This review discusses data supporting continuous kinase suppression with imatinib for the treatment of gastrointestinal stromal tumors and key issues, including response to imatinib reintroduction, effect of treatment interruption on secondary resistance to imatinib, and prognostic factors associated with sustained response to imatinib. Results from recent studies provide a strong rationale for continuous imatinib treatment for 3 years following surgical resection and long-term continuous administration in advanced or metastatic gastrointestinal stromal tumors.
Keywords: Gastrointestinal stromal tumor, Imatinib, Tyrosine kinase inhibitor, KIT, Metastatic, Adjuvant
Abstract
Background.
The oral tyrosine kinase inhibitor (TKI) imatinib has revolutionized the treatment of gastrointestinal stromal tumors (GISTs), most of which harbor oncogenic mutation in genes that encode the receptor tyrosine kinases KIT or PDGFA. Imatinib is the standard of care for patients with advanced GIST and for patients with primary GIST at significant risk of recurrence after surgery.
Design.
This review discusses data supporting continuous kinase suppression with imatinib and key issues, including response to imatinib reintroduction, effect of treatment interruption on secondary resistance to imatinib, and prognostic factors associated with sustained response to imatinib.
Results.
Long-term follow-up results of the B2222 study and updated results of the BFR14 trial demonstrate that continuous imatinib treatment in patients with advanced GIST is associated with reduced risk of progression. For patients progressing on or intolerant of imatinib, continuing therapy with TKIs sunitinib followed by regorafenib is recommended. In the adjuvant setting, final results of the trial by the Scandinavian Sarcoma Group and the Sarcoma Group of the Arbeitsgemeinschaft Internistische Onkologie demonstrate that 3 years of adjuvant imatinib, compared with 1 year, significantly reduces the risk of recurrence and improves overall survival of patients with KIT-positive GIST at high risk of recurrence.
Conclusions.
Maintenance of therapy with TKIs is the key to successful treatment of GIST. Results from recent studies provide a strong rationale for continuous imatinib treatment for 3 years following surgical resection and long-term continuous administration in advanced or metastatic GIST.
Implications for Practice:
Imatinib interruption in advanced setting results in rapid progression in the vast majority of patients and should not be recommended outside clinical trials unless patients experienced significant toxicity. The results observed in advanced GIST need to be considered also for the use of imatinib in the adjuvant setting where the optimal duration of imatinib is unknown (at least three years) and where reintroduction of imatinib is the standard of care in case of disease recurrence.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common sarcoma of the gastrointestinal tract, with an estimated incidence of 10–15 cases per 1 million people [1]. The KIT protein is expressed by 95% of GIST specimens and is a sensitive and specific histological marker for GIST [1]. KIT gain-of-function mutations contribute significantly to the molecular pathogenesis underlying most GISTs [2–5]. Approximately 70%–80% of GISTs contain an activating mutation in the KIT proto-oncogene, and 5%–10% have activating mutations in PDGFRA [6]. Mutations in KIT and PDGFRA appear to be mutually exclusive oncogenic mechanisms in GIST [2, 7].
The advent of imatinib mesylate, an orally bioavailable tyrosine kinase inhibitor (TKI) with targets that include KIT, PDGFR, and BCR-ABL [5], has revolutionized the treatment of patients with unresectable or metastatic GIST. Prior to the approval of imatinib, no systemic treatments had demonstrated a meaningful clinical benefit for patients with advanced GIST [8]; the median 2-year survival was 26% for GIST patients treated with chemotherapy in clinical trials. With the use of imatinib, 2-year survival has increased to more than 70% for patients with unresectable or metastatic GIST [9]. Given this and its favorable safety profile, the use of imatinib has been extended to the adjuvant setting for the treatment of adult patients following resection of KIT-positive GIST [10].
Despite the efficacy imatinib demonstrated in patients with advanced GIST, the majority of patients will eventually experience disease progression [11, 12]. For patients treated with first-line imatinib for advanced or metastatic GIST, the median time to progression (TTP) is approximately 24 months [1, 9, 13]. Studies in both the metastatic and adjuvant settings support the importance of maintaining continuous suppression of KIT/PDGFR kinase activity in order to delay disease progression and achieve optimal clinical outcomes. The optimal duration of imatinib therapy is unknown, yet recent reports support long-term, continuous administration of imatinib therapy in patients with advanced or metastatic GIST, and at least 3 years of imatinib is recommended by the U.S.-based National Comprehensive Cancer Network (NCCN) for patients with surgically resected tumors at high risk of recurrence [14]. This review summarizes the data supporting continuous kinase suppression in adjuvant and metastatic settings and explores other key issues including whether patients respond to imatinib reintroduction after interruption of various treatment durations, the effect of treatment interruption on secondary resistance to imatinib, and prognostic factors associated with sustained response to imatinib treatment.
The optimal duration of imatinib therapy is unknown, yet recent reports support long-term, continuous administration of imatinib therapy in patients with advanced or metastatic GIST, and at least 3 years of imatinib is recommended by the U.S.-based NCCN for patients with surgically resected tumors at high risk of recurrence.
Why Is Continuous Kinase Suppression Necessary for Treatment of GIST?
Studies have demonstrated that interruption of imatinib therapy in advanced GIST can lead to rapid disease progression, whereas long-term continuous imatinib treatment is associated with reduced risk of GIST progression (Table 1).
Table 1.
Randomized clinical studies of imatinib in advanced or metastatic GIST—beyond 1 year of treatment
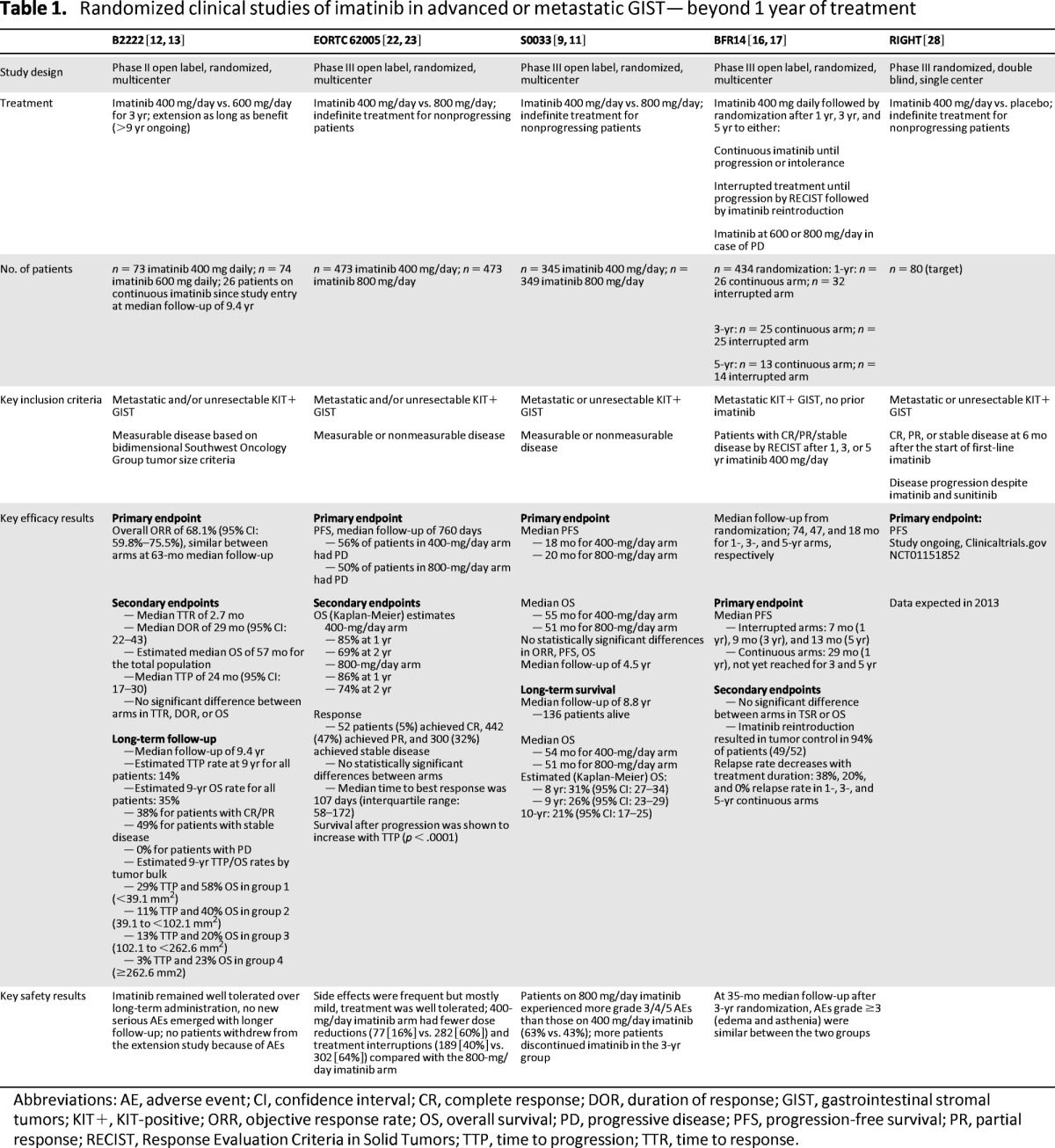
Abbreviations: AE, adverse event; CI, confidence interval; CR, complete response; DOR, duration of response; GIST, gastrointestinal stromal tumors; KIT+, KIT-positive; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; TTP, time to progression; TTR, time to response.
The BFR14 randomized phase III study conducted by the French Sarcoma Group assessed the impact of interrupting therapy after 1 year, 3 years, or 5 years of daily treatment with 400 mg of imatinib in patients with advanced GIST [15–18]. Interruption of imatinib treatment after 1 year resulted in increased disease progression in 26 of 32 patients (81%) compared with 8 of 26 patients (31%) who continued treatment after 1 year (p ≤ .0001) [15]. Similarly, progression-free survival (PFS) was significantly increased in patients who continued imatinib after 3 years; the 2-year PFS was 80% for patients in the continuation group versus 16% (p < .0001) in the interruption group (Fig. 1). Notably, the increased risk of relapse associated with imatinib interruption after 1 year or 3 years of treatment was observed even in patients who achieved complete response (CR) before randomization [15, 16]. Treatment interruption after 5 years of imatinib also resulted in rapid disease progression in the majority of patients: 45% of patients experienced disease relapse, whereas no disease progression was observed in patients randomized to the continuation arm (p = .035) [18].
Figure 1.
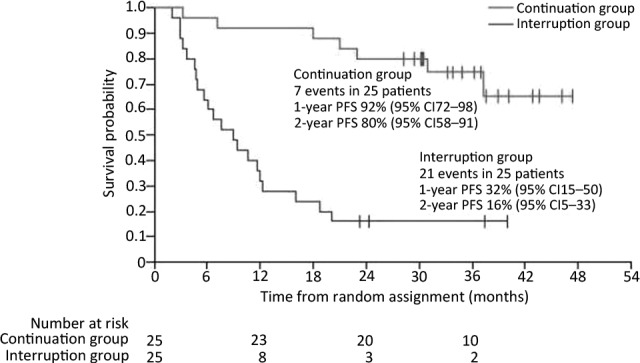
PFS in patients (n = 50) with metastatic or advanced gastrointestinal stromal tumor randomized to interrupt or continue imatinib therapy after 3 years of imatinib, with median follow-up of 35 months (95% CI: 33–38) after randomization [16]. Reproduced with permission from Elsevier [16].
Abbreviations: CI, confidence interval; PFS, progression-free survival.
Updated results reported at the American Society of Clinical Oncology 2011 annual meeting included the long-term outcome of patients randomized after 1 year (n = 58), 3 years (n = 50), or 5 years (n = 27) of imatinib therapy after median follow-up times of 74 months, 47 months, and 18 months, respectively [17]. The median PFS after 1 year, 3 years, or 5 years was only 7 months, 9 months, or 13 months after randomization to interruption, respectively, whereas median PFS was 29 months (1-year randomization) or was not reached (3- or 5-year randomization) in the continuation group [17]. These results clearly demonstrate that interruption of imatinib therapy in nonprogressing patients significantly increases the risk of progression. This held even for patients with a favorable tumor status at randomization (CR or major partial response [PR]). Consequently, interruption of imatinib in responding patients is not recommended unless they experience significant toxicities [16].
The BFR14 trial also determined whether long-term continuous imatinib therapy could have an impact on the incidence of secondary resistance. Time to secondary resistance (TSR; defined as TTP under imatinib treatment) was found to increase with duration of continuous treatment; the 2-year PFS following randomization (in the continuous treatment arm) increased from 62% (randomization at 1 year) to 80% (randomization at 3 years) [17]. Consequently, the rate of patients with relapse at 2 years after randomization decreased from 38% (after 1 year of imatinib) to 20% after 3 years of continuous treatment and to 0% after 5 years of continuous treatment [17]. Although these results indicate a clear selection for patients who are responsive to imatinib at later time points, they also demonstrate that the rate of secondary resistance decreases over time, suggesting the possibility of long-term tumor control with continuous imatinib in a significant subset of patients with advanced GIST.
Decreased risk of relapse over time with continuous imatinib therapy also was seen in patients with advanced GIST in the phase II B2222 trial (Table 1) [13]. The latest long-term results were reported at the American Society of Clinical Oncology 2011 annual meeting [12]: For all patients (N = 147), the estimated 9-year overall survival (OS) rate was 35% and the TTP rate was 14%, which was similar for patients with CR, PR (both 16%), or stable disease (17%) as best overall response. Similar to the BFR14 results, the rate of tumor progression in patients continuously treated with imatinib decreased considerably with time. Consequently, there was also a decreasing risk of death from GIST over time [12].
Similar observations were made in an earlier phase III study by the European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group, the Italian Sarcoma Group, and the Australasian Gastrointestinal Trials Group (Table 1) [19]. In this study, known as EORTC 62005, 56% of patients receiving 400 mg per day of imatinib (n = 473) and 50% of patients receiving 800 mg per day (n = 473) experienced progression at a median follow-up of 760 days [19]. Although the median follow-up was short, survival after first progression was found to increase with TTP [20]. Long-term continuous dosing also is supported by the long-term results of the randomized phase III S0033 intergroup trial [11]. Among 695 eligible patients with advanced GIST, the median survival was 54 months for patients receiving 400 mg per day and 51 months for patients receiving 800 mg per day, with a median follow-up of 8.8 years for all survivors; 136 (19.6%) patients were known to be alive for at least 8 years. OS at 8, 9, and 10 years was 31%, 26%, and 21%, respectively, and no new long-term toxicities emerged in this analysis [11].
Long-term continuous imatinib therapy appears to provide optimal tumor control compared with therapy interruption, as suggested by results from the BFR14 study. Tumor volume of residual lesions at the time of imatinib interruption was compared with tumor volume at the time of best response after imatinib reintroduction in 51 patients with progressive disease (PD) randomized to the interruption arms. Only 7 patients (41.2%) with CR observed at the time of randomization achieved a new CR as best response when imatinib was restarted, and only 14 patients (56%) with PR achieved a new PR after imatinib reintroduction [21]. These results provide strong evidence supporting long-term continuous imatinib treatment for patients with unresectable and/or metastatic KIT-positive GIST.
Continuous TKI Therapy for Advanced GIST Progressing on Imatinib
For patients with advanced GIST progressing on or with intolerance of first-line imatinib, second- and third-line treatment options are available to maintain receptor tyrosine kinase inhibition [22–25]. Sunitinib is an oral multitargeted TKI with activity toward KIT, PDGFR, VEGFR, and several other receptor tyrosine kinases [22]. Results from a phase III study demonstrated the efficacy of sunitinib as second-line therapy for advanced GIST [22]. TTP was significantly increased in sunitinib-treated patients (n = 207) compared with placebo-treated patients (n = 105) (median TTP: 27.3 vs. 6.4 weeks; hazard ratio [HR]: 0.33; 95% confidence interval [CI]: 0.23–0.47; p < .0001). In this study, sunitinib was dosed at 50 mg per day with 4 weeks on treatment followed by 2 weeks off (schedule 4/2). Sunitinib administration at a lower dose (37.5 mg per day) on a continuous daily dosing schedule was subsequently evaluated in a phase II study of patients with imatinib-resistant or -intolerant GIST (n = 60); 53% achieved a clinical benefit rate (CBR; CR plus PR plus stable disease) demonstrative of meaningful tumor control. Continuous dosing also was associated with acceptable safety [23]. The NCCN guidelines stipulate the use of sunitinib as the second-line therapy for advanced GIST at 50 mg per day on a schedule 4/2 basis or a continuous daily dose of 37.5 mg per day, at the physician's discretion [14].
Should patients fail on both imatinib and sunitinib, regorafenib, a third-line treatment option for advanced GIST, has recently been approved by the U.S. Food and Drug Administration (in February 2013). Similar to sunitinib, regorafenib is an oral multitargeted inhibitor that blocks the activity of several kinases including KIT, PDGFR, and VEGFR [24]. Regorafenib was investigated in clinical studies of patients with advanced GIST that had progressed on imatinib and sunitinib [24, 25]. In a phase III study, regorafenib significantly improved PFS when added to best supportive care, compared with placebo plus best supportive care (HR: 0.27; 95% CI 0.19–0.39; p < .0001); mean PFS was 4.8 months in regorafenib-treated patients, which was five times longer than those treated with placebo (0.9 month) [25].
For patients who have exhausted all available therapies, the NCCN guidelines recommend continuing TKI therapy as an essential component of best supportive care because discontinuing therapy may lead to accelerated tumor growth by disrupting control of any sensitive GIST clones [26]. Maintaining treatment with a TKI, even in the case of PD, may slow progression; therefore, reintroduction or continuation of treatment with a TKI to which the patient has already been exposed may be an option in individual cases [27]. A clinical trial exploring this hypothesis (Rechallenge of Imatinib in GIST Having No Effective Treatment, or RIGHT) is currently being conducted in Korea [28].
Maintaining treatment with a TKI, even in the case of PD, may slow progression; therefore, reintroduction or continuation of treatment with a TKI to which the patient has already been exposed may be an option in individual cases.
Prospects of Long-Term Continuous TKI Therapy in the Adjuvant Setting
Recent studies in resected primary GIST suggest that prolonged use of adjuvant imatinib is required to reduce the risk of recurrence (Table 2). The phase III American College of Surgeons Oncology Group's ACOSOG Z9001 study showed that 1-year adjuvant imatinib treatment was significantly more effective than placebo in reducing recurrence in patients with completely resected primary GIST (1-year recurrence-free survival [RFS]: 98% with adjuvant imatinib vs. 83% with placebo; HR: 0.35; p < .0001) [29]; however, the shape of the RFS curves in the placebo and imatinib arms (at least in high-risk GIST) suggests that 1-year adjuvant treatment may only postpone, rather than prevent, relapse. These observations required the approved drug label to stipulate that, in the adjuvant setting, the optimal treatment duration is unknown, despite the 1-year results of ACOSOG Z9001.
Table 2.
Randomized clinical studies of adjuvant imatinib in GIST—1 year of treatment and beyond
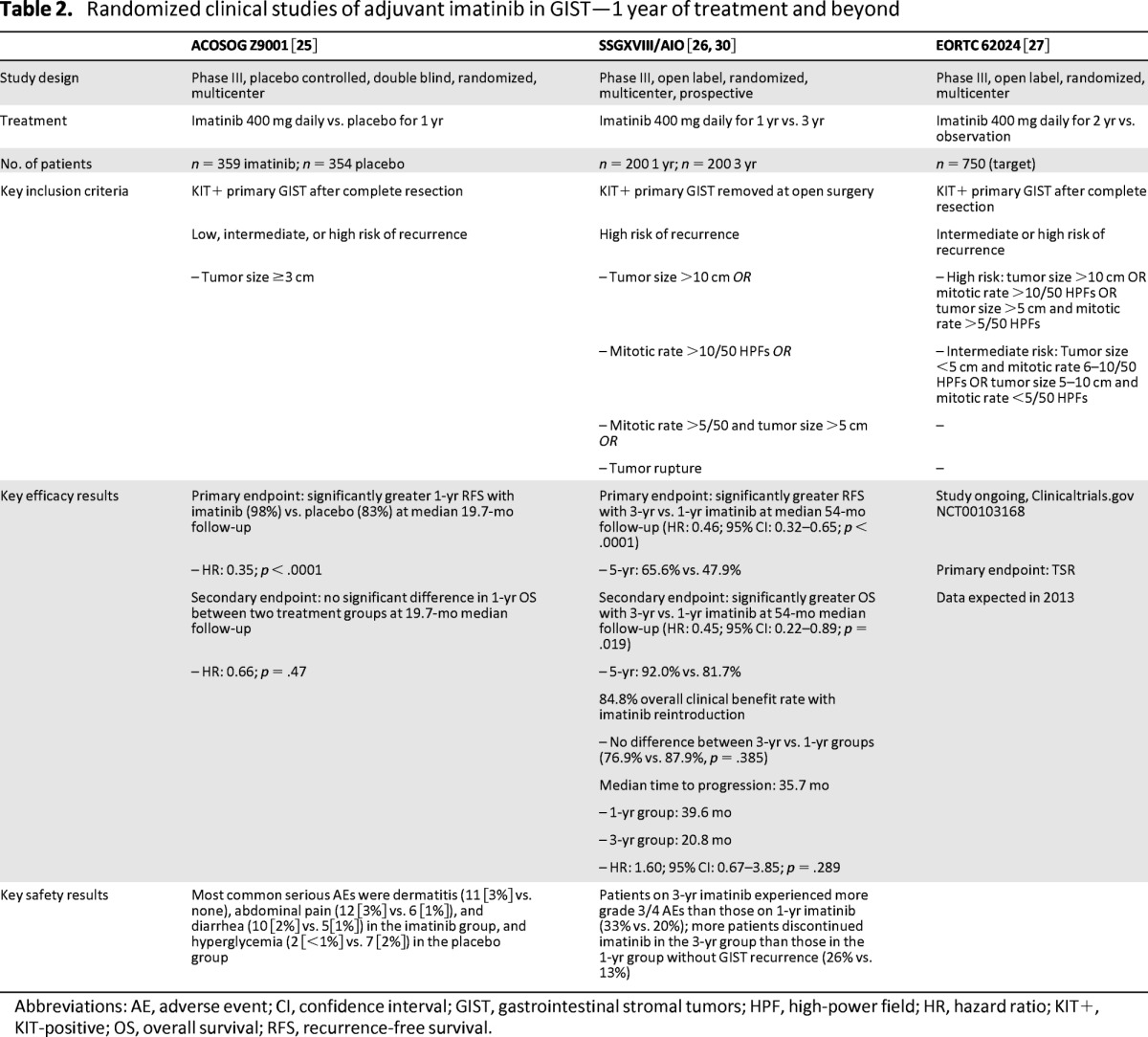
Abbreviations: AE, adverse event; CI, confidence interval; GIST, gastrointestinal stromal tumors; HPF, high-power field; HR, hazard ratio; KIT+, KIT-positive; OS, overall survival; RFS, recurrence-free survival.
The phase III study by the Scandinavian Sarcoma Group and the Sarcoma Group of the Arbeitsgemeinschaft Internistische Onkologie (the SSGXVIII/AIO study) [30] reported a reduction in the risk of recurrence and a significant improvement in OS in patients receiving 3 years of adjuvant imatinib compared with 1 year of therapy. At a median follow-up of 54 months, RFS was significantly longer for patients who received 3 years of adjuvant imatinib (n = 198) than for those who received 1-year of adjuvant imatinib (n = 199; HR: 0.46; p < .0001; 5-year RFS, 65.6% vs. 47.9%, respectively; Table 2) [30]. Patients receiving 3-year adjuvant imatinib also had significantly longer OS than those receiving 1-year adjuvant imatinib (HR: 0.45; p = .019; 5-year OS: 92.0% vs. 81.7%, respectively) [30].
Based on these results, the NCCN guidelines recommend treatment with adjuvant imatinib for at least 36 months for patients with high-risk GIST [14]. It should be noted that in both arms of the SSGXVIII/AIO trial there was a marked increase in the number of patients with recurrent disease following the discontinuation of imatinib therapy, suggesting that an even longer duration of adjuvant therapy might yield additional clinical benefit. As such, the optimal duration of adjuvant imatinib treatment has not yet been determined. Given the observations from advanced GIST trials, more prolonged treatment with adjuvant imatinib may benefit some patients at high risk of recurrence.
The goal of additional ongoing prospective randomized and nonrandomized clinical trials is to determine the clinical benefit and safety of prolonged adjuvant imatinib therapy. The phase III EORTC 62024 trial will assess no adjuvant imatinib therapy versus 2 years of adjuvant imatinib therapy (400 mg per day) in patients with either intermediate or high risk of recurrence with completely resected, localized GIST [31]. The primary endpoint for this trial is TSR, and results are anticipated during 2013. The phase II trial Post-resection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors, or PERSIST-5, with adjuvant imatinib is investigating 5 years of adjuvant imatinib therapy (400 mg per day) in patients with completely resected GIST (R0) with significant risk of recurrence, with a primary endpoint of RFS [32].
An ongoing Chinese phase II study is assessing the efficacy and safety of 5 years of adjuvant imatinib therapy in high-risk (based on modified National Institutes of Health criteria) GIST patients after resection (R0) [33]. Interim results of patients whose treatment was prematurely interrupted for toxicity reasons or patient refusal had a significantly higher tumor recurrence rate compared with patients on continuous imatinib (45.5% vs. 4.2%, respectively; p = .002). Further 5-year and longer RFS data for patients enrolled in this study are expected [33]. Results from this study need to be interpreted with caution because it is not a randomized trial and may reflect an unpredictable selection bias, and the sample size is relatively small.
These studies will contribute to a better understanding of the benefits of longer-term continuous adjuvant imatinib in patients with GIST, but there is no randomized study currently addressing treatment duration with adjuvant imatinib for longer than 3 years. Consequently, the potential benefits and harms of such treatments will likely remain controversial. An increasingly critical issue is the selection of appropriate patients for long-term imatinib exposure. Ideally, biomarkers predicting outcome beyond currently available risk assessment should be established. Moreover, additional clarity is needed to understand the benefit of reintroducing imatinib in patients who have experienced recurrence or progression.
If Imatinib Therapy Is Interrupted, Can Tumor Control Be Regained by Reintroducing Imatinib?
In the advanced or metastatic setting, the BFR14 trial assessed the efficacy of imatinib reintroduction at 400 mg per day among patients randomized to the interruption arms who progressed after therapy interruption [15–18]. Of patients who progressed after discontinuation of 1-year imatinib (n = 26), 92% achieved tumor control (CR, PR, or stable disease) when imatinib was reintroduced [15]. The majority of patients with PD after treatment interruption at 3 years and 5 years also experienced tumor control after imatinib reintroduction (100% [20 of 20] and 86% [6 of 7], respectively) [16, 17]. Overall, imatinib reintroduction led to tumor control in 94% of patients (49 of 52) [17]. Despite regaining tumor control, as discussed above, tumors did not achieve the same level of response when compared with the best response before interruption [24]. These results also emphasize the importance of maintaining continuous imatinib therapy in nonprogressing patients.
Comparatively, in the adjuvant setting, a subgroup analysis of 46 evaluable patients from the SSGXVIII/AIO study revealed an overall CBR of 84.8% from imatinib reintroduction for treating recurring GIST after prior adjuvant imatinib. No significant difference was observed in the CBR between patients assigned to adjuvant imatinib for 1 year versus 3 years (87.9% vs. 76.9%, respectively; p = 0.385) [34]. These results provide substantial evidence that imatinib reintroduction results in a high response rate in patients who have completed prior adjuvant treatment with imatinib [34]. Further analysis is needed to confirm the level of tumor response after imatinib reintroduction.
An issue of concern regarding TKI reintroduction after interruption is whether prior imatinib exposure affects the TSR. In the updated analysis of BFR14, no difference for TSR and OS was observed between the continuation and interruption arms [17]; however, this study was not powered to demonstrate any difference in OS, and the absence of difference must be interpreted with caution. Interestingly, the TTP after imatinib interruption correlated with the TSR in another subgroup analysis of the BFR14 trial: Patients who relapsed rapidly after stopping imatinib were more likely to show resistance to imatinib on reintroduction than those who did not relapse as quickly after interruption [35]. A second PD was observed in 63% of patients who relapsed in the first 6 months after imatinib interruption, compared with 35% and 30% of patients experiencing PD between 6 and 12 months and after 12 months, respectively [35].
In the aforementioned SSGXVIII/AIO subanalysis assessing imatinib reintroduction after prior adjuvant therapy, the median TTP after starting imatinib for relapsed GIST was 35.7 months. This result is similar to that observed in imatinib-naïve patients, suggesting that prior adjuvant imatinib exposure might not have an impact on TSR [34]; however, the small sample size and short follow-up times at the time of the analysis preclude any firm conclusions. The final results of the prospective randomized EORTC phase III trial specifically addressing this critical issue are expected to clarify this question and are eagerly awaited.
Prognostic Factors Associated With Long-Term Response
To determine what prognostic and predictive indicators identify those patients with advanced GIST who are most likely to respond with sustained disease control on long-term imatinib, a subanalysis of BFR14 evaluated 240 patients treated with imatinib (400 mg per day) for at least 5 years and compared characteristics data between patients who did (n = 173) or did not (n = 67) experience PD while in the study [36]. The analysis identified the following characteristics of long-term responders: female gender (p = .013), good performance status (p = .003), long delay between diagnosis and imatinib treatment (p = .018), low tumor volume at inclusion (p = .008), normal hemoglobin level at inclusion (p = .039), and normal lymphocyte count at inclusion (p = .016) [36]. Mutational analysis was available for 40 patients with prolonged response [36]: KIT exon 11 mutation was detected in 35 patients (87.5%), KIT exon 9 mutations were detected in 3 patients (7.5%), and no mutations (wild type [WT]) were detected in 2 patients (5.0%). Among GIST patients harboring an alteration of KIT exon 11, those involving codons 557–560 and 574–579 corresponded to a group of patients with prolonged PFS and a high sensitivity to imatinib. Notably, these results indicated that patients with advanced GIST harboring a KIT exon 9 mutation or WT GIST could achieve prolonged tumor control with imatinib [36].
Similar observations were recently reported in the B2222 study. Patients with a KIT exon 11 mutation (n = 16), a KIT exon 9 mutation (n = 3), a PDGFRA mutation (n = 1), or WT GIST (n = 1) were among the 26 patients who remained on continuous imatinib therapy [12]. Moreover, both 9-year PFS (p = .0051) and OS (p = .0043) significantly correlated with tumor bulk, indicating that lower tumor bulk may predict longer TTP and improved OS [12].
Pharmacokinetics and maintenance of effective imatinib plasma levels play a significant role in determining clinical outcome. In a recent retrospective analysis from the B2222 study, patients in the lowest quartile of imatinib trough levels (<1,100 ng/mL) demonstrated a shorter TTP (11.3 months) than patients in all other quartiles (>1,100 ng/mL; TTP: 30 months; p = .0029) [37]. More recent prospective evaluation of imatinib trough levels and PFS in patients with advanced GIST suggest that an even lower imatinib trough level threshold of 780 ng/mL may be sufficient to achieve extended PFS [38]. Notably, patients who have undergone major gastrectomy frequently have low imatinib trough plasma levels [39]. Consequently, factors that compromise imatinib dosing may cause deterioration of treatment outcomes [40].
Further analyses are in progress with the goal of determining whether any clinical, pathological, or molecular features might serve as markers to identify patients who are more likely to have sustained disease control on imatinib therapy.
Safety and Tolerability Considerations During Long-Term Continuous Imatinib Therapy
Consideration must be given to the management of imatinib-associated adverse events (AEs) with respect to the recommended long-term continuous daily administration of imatinib in advanced GIST and to usually 3 years of adjuvant imatinib for patients at a high risk of recurrence [14]. Imatinib is generally well tolerated, with most AEs being manageable and often transient or self-limiting [41]. The feasibility of long-term administration is demonstrated by the number of patients who remained in the BFR14, B2222, and S0033 studies after long-term follow-up [12, 17]. In the EORTC 62005 trial, most side effects occurred within the first 8 weeks of treatment [22]. In the adjuvant setting, longer-term (3-year) imatinib was also generally well tolerated, as most recently determined in the SSGXVIII/AIO trial [30]. In this study, the proportion of patients who discontinued adjuvant imatinib for reasons other than GIST recurrence was 25.8% for patients in the 3-year group and 12.6% for patients in the 1-year group; this was expected, given the longer duration of treatment and observation [30]. In addition, imatinib at 600 mg per day for 2 years was well tolerated in a phase II study of neoadjuvant and adjuvant imatinib in patients with primary or metastatic and recurrent resectable GIST (Radiation Therapy Oncology Group 0132) [42, 43].
Because of the significant increase in risk of progression following imatinib interruption, and the evidence showing that reintroduction of imatinib generally does not achieve the same level of tumor response when compared with response before interruption, it is important that imatinib-associated AEs be recognized early and managed appropriately in order to avoid significant dose interruptions and to optimize efficacy.
AEs may prompt a patient to request imatinib dose reduction or treatment interruption or may affect patient adherence to self-administered imatinib therapy, which can be a particular issue, given the chronic nature of imatinib therapy [37]. Because of the significant increase in risk of progression following imatinib interruption and the evidence showing that reintroduction of imatinib generally does not achieve the same level of tumor response when compared with response before interruption, it is important that imatinib-associated AEs be recognized early and managed appropriately in order to avoid significant dose interruptions and to optimize efficacy.
Conclusion
Continuous kinase suppression in GIST with imatinib therapy is supported by several key studies in both the metastatic and adjuvant settings. Strategies with the goal of optimizing treatment in advanced or metastatic GIST based on the optimal duration of imatinib therapy may parallel treatment strategies in the adjuvant setting. Interruption of imatinib therapy in patients responding to treatment in the metastatic setting leads to higher rates of disease progression, and longer treatment is associated with reduced rate of progression. Patients with nonprogressing advanced or metastatic GIST should be given continuous imatinib therapy, as tolerated, in order to achieve optimal clinical benefit. Sustained tumor control on continuous imatinib therapy can be achieved in a significant proportion of patients surviving past 9–10 years. For patients progressing on or intolerant of imatinib, continuing therapy with TKIs sunitinib followed by regorafenib is recommended to maintain kinase suppression. In the adjuvant setting, 3 years of imatinib is associated with overall better clinical outcome compared with 1 year in patients with high-risk GIST and is now considered to be the standard of care for patients at high risk of recurrence. Because there are currently no ongoing randomized clinical trials that address adjuvant imatinib treatment duration longer than 3 years, this will remain the standard duration of treatment in the near future for most patients who have undergone surgery for high-risk GIST.
Acknowledgments
This work was supported by Novartis Pharmaceuticals. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Dina Marenstein, Ph.D., for medical editorial assistance with this manuscript.
Author Contributions
Conception/Design: Axel Le Cesne
Manuscript writing: Axel Le Cesne, Jean-Yves Blay, Peter Reichardt, Heikki Joensuu
Final approval of manuscript: Axel Le Cesne, Jean-Yves Blay, Peter Reichardt, Heikki Joensuu
Disclosures
Axel Le Cesne: Pfizer, PharmaMAR, Novartis (C/A). Jean-Yves Blay: Novartis, GlaxoSmithKline, Roche, PharmaMAR, MSD (C/A); Novartis, GlaxoSmithKline, Roche, PharmaMAR MSD (RF). Peter Reichardt: Novartis, Pfizer, Bayer (C/A); Novartis, Pfizer, Bayer (H); Novartis (RF). Heikki Joensuu: Novartis (C/A); Novartis (H).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Cassier PA, Blay JY. Imatinib mesylate for the treatment of gastrointestinal stromal tumor. Expert Rev Anticancer Ther. 2010;10:623–634. doi: 10.1586/era.10.33. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 3.Sommer G, Agosti V, Ehlers I, et al. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the Kit receptor tyrosine kinase. Proc Natl Acad Sci U S A. 2003;100:6706–6711. doi: 10.1073/pnas.1037763100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitayama H, Kanakura Y, Furitsu T, et al. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood. 1995;85:790–798. [PubMed] [Google Scholar]
- 5.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 6.Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008;3:557–586. doi: 10.1146/annurev.pathmechdis.3.121806.151538. [DOI] [PubMed] [Google Scholar]
- 7.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg BL, Trent JC. Adjuvant and neoadjuvant imatinib therapy: Current role in the management of gastrointestinal stromal tumors. Int J Cancer. 2011;129:2533–2542. doi: 10.1002/ijc.26234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MH, Cortazar P, Justice R, et al. Approval summary: Imatinib mesylate in the adjuvant treatment of malignant gastrointestinal stromal tumors. The Oncologist. 2010;15:300–307. doi: 10.1634/theoncologist.2009-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanke CD, Rankin C, Benjamin R, et al. Long-term survival on S0033—a phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumours (GISTs). Paper presented at: 2011 European Multidisciplinary Cancer Congress; September 23–27, 2011; Stockholm, Sweden. [Google Scholar]
- 12.von Mehren M, Heinrich MC, Joensuu H, et al. Follow-up results after 9 years (yrs) of the ongoing, phase II B2222 trial of imatinib mesylate (IM) in patients (pts) with metastatic or unresectable KIT+ gastrointestinal tumors (GIST) J Clin Oncol. 2011;29:10016a. [Google Scholar]
- 13.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Center. NCCN clinical practice guidelines in oncology. Soft tissue sarcoma. [Accessed August 18, 2012]. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 15.Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107–1113. doi: 10.1200/JCO.2006.09.0183. [DOI] [PubMed] [Google Scholar]
- 16.Le Cesne A, Ray-Coquard I, Bui BN, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: An open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010;11:942–949. doi: 10.1016/S1470-2045(10)70222-9. [DOI] [PubMed] [Google Scholar]
- 17.Le Cesne A, Ray-Coquard IL, Bui Nguyen B, et al. Time to secondary resistance (TSR) after interruption of imatinib (IM) in advanced GIST: Updated results of the prospective French Sarcoma Group randomized phase III trial on long-term survival. J Clin Oncol. 2011;29:10015a. [Google Scholar]
- 18.Ray-Coquard IL, Bin Bui N, Adenis A, et al. Risk of relapse with imatinib (IM) discontinuation at 5 years in advanced GIST patients: Results of the prospective BFR14 randomized phase III study comparing interruption versus continuation of IM at 5 years of treatment: A French Sarcoma Group Study. J Clin Oncol. 2010;28:10032a. [Google Scholar]
- 19.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 20.Van Glabbeke MM, Verweij J, Casali P, et al. Type of progression in patients treated with imatinib for advanced gastrointestinal stromal tumor (GIST): A study based on the EORTC-ISG-AGITG trial 62005. J Clin Oncol. 2009;27:10536a. doi: 10.1200/JCO.2008.21.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patrikidou A, Chabaud S, Ray-Coquard I, et al. Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: Results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann Oncol. 2013;24:1087–1093. doi: 10.1093/annonc/mds587. [DOI] [PubMed] [Google Scholar]
- 22.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomized controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 23.George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959–1968. doi: 10.1016/j.ejca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 24.George S, Wang Q, Heinreich MC, et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: A multicenter phase II trial. J Clin Oncol. 2012;30:2401–2407. doi: 10.1200/JCO.2011.39.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demetri GD, Reichardt P, Kang Y, et al. Efficacy and safety of regorafenib for advanced gastrointestinal tumours after failure of imatinib and sunitinib (GRID): An international, multicenter, randomized, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(suppl 2):S1–S41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casali PG, Blay JY. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v98–v102. doi: 10.1093/annonc/mdq208. [DOI] [PubMed] [Google Scholar]
- 28.Rechallenge of imatinib in GIST having no effective treatment [identifier NCT01151852] [Accessed January 30, 2012]. Available at http://clinicaltrials.gov/ct2/show/NCT01151852.
- 29.DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joensuu H, Eriksson M, Hatrmann J, et al. Twelve versus 36 months of adjuvant imatinib (IM) as treatment of operable GIST with a high risk of recurrence: Final results of a randomized trial (SSGXVIII/AIO) J Clin Oncol. 2011;29:LBA1a. [Google Scholar]
- 31.Imatinib mesylate or observation only in treating patients who have undergone surgery for local gastrointestinal stromal tumor [identifier NCT00103168] [Accessed February 3, 2012]. Available at http://clinicaltrials.gov/ct2/show/NCT00103168.
- 32.Five year adjuvant imatinib mesylate (Gleevec®) in gastrointestinal stromal tumor (GIST) [identifier NCT00867113] [Accessed February 3, 2012]. Available at http://clinicaltrials.gov/ct2/show/NCT00867113.
- 33.Jiang WZ, Guan GX, Lu HS, et al. Adjuvant imatinib treatment after R0 resection for patients with high-risk gastrointestinal stromal tumors: A median follow-up of 44 months. J Surg Oncol. 2011;104:760–764. doi: 10.1002/jso.22010. [DOI] [PubMed] [Google Scholar]
- 34.Reichardt P, Hartmann J, Sundby Hall K, et al. Response to imatinib rechallenge of GIST that recurs following completion of adjuvant imatinib treatment—the first analysis in the SSGXVIII/AIO trial patient population. Paper presented at: 2011 European Multidisciplinary Cancer Congress; September 23–27, 2011; Stockholm, Sweden. [Google Scholar]
- 35.Le Cesne A, Ray-Coquard I, Bin Bui N, et al. Time to onset of progression after imatinib interruption and outcome of patients with advanced GIST: Results of the BFR14 prospective French Sarcoma Group randomized phase III trial. J Clin Oncol. 2010;28:10033a. doi: 10.1093/annonc/mds587. [DOI] [PubMed] [Google Scholar]
- 36.Blesius A, Cassier PA, Ray-Coquard IL, et al. Who are the long responders to imatinib (IM) in patients with advanced GIST? Results of the BFR14 prospective French Sarcoma Group randomized phase III trial. J Clin Oncol. 2011;29:10048a. [Google Scholar]
- 37.Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141–3147. doi: 10.1200/JCO.2008.20.4818. [DOI] [PubMed] [Google Scholar]
- 38.Molimard M, Bouchet S, Poulette S, et al. Definition of an imatinib trough concentration threshold in the treatment of advanced gastrointestinal stromal tumors (GIST) J Clin Oncol. 2011;29:10013a. [Google Scholar]
- 39.Yoo C, Ryu MH, Kang BW, et al. Cross-sectional study of imatinib plasma trough levels in patients with advanced gastrointestinal stromal tumors: Impact of gastrointestinal resection on exposure to imatinib. J Clin Oncol. 2010;28:1554–1559. doi: 10.1200/JCO.2009.26.5785. [DOI] [PubMed] [Google Scholar]
- 40.Mazzeo F, Duck L, Joosens E, et al. Nonadherence to imatinib treatment in patients with gastrointestinal stromal tumors: The ADAGIO study. Anticancer Res. 2011;31:1407–1409. [PubMed] [Google Scholar]
- 41.Joensuu H, Trent JC, Reichardt P. Practical management of tyrosine kinase inhibitor-associated side effects in GIST. Cancer Treat Rev. 2011;37:75–88. doi: 10.1016/j.ctrv.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic recurrent operable gastrointestinal stromal tumor (GIST): Long-term follow-up results of RTOG 0132. J Clin Oncol. 2011;29:10057a. [Google Scholar]
- 43.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): Early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42–47. doi: 10.1002/jso.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]


