Abstract
Introduction: Atherosclerosis is one of the inflammatory underlying disease associated by oxidative stress and thrombotic agents. This study aimed to evaluate the potential role of cupper oxidized low-density lipoprotein (OxLDL) and thrombin for inducing mitogen activated protein kinases (MAPKs) in platelets.
Methods: Phosphorylation of P38MAPK, Jun N-terminal Kinase (JNK), and Extracellular signal-regulated kinases (ERK1/2) and P-selectin expression were determined in lysates of washed human platelets pretreated with low doses of thrombin and cu2+-OxLDL By Enzyme-linked immunosorbent assay (ELISA). Pharmacological inhibition was performed by SB203580, PD980559 and SP6000125 for P38MAPK, ERK1/2 and JNK activity, respectively. The ratio of phosphorylated to total protein was used for normalizing the phospho proteins contents of cells.
Results: OxLDL and thrombin significantly and differentially increased P-selectin expression (P<0.05), P38MAPK (P<0.05) and c-JNK (P<0.05) and ERK1/2 (P<0.05) phosphorylation in platelets. SB 203580 and SP6000125 significantly decreased P-selectin expression in both oxidative (P<0.05) and thrombotic (P<0.05) activated platelets.
Conclusion: Our results indicated that MAPK inhibitors can reduce atherothrombotic events via alterations in P-selectin expression suggesting that these inhibitors may be useful in the inhibition of atheroma development.
Keywords: Atherosclerosis, C-Jun N-terminal Kinase Extracellular, Signal-regulated Kinase, P38-Mitogen Activated Protein Kinase, P-selectin
Introduction
Oxidized low-density lipoprotein (OxLDL) has a crucial role in thrombotic disease and atherogenesis through activation of inflammatory cells including polymorphonuclear (PMN) cells and platelets.1,2 MAPK is a well known inflammatory pathway which acts as a series of kinase package that ultimately phosphorylates MAP kinases (MAPKs).3 Three different MAPK families have been reported: the extracellular-regulated protein kinases (ERK), the c-Jun NH2 terminal kinase (JNK) and p38.4,5 P38 can increase cytokine production through numerous mechanisms such as direct phosphorylation of some transcription factors such as AP-1 or increased phosphorylation of AU-rich elements (AREs) binding proteins.4 Small molecules of p38MAPK inhibitors have been reported to relieve the synthesis of inflammatory cytokines and MMPs. The specificity of a few broadly-used p38 inhibitors has been comprehensively described.6-8 SB-203580 is of a good selectivity for p38α and β compared with the majority of kinases.6,7
Pathogenesis of atherosclerosis is partially affected by leukocyte adhesion to the vessel wall and transendothelial migration. Localized activation of the endothelium through the action of inflammatory cytokines causes leukocyte rolling.9 Platelet P-selectin has been shown to play a pivotal role in the growth of atherosclerosis and thrombosis after vessel injury.10 Thrombin has been studied widely in platelets as one of the major platelet activators.11 It has been proven that platelet activation by oxidative vs. thrombotic agents leads to the differential activation of receptors.12 Based on this difference, we designed this study for testing the effects of cu2+oxidized low density lipoprotein or thrombin on P38, ERK 1/2, c-JNK phosphorylation and P-selectin expression simultaneously. Furthermore, we used mentioned inhibitors for considering their effects on activated platelets and inhibition panel of each of them on oxidative or thrombotic stimulated platelets.
Materials and methods
Based on the Ethical Committee of the Tabriz university of medical sciences, written informed consents were obtained from the patients prior to the study. Inclusion criteria were being health, no medication for the previous thirty days, and age 35 to 55. Blood samples (10 ml) were taken from all volunteers (n=90). Major exclusion criteria after sample collection were serum P-selectin >40 ng/ml.13 The blood samples were collected in ACD [65 mM citric, 85 mM citrate, 111 mM glucose 8.1:1.9(v/v)] then were centrifuged at 200 g for 8 min to prepare platelet-rich plasma (PRP). The PRP was centrifuged at 8000 g for 10 min at 4°C. The platelet pellet was then re-suspended in a Tyrode’s buffer (140 mM NaCl, 3 mM KCl, 12 mM NaHCO3, 0.4 mM NaH2PO4, 1 mM MgCl2, 2 mM CaCl2, 5.6 mM glucose; pH 7.4) to a final concentration of 5 × 108/mL.The platelet count in PRP was estimated by Beckman Coulter (Brea, CA, USA).
The study was carried out on 3 groups of platelets: control or resting platelet (RP), 50µg/ml cu2+-oxidized LDL treated platelets (OP), 0.5U/ml Thrombin-activated platelets (TP). The commercial LDLs after dialysis against PBS (10 mM NaH3PO4, 120 mM NaCl, 2.7 mM KCl, pH 7.4) were oxidized (1 mg/ml) by 10 μM CuSO4 in PBS for 24 h at 37°C. Malondialdehyde (MDA) was measured as a lipid peroxidation index.1
Treatment by stimuli was performed for 10 min for phosphoproteins and 24 hours for P-selectin expression. Platelet aggregation was monitored on aggregometer. P-selectin expression and phosphorylation levels of p38, ERK1/2 and JNK, in OP and TP were measured in presence or absence of 5 × 103nmol/l SB203580 (Product Number S 8307), 400 nM JNK inhibitor SP6000125 (Merck) (Dietikon, Switzerland) or10 µM ERK kinase inhibitor PD980559 (Merck).
Preparation of platelets lysate
The treated platelets were lysed within lysate buffer cotaining 10 mMTris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 20 mM Na4P2O7 1 mMNaF, 0.5% Deoxycholate1 mM PMSF, 1% Triton X-100, 10% Glycerol , 0.1% SDS , and centrifuge at 13,000 rpm at 4ºC for 10 minutes to eliminate the debris then supernatant was used for assessment of variables in duplicate way. To reduce the matrix effect the lysate was diluted for all assays (at least 1:10).
Enzyme-linked immunosorbent assay (ELISA)
Phospho-P38 MAPK (pThr180/pTyr182) content of lysates was measures by phospho-P38 MAPK ELISA kit (Catalog Number.CS0020, sigma) with sensitivity <0.8 U/mL. To normalize the p-p38MAPK content of the samples we used total p38 MAPK ELISA kit (Product No PM0100, sigma) with sensitivity <16 pg/mL. Phospho-JNK1&2 ELISA kit (Product Number CS0130.sigma) and JNK 1&2 ELISA kit (Product Number CS0100, sigma) were used in order to measure and normalize phospho-JNK, respectively. Likewise, Phospho-ERK1/2 (Thr202/Tyr204), ELISA kit (Product Number 7177) and total ERK1/2 ( Product Number 7050) were purchased from cell signaling. SPSS 13 (SPSS INC., Chicago, IL, USA) was used for statistical analyses. We used Mann-Whitney U-test and Kruskal-Wallis H to compare means. All results were presented as meanx±SD. X was equal to the ratio percentage of phosphorylated to total protein except for P-selectin that was presented as mean (concentration) ± SD. P<0.05 was considered significant.
Results
Phospho-P38 and total p38 were measured in the presence or absence of stimuli, thrombin or cu2+-OxLDL and SB 203580. The mean of total p38 in any group was not statistically different from others P= 0.77. Exposure to OxLDL for 10 min significantly increased the phosphorylation of p-P38 in OP group in comparison with RP (P<0.05) (Table 1). The similar result was observed in TP group (P<0.05) (Table 1); however, thrombin was a stronger stimulus than OxLDL (Table 1).
Table 1. OxLDL and Thrombin induce phosphorylation of p38, c-JNK and ERK1/2 in platelets.
| OP | P-value | RP | P-value | TP | |
| Pselectin µg/ml | 34.40± 6.50 | 0.001 | 11.29±2.58 | 0.001 | 46.86±6.44 |
| Phospho/total ERK(%) | 29.35±4.57 | 0.001 | 13.29±4.60 | 0.001 | 64.45±4.92 |
| Phospho/total JNK (%) | 87.70±6.15 | 0.001 | 20.07±4.80 | 0.001 | 89.27±8.03 |
| Phospho/total P38 (%) | 74.44±8.73 | 0.001 | 12.33±5.04 | 0.001 | 81.52±8.46 |
| Data are shown as the means (SD), Significant P- Value < 0.05. RP=Resting Platelets, OP=Oxidized-LDL treated Platelets , TP=Thrombin treated Platelets | |||||
A common P38 inhibitor called SB203580 was utilized. Pretreatment of platelets with SB203580 (5 mmol/l) for 30 min and stimulation by ox-LDL significantly decreased ox-LDL and thrombin induced p-P38 (P<0.05, P<0.05) (Tables 2 and 3), respectively.
Table 2. Effect of SB203580, PD980559, SP6000125 on P38, ERK1/2 and JNK phosphorylation and P-selectin expression induced by OxLDL.
| OP | OP-PD | OP-SB | OP-SP | |
| P-selectin µg/ml | 34.40± 6.50 | 33.01± 6.77 | 13.63±6.00 | 13.73±4.76 |
| Phospho/total ERK(%) | 29.35±4.57 | 10.56±3.47 | ||
| Phospho/total P38 (%) | 74.44±8.73 | 12.12±3.38 | ||
| Phospho/total JNK (%) | 87.70±6.15 | 21.21±7.73 | ||
| Data are shown as the means (SD), Significant P- Value < 0.05. RP: Resting Platelets, OP: Oxidized-LDL treated Platelets, TP: Thrombin treated Platelets. SB:SB203580 , PD=PD980559, SP=SP6000125. | ||||
Table 3. Effect of SB203580 , PD980559, SP6000125 on P38, ERK1/2 and JNK phosphorylation and P-selectin expression induced by Thrombin.
| RP | RP-PD | RP-SB | RP-SP | |
| P-selectin µg/ml | 11.29±2.58 | 10.16±2.22 | 12.62±4.22 | 26.10±5.90 |
| Phospho/total ERK(%) | 13.29±4.60 | 12.62±4.22 | ||
| Phospho/total P38 (%) | 20.07±4.80 | 10.53±2.55 | ||
| Phospho/total JNK (%) | 12.33±5.04 | 26.89±5.15 | ||
| Data are shown as the means (SD), Significant P- Value < 0.05. RP: Resting Platelets, OP: Oxidized-LDL treated Platelets, TP: Thrombin treated Platelets. SB:SB203580 , PD=PD980559, SP=SP6000125. | ||||
Treatment of platelets with 0.5 units/ml thrombin produced ERK1/2phosphorylation that was different from that of platelets treated with 50pg/ml OxLDL (P<0.05). A4 to 5 fold increase was observed in p-ERK/totalERK1/2 in TP (P <0.05; Table 1) and A1.5 fold increase in OP (P<0.05; Table 1) compared with RP. We used a specific ERK1/2 kinase inhibitor PD980559. Platelets were pretreated with PD980559 (5 mmol/l) for 30 min and stimulated by OxLDL or thrombin for expression of p-ERK1/2. PD980559 significantly decreased the expression of p-ERK1/2 induced by OxLDL (P<0.05; Table 2) or Thrombin (P<0.05; Table 3).
The results about p-JNK changes in OP and TP groups were more similar to p-p38 than to p-ERK1/2. We observed significant increases in induced p-JNK by OxLDL and thrombin (P<0.05; Table 1). In presence of JNK inhibitor SP6000125, significant decrease was observed in OP and TP (P <0.05; Tables 1-2).
We measured P-selectin expression in RP, OP and TP (Table 1). The results revealed that both of these stimuli increased the expression of P-selectin in OP (P<0.05; Table 1) and TP (P<0.05; Table 1). Likewise, inhibition of p-p38 by SB203580 or p-JNK by SP6000125 decreased P-selectin expression in OP (P<0.05; Table 2); however, ERK1/2 kinase inhibitor PD980559 did not significantly decrease expression of P-selectin in OP (Table 2). Effects of PD980559, SB203580 and SP6000125 were also evaluated in TP. Similarly, PD980559, SB203580 and SP6000125 (26.10±5.90) can significantly decrease p-selectin levels (P<0.05; Table 3). A strong relationship could be observed between P-P38/total P38(%) and P-selectin in OP (r=0.65, P<0.05) or TP (r=0.75, P<0.05) (Figure 1), between P-JNK/total JNK (%) and P-selectin in OP (r=0.72, P<0.05) or TP, between P-ERK1/2/total ERK1/2(%) P-selectin in TP (r=0.79, P<0.05; Figure 2). Nevertheless, there was a weak correlation between P-ERK1/2/total ERK1/2(%) and P-selectin in OP(r=0.24, P<0.05) or between p-JNK/total JNK (%) and P-selectin in TP (r=0.20, P<0.05) by Pearson correlation (Figure 3).
Figure 1 .
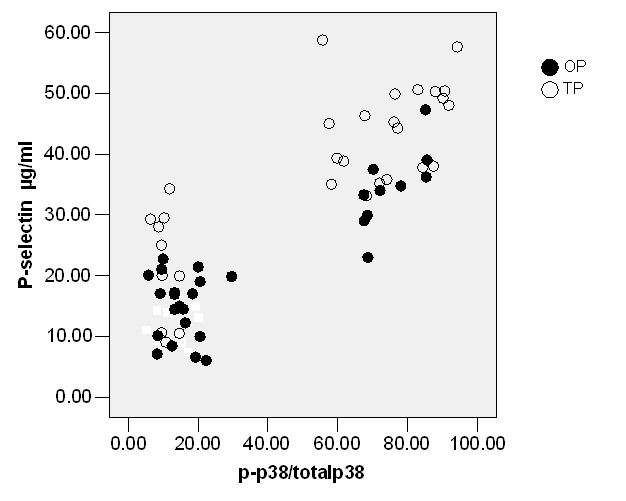
Correlation between phosph/total p38 and platelet P-selectin levels in oxidized LDL or Thrombin Threated platelets
Figure 2 .
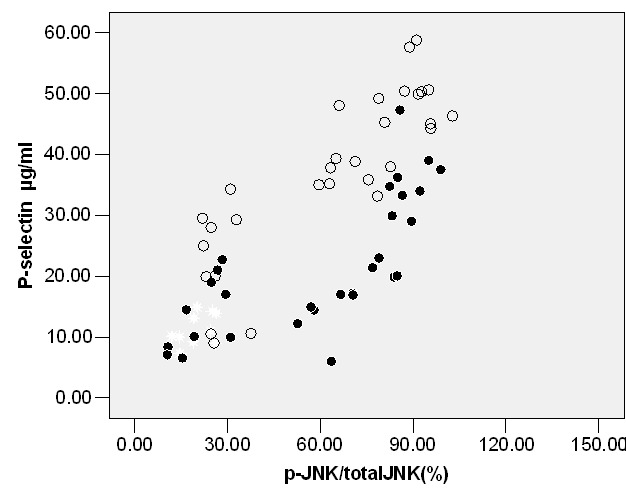
Correlation between phospho/total c-JNK and platelet P-selectin levels in oxidized LDL or Thrombin Threated platelets
Figure 3.
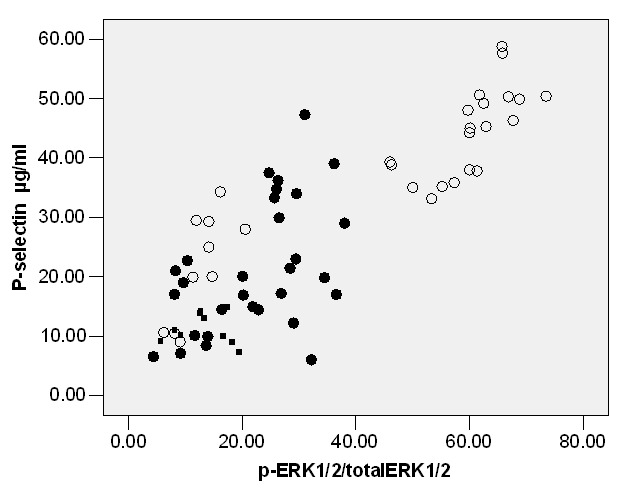
Correlation between phosph/total ERK1/2and platelet P-selectin levels in oxidized LDL or Thrombin Threated platelets
Discussion
Inflammation plays an essential role in the development of atherosclerosis.14 Polymorphonuclears, and T-cells are among the most studied inflammatory cells blamed for plaque formation15-17; however, the real role of platelets has not yet been well understood .These inflammatory cells release various cytokines such as ADP, growth factors and other internal mediators even transcription factors NF-κB.18 cell rolling, adhesion, and transendothelial migration are believed to be the cosequalae of such products . We activated human platelets with thrombin or OxLDL, and examined agonist-dependent effects on the phosphorylation of signaling proteins, and on P-selectin expression. Three major signaling proteins including JNK, ERK1/2, and p3819 were examined as to whether they were differentially affected by thrombin vs. OxLDL stimulation. Thrombin-induced signaling cascades in platelets have been intensively studied. The feature of platelet activation by the cu2+-OxLDL expectedly differed from the feature of thrombin so phosphorylation of JNK, ERK1/2 and p38 MAPK differed considerably between these agonists. This may be related to type of their receptors, as highlighted by previous studies. Some receptors have been introduced for the mediation of the cell stimulation by OxLDL for instance CD36 and toll-like receptor 4 (TLR4) in macrophages and TLR1/2 in platelets.20 However, the details of signaling pathways involving ERK1/2 or p38 remain controversial.21,22 Recent data from Rex et al. demonstrates that OxLDL-stimulation of TLR2 leads to P-selectin expression, stimulation of integrin αIIbβ3, and activation of PI3K/Akt signaling pathway.19,20 Thrombin, on the other hand, has been studied extensively in platelets. As one of the major platelet agonists, it activates the protease activated receptor 1 and 4 (PAR1/4) on the platelet surface which causes to activation of PI3K and phospholipase Cβ (PLCβ) pathways. Intracellular calcium concentrations are increased once these pathways are stimulated and platelets shape change too.21 However, many details of these signaling cascades, and the mechanisms of their downstream events remain to be elucidated. Also, greater extent of phosphorylation of both ERK1/2, p38 was observed by Thrombin compared to OxLDL. Both kinases are phosphorylated in a more rapid manner which is suggestive of their effect in inside-out signaling, when signaling events activated by an agonist lead to changes in conformation of αIIbβ3.22,23 Therefore, further expression of P-selectin is expectable as found in our study.
The second part of our study focused on inhibitors and P-selectin .We observed more or less significant correlations between P-selectin and p-38 , p-JNK or p-ERK1/2. SB203580 and SP600012 diminished P-selectin expression in OxLDL treated platelets; this, however, was not true about PD980559. In other words, all three inhibitors relieved P-selectin expression in thrombin treated platelets. This conducted us to this hypothesis that ERK1/2 is less important in oxidative stimulation in comparison with thrombin and the idea that these inhibitors probably might be counted as effective anti- atherothrombosis agents.
In conclusion, our data demonstrated that platelet stimulation with thrombin or OxLDL leads to differential activation of signaling proteins as well as P-selectin expression. These results highlight the differences in the platelet's immune vs. thrombotic responses and form the bases for further functional and mechanistic studies to better understand the platelet's role in innate immunity and inflammation.
Footnotes
Ethical issues: The protocol for the research project has been approved by the ethic committee at TUMS (Tabriz University of Medical Sciences) which is in compliance with the Helsinki Declaration.
Competing interests: The authors had no competing interests to declare in relation to this article.
References
- 1.Witztum JL, Steinberg D. The oxidative modification hypothesis of atherosclerosis: does it hold forhumans? . Trends Cardiovasc Med . 2001;11: 93–102. doi: 10.1016/s1050-1738(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 2.Daub K, Lindemann S, Langer H, Seizer P, Stellos K, Siegel-Axel D. et al. The evil in atherosclerosis: adherent platelets induce foam cell formation. Semin Thromb Hemost . 2007;33:173–8. doi: 10.1055/s-2007-969031. [DOI] [PubMed] [Google Scholar]
- 3.Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nature Reviews . 2006; 6:532–40. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- 4.Saklatvala J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr Opin Pharmacol . 2004;4:372–77. doi: 10.1016/j.coph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S, McDonnell PC, Gum RJ, Hand AT, Lee JC. Novel homologues of CSBP/p38 MAP kinase. Biochem Biophys Res Comm . 1997;235:533–8. doi: 10.1006/bbrc.1997.6849. [DOI] [PubMed] [Google Scholar]
- 6.Fabian MA, Biggs WH 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG. et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol . 2005; 23:329–36. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 7.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT. et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol . 2008; 26:127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 8.Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL, Griswold DE. Pharmacological profile of SB 20 a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther . 1996; 3580:1453–61. [PubMed] [Google Scholar]
- 9.Dong ZM, Brown AA, Wagner DD. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation . 2000;101:2290–5. doi: 10.1161/01.cir.101.19.2290. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo Y, Amano S, Furuya M, Namiki K, Sakurai K, Nishiyama M. et al. Involvement of p38alpha mitogen-activated protein kinase in lung metastasis of tumor cells. J Biol Chem . 2006; 281:36767–75. doi: 10.1074/jbc.M604371200. [DOI] [PubMed] [Google Scholar]
- 11.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S. et al. Stimulation of Toll-like receptor 2 in human platelets induces athromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res . 2009; 104:346–54. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WF, Lee JJ, Change CC, Lin KH, Wang SH, Sheu JR. Platelet protease-activated receptor (PAR)4, but not PAR1, associated with neutral sphingomyelinase responsible for thrombin-stimulated ceramide-NF-κB signaling in human platelets. Haematologica . 2013;98:793–801. doi: 10.3324/haematol.2012.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masopust J, Malý R, Andrýs C, Vališ M, Bažant J, Hosák L. Markers of thrombogenesis are activated in unmedicated patients with acute psychosis: a matched case control study. BMC Psychiatry. 2011;11:2. doi: 10.1186/1471-244X-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YG, Zhang HG, Zhang GY, Fan JS, Li XH, Liu YH. et al. Panax notoginseng saponins attenuate atherosclerosis in rats by regulating the blood lipid profile and an anti-inflammatory action. Clin Exp Pharmacol Physiol . 2008;35:1238–44. doi: 10.1111/j.1440-1681.2008.04997.x. [DOI] [PubMed] [Google Scholar]
- 15.Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Björkström NK. et al. Distinct Infiltration of Neutrophils in Lesion Shoulders in ApoE–/– Mice. Am J Pathol . 2010;177:493–500. doi: 10.2353/ajpath.2010.090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim YC, Wakelin MW, Henault L, Goetz DJ, Yednock T, Cabañas C. et al. Alpha4beta1-integrin activation is necessary for high-efficiency T-cell subset interactions with VCAM-1 under flow. Microcirculation . 2000;7:201–14. [PubMed] [Google Scholar]
- 17.Takeuchi Y, Okayama N, Imaeda K, Okouchi M, Omi H, Imai S. et al. Effects of histamine 2 receptor antagonists on endothelial-neutrophil adhesion and surface expression of endothelial adhesion molecules induced by high glucose levels. J Diabetes Complicat . 2007;21:50–5. doi: 10.1016/j.jdiacomp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Nagahama Y, Obama T, Usui M, Kanazawa Y, Iwamoto S, Suzuki K. et al. Oxidized low-density lipoproteininduced periodontal inflammation is associated with the up-regulation of cyclooxygenase-2 and microsomal prostaglandin synthase 1 in human gingival epithelial cells. Biochem Biophys Res Commun . 2011; 413:566–71. doi: 10.1016/j.bbrc.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Chan CP, Chang MC, Wang YJ, Chen LI, Tsai YL, Lee JJ. et al. Thrombin activates Ras-CREB/ATF-1 signaling and stimulates c-fos, c-jun, and c-myc expression in human gingival fibroblasts. J Periodontol . 2008; 79:1248–54. doi: 10.1902/jop.2008.070523. [DOI] [PubMed] [Google Scholar]
- 20.Rex S, Beaulieu LM, Perlman DH, Vitseva O, Blair PS, McComb ME. et al. Immune versus thrombotic stimulation of platelets differentially regulates signalling pathways, intracellular protein-protein interactions, and alpha-granule release. J Thromb Haemost . 2009;102:97–110. doi: 10.1160/TH08-08-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest . 2009; 119:136–45. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation . 2000; 101:2889–95. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- 23.Tanigawa H, Miura S, Matsuo Y, Fujino M, Sawamura T, Saku K. Dominant-negative Lox-1 blocks homodimerization of wild-type Lox-1- induced cell proliferation through exracellular signal regulated kinase ½ activation. Hypertension . 2006; 48:294–300. doi: 10.1161/01.HYP.0000229825.98545.5e. [DOI] [PubMed] [Google Scholar]
- 24.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol . 2005; 25:1213–9. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]


