Published data suggest that epidermal growth factor receptor (EGFR) addiction persists after development of acquired resistance to an EGFR tyrosine kinase inhibitor (TKI), leading many clinicians to continue the TKI with subsequent chemotherapy; however, this strategy has not been formally evaluated. We retrospectively reviewed an institutional database and identified 78 patients with advanced EGFR-mutation with acquired resistance who subsequently received chemotherapy; 34 were treated with chemotherapy and erlotinib and 44 were treated with chemotherapy alone. This study demonstrates that continuation of EGFR TKI with chemotherapy in patients with acquired resistance improves outcomes compared with switching to chemotherapy alone.
Keywords: Non-small cell lung cancer, Epidermal growth factor receptor, Protein kinase inhibitor, Drug resistance
Abstract
Purpose.
Epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer has an oncogene-addicted biology that confers sensitivity to EGFR tyrosine kinase inhibitors (TKIs). Published data suggest that EGFR addiction persists after development of TKI acquired resistance, leading many clinicians to continue TKI with subsequent chemotherapy; however, this strategy has not been formally evaluated.
Methods.
We retrospectively reviewed an institutional database to identify patients with advanced EGFR mutation with acquired resistance who subsequently received chemotherapy. Patients were classified as receiving chemotherapy with continued erlotinib or chemotherapy alone. We assessed differences in outcomes between the two strategies.
Results.
Seventy-eight patients were included, 34 treated with chemotherapy and erlotinib and 44 treated with chemotherapy alone. Objective response rate was evaluable in 57 patients and was 41% for those treated with chemotherapy and erlotinib and 18% for those treated with chemotherapy alone. After adjusting for chemotherapy regimen and length of initial TKI course, the odds ratio for the response rate was 0.20 (95% confidence interval: 0.05–0.78; p = .02) favoring treatment with chemotherapy and erlotinib. The median progression-free survival was 4.4 months on chemotherapy and erlotinib and 4.2 months on chemotherapy alone (adjusted hazard ratio = 0.79; 95% confidence interval: 0.48–1.29; p = .34). There was no difference in overall survival.
Conclusion.
This is the first study, to our knowledge, to demonstrate that continuation of EGFR TKI with chemotherapy in patients with acquired resistance improves outcomes compared with chemotherapy alone. We observed an improved response rate but no difference in progression-free survival or overall survival. A larger prospective clinical trial is needed to evaluate this promising strategy further.
Implications for Practice:
Patients with advanced EGFR-mutant non-small cell lung cancer (NSCLC) typically respond well to treatment with an EGFR tyrosine kinase inhibitor (TKI); however, acquired resistance eventually develops. It is unknown whether the continuation of the EGFR TKI along with initiation of chemotherapy results in improved patient outcomes compared to switching to chemotherapy alone. This article describes a retrospective study that compares these two practices, providing clinicians with information regarding the potential benefit to treating patients with chemotherapy plus erlotinib rather than chemotherapy alone after the development of TKI resistance. We found that continuing erlotinib with chemotherapy improves the response rate and is therefore a reasonable treatment option for patients with EGFR-mutant lung cancer acquired resistance to EGFR TKIs.
Introduction
A subgroup of patients with non-small cell lung cancer (NSCLC) has mutations in the epidermal growth factor receptor (EGFR) gene. These patients are extremely sensitive to treatment with EGFR-specific tyrosine kinase inhibitors (TKIs). The unprecedented therapeutic success of EGFR TKIs in EGFR-mutant lung cancer is the result of the oncogene-addicted biology conferred by the mutations, such that the downstream signaling pathways that promote cell growth and survival are unusually dependent on EGFR. Hence, blocking EGFR-derived signaling with a TKI leads to massive cell death. However, TKI resistance develops after a median of 10–14 months [1–4], represented clinically as tumor progression and symptomatic decline. The optimal treatment of EGFR TKI-acquired resistance (AR) is a subject currently under intense study [5].
Preclinical data suggest that a heterogeneous population of tumor cells exists at the time of AR, a subset of which remains sensitive to EGFR inhibition [6]. This has also been described clinically. When the TKI is withdrawn from patients with AR, tumor growth may accelerate and then plateau when a TKI is restarted [7, 8]. One report found that severe flare (defined as hospitalization or death) developed in 23% of patients with AR who discontinued TKI prior to starting a clinical trial after a median of 8 days [7]. Although conventional clinical practice at the time of lung cancer progression is to discontinue the current therapy and initiate a new chemotherapy, there are some settings in oncology where a targeted therapy is continued after progression. In prostate cancer, biochemical castration with gonadotropin-releasing hormone agonists is continued when a patient begins chemotherapy. A similar strategy has been evaluated in HER-2-positive breast cancer, where continuation of trastuzumab beyond disease progression in addition to chemotherapy has been shown to improve response rate, time to progression, and (in some studies) overall survival [9, 10].
We and others have recently begun to consider continuing postprogression EGFR TKI therapy during subsequent chemotherapy in our patients with EGFR-mutant NSCLC [11, 12]. Because this strategy is commonly practiced but has not been prospectively evaluated, we designed a retrospective study of patients with EGFR TKI AR to assess the benefit of continuing the EGFR TKI along with chemotherapy compared with switching therapy from a TKI to chemotherapy alone.
Materials and Methods
Patients
We reviewed internal databases from Massachusetts General Hospital and Dana-Farber Cancer Institute from August 2004 (when clinical mutation testing was initiated at our centers) through September 2011 under an institutional review board-approved protocol. Medical records of patients with stage IV NSCLC [13] harboring an EGFR mutation were reviewed to identify all those who had ever received an EGFR TKI (erlotinib, gefitinib, or experimental drug) and had received chemotherapy. Patients were included in this analysis only if AR developed to the EGFR TKI and they subsequently went on to receive chemotherapy. AR was defined per the Jackman criteria [14], mandating patients have either a documented EGFR mutation associated with TKI sensitivity and/or objective clinical benefit from treatment with an EGFR TKI, followed by disease progression while on continuous TKI. For this analysis, we included only patients with a documented clinical response to EGFR TKI or stable disease sustained for at least 6 months to focus the study population on those with the most robust evidence of EGFR oncogene addiction. Disease progression was defined as radiographic documentation of tumor growth resulting in change in therapy for reasons other than drug toxicity.
Patients were divided into those who received chemotherapy with erlotinib following the development of AR and those who received chemotherapy alone. Note that no patients were treated with gefitinib and concurrent chemotherapy, presumably because gefitinib is not commercially available in the United States. In addition, none of the patients were participating in a prospective trial comparing chemotherapy with erlotinib and chemotherapy alone. A small number of patients temporarily stopped the EGFR TKI at the time of AR, often because they were considering enrollment in a clinical trial that required drug washout. In these cases, a maximum duration of four weeks holiday from TKI was permitted for inclusion in this analysis to decrease the chance of confounding from re-treatment effect when the TKI was reintroduced [8, 14, 15]. Patients were excluded from the analysis if they discontinued TKI because of toxicity rather than disease progression, if they had evidence of small cell lung cancer histology at time of AR [16, 17], or if they had another active malignancy.
Data Collected
Electronic medical records were reviewed to record patient age, gender, race, and smoking status. Details of the treatment courses were abstracted, including initial EGFR TKI administered, length of time the patient received initial TKI (defined as time from the start of initial TKI until chemotherapy was introduced), whether erlotinib was prescribed along with chemotherapy following AR, and chemotherapy regimens administered. Eastern Cooperative Oncology Group performance status [18] and the presence of cancer-related symptoms were recorded at the time of chemotherapy initiation. For all patients, EGFR mutation status was recorded in the medical record and had been tested in a Clinical Laboratory Improvement Amendment-certified laboratory, using either direct sequencing or a polymerase chain reaction-based allele-specific assay [19, 20].
Objective response rate (RR) to chemotherapy with erlotinib or chemotherapy alone was assessed using Response Evaluation Criteria in Solid Tumors [21] by a thoracic radiologist who was blinded to patient treatment. The baseline scan was the scan obtained just prior to the start of chemotherapy. Because patients were treated off protocol, response confirmation was not required to meet criteria for response. Progression-free survival (PFS) was calculated from the date of chemotherapy initiation until clinical progression (as determined by the treating physician) or death. Overall survival (OS) was determined from the date of chemotherapy initiation until death. Those without progression or death at the last date of data extraction were censored at the date of last tumor assessment or the date they were last known to be alive, respectively. Patients for whom there was insufficient imaging data available for evaluation of response were still eligible for PFS and OS analysis.
Statistical Considerations
Baseline patient and treatment characteristics in the chemotherapy with erlotinib group and the chemotherapy alone group were compared using Fisher exact and Wilcoxon rank sum tests. Differences in RR were analyzed with Fisher exact test and a logistic regression model that included clinically relevant confounders (chemotherapy regimen and time on initial EGFR TKI). A model including all available potential confounders (including gender, race, smoking history, EGFR mutation subtype, performance status, and initial TKI prior to progression) was also assessed; however, the more parsimonious model was chosen because there was little difference between the two. We assessed for an interaction between chemotherapy regimen and physician assignment to chemotherapy with erlotinib versus chemotherapy alone; because the interaction term was not significant, it was not included in the final model. Median PFS and OS were estimated using the Kaplan-Meier method and survival between treatments was compared by log-rank test and multivariable Cox analysis using the same covariates as in the logistic regression analysis of RR. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, http://www.sas.com).
Results
Patients and Study Treatments
A total of 288 patients with EGFR-mutant lung cancer who received an EGFR TKI followed by chemotherapy were identified. Of these, 210 were excluded for the following reasons: 79 had insufficient records for review, 55 did not receive chemotherapy after AR by our criteria, 36 had insufficient initial clinical benefit with an EGFR TKI, 15 had a treatment break of more than four weeks between discontinuing TKI and initiating chemotherapy, 9 discontinued TKI because of toxicity rather than AR, 5 had chemotherapy combined with radiation, 5 had disease that transformed to small cell lung cancer at AR, 4 had another concurrent active malignancy, and 2 had TKI combined with chemotherapy from the time of TKI initiation. The remaining 78 patients were therefore included in our analysis. At the time of progression on TKI (defined as change in therapy from TKI to chemotherapy for reasons other than toxicity), 34 (44%) patients were treated with chemotherapy with erlotinib and 44 (56%) received chemotherapy alone.
The majority of patients in both the chemotherapy with erlotinib and chemotherapy alone groups were female never-smokers with a good Eastern Cooperative Oncology Group performance status (Table 1). The median age was 58. All patients had an EGFR mutation, most commonly exon 19 deletions and L585R point mutations. Although treatment assignment was not determined prospectively or by means of randomization, the treatment arms were relatively well balanced in terms of demographic and clinical features. There were slightly more women and never-smokers who received chemotherapy with erlotinib compared with chemotherapy alone.
Table 1.
Patient demographics and study treatment
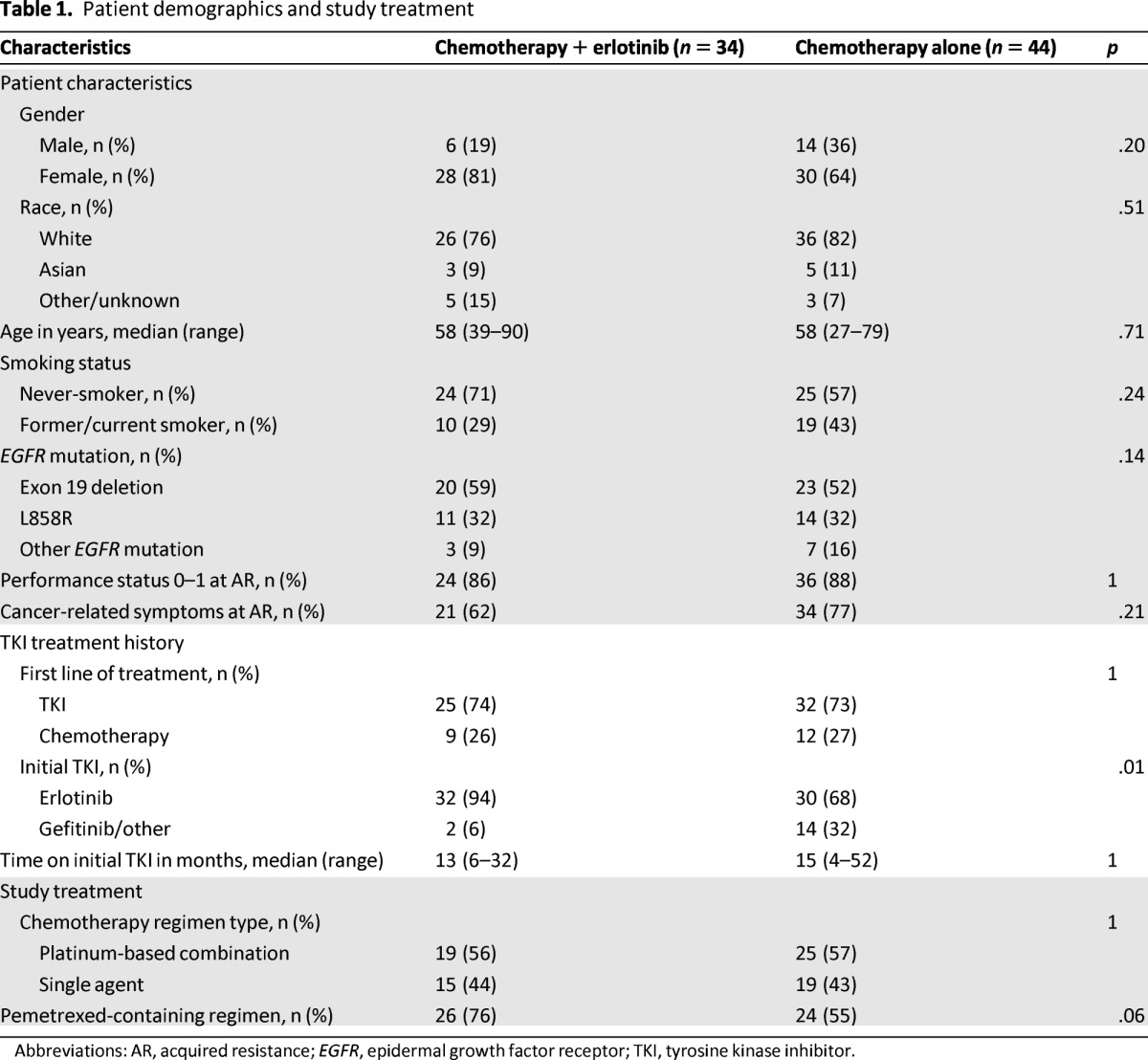
Abbreviations: AR, acquired resistance; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
The median time on initial EGFR TKI was 13 months (range 6–32 months) for the chemotherapy with erlotinib group and 15 months (range 4–52 months) for the chemotherapy alone group. EGFR TKI had been given in the first-line setting for 75% of patients (Table 1). Erlotinib was the most common choice for initial TKI, given to 94% of the chemotherapy with erlotinib group and 68% of the chemotherapy alone group. This difference likely reflects historical trends, as the majority of patients receiving gefitinib as their initial TKI started therapy before the Food and Drug Administration narrowed gefitinib's indication and limited its availability in 2005 [22]. The strategy of continuing EGFR TKI with chemotherapy has gained popularity more recently. A small number of patients (n = 2) temporarily stopped the initial TKI before restarting it along with chemotherapy. The arms were balanced in terms of proportion receiving platinum-based combination chemotherapy compared with single-agent chemotherapy. Chemotherapy regimens included pemetrexed in 76% of those who received chemotherapy with erlotinib and in 55% of those who received chemotherapy alone, again likely reflecting changing practice patterns over the study period.
Efficacy
A total of 57 patients were evaluable for response. Partial responses were observed in 12 of 29 (41%) patients in the chemotherapy with erlotinib group and in 5 of 28 (18%) patients in the chemotherapy alone group, yielding an odds ratio (OR) of 0.31 (95% confidence interval [CI]: 0.09–1.04; p = .08). After adjusting for chemotherapy regimen and time on initial TKI, the adjusted OR was 0.20 (95% CI: 0.05–0.78; p = .02) (Table 2; Fig. 1). Among those who received a platinum-based regimen, the addition of erlotinib to chemotherapy improved RR even more, with RR of 63% in the chemotherapy with erlotinib group and 21% in the chemotherapy alone group (OR = 0.16; 95% CI: 0.04–0.72; p = .02). All partial responses except two occurred in patients who received pemetrexed chemotherapy (either monotherapy or with a platinum agent). The two other responders received carboplatin, paclitaxel, and bevacizumab, one with and one without concurrent erlotinib.
Table 2.
Response to treatment

Note that total may exceed 100% because of rounding.
Abbreviations: C, chemotherapy alone; CE, chemotherapy plus erlotinib.
Figure 1.

Response to chemotherapy plus erlotinib (A) or chemotherapy alone (B). Each bar represents an individual patient and demonstrates the percent change in tumor as measured by Response Evaluation Criteria in Solid Tumors (RECIST) from baseline to best response. The dashed line represents the threshold for RECIST partial response (30% decrease). *Single-agent chemotherapy (all others platinum-based combination chemotherapy).
All 78 patients were evaluable for PFS and OS. Median PFS was not different between treatment strategies, at 4.4 months in the chemotherapy with erlotinib group and 4.2 months in the chemotherapy alone group, yielding a hazard ratio (HR) of 0.84 (95% CI: 0.52–1.38; p = .50). Stratified analyses of those who received either single-agent or combination chemotherapy did not show a significant difference in PFS with the addition of erlotinib, although there was a suggestion of benefit for chemotherapy with erlotinib compared with chemotherapy alone in the group who received single-agent chemotherapy (Fig. 2). Median OS was 14.2 months in the chemotherapy with erlotinib group and 15.0 months in the chemotherapy alone group (HR = 0.75; 95% CI: 0.41–1.39; p = .37). There was no apparent difference in OS when analyzing patients who received either single-agent or combination chemotherapy. Additionally, there was no significant difference in PFS or OS after adjusting for potential confounders. Patient numbers were too small to analyze outcomes by specific EGFR mutation type.
Figure 2.

Progression-free survival in those who received platinum-based combination chemotherapy (A) or single-agent chemotherapy (B) with and without erlotinib.
Discussion
This retrospective study is the first, to our knowledge, to examine whether patients with advanced, EGFR-mutant NSCLC with acquired resistance to EGFR TKIs benefit from continued EGFR suppression by TKI administration along with chemotherapy compared to receiving chemotherapy alone. We found that chemotherapy with erlotinib more than doubled the response rate compared with chemotherapy alone, and this difference was both statistically significant (p = .02) and clinically meaningful (RR = 41% with chemotherapy with erlotinib and 18% with chemotherapy alone). The benefit appeared to be confined primarily to patients receiving platinum-based combination chemotherapy, with an observed RR of 63% among those treated with chemotherapy and erlotinib compared with 21% when TKI was discontinued and replaced with chemotherapy. However, this dramatic improvement in RR did not translate to a difference in PFS or OS among our cohort.
The strategy of continuing EGFR TKI when initiating chemotherapy after the development of AR is supported by preclinical studies in EGFR-mutant cell lines and mouse models. When TKI-sensitive cell lines were made resistant to TKI in vitro, a heterogeneous population of TKI-sensitive and TKI-resistant cells was observed [6]. Moreover, discontinuation of the TKI allows for expansion of the TKI-sensitive subpopulation, analogous to the clinical flare of rapid tumor progression observed in some patients after discontinuation of EGFR TKI therapy [7]. In vivo and in vitro studies of cisplatin or pralatrexate (an antifolate chemotherapy agent similar to pemetrexed) with or without erlotinib in cancers with AR demonstrated that the combination of chemotherapy and TKI results in slower growth than chemotherapy alone [6].
Several prospective randomized trials have previously examined the benefit of combining an EGFR TKI and chemotherapy. In unselected patients with advanced NSCLC, four phase III trials have found the combination of TKI and platinum-based chemotherapy to be no better than first-line chemotherapy alone [23–27]. Other investigators, concerned about antagonism between the combination of chemotherapy and EGFR inhibition, have proposed that pharmacodynamic separation of chemotherapy and erlotinib might improve the effectiveness of the combination; to date, randomized phase II and III studies of this approach have not provided a clear path for development [28–31]. A study by the Cancer and Leukemia Group B found that the addition of carboplatin and paclitaxel to first-line erlotinib was no better than erlotinib alone in never/light smokers with advanced lung adenocarcinoma, nor was there a discernible difference in the subset of patients with EGFR-mutant lung cancers [27]. In the population of patients with AR to an EGFR TKI, a single-arm trial of pemetrexed plus erlotinib or gefitinib demonstrated that the combination resulted in a response rate of 25.9% and PFS of 7 months [32]. Another prospective study in patients who had derived clinical benefit and then had disease progression on erlotinib found no PFS or OS benefit in combining erlotinib with single-agent chemotherapy compared with chemotherapy alone; however, only a subset of patients had a documented EGFR mutation and the study was stopped prematurely because of poor accrual and was therefore likely underpowered [33]. Taken together, these trials suggest that combining EGFR TKIs with chemotherapy is feasible and tolerable, without clear clinical evidence of antagonism. We believe this has greatly influenced the willingness of clinicians to begin to use EGFR TKIs plus chemotherapy in patients with EGFR-mutant lung cancer and AR in recent years.
Our study must be interpreted with caution, particularly given the inherent bias in retrospective studies. The decision whether to continue an EGFR TKI or not along with chemotherapy was made at the discretion of the treating physician and not by randomization. Although adoption of this strategy was influenced by emerging trends in patient care over the time period of our review, there were likely individual patient characteristics that also influenced the treatment decision and could have confounded the outcome. We attempted to minimize confounding by controlling for two of the most influential clinical factors of outcome: use of combination chemotherapy versus single-agent chemotherapy and duration of benefit from initial TKI. We observed significant benefit from the chemotherapy with erlotinib strategy after controlling for these factors.
An important limitation to this study is the inclusion of only those patients who were able to receive chemotherapy after AR. Although necessary from a data extraction perspective, this could have omitted patients who discontinued TKI with the plan to receive chemotherapy, but who had a severe flare resulting in hospitalization or death before actually receiving the intended treatment. These patients would not have been captured by our inclusion methods and might have biased the chemotherapy alone arm to have falsely improved outcomes compared with an intent-to-treat prospective design. As tumor flare has been estimated to occur in approximately 20% of patients who discontinue TKI to start a subsequent therapy [7], our methods may have decreased the difference observed between the two groups by omitting those who did not receive chemotherapy because of flare. It is important to note that the 18% RR we found in the chemotherapy alone arm was consistent with the published experience of Wu et al., who found a 15% RR among 41 patients with EGFR-mutant lung cancer switched to cytotoxic chemotherapy after receipt of first-line gefitinib [34].
Conclusion
Our data suggest that continuing an EGFR TKI at the time of chemotherapy initiation may be a valuable treatment strategy for patients with EGFR-mutant lung cancer and AR to EGFR TKI whose tumors remain oncogene addicted. Given the lack of prospective randomized data, this is not a strategy that should be instituted in all cases, but it could be considered in individual patients, particularly those with symptomatic progression in whom a higher response rate may be clinically beneficial. We do not advocate for the continuation of EGFR TKI in patients whose tumors are wild type for EGFR because these cancers do not harbor the same oncogene-addicted biology. Several prospective clinical trials will address the efficacy of continued TKI post-progression: a phase II trial is planned that will randomly assign patients with AR on first-line EGFR TKIs to platinum/pemetrexed/erlotinib or platinum/pemetrexed, and an ongoing phase III randomized, double-blind trial has a similar design but uses gefitinib instead of erlotinib (NCT01544179). We eagerly await the results of these studies.
Acknowledgments
This work was supported in part by the Conquer Cancer Foundation of the American Society of Clinical Oncology and by the National Cancer Institute (P50-CA090578).
This study was presented in part as a poster discussion at the 2012 American Society of Clinical Oncology annual meeting, Chicago, IL.
Author Contributions
Conception/Design: Sarah B. Goldberg, Geoffrey R. Oxnard, Lecia V. Sequist
Provision of study material or patients: Sarah B. Goldberg, David M. Jackman, Lecia V. Sequist
Collection and/or assembly of data: Sarah B. Goldberg, Geoffrey R. Oxnard, David M. Jackman
Data analysis and interpretation: Sarah B. Goldberg, Subba R. Digumarthy, Alona Muzikansky, Inga T. Lennes, Lecia V. Sequist
Manuscript writing: Sarah B. Goldberg, Geoffrey R. Oxnard, Subba R. Digumarthy, Alona Muzikansky, David M. Jackman, Inga T. Lennes, Lecia V. Sequist
Final approval of manuscript: Sarah B. Goldberg, Geoffrey R. Oxnard, Subba R. Digumarthy, Alona Muzikansky, David M. Jackman, Inga T. Lennes, Lecia V. Sequist
Disclosures
Sarah B. Goldberg: Boehringer Ingelheim (C/A), Geoffrey R. Oxnard: Astra Zeneca (C/A); Boehringer Ingelheim (C/A); Genentech (C/A); Chugai (H), David M. Jackman: Foundation Medicine (C/A); Genentech (C/A); Infinity (C/A); Chugai (H), Lecia V. Sequist: AstraZeneca (C/A); Boehringer Ingelheim (C/A); Clovis Oncology (C/A); GlaxoSmithKline (C/A); Merrimack Pharmaceuticals (C/A), The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 2.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Oxnard GR, Arcila ME, Chmielecki J, et al. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17:5530–5537. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chmielecki J, Foo J, Oxnard GR, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3:90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: Implications for clinical trial design. Clin Cancer Res. 2011;17:6298–6303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5155. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 9.Extra JM, Antoine EC, Vincent-Salomon A, et al. Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: The observational Hermine study. The Oncologist. 2010;15:799–809. doi: 10.1634/theoncologist.2009-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A german breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. Non-Small Cell Lung Cancer, Version 3. [Accessed September 9, 2013]. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.
- 12.Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17:1616–1622. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 14.Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357–360. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxnard GR, Janjigian YY, Arcila ME, et al. Maintained sensitivity to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer recurring after adjuvant erlotinib or gefitinib. Clin Cancer Res. 2011;17:6322–6328. doi: 10.1158/1078-0432.CCR-11-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17:1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 19.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: A clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sequist LV, Joshi VA, Janne PA, et al. Epidermal growth factor receptor mutation testing in the care of lung cancer patients. Clin Cancer Res. 2006;12:4403s–4408s. doi: 10.1158/1078-0432.CCR-06-0099. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Blackhall F, Ranson M, Thatcher N. Where next for gefitinib in patients with lung cancer? Lancet Oncol. 2006;7:499–507. doi: 10.1016/S1470-2045(06)70725-2. [DOI] [PubMed] [Google Scholar]
- 23.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 24.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial–INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial–INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 26.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 27.Jänne PA, Wang X, Socinski MA, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 Trial. J Clin Oncol. 2012;30:2063–2069. doi: 10.1200/JCO.2011.40.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch FR, Kabbinavar F, Eisen T, et al. A randomized, phase II, biomarker-selected study comparing erlotinib to erlotinib intercalated with chemotherapy in first-line therapy for advanced non–small-cell lung cancer. J Clin Oncol. 2011;29:3567–3573. doi: 10.1200/JCO.2010.34.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riely GJ, Rizvi NA, Kris MG, et al. Randomized phase II study of pulse erlotinib before or after carboplatin and paclitaxel in current or former smokers with advanced non–small-cell lung cancer. J Clin Oncol. 2009;27:264–270. doi: 10.1200/JCO.2008.17.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y-L, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): A randomised, double-blind trial. Lancet Oncol. 2013;14:777–786. doi: 10.1016/S1470-2045(13)70254-7. [DOI] [PubMed] [Google Scholar]
- 31.Aerts JG, Codrington H, Burgers S, et al. A randomized phase II study comparing erlotinib (E) versus E alternating with chemotherapy in relapsed non-small cell lung cancer (NSCLC) patients. The NVALT10 study. Ann Oncol. 2013 Aug 28; doi: 10.1093/annonc/mdt341. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura N, Okishio K, Mitsuoka S, et al. Prospective assessment of continuation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of pemetrexed. J Thorac Oncol. 2013;8:96–101. doi: 10.1097/JTO.0b013e3182762bfb. [DOI] [PubMed] [Google Scholar]
- 33.Halmos B, Pennell NA, Otterson GA, et al. Erlotinib beyond progression study: Randomized phase II study comparing chemotherapy plus erlotinib with chemotherapy alone in EGFR tyrosine kinase inhibitor (TKI)-responsive, non-small cell lung cancer (NSCLC) that subsequently progresses. J Clin Oncol. 2013;31(suppl) abstr 8114. [Google Scholar]
- 34.Wu JY, Shih JY, Yang CH, et al. Second-line treatments after first-line gefitinib therapy in advanced nonsmall cell lung cancer. Int J Cancer. 2010;126:247–255. doi: 10.1002/ijc.24657. [DOI] [PubMed] [Google Scholar]


