Abstract
Background.
Peripheral neuropathy (PN) is a recognized side effect of microtubule-targeting agents and the most clinically relevant toxicity observed with the epothilone sagopilone (SAG). Studies suggest that acetyl-L-carnitine (ALC) may prevent chemotherapy-induced PN. We conducted a prospective, placebo (PBO)-controlled, double-blind, randomized trial to investigate the safety and efficacy of ALC for the prevention of SAG-induced PN.
Methods.
Patients with ovarian cancer (OC) or castration-resistant prostate cancer (CRPC) and no evidence of neuropathy received SAG (16 mg/m2 intravenously over 3 hours every 3 weeks) with ALC (1,000 mg every 3 days) or placebo (PBO). The primary endpoint was incidence of PN within six or fewer cycles in both treatment groups.
Results.
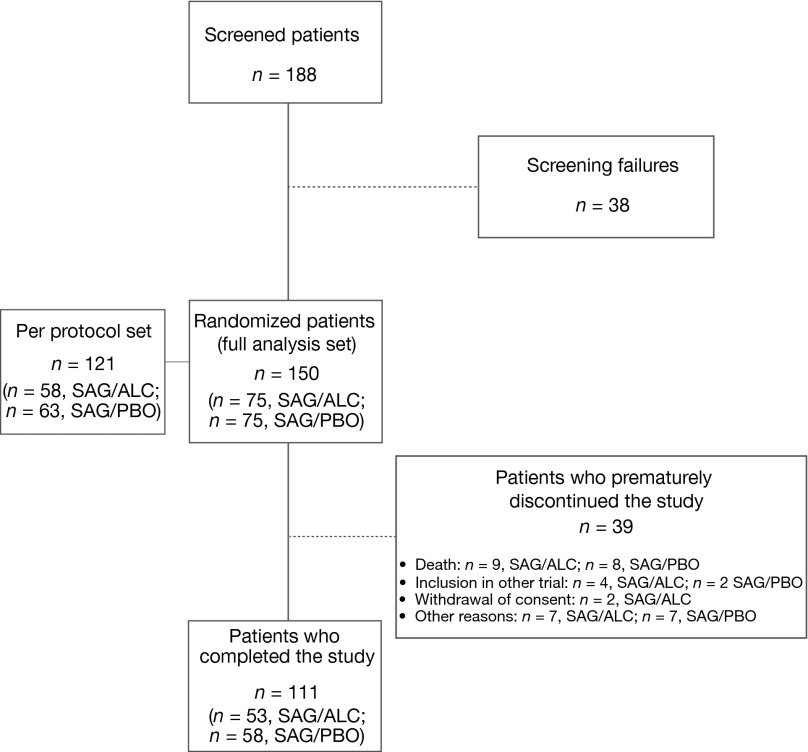
Overall, 150 patients enrolled (98 OC patients, 52 CRPC patients), with 75 per treatment arm. No significant difference in overall PN incidence was observed between treatment arms. The incidence of grade ≥3 PN was significantly lower in the ALC arm in OC patients. Median duration of neuropathy was similar between treatment arms. The best overall response (according to the modified Response Evaluation Criteria in Solid Tumors), response according to tumor markers, time-to-event variables, and discontinuations because of adverse events (AEs) were comparable between treatment arms.
Conclusion.
Administration of ALC with SAG did not result in a significant difference in overall PN incidence compared with a PBO. OC patients in the SAG/ALC arm had a significantly lower incidence of grade 3 or 4 PN compared with OC patients in the SAG/PBO arm.
Author Summary
Discussion
In this first randomized, prospective study of ALC for the prevention of chemotherapy-induced PN, no significant difference was observed in PN incidence between the two treatment arms. Consistent with previous clinical findings, however, ALC appeared to significantly reduce the incidence of high-grade neuropathy. Consequently, ALC given concurrently with SAG appears to significantly reduce the intensity of neurotoxicity but not the incidence.
The reasons for the greater reduction in high-grade neuropathy in patients with recurrent OC, compared with those with CRPC, are not entirely clear. Almost all patients in this study had received prior taxane-based therapy, and it is possible that some patients developed taxane-induced PN diagnosed only after taxane cessation. A previously published SAG clinical study of patients with OC, however, suggested that SAG-induced PN does not appear to correlate with prior taxane treatment [1].
Our study supports previously reported safety findings that demonstrated SAG to be well tolerated by patients with CRPC or OC. PN was the most commonly reported grade ≥3 AE in our study, as also reported in earlier studies [1–6]. Incidence and types of AEs were broadly similar across treatment arms, with slightly more serious AEs and grade 3 or 4 AEs reported in the SAG/PBO arm. Few patients reported ALC-related AEs, and, consistent with previous studies, ALC appears to be very well tolerated [7, 8]. In our study, there was no notable difference in response, progression-free survival, or time to progression between the two treatment arms. This finding suggests that, consistent with previous clinical reports in patients treated with taxanes, ALC did not appear to compromise the antitumor activity of SAG [7, 8].
Figure 1.
CONSORT diagram.
Abbreviations: ALC, acetyl-L-carnitine; PBO, placebo; SAG, sagopilone.
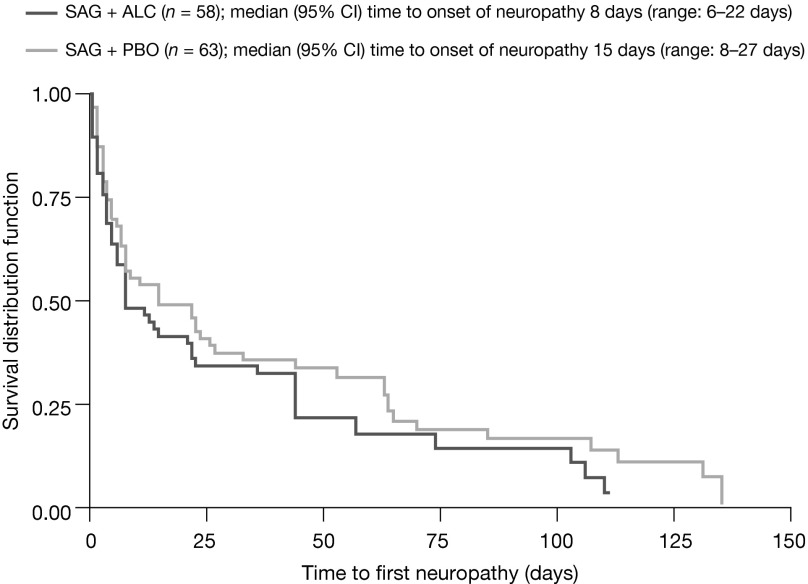
Figure 2.
Time to recovery from neuropathy (per protocol set).
Abbreviations: ALC, acetyl-L-carnitine; CI, confidence interval; PBO, placebo; SAG, sagopilone.
In conclusion, our study findings are encouraging regarding the therapeutic role of ALC in combination with SAG.
Supplementary Material
Footnotes
Access the full results at: Campone-13-61.theoncologist.com
ClinicalTrials.gov Identifier: NCT00751205 Sponsor(s): Bayer HealthCare Pharmaceuticals
Principal Investigator: Mario Campone IRB Approved: Yes
Editor's Note: See the accompanying commentary on pages 1151-1152 of this issue.
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.