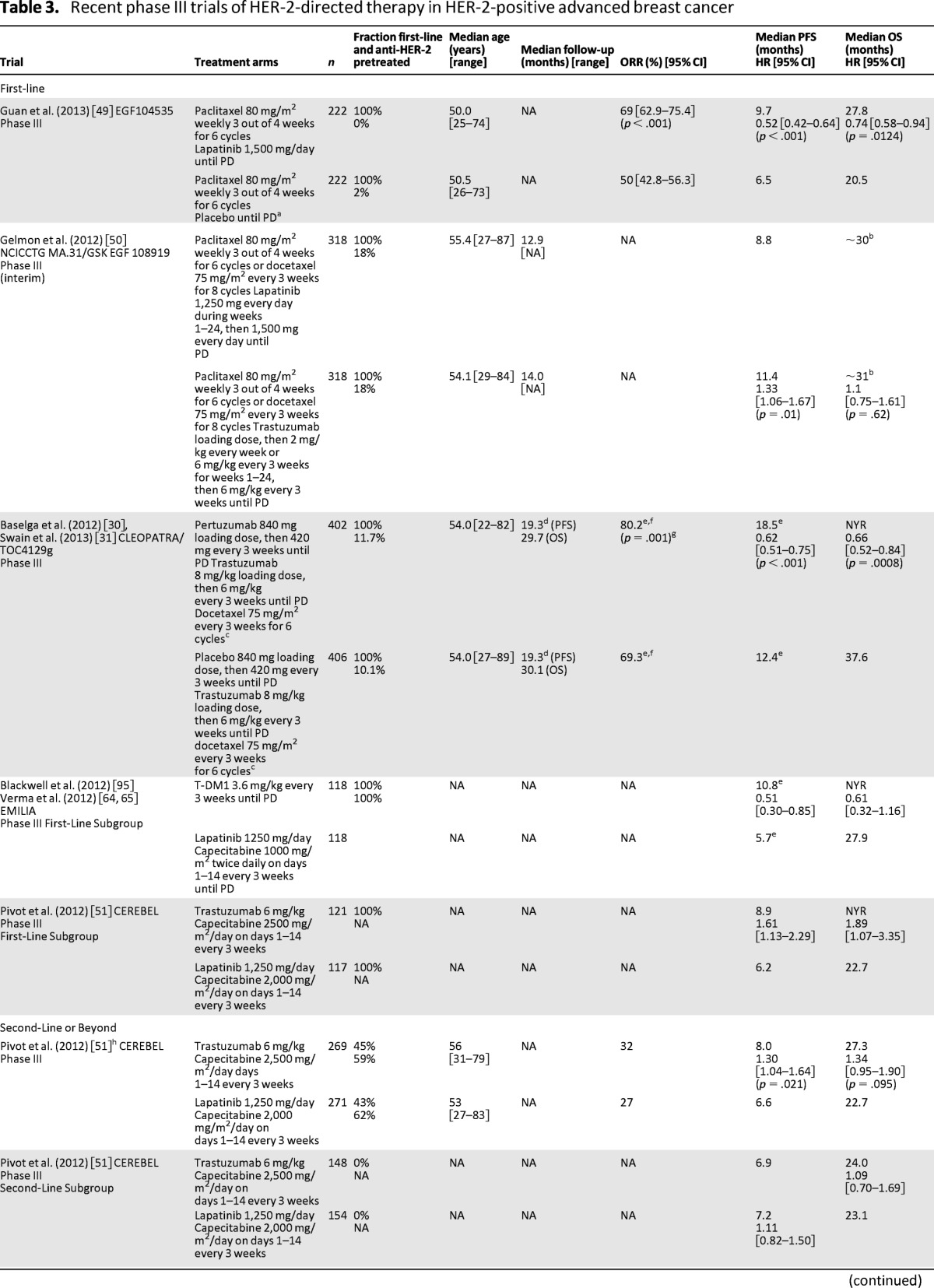

a Crossover to lapatinib monotherapy was allowed after progression.
bAs estimated from Kaplan-Meier curves.
cRecommended minimum number of cycles.
dKaplan-Meier estimate.
e Independent assessment.
fResponse-evaluable population: pertuzumab–trastuzumab plus docetaxel, n = 343; placebo–trastuzumab plus docetaxel, n = 336.
gp value associated with difference in ORR.
hOverall outcomes consisting of a mix of first and second-line (or beyond) patients; majority (56%) second-line.
iResponse-evaluable population: n = 389 for lapatinib plus capecitabine, n = 397 for T-DM1.
jPatients had received a median of 4.5 prior chemotherapy regimens (all settings) and a median of 3 prior trastuzumab regimens for advanced breast cancer.
k The median length of time on study was 12.8 months for patients receiving trastuzumab plus lapatinib and 8.7 months for patients treated with lapatinib alone.
l The main PFS and OS analyses included all randomly assigned patients with strata verified by data (n = 291), PFS and OS results from intent-to-treat population (n = 296) were consistent with results from main analyses.
mCrossover to the trastuzumab plus lapatinib arm was allowed after progression.
nPatients had received a median of 4 prior chemotherapy regimens (all settings) and a median of 3 prior trastuzumab regimens for advanced breast cancer.
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not available; NYR, not yet reached; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; T-DM1, trastuzumab entamsine.
The international phase III NCICCTG MA.31/EGF108919 trial randomly assigned 652 patients with HER-2-positive ABC to lapatinib 1,250 mg daily plus taxane chemotherapy (six cycles of paclitaxel 80 mg/m2 weekly for three out of four weeks or eight cycles of docetaxel 75 mg/m2 every three weeks) followed by lapatinib monotherapy (1,500 mg daily) until progression or trastuzumab (4 mg/kg loading dose then 2 mg/kg weekly or 6 mg/kg every three weeks for first 24 weeks) plus taxane chemotherapy followed by trastuzumab monotherapy (6 mg/kg every three weeks) until progression [50]. The primary endpoint was PFS. Baseline patient and disease characteristics were well balanced across treatment arms, with a minority of patients having prior adjuvant HER-2-directed therapy (18% in both arms). At the event-driven interim analysis, with a median follow-up of 13.6 months, patients on the lapatinib arm (n = 318) experienced a significantly shorter median PFS (8.8 months vs. 11.4 months; HR = 1.33; 95% CI = 1.06–1.67; p = .01) (Table 3) and comparable OS (HR = 1.1; 95% CI = 0.75–1.61; p = .62) compared with the trastuzumab-containing regimens (n = 318) [50]. Lapatinib was associated with higher rates of treatment discontinuation caused by toxicity (17.8% vs. 10.6%), and grade 3/4 diarrhea and rash (19.3% vs. 1.3% and 8.9% vs. 0.3%; p < .001), but fewer reductions in left ventricular ejection fraction (decrease of ≥20% at 36 weeks, 0% vs. 2%). The final analysis and correlative translational studies are pending. However, these initial results suggest that lapatinib plus taxane therapy should generally not be considered standard first-line therapy. Findings from this trial confirm results from the CEREBEL [51] and NEO-ALTTO trials [26] and suggest improved outcomes with trastuzumab-based chemotherapy combinations.
Dual HER-2 Blockade
Pertuzumab is a HER-2-directed humanized monoclonal antibody that inhibits heterodimerization, specifically that associated with HER-2–HER-3 dimerization [52]. When combined with chemotherapy, dual HER-2 inhibition with pertuzumab–trastuzumab was more active than single HER-2 inhibition with either pertuzumab or trastuzumab [27]. The double-blind, placebo-controlled, phase III CLEOPATRA trial evaluated the efficacy and safety of docetaxel in combination with pertuzumab–trastuzumab compared with trastuzumab alone as first-line therapy for patients with HER-2-positive ABC [30]. All 808 patients were treated with trastuzumab (8 mg/kg loading dose then 6 mg/kg every three weeks) plus docetaxel (75 mg/m2 every three weeks for six cycles), and half of patients were randomly assigned to also receive either pertuzumab (840 mg loading dose then 420 mg every three weeks) or a matching placebo. HER-2-directed therapy was continued until disease progression. The primary endpoint was independently assessed PFS. Disease characteristics were well balanced between treatment arms; a total of 53% of the patients enrolled in this trial had not received prior chemotherapy and approximately 89% had not received prior trastuzumab. At a median follow-up of 19.3 months, median PFS was 6.1 months longer for patients on the pertuzumab arm compared with those on the placebo arm (18.5 months vs. 12.4 months; HR = 0.62; 95% CI = 0.51–0.75; p < .001) (Table 3). The PFS benefit was consistent across all predefined subgroups, including patients who had received prior chemotherapy with or without trastuzumab (HR = 0.62; 95% CI = 0.35–1.07 and HR = 0.60; 95% CI = 0.43–0.83, respectively) and those who had both hormone receptor-positive (HR = 0.72; 95% CI = 0.55–0.95) (Table 1) and hormone receptor-negative (HR = 0.55; 95% CI = 0.42–0.72) disease. At a median follow-up of 30 months, the updated analysis also confirmed a significant increase in OS in the pertuzumab arm (median OS: not yet reached vs. 37.6 months; HR = 0.66; 95% CI = 0.52–0.84; p = .0008) compared with the control arm [31]. Treatment discontinuation because of AEs was comparable across arms (6.1% vs. 5.3%) [53]. Grade 3/4 febrile neutropenia and diarrhea occurred more often in the pertuzumab arm (13.8% vs. 7.6% and 7.9% vs. 5.0%, respectively), and left ventricular systolic dysfunction was less frequently reported in the pertuzumab-treated group (all grades: 4.4% vs. 8.3%; grade 3/4: 1.2% vs. 2.8%) [54]. The median time to symptom progression based on the Trial Outcome Index-Physical/Functional/Breast composite score of the Functional Assessment of Cancer Therapy–Breast was similar across pertuzumab and placebo arms (18.4 weeks vs. 18.3 weeks; HR = 0.97; 95% CI = 0.81–1.16; p = .7161) [55]. This study confirms that dual HER-2 blockade is both effective and safe. Although there is some indication of benefit of weekly paclitaxel in combination with pertuzumab and trastuzumab [56], the merits of chemotherapy companions other than docetaxel for dual HER-2 inhibition remain unclear. Ongoing trials investigating alternative chemotherapies may prove helpful in identifying chemotherapy partners, as well as providing further evidence for the benefits of this regimen in patients receiving prior chemotherapy and trastuzumab [56–58].
Companion Biologics to HER-2-Directed Therapy
Two trials evaluated the benefits of adding bevacizumab, a monoclonal antibody directed against the vascular endothelial growth factor, to HER-2-directed therapy in first-line ABC. At a median follow-up of approximately 26 months, the phase III AVEREL trial (n = 424) failed to show a significant improvement in either the primary endpoint of investigator-assessed PFS (median: 13.7 months vs. 16.5 months; HR = 0.82; 95% CI = 0.65–1.02; p = .0775) (Table 4) or OS (HR = 1.01; 95% CI = 0.74–1.38; p = .9543) with the addition of bevacizumab (15 mg/kg every three weeks) to therapy with trastuzumab–docetaxel every three weeks [59]. Results of the smaller E1105 trial (n = 96) observed a similar lack of improvement in median PFS (11.1 months vs. 12.2 months; HR = 0.65; 95% CI = 0.39–1.08; p = .10) (Table 4) when bevacizumab (10 mg/kg every two weeks) was added to a 24-week trastuzumab–paclitaxel-based induction regimen followed by trastuzumab plus bevacizumab (15 mg/kg every three weeks) maintenance [60]. These findings suggest that the addition of bevacizumab to HER-2-directed therapy does not confer any significant clinical benefit. There is also significant interest in blocking the mammalian target of rapamycin (mTOR) pathway, as it has been described as a key resistance pathway for HER-2-directed therapies. Ongoing trials such as the phase III BOLERO 1 and 3 trials [61, 62] will evaluate the merits of adding the mTOR inhibitor, everolimus, to HER-2-directed therapy in ABC.
Table 4.
Phase III trials of biologic companions to HER-2-directed therapy in HER-2-positive advanced breast cancer
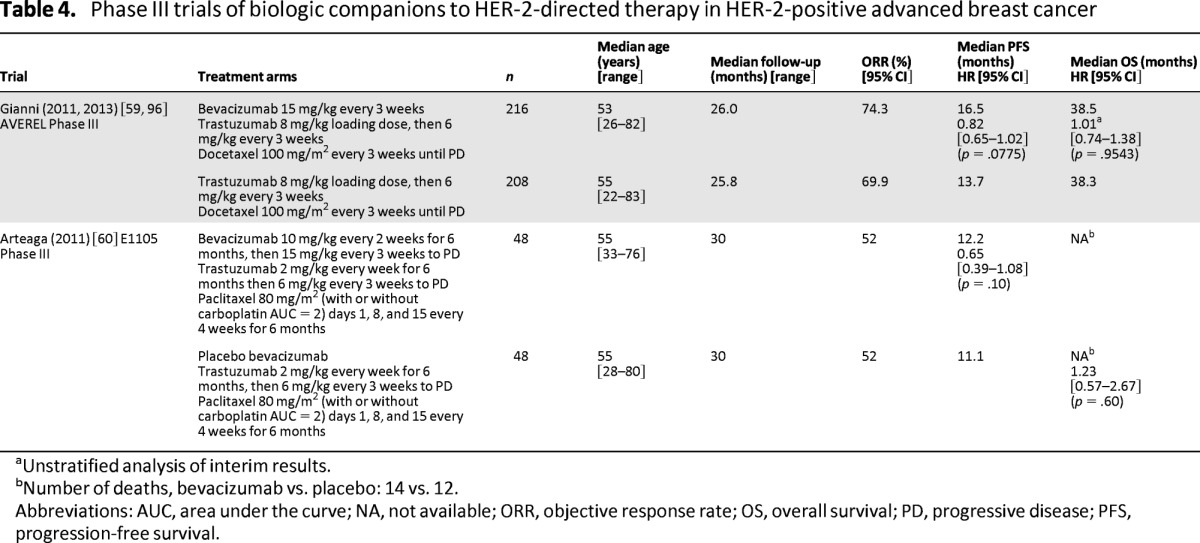
aUnstratified analysis of interim results.
bNumber of deaths, bevacizumab vs. placebo: 14 vs. 12.
Abbreviations: AUC, area under the curve; NA, not available; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
HER-2-Directed Therapy for HER-2-Positive ABC: Second-Line and Beyond
HER-2-Directed Therapies
The international, open-label, phase III CEREBEL/EGF111438 trial evaluated lapatinib compared with trastuzumab in combination with capecitabine for the first- and second-line treatment of HER-2-positive ABC. The trial randomly assigned 540 patients to oral lapatinib plus capecitabine (1,250 mg once daily plus 1,000 mg/m2 twice daily on days 1–14, every three weeks) or trastuzumab plus capecitabine (8 mg/kg load then 6 mg/kg every three weeks plus 1,250 mg/m2 twice daily on days 1–14 every three weeks) [51]. The primary endpoint was incidence of central nervous system first progression. Secondary endpoints included investigator-assessed PFS, OS, and safety. The incidence of central nervous system metastasis as site of first progression was 3% for the lapatinib arm (n = 251) and 5% for the trastuzumab arm (n = 250; OR = 0.65; 95% CI = 0.26–1.63; p = .360 [not significant]) with median PFS favoring the trastuzumab combination (8.0 months vs. 6.6 months; HR = 1.3; 95% CI = 1.04–1.64; p = .021) (Table 3). The PFS advantage was observed in trastuzumab-naive and first-line treatment subgroups but not in those previously treated for ABC (56%) or those who had received prior trastuzumab-based therapy (60%). A trend toward improved median OS (27.3 months vs. 22.7 months; HR = 1.34; 95% CI = 0.95–1.90; p = .095) was also observed. The incidence of serious AEs and AEs leading to treatment discontinuation was low overall and comparable across treatment arms (trastuzumab vs. lapatinib arms, 17% vs. 13% and 13% vs. 11%, respectively). The most common grade 3/4 AE, palmar-plantar erythrodysesthesia, was more frequently observed in patients on the trastuzumab arm than in those on the lapatinib arm (15% vs. 9%). Although the central nervous system was the site of first progression in numerically fewer patients on the lapatinib arm, the difference was not statistically significant. Observations of potential inferiority with lapatinib-containing regimens from CEREBEL are generally consistent with those of MA31 [50] and NEO-ALTTO [26].
HER-2-Antibody–Drug Conjugates
TDM1, an antibody–drug conjugate with demonstrated activity in phase II trials [63], was evaluated in patients with HER-2-positive disease who were previously treated with trastuzumab and taxane in the international, open-label, phase III EMILIA trial. Trastuzumab emtansine (T-DM1) combines the microtubule-inhibitor DM1 with trastuzumab using a stable linker. The trial compared treatment with T-DM1 to treatment with lapatinib plus capecitabine for patients with HER-2-positive ABC who were previously treated with trastuzumab and a taxane [64]. A total of 991 patients were randomly assigned to oral lapatinib (1,250 mg once daily) plus capecitabine (1,000 mg/m2 twice daily on days 1–14 every three weeks) or T-DM1 (3.6 mg/kg every three weeks) until disease progression. Postprogression treatment crossover to T-DM1 was not permitted until final OS results were reported in October 2012. The co-primary endpoints were independently assessed PFS, OS, and safety. At a median follow-up of approximately 13 months, T-DM1 was associated with a significant improvement in median PFS compared with lapatinib plus capecitabine (9.6 months vs. 6.4 months; HR = 0.65; 95% CI = 0.55–0.77; p < .001) (Table 3). This benefit was observed across most clinically relevant subgroups, including patients receiving prior systemic therapy for ABC with or without trastuzumab, those receiving earlier and later lines of therapy, and those with hormone receptor-positive (HR = 0.72; 95% CI = 0.58–0.91) (Table 1) and hormone receptor-negative (HR = 0.56; 95% CI = 0.44–0.72) tumors. At a median follow-up of approximately19 months, T-DM1 led to a significant median OS gain of 5.8 months compared with lapatinib plus capecitabine (30.9 months vs. 25.1 months; HR = 0.682; 95% CI = 0.55–0.85; p = .0006) [65]. The frequency of AEs leading to discontinuation of study drug was higher on the lapatinib arm (10.7% vs. 5.9%) [65], as was the incidence of grade 3/4 AEs (57.0% vs. 40.8%). The most common grade 3/4 AEs were diarrhea and palmar-plantar erythrodysesthesia (20.7% vs. 1.6% and 16.4% vs. 0%, respectively) on the lapatinib arm and thrombocytopenia (0.2% vs. 12.9%) and elevated hepatic transaminases (aspartate transaminase, 0.8% vs. 4.3%; alanine aminotransferase, 1.4% vs. 2.9%) on the T-DM1 arm [64]. The bleeding event rate was higher with T-DM1 versus lapatinib plus capecitabine, but grade 3/4 events were low in both arms (29.8% vs. 15.8% and 1.4% vs. 0.8%, respectively). The median time to a decrease of five points or more in the Trial Outcome Index-Physical/Functional/Breast composite score of the Functional Assessment of Cancer Therapy–Breast score was delayed in the T-DM1 group compared with the lapatinib group (7.1 months vs. 4.6 months; HR = 0.80; 95% CI = 0.67–0.95; p = .012). This study confirms that T-DM1 is effective and safe with less toxicity than the lapatinib plus capecitabine combination. This regimen has recently been approved for use by the Food and Drug Administration in the United States [46, 66, 67]. The role of T-DM1 in both earlier and subsequent lines of therapy will be elucidated once the results of the MARIANNE and TH3RESA trials are available [68, 69].
Dual HER-2 Blockade
The open-label, phase III EGF104900 trial evaluated lapatinib alone or in combination with trastuzumab for patients with HER-2-positive ABC progressing on prior trastuzumab-based therapy [70, 71]. A total of 296 heavily pretreated patients receiving a median of three prior trastuzumab-based ABC regimens (range: 0–13) were randomly assigned to oral lapatinib alone (1,500 mg daily), or lapatinib (1,000 mg daily) with trastuzumab (2 mg/kg every week after 4 mg/kg loading dose). Crossover to the combination arm was permitted on disease progression. The primary endpoint was investigator-assessed PFS. At the time of the final efficacy analysis, patients on the combination arm experienced significant improvements in median PFS (2.6 months vs. 1.9 months; HR = 0.74; 95% CI = 0.58–0.94; p = .011) (Table 3) and median OS (14 months vs. 9.5 months; HR = 0.74; 95% CI = 0.57–0.97; p = .026) compared with those receiving single-agent lapatinib. In an exploratory analysis, a statistically significant OS advantage for dual HER-2-directed therapy over lapatinib alone was observed in the estrogen receptor-negative subgroup but not in the estrogen receptor-positive subgroup, and in patients who had received between one and three prior trastuzumab therapies but not in patients who had received four or more prior lines of trastuzumab. Treatment with trastuzumab–lapatinib was associated with higher rates of treatment discontinuation resulting from AEs compared with lapatinib alone (11% vs. 6%) [70] as well as a higher rate of serious AEs (26% vs. 16%) [71]. Ten patients in the combination arm and three in the monotherapy arm met the protocol-defined criteria for a serious cardiac event [71]. Dual HER-2 blockade may be an appropriate treatment option for patients with progressive HER-2-positive ABC despite multiple lines (one–three) of prior HER-2-directed therapy or for patients who cannot tolerate chemotherapy.
Dual HER-2 blockade with pertuzumab–trastuzumab in combination with docetaxel improves median OS in predominantly trastuzumab-naive patients and trends toward improved PFS in trastuzumab-pretreated patients [30, 31]. This approach is therefore suggested for first-line disease regardless of prior trastuzumab exposure, as long as there is a disease-free interval of at least one year, per the inclusion criteria for the CLEOPATRA study.
Discussion
Optimal HER-2-Directed Therapy Sequencing
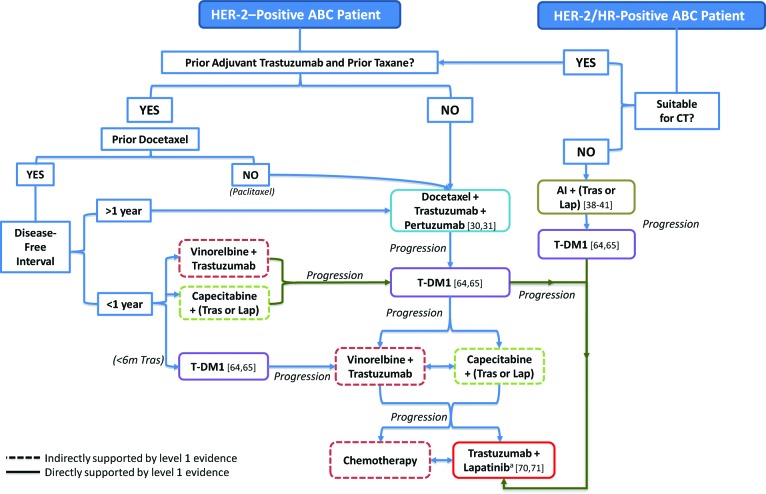
The optimal sequencing of HER-2-directed combinations in ABC is a topic of considerable debate. For patients who have both HER-2-positive and hormone receptor-positive disease, the addition of HER-2-directed therapy to standard endocrine therapy has been associated with modest gains in PFS but no OS gains compared with endocrine monotherapy [39–41]. Endocrine HER-2-directed combination therapy may therefore be recommended for patients with indolent low-volume disease or for those unsuitable for chemotherapy (Fig. 2) [72].
Figure 2.
Optimal HER-2–directed therapy sequencing for HER-2–positive ABC.
aTrastuzumab-lapatinib may also be considered in earlier lines of treatment as a less toxic option for low-burden disease; for example, after progression on T-DM1 but prior to chemotherapy-containing regimens.
Abbreviations: ABC, advanced breast cancer; AI, aromatase inhibitor; CT, chemotherapy; HER-2, human epidermal growth factor receptor 2; HR, hormone receptor; Lap, lapatinib; T-DM1, trastuzumab emtansine; Tras, trastuzumab.
There remains a degree of uncertainty regarding optimal first-line HER-2-directed chemotherapy combinations, as most available data are limited to trastuzumab-naive populations, and the proportion of first-line patients presenting with prior adjuvant trastuzumab-taxane exposure is steadily increasing [73, 74]. Given, however, the overall merits of continued HER-2 blockade in ABC [16, 17, 51, 64, 65, 70, 71, 75–77], use of first-line HER-2-directed therapy is considered standard practice regardless of prior trastuzumab exposure. Dual HER-2 blockade with pertuzumab–trastuzumab in combination with docetaxel improves median OS in predominantly trastuzumab-naive patients and trends toward improved PFS in trastuzumab-pretreated patients [30, 31]. This approach is therefore suggested for first-line disease regardless of prior trastuzumab exposure, as long as there is a disease-free interval of at least one year, per the inclusion criteria for the CLEOPATRA study (Fig. 2). There are few data demonstrating a benefit for dual-HER-2 inhibition in patients receiving prior docetaxel and having disease recurrence within one year after (neo)adjuvant therapy. For these patients, the use of TDM1, which has demonstrated a benefit in the treatment of first-line patients who have disease recurrence within six months of prior trastuzumab [64, 65], or trastuzumab in combination with a non-taxane alternative, either vinorelbine or capecitabine, is suggested.
In the second-line setting, the use of T-DM1, which improved median OS compared with standard lapatinib plus capecitabine therapy in HER-2-positive ABC, is recommended [64, 65]. For patients who have disease progression on either first- or second-line T-DM1, continued HER-2-blockade with chemotherapy plus HER-2-directed therapy (e.g., vinorelbine plus trastuzumab, capecitabine plus trastuzumab, or capecitabine plus lapatinib) should be considered. Finally, for those who have progressed on capecitabine or vinorelbine in combination with a HER2-directed agent, treatment with trastuzumab–lapatinib can be considered, although use of this regimen may be more beneficial in patients receiving fewer than four lines of prior trastuzumab [71].
Central Nervous System Metastases
Recent meta-analyses of randomized trastuzumab trials have reported an increased risk for central nervous system metastases as first only or general site of relapse in patients who had relapse after receiving adjuvant trastuzumab compared with chemotherapy alone [78–81]. Treatment with lapatinib and capecitabine has been associated with a lower incidence of central nervous system metastases compared with capecitabine alone [75]; however, recent findings of the CEREBEL trial demonstrate no difference in the incidence of brain metastasis as a first site of progression (3% vs. 5%; OR = 0.65; 95% CI = 0.26–1.63; p = .360) between lapatinib–capecitabine and trastuzumab–capecitabine therapy [51].
Surgery, stereotactic radiosurgery, and whole brain radiotherapy have important roles in the management of central nervous system metastases for patients with HER-2-positive ABC. Selection and proper timing of these therapies depends on the extent of central nervous system disease, the status of the systemic disease, and the availability of systemic treatment options [82]. In a small phase II, single-center trial for patients with HER-2-positive ABC with diffuse central nervous system metastases not previously treated with whole brain radiotherapy, lapatinib plus capecitabine was active (ORR, 65.9%) when administered as initial therapy [83], allowing treatment of both central nervous system and extra-central nervous system metastases and delaying whole brain radiotherapy. Results from a planned randomized trial comparing lapatinib plus capecitabine to whole brain radiotherapy are awaited [83].
Cardiac Safety
Increased rates of cardiac dysfunction with the addition of trastuzumab to chemotherapy compared with controls in initial HER-2-directed therapy trials have highlighted cardiac safety as an important clinical issue in the management of HER-2-positive BC [9, 84]. Despite initial concerns, relatively low rates of cardiotoxicity (grade 3 left ventricular systolic dysfunction: 0%–2.8%) have been reported for standard or emerging HER-2-directed therapies in the HER-2-positive ABC setting [30, 50, 51, 64].
Adequate cardiac monitoring remains an integral part of cardiac safety. There is currently limited guidance available from published guidelines and product monographs specifically addressing the cardiac monitoring of patients with HER-2-positive ABC who are receiving HER-2-directed therapy. In the absence of comprehensive guidelines specific for this setting, it is recommended that cardiac function testing (either with two-dimensional echocardiogram or multigated acquisition scan) be performed prior to initiating HER-2-directed therapy. Serial cardiac monitoring is not routinely required over the course of HER-2-directed therapy in the advanced setting because the benefit of anti-HER-2 therapy outweighs the low cardiac risk. However, if there are clinical concerns related to symptoms or signs of cardiac dysfunction or if there are plans to change treatment to another HER-2-directed regimen, a cardiac assessment consisting of cardiac function testing, along with a clinically relevant examination, is recommended. Should clinical congestive heart failure be suspected or a significant decline in cardiac function from baseline be noted, consultation with a cardiologist is recommended and HER-2-directed therapy should be held until cardiac function is optimized.
There is currently limited guidance available from published guidelines and product monographs specifically addressing the cardiac monitoring of patients with HER-2-positive ABC who are receiving HER-2-directed therapy. In the absence of comprehensive guidelines specific for this setting, it is recommended that cardiac function testing (either with two-dimensional echocardiogram or multigated acquisition scan) be performed prior to initiating HER-2-directed therapy.
Correlative Data
Extensive correlative biomarker research has been undertaken in both early and advanced settings to identify those with HER-2-positive disease and subpopulations of those patients who would benefit preferentially from HER-2-directed therapy. These include subgroups defined by levels of HER-2 expression or amplification (by immunohistochemistry or fluorescence in situ hybridization, respectively) [42, 75, 85, 86], levels of HER-2 messenger RNA (mRNA) [87–89], levels of HER-2 extracellular domain [75], levels of progesterone receptor, and c-myc levels [90]. Chromosome 17 polysomy [85, 86], phosphoinositide 3-kinase mutation, and phosphatase and tensin homolog loss [91] have also been assessed for potential to predict response to HER-2-directed treatment. Of these, only a few have shown predictive value. Increased levels of HER-2 mRNA have been associated with benefit from neoadjuvant trastuzumab for patients with estrogen receptor-positive disease [87–89], and c-myc amplification or negative progesterone receptor status predicts benefit from neoadjuvant trastuzumab [90]. Recent biomarker analyses from the EMILIA study suggest that patients with tumors expressing high HER-2 mRNA levels derived even greater OS benefit from T-DM1 [92]. Patients treated with lapatinib plus capecitabine with phosphoinositide 3-kinase catalytic subunit alpha (PIK3CA) mutations had worse clinical outcomes than those with wild-type PIK3CA, whereas those receiving treatment with T-DM1 were unaffected by PIK3CA mutation status [92]. Although these findings may inform future trial design, application in clinical practice is not supported. Analyses from recent phase III trials of dual HER-2-directed regimens have also failed to identify biomarkers able to predict the benefit of adding a second HER-2-directed agent [27, 93, 94].
Conclusion
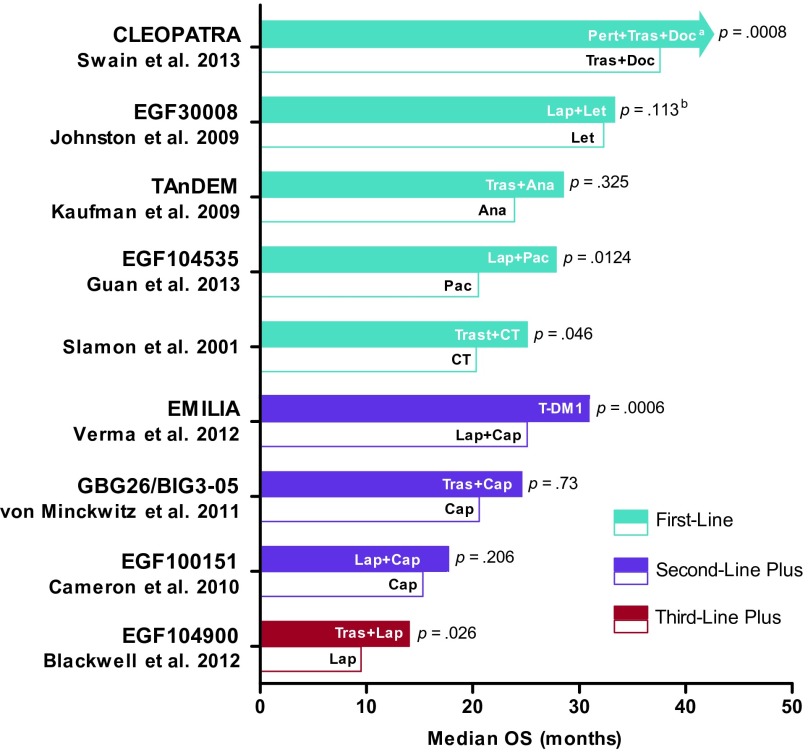
Continued research into HER-2-directed therapy has led to important clinical developments across all lines of treatment for ABC, producing unprecedented improvements in survival (Fig. 3). Median OS in first-line patients receiving dual HER-2-directed therapy is >3.5 years [31]; for second-line patients receiving T-DM1, median OS is approximately 2.5 years [65]; and for late-stage patients receiving lapatinib plus trastuzumab, median OS is 14 months [71]. These discoveries, coupled with strategies still under investigation, promise to reshape the landscape of HER-2-positive ABC, significantly improving the lives of women with this once poor-prognosis disease.
Figure 3.
Therapeutic milestones in the management of HER-2-positive advanced breast cancer.
aMedian OS of pertuzumab arm not yet reached at approximately 48 months. Median follow-up was approximately 30 months in both arms and 69% of planned events had occurred at the time of final analysis.
bFewer than 50% of OS events recorded at time of analysis.
Abbreviations: Ana, anastrozole; Cap, capecitabine; CT, chemotherapy; Doc, docetaxel; Lap, lapatinib; Let, letrozole; OS, overall survival; Pac, paclitaxel; Pert, pertuzumab; T-DM1, trastuzumab emtansine; Tras, trastuzumab.
Acknowledgments
Funding for this manuscript was provided by Hoffmann-La Roche Ltd. We thank Ilidio Martins of Kaleidoscope Strategic for his research assistance in preparing the review.
Author Contributions
Conception/Design: Sunil Verma, Anil A. Joy, Daniel Rayson, Deanna McLeod, Christine Brezden-Masley, Jean-François Boileau, Karen A. Gelmon
Provision of study material or patients: Sunil Verma
Collection and/or assembly of data: Sunil Verma, Anil A. Joy, Daniel Rayson, Deanna McLeod, Christine Brezden-Masley, Jean-François Boileau, Karen A. Gelmon
Data analysis and interpretation: Sunil Verma, Anil A. Joy, Daniel Rayson, Deanna McLeod, Christine Brezden-Masley, Jean-François Boileau, Karen A. Gelmon
Manuscript writing: Sunil Verma, Anil A. Joy, Daniel Rayson, Deanna McLeod, Christine Brezden-Masley, Jean-François Boileau, Karen A. Gelmon
Final approval of manuscript: Sunil Verma, Anil A. Joy, Daniel Rayson, Deanna McLeod, Christine Brezden-Masley, Jean-Fran 155 ois Boileau, Karen A. Gelmon
Disclosures
Sunil Verma: AstraZeneca (C/A); GlaxoSmithKline (C/A); Novartis (C/A); Roche (C/A); AstraZeneca (H); Novartis (H); Roche (H); Roche (RF); Sanofi-Aventis (RF); Anil A. Joy: AstraZeneca (C/A); GlaxoSmithKline (C/A); Novartis (C/A); Roche (C/A); AstraZeneca (H); GlaxoSmithKline (H); Novartis (H); Roche (H); AstraZeneca (RF); GlaxoSmithKline (RF); Novartis (RF); Roche (RF); Daniel Rayson: Roche (H); Roche (RF); Deanna McLeod: Hoffman-LaRoche (RF); Christine Brezden-Masley: Hoffman-LaRoche (C/A); Hoffman-LaRoche (H); Hoffman-LaRoche (RF); Jean-François Boileau: Roche (C/A); Roche (H); Karen A. Gelmon: Novartis (C/A); Pfizer (C/A); Roche (C/A); GlaxoSmithKline (RF); Pfizer (RF); Roche (RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Sledge GW., Jr The role of chemotherapy for metastatic breast cancer. Hematol Oncol Clin North Am. 1999;13:415–434. doi: 10.1016/s0889-8588(05)70063-0. [DOI] [PubMed] [Google Scholar]
- 3.Dawood S, Broglio K, Ensor J, et al. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21:2169–2174. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. The Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 7.Witton CJ, Reeves JR, Going JJ, et al. Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. Trastuzumab approval letter. 1998. [Accessed April 26, 2013]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/1998/trasgen092598L.pdf.
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 11.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 12.NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V. 1.2002. [Accessed January 14, 2013]. Available at http://www.nccn.org.
- 13.NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V. 1.2012. [Accessed January 14, 2013]. Available at: http://www.nccn.org.
- 14.US Food and Drug Administration. Lapatinib approval letter. 2007. [Accessed April 26, 2013]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2007/022059s000ltr.pdf.
- 15.NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V. 2.2008. [Accessed January 14, 2013]. Available at: http://www.nccn.org.
- 16.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Schwedler K, Schmidt M, et al. Trastuzumab beyond progression: Overall survival analysis of the GBG 26/BIG 3–05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011;47:2273–2281. doi: 10.1016/j.ejca.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 18.NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V. 2.2011. [Accessed January 14, 2013]. Available at: http://www.nccn.org.
- 19.Mackey J, Kaufman B, Clemens M, et al. Trastuzumab prolongs progression-free survival in hormone-dependent and HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2006;100(suppl 1):S5–S6. [Google Scholar]
- 20.Chu I, Blackwell K, Chen S, et al. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005;65:18–25. [PubMed] [Google Scholar]
- 21.Leary A, Martin L, Lykkesfeldt A, et al. Enhancing endocrine responsiveness using the dual EGFR/HER2 tyrosine kinase inhibitor lapatinib in cell models of endocrine resistance. Breast Cancer Res Treat. 2006;100(suppl 1):S29. [Google Scholar]
- 22.Chu QS, Cianfrocca ME, Goldstein LJ, et al. A phase I and pharmacokinetic study of lapatinib in combination with letrozole in patients with advanced cancer. Clin Cancer Res. 2008;14:4484–4490. doi: 10.1158/1078-0432.CCR-07-4417. [DOI] [PubMed] [Google Scholar]
- 23.Witters L, Engle L, Lipton A. Restoration of estrogen responsiveness by blocking the HER-2/neu pathway. Oncol Rep. 2002;9:1163–1166. [PubMed] [Google Scholar]
- 24.Kunisue H, Kurebayashi J, Otsuki T, et al. Anti-HER2 antibody enhances the growth inhibitory effect of anti-oestrogen on breast cancer cells expressing both oestrogen receptors and HER2. Br J Cancer. 2000;82:46–51. doi: 10.1054/bjoc.1999.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcom PK, Isaacs C, Harris L, et al. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res Treat. 2007;102:43–49. doi: 10.1007/s10549-006-9307-8. [DOI] [PubMed] [Google Scholar]
- 26.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 28.Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: Results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30:1989–1995. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- 29.Holmes F, Nagarwala Y, Espina V, et al. Correlation of molecular effects and pathologic complete response to preoperative lapatinib and trastuzumab, separately and combined prior to neoadjuvant breast cancer chemotherapy. J Clin Oncol. 2011;29(15S) abstract 506. [Google Scholar]
- 30.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013 doi: 10.1093/annonc/mdt182. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Pegram M, Yeon C, Ku N, et al. Phase I combined biological therapy of breast cancer using two humanized monoclonal antibodies directed against HER2 proto-oncogene and vascular endothelial growth factor (VEGF) Breast Cancer Res Treat. 2004;88(suppl 1):S124–S125. [Google Scholar]
- 34.Klos KS, Wyszomierski SL, Sun M, et al. ErbB2 increases vascular endothelial growth factor protein synthesis via activation of mammalian target of rapamycin/p70S6K leading to increased angiogenesis and spontaneous metastasis of human breast cancer cells. Cancer Res. 2006;66:2028–2037. doi: 10.1158/0008-5472.CAN-04-4559. [DOI] [PubMed] [Google Scholar]
- 35.Konecny GE, Meng YG, Untch M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10:1706–1716. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- 36.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 37.Junttila TT, Li G, Parsons K, et al. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128:347–356. doi: 10.1007/s10549-010-1090-x. [DOI] [PubMed] [Google Scholar]
- 38.Huober J, Fasching PA, Barsoum M, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer — results of the eLEcTRA trial. Breast. 2012;21:27–33. doi: 10.1016/j.breast.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Johnston S, Pippen J, Jr., Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: Results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 41.Schwartzberg LS, Franco SX, Florance A, et al. Lapatinib plus letrozole as first-line therapy for HER-2+ hormone receptor-positive metastatic breast cancer. The Oncologist. 2010;15:122–129. doi: 10.1634/theoncologist.2009-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24:2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 43.Valero V, Forbes J, Pegram MD, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): Two highly active therapeutic regimens. J Clin Oncol. 2011;29:149–156. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 44.Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: The HERNATA study. J Clin Oncol. 2011;29:264–271. doi: 10.1200/JCO.2010.30.8213. [DOI] [PubMed] [Google Scholar]
- 45.Burstein HJ, Keshaviah A, Baron AD, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: The trastuzumab and vinorelbine or taxane study. Cancer. 2007;110:965–972. doi: 10.1002/cncr.22885. [DOI] [PubMed] [Google Scholar]
- 46.NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V. 3.2013. [Accessed August 14, 2013]. Available at: http://www.nccn.org.
- 47.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 48.Inoue K, Nakagami K, Mizutani M, et al. Randomized phase III trial of trastuzumab monotherapy followed by trastuzumab plus docetaxel versus trastuzumab plus docetaxel as first-line therapy in patients with HER2-positive metastatic breast cancer: The JO17360 Trial Group. Breast Cancer Res Treat. 2010;119:127–136. doi: 10.1007/s10549-009-0498-7. [DOI] [PubMed] [Google Scholar]
- 49.Guan Z, Xu B, Desilvio ML, et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. J Clin Oncol. 2013;31:1947–1953. doi: 10.1200/JCO.2011.40.5241. [DOI] [PubMed] [Google Scholar]
- 50.Gelmon K, Boyle F, Kaufman B, et al. Open-label phase III randomized controlled trial comparing taxane-based chemotherapy (Tax) with lapatinib (L) or trastuzumab (T) as first-line therapy for women with HER2+ metastatic breast cancer: Interim analysis (IA) of NCIC CTG MA. 31/GSK EGF 108919 Oral presentation and abstract presented at: ASCO annual meeting; June 3, 2012; Chicago, IL. [Accessed January14, 2013]. Available at: http://am.asco.org/sites/chicago2013.asco.org/files/Annual%20Meeting%20Program.pdf (see Breast Cancer, Oral abstract session–ER/HER2) [Google Scholar]
- 51.Pivot X, Semiglazov V, Żurawski B, et al. CEREBEL (EGF111438): An open label randomized phase III study comparing the incidence of CNS metastases in patients (pts) with HER2+ metastatic breast cancer (MBC), treated with lapatinib plus capecitabine (LC) versus trastuzumab plus capecitabine (TC). Oral presentation and abstract presented at: European Society for Medical Oncology; October 1, 2012; Vienna, Austria. [Accessed January 14, 2013]. ESMO Oral Presentation, 2012. Available at: http://abstracts.webges.com/myitinerary/session-128.html?congress=esmo2012. [Google Scholar]
- 52.Capelan M, Pugliano L, De Azambuja E, et al. Pertuzumab: New hope for patients with HER2-positive breast cancer. Ann Oncol. 2013;24:273–282. doi: 10.1093/annonc/mds328. [DOI] [PubMed] [Google Scholar]
- 53.Baselga J, Cortes J, Im S-A, et al. Adverse events with pertuzumab and trastuzumab: Evolution during treatment with and without docetaxel in CLEOPATRA. J Clin Oncol. 2012;30(suppl) abstr 597. [Google Scholar]
- 54.Swain SM, Ewer MS, Cortes J, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: A randomized, double-blind, placebo-controlled phase III study. The Oncologist. 2013;18:257–264. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cortes J, Baselga J, Im Y-H, et al. Quality of life assessment in CLEOPATRA, a phase III study combining pertuzumab with trastuzumab and docetaxel in metastatic breast cancer. J Clin Oncol. 2012;30(suppl) doi: 10.1093/annonc/mdt274. abstr 598. [DOI] [PubMed] [Google Scholar]
- 56.Datko F, D'Andrea G, Dickler M, et al. Phase II study of pertuzumab, trastuzumab, and weekly paclitaxel in patients with metastatic HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2012;30(27 suppl):134. [Google Scholar]
- 57.Bachelot T, Ciruelos E, Peretz-Yablonski T, et al. A single-arm phase IIIb study of pertuzumab and trastuzumab with a taxane as first-line therapy for patients with HER2-positive advanced breast cancer (PERUSE) Cancer Research. 2012;72(24 supplement) abstract OT1–1-02. [Google Scholar]
- 58.Perez E, Lopez-Vega J, Del Mastro L, et al. A combination of pertuzumab, trastuzumab, and vinorelbine for first-line treatment of patients with HER2-positive metastatic breast cancer: An open-label, two-cohort, phase II study (VELVET) J Clin Oncol. 2012;30(suppl) abstr TPS653. [Google Scholar]
- 59.Gianni L, Romieu GH, Lichinitser M, et al. AVEREL: A randomized phase III trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31:1719–1725. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- 60.Arteaga C, Mayer I, O'Neill A, et al. A randomized phase III double blinded placebo controlled trial of first line chemotherapy and trastuzumab with or without bevacizumab for patients with HER2/neu overexpressing metastatic breast cancer (HER2+ MBC): A trial of the Eastern Cooperative Oncology Group (E1105) J Clin Oncol. 2012;30(suppl) abstr 605. [Google Scholar]
- 61.ClinicalTrials.gov. Everolimus in combination with trastuzumab and paclitaxel in the treatment of HER2 positive locally advanced or metastatic breast cancer (BOLERO-1) [Accessed May 21, 2013]. Available at: http://clinicaltrials.gov/show/NCT00876395.
- 62.ClinicalTrials.gov. Daily everolimus in combination with trastuzumab and vinorelbine in HER2/neu positive women with locally advanced or metastatic breast cancer (BOLERO-3) [Accessed May 21, 2013]. Available at: http://clinicaltrials.gov/show/NCT01007942.
- 63.Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–1163. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]
- 64.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verma S, Miles D, Gianni L, et al. Updated overall survival results from EMILIA, a phase 3 study of trastuzumab emtansine (T-DM1) vs capecitabine and lapatinib in HER2-positive locally advanced or metastatic breast cancer. Oral presentation and abstract presented at: European Society for Medical Oncology; October 1, 2012; Vienna, Austria. [Accessed January 14, 2013]. Available at http://abstracts.webges.com/myitinerary/session-128.html?congress=esmo2012. [Google Scholar]
- 66.US Food and Drug Administration. KADCYLA(ado-trastuzumab emtansine) prescribing information. [Accessed March 4, 2013]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf.
- 67.US Food and Drug Administration. FDA approves new treatment for late-stage breast cancer. 2013. [Accessed March 4, 2013]. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm340704.htm.
- 68.Ellis P, Barrios C, Im Y, et al. MARIANNE: A phase III, randomized study of trastuzumab-DM1 (T-DM1) with or without pertuzumab (P) compared with trastuzumab (H) plus taxane for first-line treatment of HER2-positive, progressive, or recurrent locally advanced or metastatic breast cancer (MBC) J Clin Oncol. 2011;29(15 suppl) abstract TPS102. [Google Scholar]
- 69.ClinicalTrials.gov. A study of trastuzumab emtansine in comparison with treatment of physician's choice in patients with HER2-positive breast cancer who have received at least two prior regimens of HER2-directed therapy (TH3RESA) [Accessed February 19, 2013]. Available at: http://clinicaltrials.gov/show/NCT01419197.
- 70.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 71.Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: Final results from the EGF104900 study. J Clin Oncol. 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 72.Cardoso F, Harbeck N, Fallowfield L, et al. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii11–19. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 73.Freedman RA, Hughes ME, Ottesen RA, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer. 2013;119:839–846. doi: 10.1002/cncr.27831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tjan-Heijnen V, Seferina S, Lobbezoo D, et al. Real-world use and effectiveness of adjuvant trastuzumab in 2665 consecutive breast cancer patients. Cancer Research. 2012;72(24) supplement 3 abstract P5–21-04. [Google Scholar]
- 75.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 76.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 77.Cameron D, Casey M, Oliva C, et al. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: Final survival analysis of a phase III randomized trial. The Oncologist. 2010;15:924–934. doi: 10.1634/theoncologist.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yin W, Jiang Y, Shen Z, et al. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: A meta-analysis of published randomized controlled trials. PLoS One. 2011;6:e21030. doi: 10.1371/journal.pone.0021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Viani GA, Afonso SL, Stefano EJ, et al. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: A meta-analysis of published randomized trials. BMC Cancer. 2007;7:153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dahabreh IJ, Linardou H, Siannis F, et al. Trastuzumab in the adjuvant treatment of early-stage breast cancer: A systematic review and meta-analysis of randomized controlled trials. The Oncologist. 2008;13:620–630. doi: 10.1634/theoncologist.2008-0001. [DOI] [PubMed] [Google Scholar]
- 81.Olson EM, Abdel-Rasoul M, Maly J, et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24:1526–1533. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Central Nervous System Cancers V. 2.2013. [Accessed August 14, 2013]. Available at: http://www.nccn.org.
- 83.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 84.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 85.Dowsett M, Procter M, McCaskill-Stevens W, et al. Disease-free survival according to degree of HER2 amplification for patients treated with adjuvant chemotherapy with or without 1 year of trastuzumab: The HERA Trial. J Clin Oncol. 2009;27:2962–2969. doi: 10.1200/JCO.2008.19.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez EA, Reinholz MM, Hillman DW, et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol. 2010;28:4307–4315. doi: 10.1200/JCO.2009.26.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paik S. Is gene array testing to be considered routine now? The Breast. 2011;20:S87–S91. doi: 10.1016/S0960-9776(11)70301-0. [DOI] [PubMed] [Google Scholar]
- 88.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011 Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Denkert C, Huober J, Loibl S, et al. HER2 and ESR1 mRNA expression levels and response to neoadjuvant trastuzumab plus chemotherapy in patients with primary breast cancer. Breast Cancer Res. 2013;15:R11. doi: 10.1186/bcr3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gianni L, Eiermann W, Pusztai L, et al. Biomarkers as potential predictors of pathologic complete response (pCR) in the NOAH trial of neoadjuvant trastuzumab in patients (pts) with HER2-positive locally advanced breast cancer (LABC) J Clin Oncol. 2008;26(15 suppl) abstract 504. [Google Scholar]
- 91.Paik S. Controversies in ER and HER2 testing. Paper presented at: 5th Annual International Congress on the Future of Breast Cancer; August 3–6, 2006; Koala Coast, HI. [Accessed October 9, 2013]. Available at: http://www.my-medical-education.com/en/mme/index.php?page=veranstaltung&field=&event=6bf12b9a-cf90–9881-7d19–a9ce4df861e1, http://professional.fctn.org/calendar/2006-meetings/ [Google Scholar]
- 92.Baselga J, Verma S, Ro J, et al. Relationship between tumor biomarkers (BM) and efficacy in EMILIA, a phase III study of trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC). Abstract presented at: annual meeting of the American Association for Cancer Research; April 7, 2013; Washington, DC. [Accessed May 21, 2013]. Available at http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=1e9ae007-6c50-46e2-951f-4b825865e743&cKey=15187a93-e3d3-443c-81b5-24d4f5e7bf0d&mKey={9B2D28E7-24A0-466F-A3C9-07C21F6E9BC9} [Google Scholar]
- 93.Gianni L, Bianchini G, Kiermaier A. Neoadjuvant pertuzumab (P) and trastuzumab (H): Biomarker analyses of a 4-Arm randomized phase II study (NeoSphere) in patients (pts) with HER2-pos-itive breast cancer (BC) Cancer Res. 2011;71(24) supplement 3 abstract S5–1. [Google Scholar]
- 94.Baselga J, Cortés J, Im S-A, et al. Biomarker analyses in CLEOPATRA: A phase III, placebo-controlled study of pertuzumab in HER2-positive, first-line metastatic breast cancer (MBC). Oral presentation and abstract presented at: San Antonio Breast Cancer Symposium; December 4–8, 2012; San Antonio, TX. [Accessed February 19, 2013]. Available at: http://sabcs12.m2usa.com/sabcs.html?p=GS5_01. [Google Scholar]
- 95.Blackwell KL, Miles D, Gianni L, et al. Primary results from EMILIA, a phase III study of trastuzumab emtansine (T-DM1) versus capecitabine (X) and lapatinib (L) in HER2-positive locally advanced or metastatic breast cancer (MBC) previously treated with trastuzumab (T) and a taxane. J Clin Oncol. 2012;30(18 suppl):LBA1. [Google Scholar]
- 96.Gianni L, Romieu G, Lichinitser M, et al. First results of AVEREL, a randomized phase III trial to evaluate bevacizumab (BEV) in combination with trastuzumab (H) + docetaxel (DOC) as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer (LR/mBC). Abstract presented at: San Antonio Breast Cancer Symposium; December 8, 2011; San Antonio, TX. [Accessed September 12, 2013]. Available at: http://www.abstracts2view.com/sabcs11/view.php?nu=SABCS11L_3034. [Google Scholar]