Abstract
Kraft lignin (KL) is the major pollutant in black liquor. The bacterial strain Pandoraea sp. B-6 was able to degrade KL without any co-substrate under high alkaline conditions. At least 38.2 % of chemical oxygen demand and 41.6 % of color were removed in 7 days at concentrations from 1 to 6 g L−1. The optimum pH for KL degradation was 10 and the optimum temperature was 30 °C. The greatest activities of 2,249.2 U L−1 for manganese peroxidase and 1,120.6 U L−1 for laccase were detected on the third and fifth day at pH 10, respectively. Many small molecules, such as cinnamic acid, ferulic acid, 2-hydroxy benzyl alcohol, and vanillyl methyl ketone, were formed during the period of KL degradation based on GC–MS analysis. These results indicate that this strain has great potential for biotreatment of black liquor.
Keywords: Pandoraea sp. B-6, Kraft lignin, Degradation, Black liquor
Introduction
Lignin is the most abundant aromatic compound on earth and is second only to cellulose in its contribution to living terrestrial biomass [1]. The structural complexity of lignin, its high molecular weight, and its insolubility make its degradation difficult [2]. The biological degradation of lignin is critical to the biospheric carbon and oxygen cycle [3] and is responsible for much of the natural destruction of wood in use. Applications utilizing lignin-degrading organisms or isolated enzymes provide environmentally friendly technologies for the pulp and paper industry and for the treatment of many xenobiotic compounds and dyes [4].
Despite the resistance of lignin to degradation, a number of fungi are able to breakdown lignin. The best characterized degraders are white-rot fungi, in particular Phanerochaete chrysosporium and Phlebia radiata [5], although brown-rot and soft-rot fungi are also able to degrade lignin. White-rot fungi secrete phenol oxidases, including lignin peroxidase, manganese peroxidase, and laccase, that attack lignin. These enzymes act through radical reactions [6]. Basidiomycetous fungi are the most efficient lignin degraders currently known, but there is as yet no commercial biocatalytic process for lignin depolymerization [7]. Bacteria also have versatile pathways for degradation of aromatic substances, from simple phenols to highly complex lignin and related xenobiotic substances.
Bacterial degradation is also attractive in part because fungal lignin degradation results in the formation of low molecular weight, mostly aromatic carboxylic acids, that may be further metabolized by bacteria [8]. There are literature reports of lignin degradation by a number of bacterial species [9–12]. Recent work also indicates that Sphingomonas paucimobilis SYK-6, known to degrade lignin-derived aromatic compounds, degrades lignin [6]. More than 10 lignin-degrading bacteria from several genera of Serratia, Citrobacter, Klebsiella, Paenibacillus, Aneurinibacillus, and Bacillus were isolated from the sludge of pulp paper mill and investigated for the ability to degrade lignin [13, 14] and lignin degradation products [15, 16]. Some of those bacteria have been used in the treatment of black liquor [17–19]. The structure of natural lignin is very complex and intact lignin is not commercially available. Because of similarities to natural lignin, kraft lignin (KL) has been widely used for lignin-related studies. KL is also the major by-product in the relatively high alkaline effluent (black liquor) generated by the pulp and paper industry [20]. Although several bacterial strains had been reported to degrade KL, only a few strains are able to degrade KL from pulp and paper industry waste [17, 18].
The Gram-negative bacterial strain Pandoraea sp. B-6 was isolated from eroded bamboo slip steeping fluid derived from Kingdom Wu during the Three-Kingdoms Dynasty of ancient China (A.D. 220–280). Pandoraea sp. B-6 can use KL and lignin derivatives as its sole carbon sources under high alkaline conditions. In this study, the abilities of Pandoraea sp. B-6 and extracellular ligninolytic enzymes to degrade KL were investigated. The low molecular weight compounds resulted from KL degradation were identified by GC–MS analysis. The partial 16S rRNA gene sequence of the microorganism has been deposited in GenBank with the accession number of JN128829.1. This work indicates that Pandoraea sp. B-6 has significant potential for use in lignin degradation and black liquor biotreatment.
Materials and methods
Bacterial strain and culture conditions
The bacterial strain Pandoraea sp. B-6 was isolated from the steeping fluid of eroding bamboo slips [21]. The bacteria were grown in the Luria–Bertani broth medium at 30 °C with shaking at 120 rpm until the optical density at 600 nm (OD600) of inoculum reached approximately 1.0. Two-millimeter aliquots of this culture were aseptically inoculated into triplicate flasks containing 100 ml sterile KL mineral salt medium (3 g KL, 2 g, (NH4)2SO4, 1 g K2HPO4, 1 g KH2PO4, 0.2 g MgSO4, 0.1 g CaCl2, 0.05 g FeSO4, 0.02 g MnSO4 in 1 litre distilled water, pH 7.0). The flasks were incubated at 30 °C with shaking speed of 120 rpm for 7 days. These cultures were used to investigate the effects of the temperature, pH, and initial KL concentration on KL degradation by Pandoraea sp. B-6. The pH values were monitored with a pH meter during adjustment of buffer to required pH.
Bacterial growth and COD measurements
Degradation experiments were carried out on a rotary shaker (120 rpm) under aerobic conditions at 30 °C, pH 10 for 7 days. The rate of Pandoraea sp. B-6 growth was determined by measuring the OD600 of cultured samples withdrawn at intervals on a Hitachi U-4100 spectrophotometer using centrifuged uninoculated medium as a control in 1.0-cm cuvette path length cells. The control and cultured samples were centrifuged at 10,000 rpm for 10 min to remove biomass and suspended solids, and the chemical oxygen demand (COD) of the supernatant was measured by the fast digestion-spectrophotometric method (the Environmental Protection Industry Standard of the People’s Republic of China, HJ/T 399-2007).
Measurement of the color removal
The intensity of color, before and after incubation, was determined by the standard method of the Canadian Pulp and Paper Association [22]. The amount of color present was determined spectrophotometrically and was related to the absorbance of a PtCo standard solution at the same wavelength. The samples were centrifuged at 10,000 rpm for 10 min to remove suspended solids. The pH of the supernatant was then adjusted to 7.6, and thereafter the absorbance at 465 nm against distilled water was measured using the spectrophotometer. The absorbance values (A) were then transformed into color units (PtCo) as follows:
where A 1 corresponds to the A 465 of a 500-CU platinum-cobalt standard solution (0.132); and A 2 is the absorbance of the effluent sample.
Determination of the ligninolytic enzyme activity
An 1-ml aliquot of culture supernatant, prepared by centrifugation (10,000 rpm for 10 min), was used to determine the activity of laccase, lignin peroxidase, and manganese peroxidase. Laccase activity was determined using 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) as substrate with monitoring at 420 nm (ε 420 = 36,000 mol−1 cm−1) [23]. Lignin peroxidase activity was determined by the peroxide-dependent oxidation of 2 mM veratryl alcohol to veratraldehyde followed at 310 nm (ε 310 = 9.300 mol−1 cm−1) [24]. Manganese peroxidase activity was assayed by monitoring the oxidation of 2,6-dimethoxyphenol (2, 6-DMP) to coerulignone at 469 nm (ε 469 = 49,600 mol−1 cm−1) [25]. The value of ε was obtained using the Beer–Lambert law:
where log(I 0 /I) is absorbance, ε is the proportional coefficient of optical absorption, C is the concentration of the sample, and l is the optical path length of the cuvette.
Analysis of small molecule intermediates from KL degradation through GC–MS
Cultured samples of bacteria containing 1 g L−1 KL in the medium were periodically withdrawn and centrifuged at 12,000 rpm for 10 min. Supernatants were acidified to pH 2.0 with 6 mmol L−1 HCl and then extracted with equal volume of ethyl acetate. Three portions of the extraction were collected, dehydrated over anhydrous Na2SO4, filtered though filter paper, and evaporated at 40 °C under vacuum on a rotary vacuum evaporator. Then, 0.1 ml dioxane and 0.01 ml pyridine were added to the samples followed by silylation with 0.05 ml trimethylsilyl (TMS). The mixture was heated at 60 °C for 15 min with periodic shaking to dissolve residues. GC–MS analysis of organic extracts residues was conducted using the method reported previously [26]. The identification of low molecular weight compounds as TMS derivatives derived from bacterial degradation was done by comparing their mass spectra with that of the National Institute of Standards and Technology (NIST) library available through the instrument manufacturer and also by comparing the retention time (RT) with those of authentic compounds when available.
Results and discussion
Effects of temperature and pH on KL degradation by Pandoraea sp. B-6
The degradation of KL (3 g L−1) by Pandoraea sp. B-6 as a function of temperature is shown in Fig. 1a. The extent of KL degradation was significantly different at 15 °C than at 45 °C. The optimum temperature for degradation under these conditions was 30 °C. Approximately, 54.5 % of KL remained after 7 days in the bacterial culture at 30 °C. Degradation efficiency decreased dramatically at both higher and lower temperatures with only 5.4 % degraded after 7 days at 45 °C.
Fig. 1.
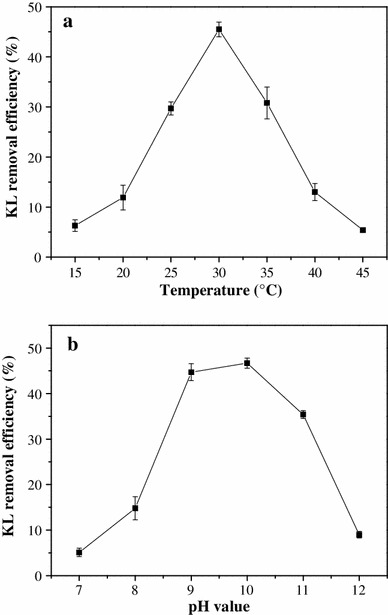
Effect of temperature (a) and pH (b) on kraft lignin degradation by Pandoraea sp. B-6. Data are presented as mean of three replicates with SE
The effect of pH on KL degradation is shown in Fig. 1b. The efficiency of KL (1 g L−1) was higher under alkaline conditions (pH 9–11) than at lower pH values. There was no significant difference in the amount of KL degradation at pH 9 vs. 10, with 44.7 and 46.7 % KL remaining after 7 days, respectively. The optimum value was 10.
Treatment of bleached kraft mill effluent (black liquor) with white-rot fungi has been well characterized [17]. The fungi require low pH (4–5) for growth and enzyme activity. The solubility of high molecular weight lignin and derivatives are reduced at low pH compared to alkaline pH, and the pH values of pulp and paper effluent are generally high (10–13). Thus, it would be most economical to develop a degradation method that is effective at alkaline pH. Previously reported optimum pH values for KL lignin degradation by bacterial strains varied from 7 to 8.5. For example, the optimum pH of Aneurinibacillus aneurinilyticus was 7.6 [15], that for Comamonas sp. B-9 was 7 [26], and that for a Bacillus strain was 7.6 [17]. For Streptomyces strains, pH optimum ranged from 7.8 to 8.5 [27]. Our results indicate that Pandoraea sp. B-6, with its optimum KL degradation activity at high pH, has a significant advantage for application in black liquor biotreatment compared with other microorganisms.
Bacterial growth and KL degradation
In order to determine the optimal KL concentration for the degradation reaction, Pandoraea sp. B-6 growth was evaluated under seven initial KL concentrations ranging from 1 to 6 g L−1. Pandoraea sp. B-6 grew fairly well at concentrations from 1 to 6 g L−1 (Fig. 2a). The OD value of the cultured sample increased with increasing initial KL concentration. KL degradation at each initial concentration surpassed 38 % after days in culture (Fig. 2b), but there was no obvious correlation between the initial concentration and extent of KL degradation. The highest percent degradation was 46.5 % at the initial KL concentration of 3 g L−1; the greatest degradation capacity was 2.52 g L−1 for the initial KL concentration of 6 g L−1.
Fig. 2.
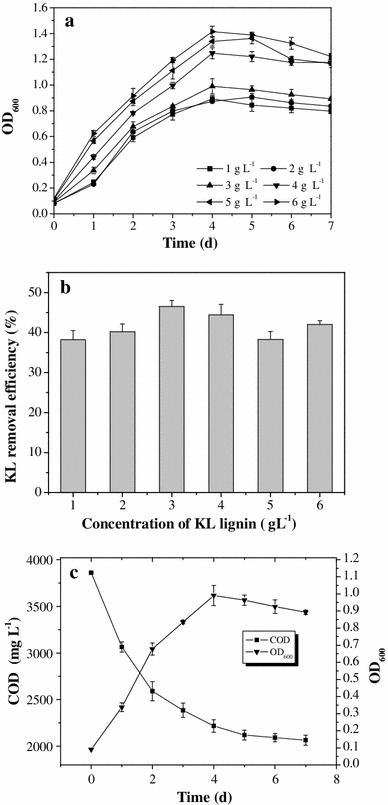
Pandoraea sp. B-6 growth and kraft lignin degradation. a Pandoraea sp. B-6 growth in different initial concentration, b kraft lignin removal rate by Pandoraea sp. B-6 on day 7 in different initial concentration, and c Pandoraea sp. B-6 growth and COD reduction in 2 g L−1 kraft lignin. Data are presented as mean of three replicates with SE
The growth of Pandoraea sp. B-6 and its ability to degrade KL in the nutrient medium with the KL concentration of 3 g L−1 were investigated in detail. The result is shown in Fig. 2c. Pandoraea sp. B-6 growth was rapid during the first 2 days in culture and reached the maximum on the fourth day. KL degradation occurred mainly during the first 2 days of culture during the exponential growth phase. Accordingly, the maximum KL removal rate of 795.7 mg L−1 day−1 was recorded during this period. From the third day, the removal rate decreased gradually, but KL degradation continued. The COD value was 3,860 mg L−1 on the first day of culture and was 2,065.1 mg L−1 on the seventh day. These results with Pandoraea sp. B-6 differ from those of other bacteria such as Citrobacter strains. Other strains initially use glucose and peptone as carbon source and subsequently utilize lignin as a co-metabolite [18]. KL can be used as the sole carbon source by Pandoraea sp. B-6; accordingly, it was metabolized during the initial growth stage to provide carbon and energy sources. Like KL degradation by Pandoraea sp. B-6, Comamonas sp. B-9 [26] and Streptomyces viridosporus [11] metabolize KL during the initial growth phase and also have a high capacity for KL degradation. These bacterial species metabolize KL throughout the whole life cycle for their growth; therefore, the efficiency and total amount of lignin degradation are higher than with strains that use lignin as a co-metabolite. Pandoraea sp. B-6 can be directly used for treatment of black liquor without need for a supplementary carbon source that would increase the COD load of the system.
KL decolorization
The decolorization of KL at different initial concentrations at pH 10 after 7 days in culture with Pandoraea sp. B-6 is shown in Fig. 3. Percent decolorization at initial KL concentrations from 1 to 6 g L−1 ranged from 41.6 to 47.9 %. The difference was <7 %, indicating that the effect of initial KL concentration on the Pandoraea sp. B-6 decolorization is negligible. The percent decolorization of different initial concentrations of KL at pH 10 over 7 days in culture is shown in Table 1. The decolorization of KL was mainly observed in the first 2 days, consistent with fast bacterium growth and COD reduction. Moreover, like COD reduction, KL decolorization was continued after the second day but at significantly decreased levels. Initially, the color of the culture ranged from 2,651.5 to 18,333.3 CU but dropped to 1,474.2 CU for the lowest KL concentration and to 1,0431.7 CU for the highest KL concentration on day 7. The decolorization rates of some bacterial strain, such as Bacillus sp. and Aneurinibacillus aneurinilyticus [13, 15] are reportedly more than 50 %. Although these decolorization rates are higher than that of Pandoraea sp. B-6, the KL concentrations in cultures of the other strains were <0.6 g L−1, much lower than the 3 g L−1 KL used in the present study.
Fig. 3.

Color removal rate by Pandoraea sp. B-6 on day 7 in different initial concentrations of kraft lignin. Data are presented as mean of three replicates with SE
Table 1.
Color removal (change in color units) in six different initial concentration of KL during the process of KL degradation by Pandoraea sp. B-6
| Time (days) | Different initial concentrations of KL | |||||
|---|---|---|---|---|---|---|
| 1 g L−1 | 2 g L−1 | 3 g L−1 | 4 g L−1 | 5 g L−1 | 6 g L−1 | |
| 1 | 2,651.5 | 5,643.9 | 8,712.1 | 11,515.2 | 15,075.8 | 18,333.3 |
| 2 | 2,324.8 ± 63.0 | 4,980.9 ± 82.6 | 7,783.0 ± 87.4 | 9,914.6 ± 166.1 | 13,369.0 ± 268.6 | 16,267.27 ± 203.7 |
| 3 | 1,801.0 ± 73.5 | 4,021.8 ± 70.5 | 6,090.5 ± 62.1 | 7,951.6 ± 138.1 | 10,985.5 ± 128.4 | 12,581.5 ± 171.3 |
| 4 | 1,661.7 ± 47.5 | 3,549.5 ± 58.1 | 5,578.3 ± 50.1 | 7,545.9 ± 126.2 | 10,207.4 ± 273.5 | 11,683.4 ± 250.3 |
| 5 | 1,571.0 ± 64.3 | 3,338.0 ± 54.9 | 5,339.9 ± 83.4 | 7,220.3 ± 84.6 | 9,702.3 ± 133.4 | 11,484.83 ± 187.1 |
| 6 | 1,498.3 ± 42.5 | 3,269.5 ± 101.9 | 4,839.5 ± 61.7 | 6,398.6 ± 120.6 | 9,054.8 ± 127.4 | 11,044.65 ± 257.5 |
| 7 | 1,474.2 ± 55.0 | 3,194.5 ± 39.8 | 4,539.0 ± 72.6 | 6,229.7 ± 100.7 | 8,804.2 ± 130.4 | 10,431.7 ± 65.8 |
Bioassay of enzymes related to KL degradation
Three major extracellular enzymes, including lignin peroxidase, manganese peroxidase, and laccase, carry out lignin degradation and have been well characterized in microorganisms [2]. The activity of these three enzymes from Pandoraea sp. B-6 was investigated at different pH values. Results after 3 days in culture of an initial KL concentration of 3 g L−1 KL are shown in Fig. 4a. No obvious lignin peroxidase activity was observed at any pH value. The activities of manganese peroxidase and laccase were observed at pH values from 8 to 11, with the maximum values of 2,274 and 537.4 U L−1, respectively, at pH 10. The activities of these two enzymes were almost undetectable at pH 7. These results indicate that manganese peroxidase and laccase are basophilic enzymes, although activities were suppressed pH 12.
Fig. 4.
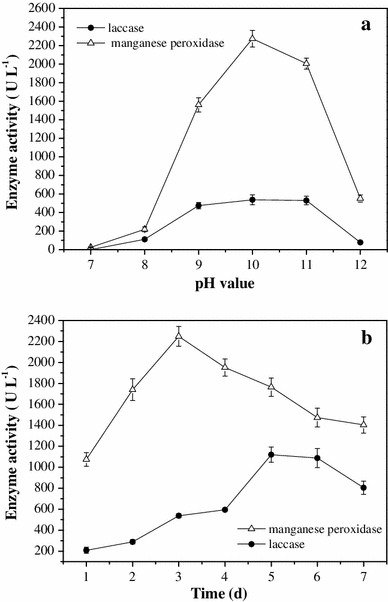
The activity of manganese peroxidase and laccase during the process of kraft lignin degradation by Pandoraea sp. B-6. a The activity of manganese peroxidase and laccase at different pH values on day 3. b The activity of manganese peroxidase and laccase during 7 days at pH 10. Cell-free supernatants were used as enzyme source for ligninolytic enzyme assays. Values are mean of three replicates with SE
The activities of manganese peroxidase and laccase during a time course of KL (3 g L−1) degradation are shown in Fig. 4b. The manganese peroxidase activity increased significantly during the initial 3 days with the maximum of 2,249.2 U L−1 on the third day, decreasing thereafter. The laccase activity was maintained at a low level through the first 2 days. A significant increase in activity was observed on the third day with the maximum of 1,120.6 U L−1 on day 5. These results indicate that manganese peroxidase plays a crucial role in KL degradation by Pandoraea sp. B-6 beginning on the first day of culture; whereas laccase mainly functions in once the exponential growth phase is almost over. This is similar to the conclusions from previous reports regarding enzymes responsible for KL degradation in other bacterial species [28, 29]. It may be that the secretion of ligninolytic enzymes by Pandoraea sp. B-6 occurs mostly during primary metabolism. As Pandoraea sp. B-6 is a Gram-negative bacterium, large lignin polymer molecules cannot be passively taken into the cell. Therefore, Pandoraea sp. B-6 produces extracellular ligninolytic enzymes to degrade the lignin polymer into fragments that can traverse the bacterial membrane. In addition, no obvious activity of lignin peroxidase was observed during the course of KL degradation, indicating that lignin peroxidase was not produced by Pandoraea sp. B-6. Some white-rot fungi and bacteria, such as Dichomitus squalens, Lentinula edodes [8], and Comamonas sp. B-9 [26], that are lignin degraders simultaneously produce manganese peroxidase and laccase, but did not secrete detectable levels of lignin peroxidase. Lignin peroxidase is responsible for the oxidation of non-phenolic syringyl and biphenyl model compounds in certain types of lignin (like hardwood) and subsequent ring cleavage [8]. The mechanism of lignin biodegradation in the absence of lignin peroxidase production is not understood. It is conceivable that the efficiency of hardwood degradation by Pandoraea sp. B-6 and the other microorganisms mentioned above is relatively low.
Pandoraea sp. B-6 produces extracellular ligninolytic enzymes to degrade the lignin polymer. Activity of ligninolytic enzymes was detected in the cell-free supernatant of bacteria grown at alkaline pH, and the level of manganese peroxidase and laccase was high in alkaline conditions. Unlike most lignin-degrading microorganisms, Pandoraea sp. B-6 produced manganese peroxidase and laccase to degrade lignin without need for an exogenous carbon source. We conclude that Pandoraea sp. B-6 can be directly used for treatment of black liquor without carbon source supplementation that increases the COD load of the system.
Metabolite characterization through GC–MS
The potential of Pandoraea sp. B-6 for the treatment of black liquor was further studied by identifying the lignin-related aromatic compounds released during KL degradation using GC–MS analysis. The total ion chromatograph (TIC) patterns corresponding to the compounds extracted with ethyl acetate from the acidified supernatants obtained from the control (uninoculated medium sample) and degraded sample are shown in Fig. 5a, b and their peak identities are listed in Table 2. In the TIC pattern of control sample (Fig. 5a), acetic acid and phenol were identified at retention times (RTs) of 8.05 and 10.56, respectively. These two compounds are the two important intermediate metabolites during the breakdown of lignin by the microorganism [30, 31]. The presence of acetic acid and phenol may be attributed to the chemical oxidation of lignin due to aeration and agitation in the uninoculated culture. Moreover, some other lignin-related compounds were also identified, suggesting that minor degradation of KL occurs during the industrial production process [32].
Fig. 5.
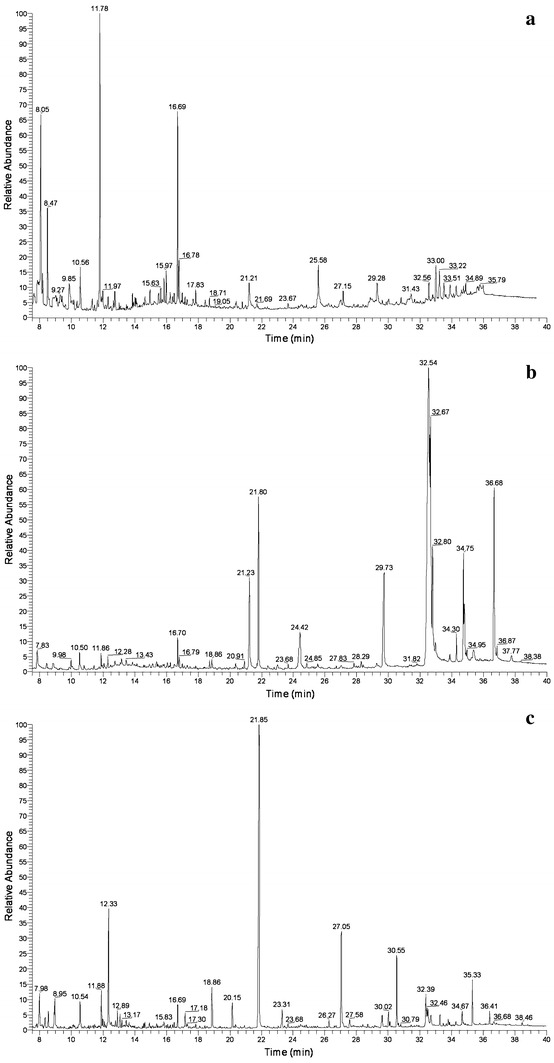
The total ion chromatograph of trimethylsilyl derivatives of compounds extracted with trichloromethane from kraft lignin medium incubated with Pandoraea sp. B-6. a 0 days; b 3 days; and c 7 days
Table 2.
Compounds identified as TMS derivatives in chloroform extract from control and bacterial degraded kraft lignin samples as given in Fig. 5
| Present in | |||||
|---|---|---|---|---|---|
| No. | RTa | Fig. 4a | Fig. 4b | Fig. 4c | Compounds |
| (0 days) | (3 days) | (7 days) | |||
| 1 | 8.05 | + | – | – | Acetic acid |
| 2 | 8.47 | + | – | – | Methyl acetate |
| 3 | 10.5 | – | + | – | Ethanedioic acid |
| 4 | 10.56 | + | – | – | Phenol |
| 5 | 11.78 | + | – | – | 3,5-Dimethyl-4-hydroxybenzaldehyde |
| 6 | 11.86 | – | + | – | Veratryl alcohol |
| 7 | 12.33 | – | – | + | 2-Hydroxy-5-methyl-p-benzoquinone |
| 8 | 16.69 | – | – | + | Cinnamic acid |
| 9 | 16.79 | – | + | – | Ethylguaiacol |
| 10 | 18.86 | – | + | + | Ferulic acid |
| 11 | 20.15 | – | – | + | 4-Hydroxy-3-methoxyphenylacetone |
| 12 | 21.23 | – | + | – | Dibutyl phthalate |
| 13 | 21.80 | – | + | – | 4-Hydroxycinnamic acid |
| 14 | 21.85 | – | – | + | Guaiacyl vinyl ketone |
| 15 | 23.31 | – | – | + | 3,4,5-Trimethoxy benzaldehyde |
| 16 | 24.42 | – | + | – | 3,4,5-Trimethoxycinnamic acid |
| 17 | 27.05 | – | – | + | 4-Hydroxy-3,5-dimethoxy benzaldehyde |
| 18 | 29.73 | – | + | – | 4-Hydroxy-3-methoxybenzoic acid |
| 19 | 30.02 | – | – | + | 2-Hydroxy benzyl alcohol |
| 20 | 30.55 | – | – | + | Hexadecanoic acid |
| 21 | 32.39 | – | – | + | Vanillyl methyl ketone |
| 22 | 34.75 | – | + | – | Lignophenols |
| 23 | 36.68 | – | + | + | 3-3′-Dihydroxy-,4,-4′ dimethoxy-6-formyl-6′-methyl-biphenyl carboxylic acid |
aRetention time
As shown in Fig. 5b, the TIC of a sample of KL inoculated with Pandoraea sp. B-6 showed a significant increase in the number of peaks after 3 days of incubation as compared to the uninoculated sample, which indicated that a large number of low molecular compounds were released from the KL polymer as a result of the presence of the bacteria. Furthermore, many peaks identified on day 3 decreased after 7 days of incubation and new peaks appeared, indicating that low molecular weight compounds released form KL polymer initially were further degraded or mineralized. Based on results of the TIC for control and degraded samples, the process of KL degradation by Pandoraea sp. B-6 was divided into two steps: the initial depolymerization of KL and the degradation of the lower molecular weight compounds.
Many low molecular weight aromatic compounds were detected in the extract of the degraded sample (Fig. 5b, c; Table 2). These included veratryl alcohol (RT 11.86), cinnamic acid (RT 16.69), ethylguaiacol (RT 16.79), ferulic acid (RT 18.86), 4-hydroxycinnamic acid (RT 21.80), 3,4,5-trimethoxy benzaldehyde (RT 23.31), and lignophenols (RT 34.75), none of which were present in the control sample. The aromatic compounds identified in extract of degraded sample were due to the radical polymerization of guaiacyl (G) units from precursor coniferyl alcohol, syringyl (S) units from precursor sinapyl alcohol, and p-hydroxyphenyl (H) units from precursor p-coumaryl alcohol. G, S, and H units are considered the basic moieties of the lignin structure [30]. Ferulic acid results from formation of ester and ether linkages by reaction of its phenolic group [33]. Cinnamic acid, which is involved in linking the lignin and hemicellulose fraction of lignocellulose, was identified in the extract degraded by Pandoraea sp. B-6. During the process of pulping alkaline extraction, most of the ester linkages are broken down, but some cinnamic acid still remains bound to the lignin by ether linkages [32]; these were cleaved by Pandoraea sp. B-6. Further, the peak at RT 21.23 was identified as dibutyl phthalate, which reportedly occurs during fungal peroxidase degradation of lignosulfonate [34] and during photo-degradation of black liquor lignin [35]. In addition to aromatic compounds, many acid-type compounds were identified in the control and inoculated sample. As in earlier reports [10, 17, 36], lignin degradation products were more often acid-type compounds than aldehyde and ketone-type molecules. It has been reported that cupric oxide degradation of native lignin from different vegetal tissues yields aldehyde-type compounds in higher abundance than ketones and acid-type compounds [37].
Conclusion
In this paper, we report in the ability of the Pandoraea strain B-6 to efficiently degrade KL. Pandoraea sp. B-6 grew well in the sterile KL mineral salt medium without any co-substrate. For KL degradation by Pandoraea sp. B-6, the optimum pH was 10 and the optimum temperature was 30 °C. In the range from pH 7 to 12, the greatest COD and color removal and the highest levels of activity of extracellular ligninolytic enzymes (manganese peroxidase and laccase) were observed at pH 10. Many intermediates formed during the process of bacterial degradation of were identified through GC–MS analysis. Our results indicate that Pandoraea sp. B-6 has significant potential for use in applications requiring for lignin degradation and for treatment of black liquor or other KL containing pollutants before their release into the environment.
Acknowledgments
This work was funded by National Funds for Distinguished Young Scientists of China (50925417), National Natural Science Foundation of China (51074191), and the Key Project of National Natural Science Foundation of China (50830301).
Contributor Information
Yan Shi, Email: shiyzy@126.com.
Liyuan Chai, Phone: +86-731-88836921, FAX: +86-731-88710171, Email: Liyuan.chai@yahoo.com.cn, Email: Lychai@csu.edu.cn.
References
- 1.Hammel KE. Fungal degradation of lignin. In: Cadish G, Giller KE, editors. Driven by nature: plant litter quality and decomposition. Wallingford: CAB International; 1997. pp. 33–45. [Google Scholar]
- 2.Perez J, Munoz-Dorado J, de la Rubia T, Martinez J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol. 2002;5(2):53–63. doi: 10.1007/s10123-002-0062-3. [DOI] [PubMed] [Google Scholar]
- 3.Crawford DL. Microbial degradation of lignin. Enzyme Microb Technol. 1980;2:11–22. doi: 10.1016/0141-0229(80)90003-4. [DOI] [Google Scholar]
- 4.Hatakka A (2001) Biodegradation of lignin. In: Hofrichter M, Steinbuchel A (eds) Biopolymers. Lignin, humic substances and coal, vol. 1. Wiley, Weinheim, p 129–180
- 5.Tuomela M, Vikman M, Hatakka A, Itavaara M. Biodegradation of lignin in a compost environment: a review. Biores Technol. 2000;72:169–183. doi: 10.1016/S0960-8524(99)00104-2. [DOI] [Google Scholar]
- 6.Masai E, Katayama Y, Fukuda M. Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotechnol Bioch. 2007;71:1–15. doi: 10.1271/bbb.60437. [DOI] [PubMed] [Google Scholar]
- 7.Bugg TDH, Ahmad M, Hardiman EM, Singh R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol. 2010;22:1–7. doi: 10.1016/j.copbio.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Tuor U, Winterhalter K, Fiechter A. Enzymes of white-rot fungi involved in lignin degradation and ecological determinants of wood decay. J Biotechnol. 1995;41:1–17. doi: 10.1016/0168-1656(95)00042-O. [DOI] [Google Scholar]
- 9.Jokela JJ, Pellinen M, Salkinoja-Salonen M, Brunow G. Biodegradation of two tetrameric lignin model compounds by a mixed bacterial culture. Appl Environ Microbiol. 1985;23:38–46. doi: 10.1128/aem.53.11.2642-2649.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta VK, Minocha AK, Jain N. Batch and continuous studies on treatment of pulpmill wastewater by Aeromonas formicans. J Chem Technol Biotechnol. 2001;76:547–552. doi: 10.1002/jctb.417. [DOI] [Google Scholar]
- 11.Ramachandra M, Crawford DL, Hertel G. Characterization of an extracellular lignin peroxidase of the lignocellulolytic actinomycete Streptomyces viridosporus. Appl Environ Microbiol. 1988;54:3057–3063. doi: 10.1128/aem.54.12.3057-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann W. Degradation of lignin by bacteria. J Biotechnol. 1990;13:119–130. doi: 10.1016/0168-1656(90)90098-V. [DOI] [Google Scholar]
- 13.Chandra R, Raj A, Purohit HJ, Kapley A. Characterisation and optimisation of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere. 2007;67:839–846. doi: 10.1016/j.chemosphere.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Chandra R, Singh S, Reddy MMK, Patel DK, Purohit HJ, Kapley A. Isolation and characterization of bacterial strains Paenibacillus sp. and Bacillus sp. for kraft lignin decolorization from pulp paper mill waste. J Gen Appl Microbiol. 2008;54:399–407. doi: 10.2323/jgam.54.399. [DOI] [PubMed] [Google Scholar]
- 15.Raj A, Chandra R, Reddy MMK, Purohit HJ, Kapley A. Biodegradation of kraft lignin by a newly isolated bacterial strain, Aneurinibacillus aneurinilyticus from the sludge of a pulp paper mill. World J Microbiol Biotechnol. 2007;23:793–799. doi: 10.1007/s11274-006-9299-x. [DOI] [Google Scholar]
- 16.Raj A, Reddy MMK, Chandra R, Purohit HJ, Kapley A. Biodegradation of kraft-lignin by Bacillus sp. isolated from sludge of pulp and paper mill. Biodegradation. 2007;18:783–792. doi: 10.1007/s10532-007-9107-9. [DOI] [PubMed] [Google Scholar]
- 17.Raj A, Reddy MMK, Chandra R. Decolourisation and treatment of pulp and paper mill effluent by lignin-degrading Bacillus sp. J Chem Technol Biotechnol. 2007;82:399–406. doi: 10.1002/jctb.1683. [DOI] [Google Scholar]
- 18.Chandra R, Abhishek A. Bacterial decolorization of black liquor in axenic and mixed condition and characterization of metabolites. Biodegradation. 2011;22:603–611. doi: 10.1007/s10532-010-9433-1. [DOI] [PubMed] [Google Scholar]
- 19.Chandra R, Abhishek A, Sankhwar M. Bacterial decolorization and detoxification of black liquor from rayon grade pulp manufacturing paper industry and detection of their metabolic products. Bioresour Technol. 2011;102:6429–6436. doi: 10.1016/j.biortech.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 20.Grover R, Marwaha SS, Kennedy JF. Studies on the use of an anaerobic baffled reactor for the continuous anaerobic digestion of pulp paper mill black liquors. Process Biochem. 1999;34:653–657. doi: 10.1016/S0032-9592(98)00138-1. [DOI] [Google Scholar]
- 21.Chai LY, Chen YH, Huang Y, Yang ZH. Diversity of culturable microorganisms from erosive bamboo slips of Kingdom Wu. J Cent South Univ (Sci Technol) 2010;41:1674–1679. [Google Scholar]
- 22.Shintain N, Sugano Y, Shoda M. Decolorization of kraft pulp bleaching effluent by a newly isolated fungus, Geotrichum candidum. Japan Wood Res Soc. 2002;48:402–408. doi: 10.1007/BF00770700. [DOI] [Google Scholar]
- 23.Nakagawa Y, Sakamoto Y, Kikuchi S, Sato T, Yano A. A chimeric laccase with hybrid properties of the parental Lentinula edodes laccases. Microbiol Res. 2010;165:392–401. doi: 10.1016/j.micres.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Orth AB, Royse DJ, Tien M. Ubiquity of lignin-degrading peroxidases among various wood-degrading fungi. Appl Environ Microbiol. 1993;59:4017–4023. doi: 10.1128/aem.59.12.4017-4023.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapicha AN, Priora BA, Bothaa A, Galkinb S, Lundellb T, Hatakkab A. Effect of lignocellulose-containing substrates on production of ligninolytic peroxidases in submerged cultures of Phanerochaete chrysosporium ME-446. Enzyme Microbiol Tech. 2004;34:187–195. doi: 10.1016/j.enzmictec.2003.10.004. [DOI] [Google Scholar]
- 26.Chen YH, Chai LY, Zhu YH, Yang ZH, Zheng Y, Zhang H. Biodegradation of kraft lignin by a bacterial strain Comamonas sp. B-9 isolated from eroded bamboo slips. J Appl Microbiol. 2012;112:900–906. doi: 10.1111/j.1365-2672.2012.05275.x. [DOI] [PubMed] [Google Scholar]
- 27.Giroux H, Vidal P, Bouchard J, Lamy F. Degradation of kraft indulin lignin by Streptomyces viridosporus and Streptomyces badius. Appl Environ Microbiol. 1988;54:3064–3070. doi: 10.1128/aem.54.12.3064-3070.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofrichter M. Review: lignin conversion by manganese peroxidase (MnP) Enzyme Microb Technol. 2002;30:454–466. doi: 10.1016/S0141-0229(01)00528-2. [DOI] [Google Scholar]
- 29.Leonowicz A, Cho NS, Luterek J, Wilkolazka A, Wojtas-Wasilewska M, Matuzewska A, Hofrichter M, Wesenberg D, Rogalski J. Fungal laccase: properties and activity on lignin. J Basic Microbiol. 2001;41:185–227. doi: 10.1002/1521-4028(200107)41:3/4<185::AID-JOBM185>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 30.Bugg TDH, Ahmad M, Hardiman EM, Rahmanpour R. Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep. 2011;28:1883–1896. doi: 10.1039/c1np00042j. [DOI] [PubMed] [Google Scholar]
- 31.Hernes PJ, Benner R. Photochemical and microbial degradation of dissolved lignin phenols: implications for the fate of terrigenous dissolved organic matter in marine environments. J Geophys Res. 2003;108:1–9. doi: 10.1029/2002JC001421. [DOI] [Google Scholar]
- 32.Hernandez M, Rodriguez J, Perez MI, Ball AS, Arias ME. 13C NMR cross polarization and magic angle spinning (CPMAS) and gas chromatography/mass spectroscopy analysis of the products from a soda pulp mill effluent decolourised with two Streptomyces strains. Appl Microbiol Biotechnol. 1997;74:272–278. [Google Scholar]
- 33.Jeffries TW. Biodegradation of lignin-biochemistry of lignin-carbohydrate complexes. Biodegradation. 1990;1:163–176. doi: 10.1007/BF00058834. [DOI] [Google Scholar]
- 34.Shin KS, Lee YJ. Depolymerisation of lignosulfonate by peroxidase of the white-rot basidiomycete, Pleurotus ostreatus. Biotechnol Lett. 1999;21:585–588. doi: 10.1023/A:1005591027897. [DOI] [Google Scholar]
- 35.Ksibi M, Amor SB, Cherif S, Elaloui E, Houas A, Elaloui M. Photodegradation of lignin from black liquor using UV/TiO2 system. J Photochem Photobiol. 2003;154:211–218. doi: 10.1016/S1010-6030(02)00316-7. [DOI] [Google Scholar]
- 36.Hernandez M, Hernandez-Coronado MJ, Montiel MD, Rodriguez J, Arias ME. Analysis of alkali-lignin in a paper mill effluent decolourised with two Streptomyces strains by gas chromatography-mass spectrometry after cupric oxide degradation. J Chromatogr. 2001;919:389–394. doi: 10.1016/S0021-9673(01)00813-5. [DOI] [PubMed] [Google Scholar]
- 37.Hedges JI, Ertel JR. Characterization of lignin by gas capillary chromatography of cupric oxide oxidation products. Anal Chem. 1982;54:174–178. doi: 10.1021/ac00239a007. [DOI] [Google Scholar]


