The impact of multifocal (MF) or multicentric (MC) breast cancer on locoregional (LR) control rates is unknown. This study found that MF and MC disease are not independent risk factors for LR recurrence. Patients with MF and MC breast cancer had rates of LR control similar to those of their unifocal counterparts. These data suggest that breast conservation therapy is a safe option for patients with MF tumors and that MF or MC disease alone is not an indication for postmastectomy radiation therapy.
Keywords: Multifocal, Multicentric, Breast cancer, Locoregional, Outcomes
Abstract
Background.
The impact of multifocal (MF) or multicentric (MC) breast cancer on locoregional (LR) control rates is unknown.
Methods.
MF was defined as two or more separate invasive tumors in the same quadrant of the breast. MC was defined as two or more separate invasive tumors occupying more than one quadrant of the same breast. Patients were categorized by LR treatment: breast conservation therapy (BCT; n = 256), mastectomy (n = 466), or mastectomy plus postmastectomy radiation therapy (PMRT; n = 184). All patients with MC disease had mastectomy (10 patients treated with BCT for MC disease were excluded). The Kaplan-Meier product limit method was used to calculate 5-year LR control rate. Cox proportional hazards models were used to determine independent associations of multifocality or multicentricity with LR control.
Results.
A total of 906 patients had either MF disease (n = 673) or MC disease (n = 233). With median follow-up of 52 months, the 5-year LR control rate was 99% for MF, 96% for MC, and 98% for unifocal tumors (p = .44). Subset analysis revealed no difference in LR control regardless of the LR treatment (p = .67 for BCT, p = .37 for mastectomy, p = .29 for mastectomy plus PMRT). There were five in-breast recurrences after BCT in the MF group. MF and MC did not have an independent impact on LR control rate on multivariate analysis.
Conclusion.
MF and MC disease are not independent risk factors for LR recurrence. Patients with MF and MC breast cancer had rates of LR control similar to those of their unifocal counterparts. These data suggest that BCT is a safe option for patients with MF tumors and that MF or MC disease alone is not an indication for PMRT.
Implications for Practice:
This work reviews the risk of locoregional recurrences in patients with breast cancer treated with different approaches, as currently recommended by standard guidelines. We looked at 2,816 cases with unifocal disease, 673 cases with multifocal (MF) disease, and 233 cases with multicentric (MC) disease. We showed that MF and MC breast cancers are not independently associated with increased locoregional recurrence rates and that breast conservation therapy is a safe option for patients with MF tumors. MF or MC disease alone is not an indication for postmastectomy radiation therapy.
Introduction
Multifocal (MF) and multicentric (MC) breast cancers are relatively common clinical entities, with incidence in the literature ranging from 6% to 60% and large variability because of differences in definitions used, inclusion or exclusion of in situ disease, and method of pathologic sampling [1, 2]. As advances in preoperative imaging continue, the number of MF and MC tumors identified increases [3–5], and better guidelines for their management are needed. In particular, questions still exist regarding the optimal locoregional (LR) therapy for MF and MC breast cancer.
Tumor size has long been recognized as a strong predictor of LR recurrence (LRR) [6–8], and it would seem logical that the presence of more than one synchronous unilateral tumor would also be a risk factor for LRR; however, studies have shown conflicting results. In the absence of compelling evidence to dictate otherwise, the convention according to current TNM staging guidelines has been to stage MF and MC patients according to the diameter of the largest lesions, without taking other foci of disease into consideration [9]. This approach assumes that prognosis is dependent only on the largest lesion and the presence and extent of lymph node involvement. In addition, although the location and distance between tumors often dictates surgical strategies, MF and MC alone are not standard indications for postmastectomy radiation therapy (PMRT).
We have previously shown in the same large, single-institution cohort that, although associated with a number of poor prognostic factors (e.g., higher T-stage, increased incidence of nodal involvement, higher grade and more lymphovascular invasion [LVI]), the presence of MF or MC disease alone is not an independent predictor of relapse-free survival (both local and distant combined), breast cancer-specific survival, or overall survival [10]. The purpose of this study is to further analyze the effect of multifocality and multicentricity on the LRR rate with respect to different LR treatment modalities.
Materials and Methods
Patient Selection
Using the Breast Cancer Management System database of the University of Texas MD Anderson Cancer Center (MDACC), we retrospectively identified all patients diagnosed with invasive breast cancer between 1997 and 2010. We excluded patients with metastatic disease at diagnosis and those treated with neoadjuvant chemotherapy, leaving 6,735 patients. We excluded an additional 2,811 patients who did not have MF and MC information, pathological tumor size, or nodal status available. We further excluded 192 patients who did not receive adjuvant radiation therapy after breast conserving surgery because all patients who underwent conservative surgery received radiation therapy. Another 10 patients who were treated with breast conservation therapy (BCT) for MC disease against physician advice were also excluded.
MF was defined as two or more separate invasive tumors in the same quadrant of the breast. MC was defined as two or more separate invasive tumors occupying more than one quadrant of the same breast. If patients had both MF and MC disease, they were classified as MC. Patients who had MF or MC in situ disease only were excluded. Determinations were made by pathology review only; clinical and radiographic data were not considered. All pathology specimens were independently reviewed by dedicated breast pathologists at MDACC at the time of initial treatment. A total of 3,722 patients were included in the analysis. Of these, 2,816 patients (76%) had unifocal (UF) breast cancer, and 906 patients (24%) had MF (n = 673) or MC (n = 233) breast cancer in their pathology specimens. LRR was defined after BCT as recurrence in either the ipsilateral breast or regional lymph nodes (axillary, infraclavicular, supraclavicular, or internal mammary), and after mastectomy as recurrence in the ipsilateral chest wall or regional lymph nodes (axillary, infraclavicular, supraclavicular, or internal mammary). The institutional review board of the University of Texas MDACC approved the retrospective study.
Statistical Methods
Patients were categorized as having UF, MF, or MC breast cancers. The MF and MC tumors were analyzed as separate entities and as a group (MF/MC). Patient characteristics including age, menopausal status, race, tumor size, nodal status, histology, nuclear grade, presence of LVI, tumor subtype, LR treatment modality, surgical margin status, LRR location, and adjuvant systemic treatment (chemotherapy and hormonal therapy) were tabulated and compared between groups by the chi-square test. Time to LRR was measured from the date of diagnosis to the date of the first documented LRR (regardless of whether it occurred first or after a systemic recurrence); patients not experiencing the event were censored at last follow-up. Five-year LR control rate was calculated using the Kaplan-Meier product limit method; groups were compared with the log-rank statistic. A subgroup analysis was performed by LR therapy (BCT, mastectomy only, and mastectomy followed by PMRT). Cox proportional hazards models were used to determine the association of MF, MC, and the combination of the MF and MC groups with LR control after adjustment for other patient and disease characteristics. A p value <.05 was considered statistically significant; all tests were two-sided. Statistical analyses were carried out using SAS 9.1 (SAS Institute, Inc., Cary, NC) and S-Plus 7.0 (Insightful Corp., Seattle, WA).
Results
Patient and Tumor Characteristics
MF or MC disease was seen in 906 patients, or 24% of the total patient population; 673 (18.1%) had MF and 233 (6.3%) had MC tumors (Table 1). As we have reported previously, in comparison with patients with UF disease, patients with MF or MC breast cancer were younger and thus more likely to be premenopausal [10]. They had higher T stages and N stages. Histologically, MF and MC tumors were associated with more grade 3 disease, more LVI, lobular differentiation, and HER2 positivity. In terms of treatment received, these patients were more likely to undergo mastectomy, with or without radiation (p < .001). More patients with MF and MC tumors received adjuvant chemotherapy (p < .001), but there was no difference in the proportion of patients who received adjuvant hormonal therapy (p ≥ .40) [10]. In terms of LR therapy, 62.4% of UF tumors were treated with BCT compared with 38% of MF tumors (p < .001). In addition, 31.7% of UF tumors were treated with mastectomy alone compared with 48.1% of MF tumors (p < .001) and 60.9% of MC tumors (p < .001). Only 5.9% of UF tumors were treated with mastectomy and PMRT compared with 13.8% of MF tumors (p < .001) and 39.1% of MC tumors (p < .001). In the UF group, 87 patients (3.1%) had close (<2 mm) or positive surgical margins (including invasive cancer and ductal carcinoma in situ but not lobular carcinoma in situ) compared with 5.3% in the MF group (p = .007), 3.4% in the MC group (p = .70), and 4.9% in the MF/MC group (p = .02). The low rate of positive margins in the MC group likely represents the fact that all of these patients were treated with mastectomy.
Table 1.
Treatment and site of recurrence among all patients
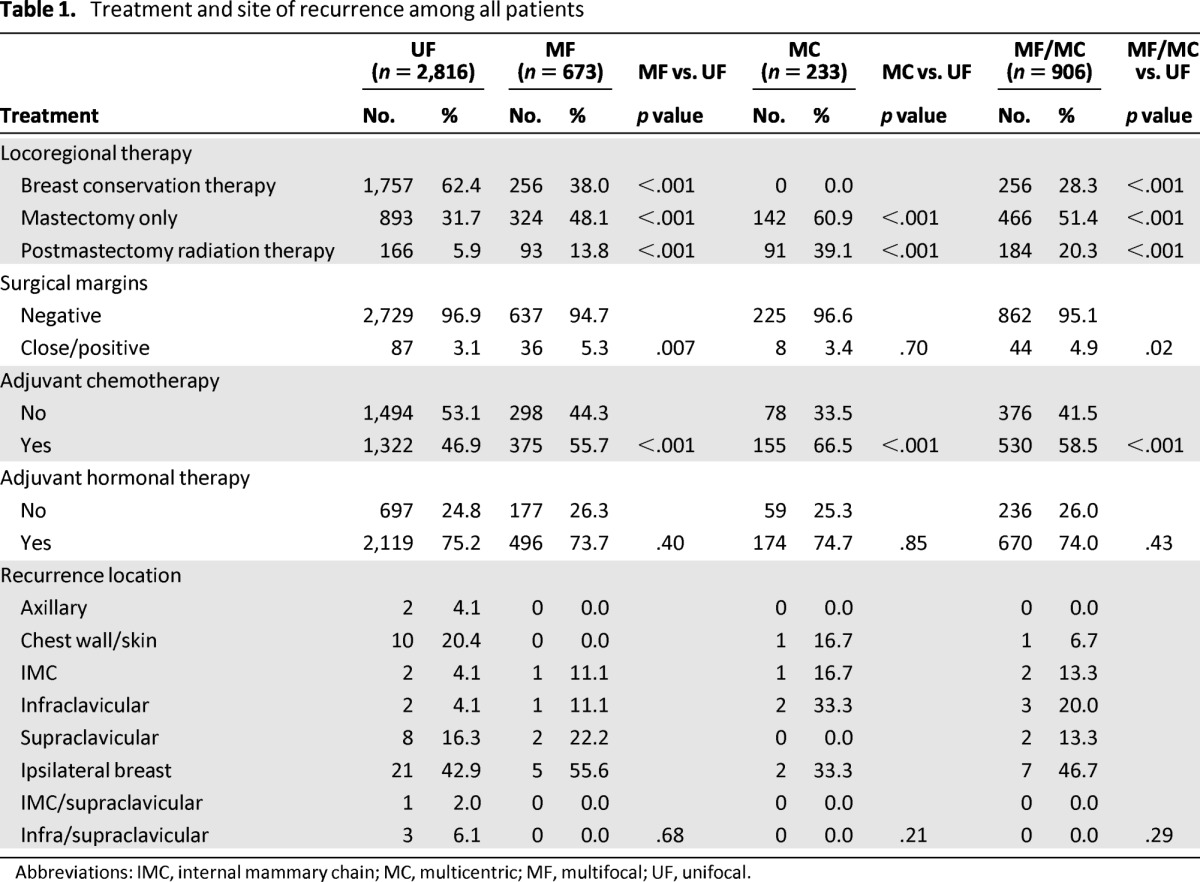
Abbreviations: IMC, internal mammary chain; MC, multicentric; MF, multifocal; UF, unifocal.
LR Control
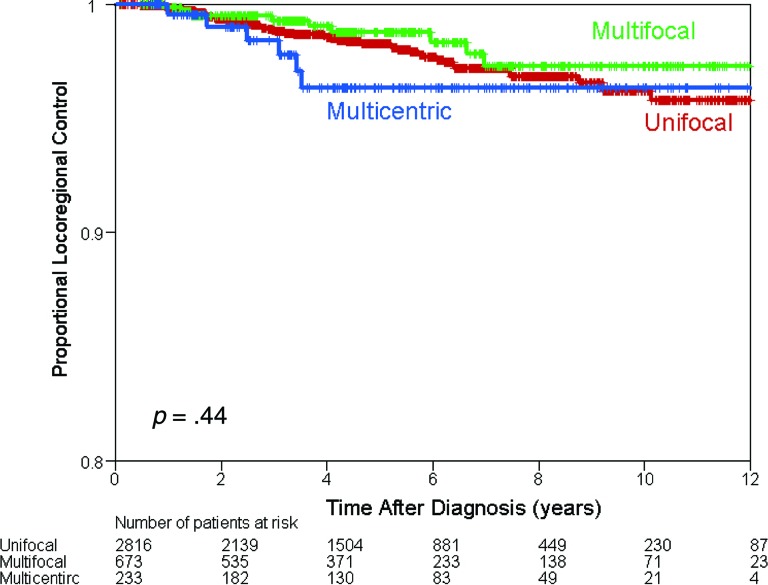
The median follow-up among all patients was 52 months (range: 1–162 months). Sixty-four patients (1.7%) had LRR: 49 (1.7%) in the UF group, 9 (1.3%) in the MF group, and 6 (2.6%) in the MC group (Table 2). There was no difference in the 5-year LR control rate among the UF group (98%), the MF group (99%), and the MC group (96%) (p = .44) (Fig. 1).
Table 2.
Five-year locoregional control estimates by patient and clinical characteristics among all patients
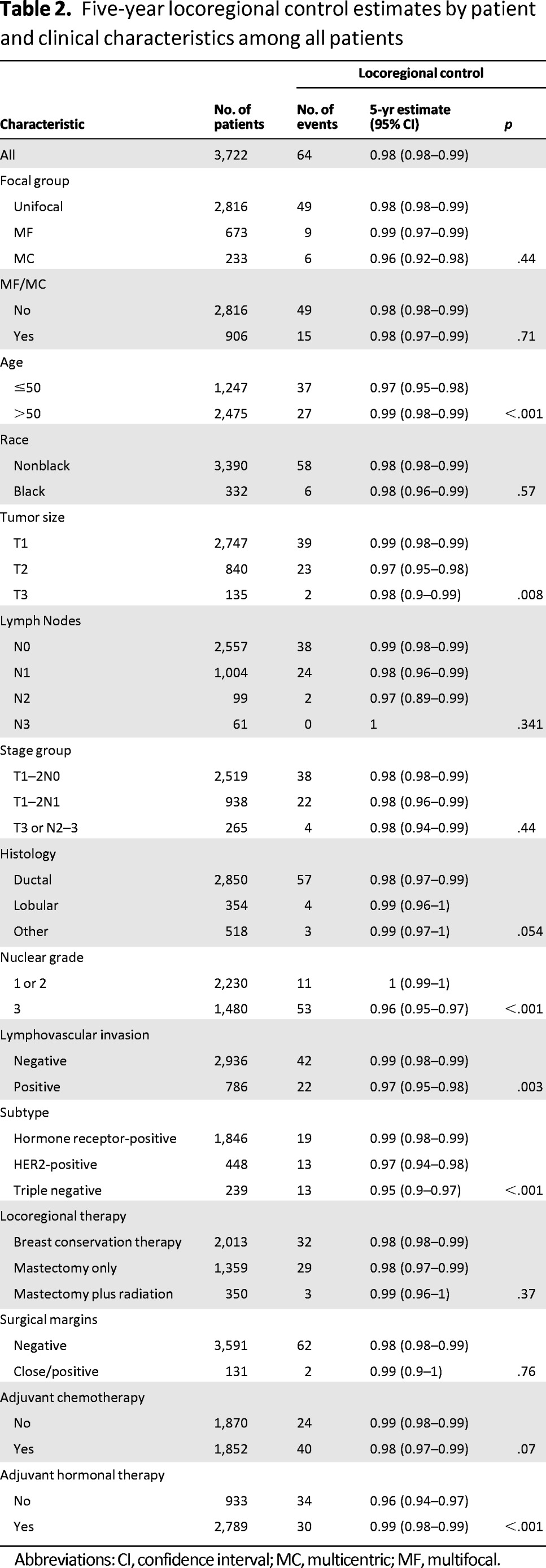
Abbreviations: CI, confidence interval; MC, multicentric; MF, multifocal.
Figure 1.
Kaplan-Meier estimates of locoregional control among all patients.
The sites of LRR are detailed in Table 1. There was no difference in terms of recurrence location among the UF, MF, and MC groups (p ≥ .21). Specifically, there were only 5 (1.95%) in-breast recurrences after BCT in the MF group compared with 18 (1.02%) in the UF group.
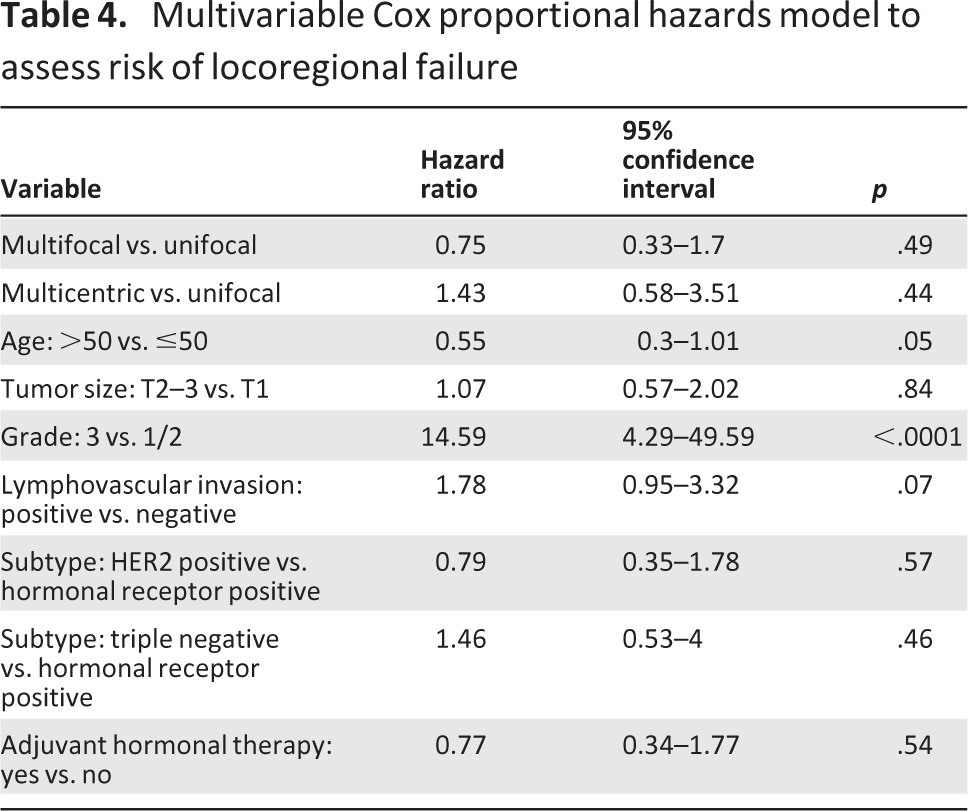
To evaluate the independent impact of MF and MC on LRR, a multivariate Cox proportional hazards model was applied. Risk factors that were significant on univariate analysis (age, tumor size, grade, LVI, histologic subtype, adjuvant hormonal therapy) were evaluated (Table 2). The results are shown in Tables 3 and 4. On multivariate analysis, only age ≤50, high tumor grade (grade 3), and the presence of LVI (for MC tumors only) independently increased the risk of LRR. MF tumors (hazard ratio [HR]: 0.73; 95% confidence interval [CI]: 0.32–1.67; p = .46), MC tumors (HR: 1.35; 95% CI: 0.55–3.34; p = .51), and combined MF/MC tumors (HR: 0.95; 95% CI: 0.49–1.83; p = .88) were not independent predictors of LRR.
Table 3.
Multivariable Cox proportional hazards models by multifocality, multicentricity, or both on locoregional control
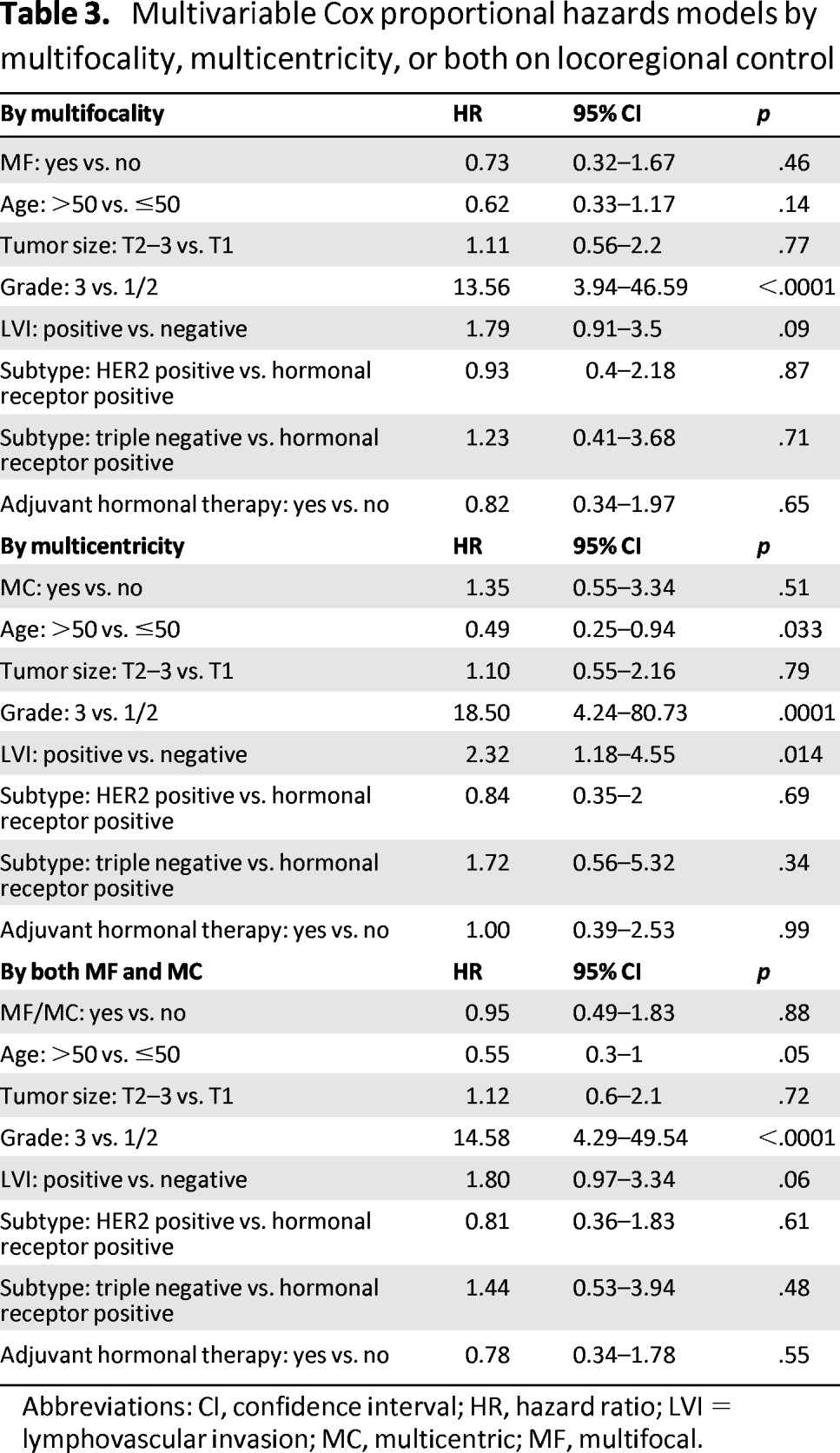
Abbreviations: CI, confidence interval; HR, hazard ratio; LVI = lymphovascular invasion; MC, multicentric; MF, multifocal.
Table 4.
Multivariable Cox proportional hazards model to assess risk of locoregional failure
Subset Analyses
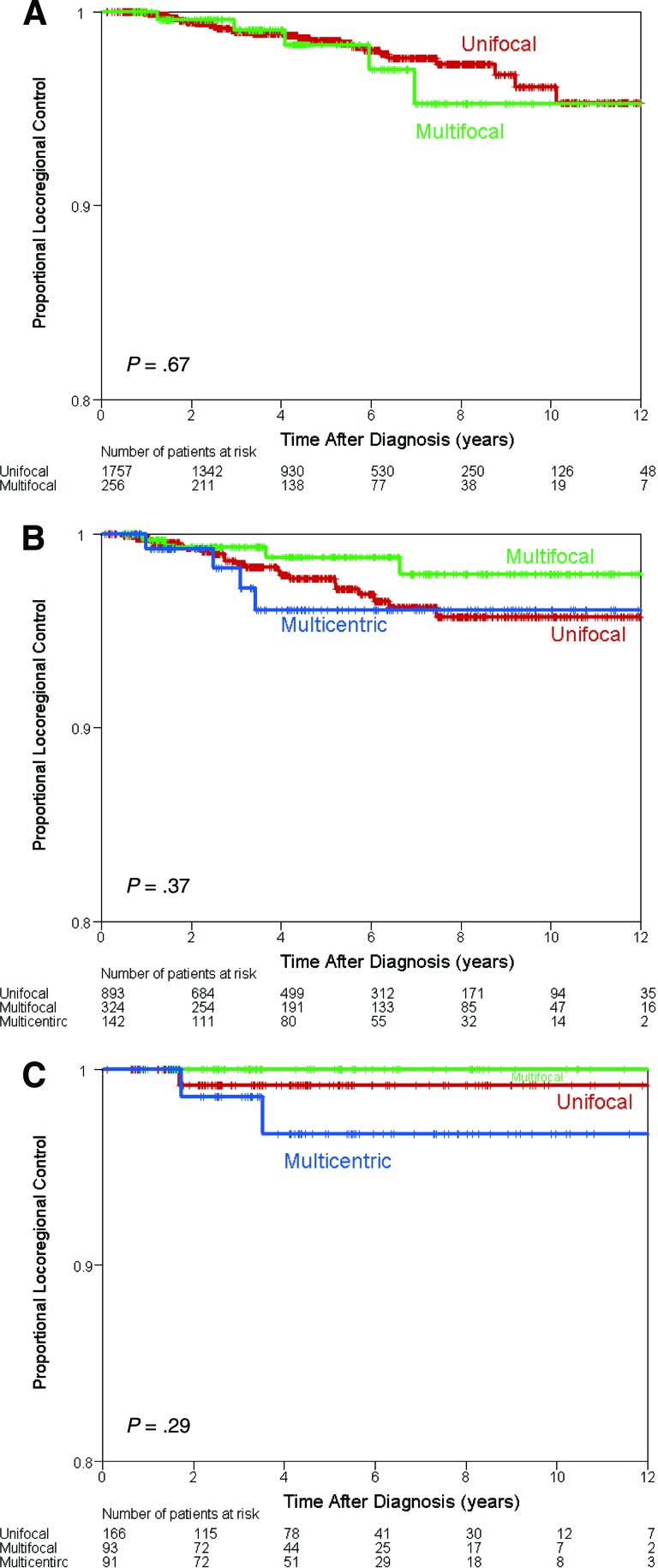
A subset analysis was performed based on the LR treatment modality received. There was no difference in the LR control between the MF/MC and UF groups treated with BCT (p = .67), mastectomy alone (p = .37), or PMRT (p = .29) (Fig. 2A–2C).
Figure 2.
Kaplan-Meier estimates of locoregional control by locoregional treatment modality. (A): Breast conserving surgery followed by adjuvant radiation. (B): Mastectomy alone. (C): Mastectomy plus postmastectomy radiation therapy.
Discussion
In this large, single-institution cohort, MF and MC disease were not independent risk factors for LRR. This was true regardless of the LR treatment modality received. Several previous studies had similar findings. In an early study, Fowble et al. reported on 88 patients with stage I–II clinically or radiographically gross MC breast cancer (n = 57) or diffuse microcalcifications (n = 31), all of whom were treated with modified radical mastectomy and 15 of whom received PMRT. After a median follow-up of 4 years, no difference was seen in the rate of isolated LRR (8% vs. 7%, no p value given) [11]. Oh et al. reviewed 97 patients with clinically diagnosed MF/MC breast cancer who were subsequently treated with neoadjuvant anthracycline-based chemotherapy followed by BCT, mastectomy, or mastectomy plus PMRT and found no difference in the 5-year rate of LRR (UF 10% vs. MF/MC 7%, p = .78), regardless of the LR treatment modality received [12]. In a population similar to ours, Cabioglu et al. examined 1,322 patients with T1–3 invasive breast cancer who were not treated with neoadjuvant chemotherapy [13]. In their study, 147 (11%) had MF or MC tumors (not distinguished as separate entities), defined as more than one simultaneous macroscopically separated tumor in the same breast on surgical pathology evaluation. Of the MF/MC patients, 30 underwent BCT and 117 underwent mastectomy; 77 received PMRT. With a median follow-up of 55 months, the rate of LRR was not significantly different between those with the MF/MC tumors (5.4%) and the UF tumors (3.7%) (p = .36). Huang et al. reviewed 84 patients with stage II–IV (ipsilateral supraclavicular adenopathy) MF and MC breast cancer (defined on pathology as either microscopic MF/MC disease or macroscopic MF/MC disease) who were all treated with neoadjuvant chemotherapy, modified radical mastectomy, and PMRT. No difference was found in the rate of LRR after a median follow-up of 70 months (p < .05) [14]. This represents a different (and higher risk) patient population because these patients had residual MF or MC disease after treatment with neoadjuvant chemotherapy; however, the findings were still similar.
In contrast to our findings, Katz et al. reported a study of 149 patients with stage II and IIIa MF/MC breast cancer, all treated with mastectomy (without PMRT) and adjuvant anthracycline-based chemotherapy [15]. Tumors were classified as MF (two or more separate areas of invasive carcinoma within the same quadrant and/or <4 cm apart by mammography or gross pathology evaluation), microscopic MC, and gross MC (two or more areas of invasive disease in more than one quadrant of the breast and separated by ≥4 cm by clinical or pathologic analysis). Of note, patients with more than one microscopic focus of invasive disease in the same breast quadrant were classified as unicentric. When considered as a separate entity, gross MC disease was found to have an increased rate of LRR at 10 years (37% vs. 17%, p = .01), a difference that persisted in multivariate analysis (p = .0001). This difference in outcomes is likely because of the difference in patient populations (our cohort included patients with stage I breast cancer), and the more stringent definition of MC that was used in the study by Katz et al. Weissenbacher et al. reported on 288 patients with early stage MF and MC breast cancer compared with a matched cohort of patients with UF disease [16]. MF and MC disease was defined clinically and radiographically and thus likely represented a similar population to the gross MC group of Katz et al. After a median follow-up of 70 months, MF and MC patients had an LRR rate of 17.4% compared with 7.3% for UF tumors (p < .001). Of note, 43.1% were treated with BCT (compared with 50.3% in the UF group).
In our cohort, 256 patients underwent BCT for MF breast cancer and 1,757 underwent BCT for UF disease. There were 5 (1.95%) in-breast recurrences after BCT in the MF group compared with 18 (1.02%) in the UF group. There was no difference in LRR (including nodal basins) between the MF and UF groups treated with this modality. Several small, early studies showed that patients with MF or MC disease have an increase in LRR following BCT [11, 17–20] (LRR ranged from 23% to 40% at 5-year follow-up in these trials). Based on these trials, mastectomy became the standard of care for women with MF or MC disease. As diagnostic tools advance, MF and MC tumors are more commonly diagnosed, and cancers that previously would have been classified as UF now can be detected as MF or MC. In addition, LR treatment modalities have improved significantly over the past decade. More recent studies reflect these advances in diagnosis and treatment.
In their series of 97 patients with clinically diagnosed MF/MC breast cancer who were subsequently treated with neoadjuvant chemotherapy, Oh et al. reported no in-breast recurrences for patients treated with BCT for MF disease [12]. Baumann et al. retrospectively reviewed the charts of 22 women with an average follow-up of 3.5 years, with only 1 patient (4.5%) experiencing an in-breast recurrence [21]. Min et al. reviewed 251 stage II/III (node-positive) patients who had LRR after neoadjuvant therapy and BCT. Of these, 74 had clinical MF (cMF) disease at presentation and 11 had residual MF disease on pathology evaluation (pMF) after primary systemic therapy. The median follow-up was 55 months. There was a trend toward worse 5-year LRR-free survival (unicentric, 91%; cMF, 86%; pMF, 82%; p = .15) and ipsilateral breast tumor recurrence-free survival (unicentric, 92%; cMF, 89%; pMF, 82%; p = .23) for the patients with clinically and pathologically MF disease, but it was not statistically significant [22].
Debate continues as to whether BCT is an appropriate option for women with MC disease. This question was not addressed in this analysis because it is not currently routine practice at our institution; therefore, numbers were insufficient to make any conclusions.
PMRT has been shown in randomized controlled trials to reduce the rate of LRR and can improve overall survival rates in high-risk patients [23, 24]; however, the presence of MF and/or MC disease has not been identified as a high-risk feature mandating PMRT. In our subgroup analysis, patients with MF and MC disease had similar LRR after mastectomy, regardless of whether or not they received PMRT. Although Katz et al. [15] showed an association between MF/MC disease and LRR in patients treated with mastectomy and adjuvant anthracycline-based chemotherapy, none of these patients received PMRT; therefore, it is unknown if its addition would have significantly negated some of that risk. In addition, in a subset analysis of 23 patients with T1–2 tumors with only one, two, or three involved lymph nodes, they found no independent impact of MC disease on the risk of LRR. Similar to our findings, Oh et al. found in their series of 97 patients with clinical MF/MC disease at the time of diagnosis (who were subsequently treated with neoadjuvant chemotherapy) that there was no difference in the LR control rate between patients treated with mastectomy alone (n = 44) and those treated with mastectomy plus PMRT (n = 33) (91% vs. 93%, respectively) [12].
This study is limited by its retrospective nature and thus possible treatment biases. In addition, one of the biggest obstacles in interpreting the current literature on MF and MC breast tumors is the lack of a standard definition. These data apply to patients with MF and MC disease diagnosed on initial surgical pathology, and caution should be taken when applying the data to patients with MF or MC disease based on a different definition or after neoadjuvant chemotherapy.
Conclusion
Patients with MF and MC breast cancer had rates of LR control similar to those of their UF counterparts when receiving appropriate local treatment modalities. Our data suggest that BCT is a safe option for patients with MF tumors and that MF or MC disease alone is not an indication for PMRT. Future research should focus on the feasibility and safety of BCT for MC tumors.
Acknowledgments
This work was supported in part by National Cancer Institute 1K23CA121994–01, National Cancer Institute Breast Specialized Program for Research Excellence (Developmental Grant) P50-CA116199 (to A.M.G.) and by the National Cancer Institute through the University of Texas MD Anderson's Cancer Center support grant (P30 CA016672). The MD Anderson Breast Cancer Management System and the Breast Tumor Bank are supported in part by the Nelly B. Connally Breast Cancer Research Fund.
Author Contributions
Conception/Design: Gabriel N. Hortobágyi, Ana M. Gonzalez-Angulo, Vicente Valero
Provision of study material or patients: Limin Hsu, Ana M. Gonzalez-Angulo
Collection and/or assembly of data: Funda Meric-Bernstam, Thomas A. Buchholz, Ana M. Gonzalez-Angulo
Data analysis and interpretation: Siobhan P. Lynch, Xiudong Lei, Hong Zhang, Ana M. Gonzalez-Angulo
Manuscript writing: Ana M. Gonzalez-Angulo
Final approval of manuscript: Ana M. Gonzalez-Angulo
Disclosures
Gabriel N. Hortobágyi: Allergan, Genentech, Novartis, AstraZeneca, Sanofi Aventis (C/A); Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Yerushalmi R, Kennecke H, Woods R, et al. Does multicentric/multifocal breast cancer differ from unifocal breast cancer? An analysis of survival and contralateral breast cancer incidence. Breast Cancer Res Treat. 2009;117:365–370. doi: 10.1007/s10549-008-0265-1. [DOI] [PubMed] [Google Scholar]
- 2.Egan RL. Multicentric breast carcinomas: Clinical-radiographic-pathologic whole organ studies and 10-year survival. Cancer. 1982;49:1123–1130. doi: 10.1002/1097-0142(19820315)49:6<1123::aid-cncr2820490610>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson LS, Given-Wilson R, Hall T, et al. Increasing the diagnosis of multifocal primary breast cancer by the use of bilateral whole-breast ultrasound. Clin Radiol. 2005;60:573–578. doi: 10.1016/j.crad.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Sardanelli F, Giuseppetti GM, Panizza P, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in Fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR Am J Roentgenol. 2004;183:1149–1157. doi: 10.2214/ajr.183.4.1831149. [DOI] [PubMed] [Google Scholar]
- 5.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: Systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248–3258. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 6.Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: Implications for postoperative irradiation. J Clin Oncol. 2000;18:2817–2827. doi: 10.1200/JCO.2000.18.15.2817. [DOI] [PubMed] [Google Scholar]
- 7.Fowble B, Gray R, Gilchrist K, et al. Identification of a subgroup of patients with breast cancer and histologically positive axillary nodes receiving adjuvant chemotherapy who may benefit from postoperative radiotherapy. J Clin Oncol. 1988;6:1107–1117. doi: 10.1200/JCO.1988.6.7.1107. [DOI] [PubMed] [Google Scholar]
- 8.Recht A, Gray R, Davidson NE, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: Experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689–1700. doi: 10.1200/JCO.1999.17.6.1689. [DOI] [PubMed] [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. [Google Scholar]
- 10.Lynch SP, Lei X, Chavez-Macgregor M, et al. Multifocality and multicentricity in breast cancer and survival outcomes. Ann Oncol. 2012;23:3063–3069. doi: 10.1093/annonc/mds136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowble B, Yeh IT, Schultz DJ, et al. The role of mastectomy in patients with stage I-II breast cancer presenting with gross multifocal or multicentric disease or diffuse microcalcifications. Int J Radiat Oncol Biol Phys. 1993;27:567–573. doi: 10.1016/0360-3016(93)90381-5. [DOI] [PubMed] [Google Scholar]
- 12.Oh JL, Dryden MJ, Woodward WA, et al. Locoregional control of clinically diagnosed multifocal or multicentric breast cancer after neoadjuvant chemotherapy and locoregional therapy. J Clin Oncol. 2006;24:4971–4975. doi: 10.1200/JCO.2006.07.6067. [DOI] [PubMed] [Google Scholar]
- 13.Cabioglu N, Ozmen V, Kaya H, et al. Increased lymph node positivity in multifocal and multicentric breast cancer. J Am Coll Surg. 2009;208:67–74. doi: 10.1016/j.jamcollsurg.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Huang EH, Tucker SL, Strom EA, et al. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:351–357. doi: 10.1016/j.ijrobp.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 15.Katz A, Strom EA, Buchholz TA, et al. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys. 2001;50:735–742. doi: 10.1016/s0360-3016(01)01500-0. [DOI] [PubMed] [Google Scholar]
- 16.Weissenbacher TM, Zschage M, Janni W, et al. Multicentric and multifocal versus unifocal breast cancer: Is the tumor-node-metastasis classification justified? Breast Cancer Res Treat. 2010;122:27–34. doi: 10.1007/s10549-010-0917-9. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz JM, Jacquemier J, Amalric R, et al. Breast-conserving therapy for macroscopically multiple cancers. Ann Surg. 1990;212:38–44. doi: 10.1097/00000658-199007000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson LD, Beinfield M, McKhann CF, et al. Conservative surgery and radiation in the treatment of synchronous ipsilateral breast cancers. Cancer. 1993;72:137–142. doi: 10.1002/1097-0142(19930701)72:1<137::aid-cncr2820720126>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Leopold KA, Recht A, Schnitt SJ, et al. Results of conservative surgery and radiation therapy for multiple synchronous cancers of one breast. Int J Radiat Oncol Biol Phys. 1989;16:11–16. doi: 10.1016/0360-3016(89)90004-7. [DOI] [PubMed] [Google Scholar]
- 20.Hartsell WF, Recine DC, Griem KL, et al. Should multicentric disease be an absolute contraindication to the use of breast-conserving therapy? Int J Radiat Oncol Biol Phys. 1994;30:49–53. doi: 10.1016/0360-3016(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 21.Bauman L, Barth RJ, Rosenkranz KM. Breast conservation in women with multifocal-multicentric breast cancer: Is it feasible? Ann Surg Oncol. 2010;17(suppl 3):325–329. doi: 10.1245/s10434-010-1247-1. [DOI] [PubMed] [Google Scholar]
- 22.Min SY, Lee SJ, Shin KH, et al. Locoregional recurrence of breast cancer in patients treated with breast conservation surgery and radiotherapy following neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2011;81:e697–e705. doi: 10.1016/j.ijrobp.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 24.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]