Abstract
The general trends in synthetic bone grafting materials are shifting towards approaches that can illicit osteoinductive properties. Pharmacologics and biologics have been used in combination with calcium phosphate (CaP) ceramics, however, recently have become the target of scrutiny over the safety. The importance of trace elements in natural bone health is well documented. Ions, e.g. lithium, zinc, magnesium, manganese, silicon, strontium etc. have shown to increase osteogenesis and neovascularization. Incorporation of dopants into CaPs can provide a platform for safe and efficient delivery in clinical applications where increased bone healing is favorable. This review highlights use of trace elements in CaP biomaterials, and offers an insight into the mechanisms of how metal ions can enhance both osteogenesis and angiogenesis.
Keywords: Osteogenesis, Angiogenesis, Calcium phosphates, Trace elements, Bone remodeling
Can trace metal ions be an alternative to biologics in bone tissue engineering?
Bone typically contains about 70% inorganic calcium phosphate (CaP) with the remainder mostly comprised of organic collagen matrix. It is no surprise that some of the most successful synthetic materials used in orthopedic implant technologies are CaP based. CaP materials are used in a variety of orthopedic applications ranging from cements, to metal implant coatings and, most commonly, bone void fillers. They are a bioresorbable material, which means that over time they will break down and be replaced with natural tissue. CaPs range in composition, which is the main determinant of their application. The Ca:P ratio plays a critical role in the stability and overall resorptive capabilities [1].
One of the many beneficial properties of CaP materials in orthopedic applications is that they demonstrate osteoconductive properties. Essentially, CaPs act as scaffold in which they facilitate new bone formation by allowing the migration, attachment and proliferation of bone forming cells. Recent trends in technology, however, focus on the incorporation of materials that extends the performance of CaPs to have osteoinductive properties, or the ability to actually stimulate new bone formation. It is important to recognize that the ability to stimulate osteogenesis, or bone growth, has to also couple with angiogenesis, or blood vessel formation. The two processes are intricately linked and osteogenesis would not be possible without angiogenesis [2,3]. The first commercial synthetic product to offer the promise of osteoinductivity and angiogenesis was Medtronic's recombinant human bone morphogenic protein-2 (rhBMP-2) Infuse product. While fantastic results have been achieved in many applications ranging from lumbar (lower) spinal fusion, craniomaxilofacial and dental applications, long bone grafting applications, the apparent off label use in cervical (upper) spinal fusion applications has led to troubling issues regarding the safety and efficacy of the product. rhBMP is a growth factor that has been proven to induce bone and cartilage growth [4,5]. Pharmacologics and biologics, such as BMP-2, have been used in combination with calcium phosphate (CaP) ceramics, however, recently have become the target of scrutiny over the safety and the FDA has become increasingly resistant to approve the use of such materials. The major concerns have related to ectopic, or unwanted bone formation where in certain situations can lead to the very serious side effects [6–8]. The clinical successes of rhBMP products have led to a flurry of research into the incorporation of other potentially beneficial growth hormones and biologics into orthopedic materials. Compounds of interest include transforming growth factor β (TGF-β), fibroblast growth factor (FGF), insulin like growth factor (IGF), vascular endothelial growth factor (VEGF) and a variety of bisphosphonates (BPs) [1]. With all of the negative attention drawn to these biological compounds, however, the FDA has taken a harder stance on the approval of such devices making commercial viability much more difficult and expensive.
An alternative and potentially safer strategy has been to look past the addition of biologics and investigate a more natural approach. The major CaP component of bone is not a homogenous material. Bone incorporates various nutrients (for instance Na+, Mg2+, Zn2+, Si4+, Sr2+etc.) in the form of trace elements. These trace elements have been found to play absolutely vital roles in the formation, growth and repair of bone (Table 1). Various studies have also demonstrated that the addition of trace elements to CaP materials can lend controlled degradation, increase the mechanical strength of the materials and positively influence the biological response [9–12].
Table.
Role of metal ions and their mechanism of action
| What role? | Mechanism of action | |
|---|---|---|
| Li+ | osteogenesis | ➢ Inhibit glycogen synthase kinase3 (GSK3), which is a negative regulator of the Wnt signaling pathway [22,23]. ➢ Activates β-catenin mediated T cell factor (TCF) dependent transcription during bone and cartilage fracture healing [24]. β-catenin is known for its central role as signaling mediator in canonical Wnt signaling pathway |
| Zn2+ | osteogenesis | ➢ In the cellular microenvironment zinc is thought to stop the osteoclastic resorption process and stimulate the osteoblastic bone building process [33,34], |
| Mg2+ | angiogenesis | ➢ Magnesium induces nitric oxide production in endothelial cells which is essentially the same mechanism that VEGF uses to induce angiogenesis [37,38] |
| Sr2+ | osteogenesis | ➢ Strontium stimulates bone formation by a dual mode of action: stimulatory role on bone forming osteoblast cells, and inhibitory role on bone resorbing osteoclast cells. ➢ It activates calcium sensing receptor (CaSR) and downstream signalling pathways. This promotes osteoblast proliferation, differentiation and survival, while at the same time induces apoptosis in osteoclast cells resulting in decreased bone resorption [47]. ➢ Activation of the CaSR in osteoblasts [43,44] simultaneously increases osteoprotegerin (OPG) production and decreases receptor activator of nuclear factor kappa beta ligand (RANKL) expression [45]. ➢ OPG is a protein that inhibits RANKL induced osteoclastogenesis by operating as a decoy receptor for RANKL [46]. |
| Cu+ | angiogenesis | ➢ Copper induced angiogenesis is probably caused by the upregulation of VEGF expression [55,57]. Copper induced toxicity: At high concentration, copper can generate reactive oxygen species (ROS) in presence of superoxide radical anions (*O2−). These ROS induce oxidative damage to cells through DNA strand breaks and oxidation of bases [58,59]. |
| Co2+ | angiogenesis | ➢ It is believed that Co2+ ions induce hypoxia on the cellular level by stabilizing the hypoxia-inducible factor (HIF)-1α [65,66]. ➢ Cell compensate to this hypoxic environment by expressing genes (such as vascular endothelial growth factor (VEGF) and erythropoietin (EPO)) that promote neovascularization and angiogenesis. Cobalt induced toxicity: Like copper, cobalt also causes oxidative damage to cells by ROS [60]. Increased soluble Co2+ ions level might cause serious adverse reactions to the surrounding tissues as well as systemic toxicity. Co2+ ions can activate and increase bone resorbing osteoclast cells differentiation resulting in osteolysis aseptic implant loosening [61]. |
| Osteogenesis/angiogenesis | ➢ Thought to play a role in the upregulation of transforming growth factor beta (TGF-β) and VEGF [67] | |
| Mn+2/+3 | Osteogenesis | ➢ Is thought to have implications in the parathyroid hormone (PTH) signaling pathway, a key regulator of calcium [87]. ➢ Manganese superoxide dismutase (MnSOD) is believed to neutralize the formation of reactive oxygen species, which contribute to increased osteoclastogenesis and decreased osteoblastogenesis [89] |
| Si+4 | Osteogenesis/Angiogenesis | ➢ Has been shown to induce angiogenesis by upregulating nitric oxide synthase (NOS) leading to increased VEGF production [83]. ➢ Osteogenic mechanisms are not well understood, but has been shown to play a vital role in the mineralization process [73] |
Pharmacologics and biologics are all surface loaded and exhibit a burst release effect with very little long term benefit. While the specific action of many of these trace elements is still largely a mystery, it has been a hotbed for research and the potential for commercialization is high. In fact, many studies have shown that the addition of trace elements will naturally increase production of important growth factors such as BMP-2 and VEGF [13,14]. Because these inorganic additives are largely incorporated into the body on a dietary basis and are used in many important processes in the body, there are distinctive regulatory mechanisms that inherently deem their use as additives in CaPs safer than the addition of pharmacologics or biologics.
Incorporation of trace metallic elements into tissue engineering constructs offer low cost, longer shelf-life with low regulatory burden and low risk as compared to biologics. Due to these added benefits, trace metallic elements delivery as therapeutic agents is getting significant attention for the tissue engineering and regenerative medicine applications [15–17]. The focus of this review is trace elements in CaPs, which have shown significant osteogenic or angiogenic capability in bone tissue engineering. The purpose of this article is to review the use of trace elements as additives to CaP biomaterials and to offer insight into the mechanism of how these next generation biomaterials can enhance both osteogenesis and angiogenesis for faster patient healing times and high surgical success rates.
Monovalent Cations
Inclusion of monovalent metallic ions such as sodium (Na+), potassium (K+), lithium (Li+), and silver (Ag+) into CaP bioceramics have been explored. The interest for Ag+ ion is because of its antibacterial property. Substitution of Na+, K+ and Li+ monovalent cations into β-tricalcium phosphate (β-TCP) gives some thermal stability [18]. Among these monovalent ions, Li+ is the ion of interest that brought attention due to its role in osteogenesis. Lithium is a fairly new additive of interest to bone substitute. Traditionally, it is given as a medication to treat bipolar and other psychiatric disorders. Several studies have noted that lithium treatment may result in a hyperparathyroid condition that is often mild and reversible [19,20]. What is interesting is that hyperparathyroidism has been linked to increased rates of bone loss, but the opposite has also been found true in a study [21], where 75 patients treated with lithium were found to exhibit significantly greater bone mass in several areas compared to 75 normal participants. While these results were not entirely expected from this study, preliminary findings suggested that patients treated with lithium experienced a state of lower bone turnover. Other research has noted lithium's ability to inhibit glycogen synthase kinase3 (GSK3), which is a negative regulator of the Wnt signaling pathway [22,23]. β-catenin is known for its central role as signaling mediator in canonical Wnt signaling pathway, and it has been shown that Li+ activates β-catenin mediated T cell factor (TCF) dependent transcription during bone and cartilage fracture healing [24]. The Wnt signaling pathway is one of the most important signal cascades bone formation and the bone remodeling process [25,26]. Figure 1 presents aschematic demonstration of the importance of Wnt signaling in the various aspects of the bone formation process [27]. Research has also demonstrated a direct link between BMP production and an activated Wnt signaling pathway in osteoblasts [28,29]. Lithium containing CaP coatings on Ti6Al4V has shown increased cellular attachment and proliferation in a human osteosarcoma cell line [30]. While β-catenin stabilization favors osteoblastic differentiation once precursor cells are committed to the osteoblast lineage, the same could interfere with the osteogenic differentiation of mesenchymal cells [24]. Thus, the activation of β-catenin signaling by Li+ shows its great effectiveness for fracture healing only when the fracture is treated by lithium treatment. In this sense, lithium has the potential to be a candidate of interest for inclusion into CaP bone substitutes for orthopedic implant applications.
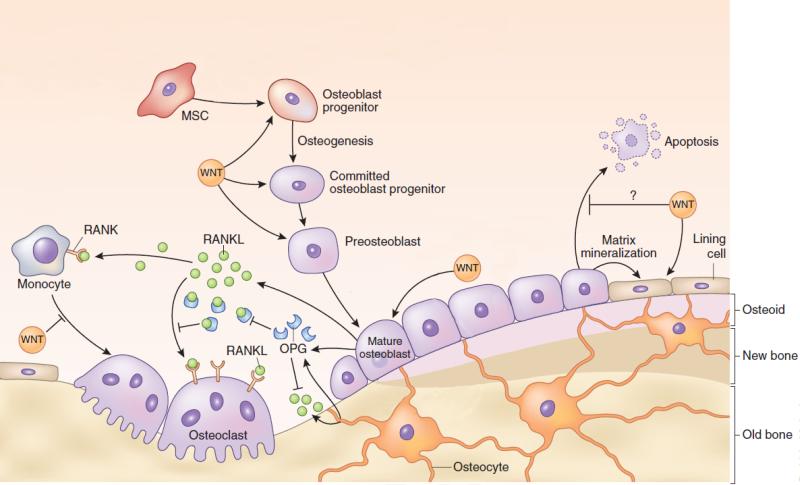
Figure 1.
Schematic demonstrating the important role of WNT in the various aspects of the bone formation process. This figure also defines the relationship between osteoblasts and osteoclasts in the bone remodeling process. Osteoblasts produce RANKL which stimulates osteoclastogenesis. At the same time, however, osteoblasts also produce OPG which competitively binds to RANKL to help decrease osteoclastogenesis. The balance of OPG:RANKL is a very important factor in healthy bone formation. This figure has been modified from its original version. Adapted with permission from reference [27].
Bivalent Cations
Many bivalent trace metallic ions have demonstrated their beneficial effects in bone tissue engineering applications. Substantial evidence from literature shows that Zn2+, Mg2+, Sr2+, Cu2+ and Co2+ ions play a vital role either in osteogenesis or in angiogenesis or in both cases. Zinc (Zn2+) is considered an important essential trace element in human development. There are several important metalloenzymes that utilize zinc for structure, catalytic or regulatory actions. One such enzyme that is absolutely vital for the maturation of new bone formation is alkaline phosphatases (ALP). ALPs are glycosyl-phosphatidylinositol anchored, Zn2+ metallated glycoproteins that are released during the maturation process of the osteoblastic cell life cycle and help to catalyze the hydrolysis of phosphomonoesters into inorganic phosphates [31]. Essentially, their role is to create an alkaline environment that favors the precipitation and subsequent mineralization of these inorganic phosphates onto the extracellular matrix that the osteoblasts produce. Dietary deficiencies in zinc are related to several skeletal developmental defects and may have a role in the prevention of osteoporosis. During the natural bone remodeling process, zinc is released from the bone [32]. The excess amount of zinc in the microenvironment due to this release is thought to stop the osteoclastic resorption process and stimulate the osteoblastic bone building process [33,34], which makes it an ion of high interest. Zinc doped CaP materials have shown increased osteoblastic response in vitro as well as new bone formation in vivo [10,35]. Zinc phosphate cements are some of the oldest and most widely used cements in the dental industry.
Magnesium (Mg2+) is an essential element and the 10th most abundant element in the human body, with about 65% of total body magnesium contained in bone and teeth [36]. High doses of magnesium in vitro were correlated to responses that suggested magnesium plays a direct and vital role in maintaining vascular function [37,38]. The presence of Mg2+ induced nitric oxide production in endothelial cells which is essentially the same mechanism that VEGF uses to induce angiogenesis [37,38] as shown in Figure 2 [39]. In a yearlong animal study, a group reported that prolonged magnesium deficiency directly resulted in osteoporosis [40]. Researchers have also found that by doping CaP materials with magnesium, the densification is improved as well as osteoblastic cellular attachment, proliferation and ALP production [41]. In vivo studies have noted that hydroxyapatite, a specific composition and phase of CaP, doped with magnesium phosphate in a femoral bone defect showed greater osteogenic properties when compared to a pure control [42]. Magnesium has been used clinically in magnesium phosphate bone cements and in several different bioglass compositions.
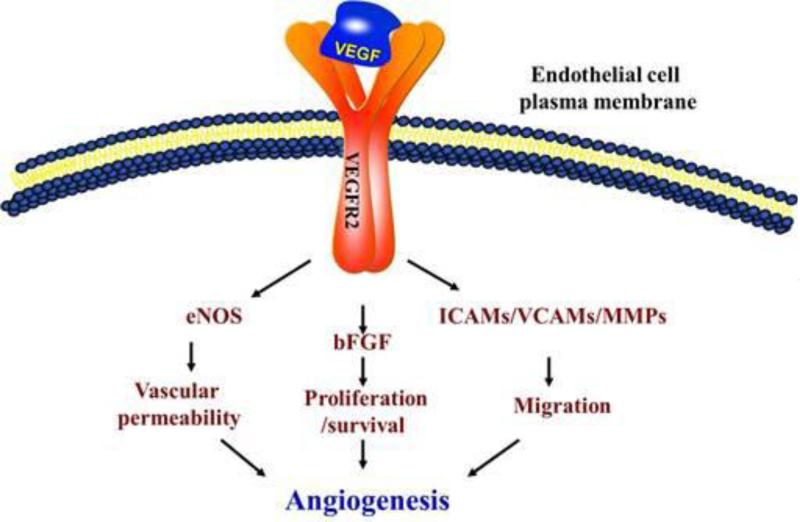
Figure 2.
Schematic representation of the mechanisms VEGF activates in endothelial cells to promote angiogenesis. VEGF released by osteoblastic (and other) cells will activate the transmembrane VEFGR2 receptors in endothelial cells, which in turn will activate several pathways responsible for angiogenesis, including eNOS, basic fibroblast growth factor (bFGF), intercellular adhesion molecules (ICAMS), vascular cell adhesion proteins (VCAM) and matrix metalloproteinases (MMPs). Adapted with permission from reference [39].
Strontium (Sr2+) is a non-essential element, which is about 0.035 % of its calcium content in our skeleton system. Sr2+ has bone seeking behavior and has been shown to enhance bone regeneration when incorporated into the synthetic bone graft. Essentially, because it is very similar in size and charge to Ca2+, it is thought to displace Ca2+ ions in osteoblastic mediated processes. Researchers have identified that strontium likely stimulates bone formation by a dual mode of action. First, it activates the calcium sensing receptor (CaSR) in osteoblasts [43,44], which simultaneously increases osteoprotegerin (OPG) production, and decreases receptor activator of nuclear factor kappa beta ligand (RANKL) expression [45]. OPG is a protein that inhibits RANKL induced osteoclastogenesis by operating as a decoy receptor for RANKL [46]. The OPG/RANKL ratio, then, can be a powerful regulator of bone resorption and osteoclastogenesis. Figure 3(a) presents a schematic of how strontium plays its stimulatory role on bone forming osteoblast cells, and inhibitory role on bone resorbing osteoclast cells. A detail mechanism for osteoblastogenesis activation by strontium is shown in Figure 3(b) [47]. In the UK, strontium is utilized in the form of strontium ranelate as a prescriptive treatment for osteoporosis in post-menopausal women. Phase III clinical trials that began in 2000 investigated the efficacy of strontium ranelate in reducing vertebral fractures and peripheral fractures, including hip fractures. After 3 years, patients treated with strontium ranelate showed significant reduction in vertebral fractures (41%) and hip fractures (36%) compared to patients treated with placebo [48]. Several studies have shown positive effects of the addition of strontium to CaP materials both in vitro and in vivo. Weak bonding is a primary reason for failure of a revision total hip arthroplasty (THA) surgery. In a study, a strontium doped HA based bone cement was used in a goat model to investigate the outcomes of revisional THA surgeries compared to a conventional poly(methyl methacrylate) (PMMA) cement. After 9 months, the strontium containing HA cement was found to have better mechanical bonding than the PMMA [49]. Strontium doped CaP material was found to increase endothelial cellular proliferation and tubule formation, both characteristics of angiogenic abilities[50]. Strontium doped material also showed between 5% and 10% increased new bone growth in vivo over 16 weeks when compared to a similar material without strontium as shown in Figure 4 [51]. There is no data available showing induced toxicity caused by the addition of strontium to biomaterials. While strontium is not used in any US devices, a company RepRegen is already marketing a grafting product StronBone that contains strontium in the UK.
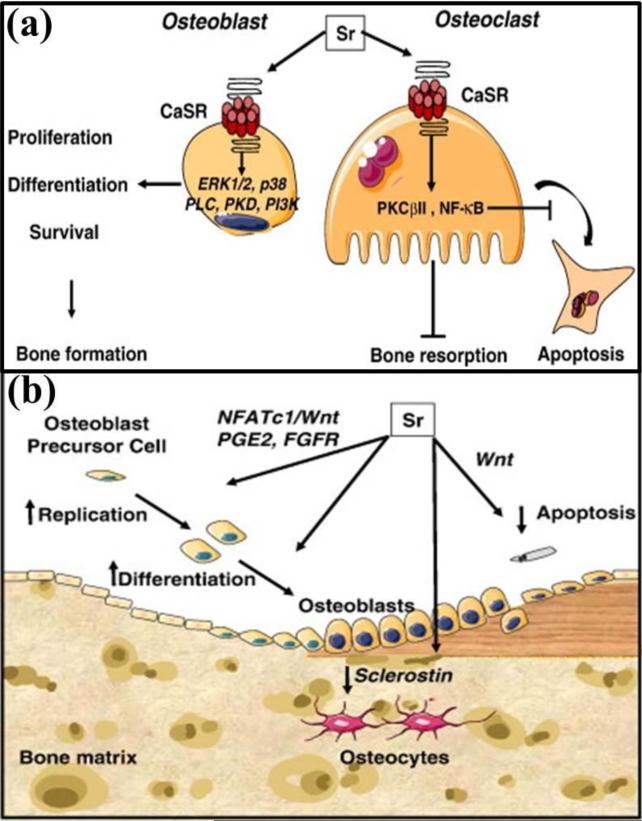
Figure 3.
(a) A schematic showing the dual mechanism of action by strontium (Sr): stimulatory role on bone forming osteoblast cells, and inhibitory role on bone resorbing osteoclast cells. Sr demonstrates its stimulatory effect on osteoblast cells through the activation of calcium sensing receptor (CaSR) and downstream signalling pathways, which promotes osteoblast proliferation, differentiation and survival. While the same CaSR and downstream signalling pathways activation by Sr in osteoclast cells induce apoptosis resulting in decreased bone resorption. Adapted with permission from reference [47]; (b) A schematic showing how Sr activates osteoblastogenesis: Sr induces increased production of nuclear factor of activated Tc (NFATc)/Wnt signaling, prostaglandin E2 (PGE2), activation of fibroblast growth factor receptor (FGFR) in osteoblastic cells, and reduction of the sclerostin expression (a Wnt antagonist produced by osteocytes). Adapted with permission from reference [47].
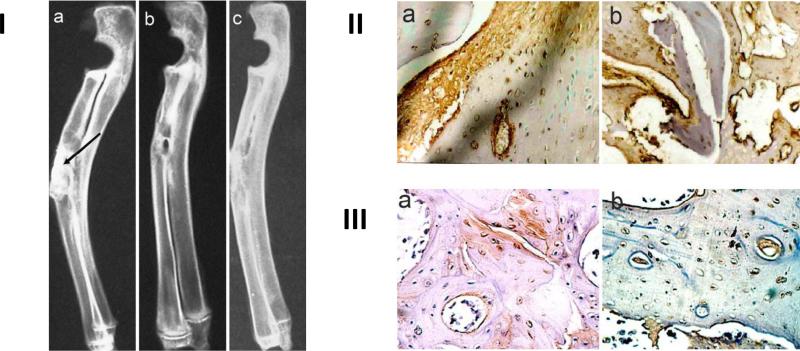
Figure 4.
I – (a) Shows arrow pointing to defect site in rabbit radiuses directly after implantation. Radiodensity is much greater than that of surrounding natural bone tissue. (b) Shows defect site after 16 weeks implantation with strontium doped calcium polyphosphate (SCPP). Implant is completely integrated into surrounding tissue as evidenced by similar radio opacity. (c) Shows defect site after 16 weeks implantation with a pure calcium polyphosphate (CPP) graft. Implantation site is much more radio dense than the surrounding bone tissue. II – (a) SCPP and (b) CPP tissue sections after 2 weeks stained for collagen I. Brown color indicates collagen formation. III – (a) SCPP and (b) CPP tissue sections after 2 weeks implantation stained for BMP-2. The red/brown color indicates BMP-2. Adapted with permission from reference [51].
Copper (Cu), which is an essential trace element with its highest abundance in the liver tissue is known for its stimulatory effect on endothelial cells towards angiogenesis [16]. Cu functions as a cofactor and is an important component for structural and catalytic properties of many enzymes. Although copper can exist in its oxidized cupric (Cu2+) or reduced cuprous (Cu+) state, the bivalent Cu2+ has attracted much attention for its role in angiogenic processes. Cu2+ ions loaded with as low as 22 ng in a dicalcium phosphate dehydrate (DCPD) scaffold has shown improved in vivo vascularization compared to control DCPD scaffold without any copper ions [52]. Enhanced activity and proliferation of osteoblastic cells were observed, when 0.56 μg Cu2+/cm2 was loaded on calcium phosphate cement (CPC) scaffolds (Brushite: CaHPO4.2H2O) [53]. Copper induced angiogenesis is also extensively studied in the systems other than CaPs. Increased blood vessel formation is reported when 560 ng CuSO4 was loaded per collagen scaffold [54]. In the same study, CuSO4 in combination with 200 ng FGF-2 showed a synergistic effect on microvessel infiltration into the scaffold, whereas microvessel area was significantly lower in the scaffold with 200 ng FGF-2 alone. Mesoporous bioactive glass (MBG) scaffolds showed multifunctional characteristics such as angiogenesis potential, osteostimulation, and antibacterial properties when copper was doped into MBG [55]. A recent study demonstrated extended survival of human umbilical vein endothelial cells (HUVECs) in presence of 10 % CuO in a phosphate-based glass (PG) with controlled 4 ppm Cu2+ ion release as compared to control PG in absence of copper [56]. Copper induced angiogenesis is probably caused by the upregulation of VEGF expression [55,57]. There is a lack of information on extended copper release from biomaterials, and in vivo effect on the surrounding tissues. More than optimum concentration can cause copper induced cellular toxicity. Copper can generate reactive oxygen species (ROS) in presence of superoxide radical anions (*O2−). These ROS induce oxidative damage to cells through DNA strand breaks and oxidation of bases [58,59].
Cobalt (Co) is an essential element in human physiology and an integral part of vitamin B12. The wide application of cobalt–chromium hard-metal alloys (metal-on-metal articulations) in total joint arthroplasty over the last decade was because of their potential advantages, including lower wear rate and increased stability compared to conventional metal-on-polyethylene articulations. Concerns have been raised due to cobalt associated toxicity resulted from wear, corrosion, and ion leaching from CoCr implants. Like copper, cobalt also causes oxidative damage to cells by ROS [60]. Increased soluble Co2+ ions level might cause serious adverse reactions to the surrounding tissues as well as systemic toxicity. Co2+ ions can activate and increase bone resorbing osteoclast cells differentiation resulting in osteolysis aseptic implant loosening [61]. Along with the evidence of cytotoxic effect by Co2+ ions (usually at higher concentration), there are evidences that the presence of Co2+ in tissue engineering scaffolds stimulate angiogenesis. It has been reported that mesoporous bioactive glass (MBG) scaffolds doped with 2 and 5% Co2+ significantly enhanced VEGF protein secretion, HIF-1α expression, and bone-related gene expression compared to pure MBG scaffolds [62]. Bone marrow stromal cells (BMSCs) showed significant upregulation of VEGF in culture condition when treated by 100μM CoCl2 [63]. Higher degree of vascularization and enhanced osteogenesis were reported, when a collagen scaffold seeded with these CoCl2 pre-treated BMSCs were implanted subcutaneously. One study showed that hydroxyapatite nanoparticles (HA NPs) substituted with 5 to 12 wt.% Co2+ did not cause any adverse effect on epithelial caco-2 cells, whereas substantially decreased osteoblast cells in vitro [64]. Contrary to in vitro results in the same study, Co2+- HA NPs showed improved osteogenesis and angiogenesis when implanted in a rat model bone defect. It is believed that Co2+ ions induce hypoxia on the cellular level by stabilizing the hypoxia-inducible factor (HIF)-1α [65,66]. Cell compensate to this hypoxic environment by expressing genes (such as vascular endothelial growth factor (VEGF) and erythropoietin (EPO)) that promote neovascularization and angiogenesis.
Tri-/tetravalent cations
A growing body of evidence has been indicating that boron may be an essential trace element to human health, with particular focus on wound healing functions and bone health. One group has observed that isolated placental nuclei in the presence of boron synthesized functional RNA molecules that produced the proteins VEGF and TGF-β [67]. Both VEGF and TFG- β are vital to new bone formation and wound healing, with the method of action being neovascularization [68]. One study has noted markedly decreased bone formation capabilities in mice that were treated to a boron deficient diet [69]. Conversely, another study determined that in addition to a high energy diet, boron supplementation resulted in not only significantly increased bone mineral density, but also an increase in both compressive and tensile bone strength [70]. Commercially, boron can be found in certain Bioglass products (40S5B5), but not with a claim that it enhances bioactivity. It, traditionally, has been used as an additive to enhance the workability of the Bioglass products, but as new information emerges on the beneficial properties of boron these claims may change its status to a bioactive nature.
Silicon (Si), while not labeled an essential trace element to human health, has some very important functions in the human body. Silicon (Si4+) can be found in bone and connective tissues in the body [71]. It has been found to be beneficial to cartilage and glycosaminoglycan formation or function, which may affect bone formation and maintenance, cardiovascular health, and wound healing [72]. In the early stages of the biomineralization process, silicon can be found at active calcification sites [73]. During the later stages of calcification, silicon plays a direct role in mineralization in aqueous orthosilicic acid (Si(OH)4) form by inducing the precipitation of HA from electrolyte solutions[73]. Because silicon is the second most abundant element on earth, deficiencies are rare and difficult to indicate in humans, but in experimental animal models dietary silicon deficiencies can result in deformed development of bone, low collagen formation and stunted growth [71,74]. In silicon substituted CaP materials, the presence of silicon can stimulate biological activity by increasing the solubility of the material, generating a more electronegative surface and creating a finer microstructure resulting in transformation of the material surface to a biologically equivalent apatite [75]. Early uses of silicon in CaPs were investigated as a way to stabilize β-TCP at high temperatures to improve densification and prevent the formation of α-TCP, a more soluble and unstable form of CaP [76]. In a study where silicon substituted HA scaffolds were implanted in rabbits at various levels, results showed that silicon-substituted HA had increased amount of bone growth compared to the pure HA composition and also affected the osteoclastic resorption rates. Another study investigated the effects of silicon substituted HA granules in rabbit model and found similar results [77,78]. Extensive research has also been conducted on high Si content biomaterials such as bioglass [79–81]. Si has shown its stimulatory effect on cellular activities, such as proliferation and differentiation of osteoblast-like cells, mineralization of osteoblasts and osteogenic differentiation of mesenchymal stem cells [79,82]. Although the exact mechanism of induced bone regeneration by silicon is yet to be understood, it is suggested that early stage induced bone regeneration by silicon is possibly caused by the synthesis and/or stabilization of collagen, which is the most abundant protein in bone matrix [71,82]. Aside from the potential osteogenic benefits of silicon addition to CaPs, several recent studies have noted that silicon may have angiogenic capabilities as well. A In a study utilizing a non-phosphorous containing calcium silicate material, results indicated that the presence of silicon induced VEFG expression in human dermal fibroblasts, which in turn upregulated nitric oxide synthase and nitric oxide production in human endothelial cells [83]. Similarly, another study utilizing a calcium silicate in a rabbit femur defect was also able to demonstrate angiogenic effects [84]. Commercially, silicon is found in various orthopedic applications from silicate stabilized CaPs to the main component of Bioglass and a mix of the two.
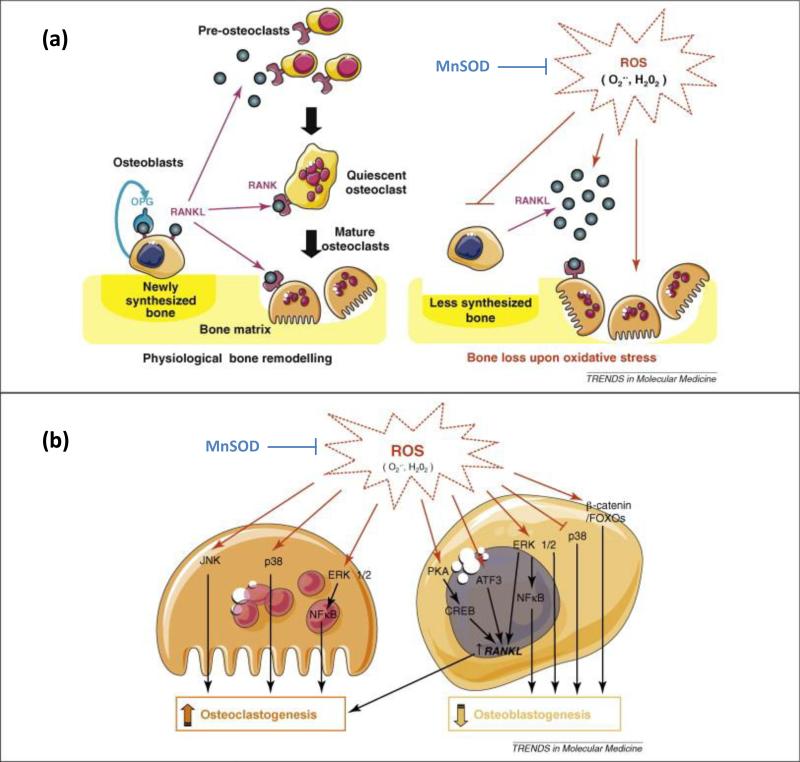
Manganese (Mn) is an essential dietary trace element and plays an important role in several metabolic pathways and cellular homeostasis. More importantly, for the purpose of this review, it has been found to play important roles in cartilage and bone formation. In one particular study, deficiencies of manganese in young chicks resulted in bone deformation and significantly decreased levels of serum of important bone markers calcitonin (CT), ALP, and tartrate resistant acid phosphatase (TRAP); conversely it showed an increase in parathyroid hormone (PTH) among others [85]. While CT and ALP are known for osteogenesis, TRAP is a chemical secreted by osteoclasts that is partially responsible for creating an acidic environment that helps to break down the bone. While it may be surprising that both osteoclast and osteoblast activities are decreased, researchers have found that manganese is essential in the binding of integrins to ligands which is the mechanism by which cells bind to substrates [86]. In the case of osteoblasts and osteoclasts, the ability to bind to fibronectin and collagen in the extracellular matrix is disrupted. According to the same study, manganese also seemed to have some role in the parathyroid hormone (PTH) signaling pathway, which is a key calcium regulator. While many studies have noted an increase with highly controlled treatments of PTH, primary hyperparathyroidism is related to catabolic effects that can lead to significant decrease in bone mineral density [87]. Another major role that manganese plays is in manganese superoxide dismutase (MnSOD). MnSOD helps protect mitochondrial components of the cell from damaging reactive oxygen species, such as superoxides, that are created during normal cellular respiration. Depleted manganese levels can increase the occurrence of oxidative stresses which has been shown to decrease bone mineral density [88]. Figure 5 shows that reactive oxygen species contribute to increased osteoclastogenesis and decreased osteoblastogenesis, while MnSOD acts to neutralize the formation of ROS [89]. Incorporation of manganese into CaP materials in low levels has been shown to stabilize heat treatment of HA and reduce the amount of calcium oxide, β-TCP, and α-TCP that can be formed at high temperatures [90,91]. Other studies have noted a markedly increased osteoblastic response including cellular adhesion and production of osteogenic compounds [90,92]. Although recent research has demonstrated the beneficial effects of manganese presence in bone biology, there are no commercial devices that currently utilize it.
Figure 5.
(a) Schematic demonstrating the normal bone remodeling process (left) between osteoblasts and osteoclasts. Reactive oxide species inhibit OPG production in osteoblasts while increasing RANKL production allowing increased osteoclastogenesis and subsequent bone resorption. MnSOD acts to neutralize the formation of ROS. (b) A more detailed look at the signaling pathways ROS has been shown to influence in both osteoclasts and osteoblasts. Adapted with permission from reference [89].
Do multiple dopants have any added benefits than single dopants?
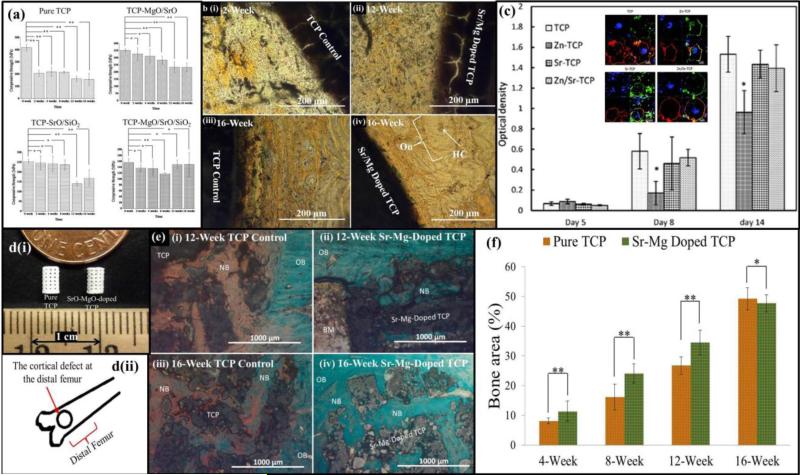
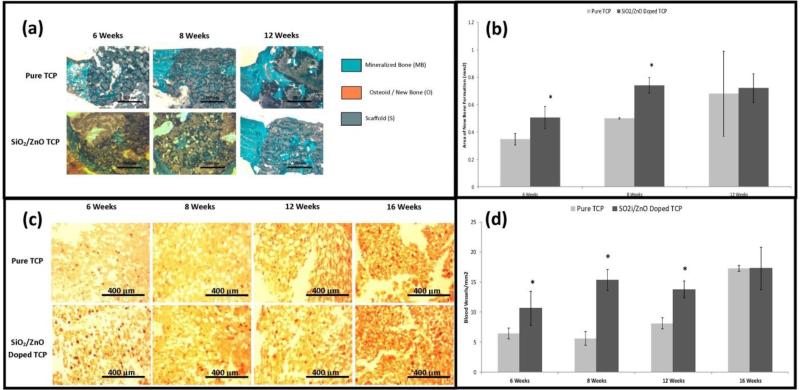
As single dopant systems have demonstrated good results, it stands to reason that using multiple dopants can be used to further increase the beneficial effects of each, within the constraints of the CaP material stability. A combination of different trace metal ions addition in calcium phosphates can alter the degradation kinetics, inhibitory effect on osteoclastogenesis, stimulatory effect on osteogenesis as shown in Figure 6 [93] [94]. In a system with a magnesium and strontium combination, results showed a desirable, gradual strength degradation rate over time in a simulated body fluid, SBF, which is an essential requirements towards efficient bone tissue engineering [93]. Results also demonstrated that accelerated defect healing demonstrated by greater degree of early bone formation and enhanced bone remodeling when compared to undoped β-TCP [93]. Decreased in vitro osteoclastogenesis was observed when zinc and strontium were doped into β-TCP [94]. Markedly accelerated early bone healing was observed in zinc and silicon doped 3D printed β-TCP scaffolds as compared to undoped β-TCP, when tested in a rat femoral defect model as shown in Figure 7 [95]. Electrical polarizability of calcium phosphates influences cellular activity, which is assessed by the stored charge capability. Negatively polarized CaP surfaces have been shown to favor osteoblast proliferation and differentiation [96]. Significantly increased stored charge density was achieved in 1 wt.% SrO and 1 wt.% MgO doped HA compared to undoped HA [97]. In phosphate free calcium silicate scaffolds, strontium and zinc substitution demonstrated enhanced expression of alkaline phosphatase, Runx-2, osteopontin, osteocalcin and bone sialoprotein when compared to pure β-TCP scaffolds [98].
Figure 6.
(a) Effect of dopants on in vitro strength degradation rate of undoped and TCP doped with 1 wt. % MgO-1 wt. % SrO, 1 wt. % SrO-0.5 wt. % SiO2 and 1 wt. % MgO- 1 wt. % SrO- 0.5 wt. % SiO2 compacts soaked for 0, 2, 4, 8, 12 and 16 weeks in SBF( **P < 0.05, *P > 0.05; n = 6). Adapted with permission from reference [93]; (b) bone remodeling after 12 and 16 weeks in undoped TCP and SrO-MgO doped TCP. A uniform bone remodeling initiation was observed after 12-week with Sr/Mg doped TCP as compared to undoped TCP. A uniform and compact interface was observed between MgO/SrO doped implant and newly remodeled bone compared to undoped TCP. Adapted with permission from reference [94]; (c) Influence of metal ions on tartrate-resistant acid phosphatase (TRAP) expression by osteoclasts (Inset: Fluorescence microscopy images of cells after 14 days culture: red: actin cytoskeleton, green: vitronectin receptor αvβ3 integrin, and blue: nucleolus). Adapted with permission from reference [95]; (d) (i) Photograph of the microwave sintered 3D printed (3DP) TCP and Sr-Mg doped TCP scaffolds for in vivo implantation, (ii) Schematic of the distal femoral cortical defect model (anterior view); (e) Photomicrograph of 3DP pure TCP implants (i and iii), and Sr/Mg doped TCP implants (ii and iv) showing the new bone formation and bone remodeling inside the interconnected macro and intrinsic micro pores of the 3DP scaffolds after 12 and 16 weeks in rat distal femoral defect model. Modified Masson Goldner's trichrome staining of transverse section. OB: old bone, NB: new bone and BM: bone marrow. Color description: dark grey/black: scaffold; orange/red: osteoid; green/bluish: new mineralized bone (NMB)/ old bone; (f) Histomorphometric analysis of bone area fraction (total newly formed bone area/total area, %) showing the new bone formation induced by pure and Sr-Mg doped TCP at different time points (**p < 0.05, *p > 0.05, n=6).
Figure 7.
I – (a) Shows the progression of new bone growth into either pure TCP or SiO2/ZnO doped TCP scaffolds over the course of 12 weeks using Goldner's Trichrome stain. (b) represents the histomorphological evaluation of same data. (c) Shows vonWillebrand factor staining to evaluate angiogenesis over the course of 16 weeks. (d) represents quantitative analysis of the same data. Adapted with permission from reference [95].
Effect of trace metallic cations in physicomechanical behavior of CaP bioceramics
Much research has gone into the study of how additives affect the physicochemical materials properties of CaPs. These changes in mechanical properties and biological responses are due to the changes in the physicochemical properties of CaPs such as phase, crystallinity, microstructure, solubility, grain size, mechanical strength and strength degradation kinetics caused by cation substitution [99]. Thus, synthesis of cation-substituted CaP ceramics has been the subject of immense interest owing to the critical roles played by these ions in the biological activity of bone. The physicochemical properties of CaP might vary depending on whether the dopant is substituting ‘Ca’ or ‘P’ site in the crystal lattice and / or staying in their respective salt form. Depending on the ionic radius, the substitution of the added dopant can either stabilize or destabilize the crystal structure of CaPs. The ionic radius of Ca2+, Sr2+, Mg2+, and Zn2+ are 0.99 Å, 1.13 Å, 0.69 Å, and 0.74 Å respectively. Sr2+ substitution for Ca2+ leads to an increase in the size of the unit cell of the β-TCP lattice, while substitution of Mg2+ for Ca2+ makes the unit cell smaller. Substitution of Mg2+ for Ca2+ stabilizes the β phase at a higher temperature by delaying the β to α phase transformation of TCP. Mg2+ substitution into hydroxyapatite (HA) reduces its crystallinity due to distortion of the crystal lattice [100]. Si4+ substitution for P5+ into CaP materials increases the solubility by introducing crystal defects. Sometimes the substitution of one element in ceramic materials is affected by the presence of another dopant [99].
Critical Issues
There are substantial evidences showing that inorganic trace metallic cations can elicit added physicomechanical and biological benefits, when added to tissue engineering constructs. Although a few of the cations discussed in this review can cause toxicity beyond a certain dose, most of these cations remain non-harmful even at considerably high concentration [17]. Biodegradation and dissolution of CaPs or even PGs and BGs vary from composition to composition. One of the major concerns regarding the in vivo metal ions delivery is the amount of ions getting released, and whether the released amount is within the therapeutic level to have any positive influence [15,101]. We need to keep in mind what type of chemical compound we are using for metal ion doping into biomaterials. Because, sometimes the entire chemical entity might be toxic to the host tissue, even though the cation itself is not toxic [16]. There is very little information available about the bioavailability of the inorganic metallic ions, when released in vivo from biomaterials. Current understanding relating to the mechanism of action of metallic ions at the cellular level is not well documented. In spite of these current limitations, already available evidences suggest that further investigation with trace metallic cation doped CaP bioceramics is going to be very worthy and timely act in bone tissue engineering for early healing.
The Future of Trace Metal Ions in Bone Tissue Engineering
The use of CaP materials for orthopedic procedures signaled the change of an era of implants. Technologies shifted from the use of autografts and allografts in bone grafting applications, to a synthetic that could offer the promise of an alternative that could avoid additional surgical techniques or use of cadaver material. In coating applications, the use of CaPs changed bioinert implants to a bioactive platform that allowed bone tissue ingrowth onto the surface of the implants allowing for a true biological fixation. As scientists and researchers discover more about how the biology of healthy bone works, they offer the promise of another paradigm shift from materials that are simply osteoconductive, to materials that are osteoinductive. The recent uses of growth factors have proven the usefulness of manipulating synthetic materials to achieve these goals, besides related cost, safety, stability have been questioned in recent years.
The use of important trace elements in bone biology in CaP materials have been shown to alter the healing states of bone and bone growth in a manner that has inherent control mechanisms. This research may offer the promise of osteoinductivity without the potential harmful side effects known with pharmacologics and biologics. While preliminary research into this area has been promising, there is still much that is unknown about how these ions function naturally. Future research will begin to develop deeper into the mechanistic behavior of these trace elements to gain a better understanding of how they can be used to benefit the bone healing and growing process. This information, then, can be used to design smarter CaP implant materials that offer a targeted healing approach at a defect site. The potential for design is vast, ranging from a biologically relevant “cocktail” of nutrients released throughout the lifetime of the implant to a functionally graded material that may first stimulate angiogenesis and then as it degrades shift to a more osteogenic nature, releasing the nutrients as they are needed.
Highlights.
The future of bone grafting materials lies in osteoinductive capabilities
Bone requires a variety of important ions to maintain healthy functionality
Addition of important ions to calcium phosphate materials can have beneficial effects
Box 1 Outstanding Questions.
What long term effects do the trace elements have on physical, chemical and biological properties of calcium phosphate based bone replacement materials?
Can trace metal ions be an alternative to biologics in bone tissue engineering? and / or
Do the trace elements have any direct effect on osteogenesis and angiogenesis?
What are the underlying mechanisms that dopants may affect in osteogenesis and angiogenesis and can they be made to be more effective?
Acknowledgement
The authors gratefully acknowledge financial support from National Institute of Health (NIH), NIBIB, under the Grant No. NIH-R01-EB-007351.
Glossary
- Osteoblasts
These cells are mononuclear cells that originate from mesenchymal progenitors. Their primary responsibility is the production and mineralization of the bone matrix. They synthesize collagen and glycoproteins and regulate the local calcium and phosphorous levels that are a key factor in the biomineralization process.
- Osteoclast
Multinucleated bone cells associated with the breakdown and resorption of mineralized bone tissue through enzymatic actions.
- Osteoconductivity
Is a materials property that refers to the ability of a material to allow unimpeded bone growth onto or throughout.
- Osteoinductivity
Is a materials property that refers to the ability of the material to actively stimulate new bone formation.
- Osteogenesis
The process of bone growth and regeneration.
- Angiogenesis
The process of new blood vessel formation. Newly formed blood vessels are involved in nutrient supply and transport of macromolecules during bone repair and regeneration.
- Bone morphogenetic protein (BMP)
A growth factor that is critical in embryonic skeletal development, bone formation, maturation, and repair.
- Extracellular matrix (ECM)
Self-assembled macromolecules generally consisting of collagens, noncollagenous glycoproteins, hyaluronan, and proteoglycans. It works as a reservoir for different cytokines and growth factors and becomes the keystone for mineralized bone.
- Trace Eelements
Often referred to as micronutrients consist of any chemical element that is required by living organisms in minute amounts. Many trace elements are utilized in cell-produced vital enzymes and are generally categorized in three categories: Essential – includes iron, zinc, sodium, magnesium, copper, cobalt, chromium, fluorine, iodine, manganese, molybdenum and selenium, Probably Essential – includes nickel, tin, vanadium, silicon, boron and Non-Essential – includes aluminum, arsenic, barium, bismuth, bromine, cadmium, germanium, gold, lead, lithium, mercury, rubidium, silver, strontium, titanium and zirconium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Bose S, Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012;8:1401–21. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carano RAD, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8:980–9. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 3.Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000;10:223–8. doi: 10.1016/s1050-1738(00)00074-8. [DOI] [PubMed] [Google Scholar]
- 4.Geiger M. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–29. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Tamai N, Myoui A, Hirao M, et al. A new biotechnology for articular cartilage repair: subchondral implantation of a composite of interconnected porous hydroxyapatite, synthetic polymer (PLA-PEG), and bone morphogenetic protein-2 (rhBMP-2). Osteoarthritis Cartilage. 2005;13:405–17. doi: 10.1016/j.joca.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Shields LBE, Raque GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542–7. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 7.Boraiah S, Paul O, Hawkes D, et al. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop. 2009;467:3257–62. doi: 10.1007/s11999-009-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luca L, Rougemont A-L, Walpoth BH, et al. The effects of carrier nature and pH on rhBMP-2-induced ectopic bone formation. J Controlled Release. 2010;147:38–44. doi: 10.1016/j.jconrel.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Bandyopadhyay A, Bernard S, Xue W, et al. Calcium Phosphate-Based Resorbable Ceramics: Influence of MgO, ZnO, and SiO2 Dopants. J Am Ceram Soc. 2006;89:2675–88. [Google Scholar]
- 10.Li X, Sogo Y, Ito A, et al. The optimum zinc content in set calcium phosphate cement for promoting bone formation in vivo. Mater Sci Eng C Mater Biol Appl. 2009;29:969–75. doi: 10.1016/j.msec.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohner M. Silicon-substituted calcium phosphates–a critical view. Biomaterials. 2009;30:6403–6. doi: 10.1016/j.biomaterials.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Fielding GA, Bandyopadhyay A, Bose S. Effects of SiO2 and ZnO doping on mechanical and biological properties of 3D printed TCP scaffolds. Dent Mater. doi: 10.1016/j.dental.2011.09.010. Published Online First: November 2011. doi:10.1016/j.dental.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanai Y, Tokuda H, Yasuda E, et al. Up-regulation by zinc of FGF-2-induced VEGF release throughenhancing p44/p42 MAP kinase activation in osteoblasts. Life Sci. 2006;80:230–4. doi: 10.1016/j.lfs.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Gao T, Aro HT, Ylänen H, et al. Silica-based bioactive glasses modulate expression of bone morphogenetic protein-2 mRNA in Saos-2 osteoblasts in vitro. Biomaterials. 2001;22:1475–83. doi: 10.1016/s0142-9612(00)00288-x. [DOI] [PubMed] [Google Scholar]
- 15.Mouriño V, Cattalini JP, Boccaccini AR. Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments. J R Soc Interface. doi: 10.1098/rsif.2011.0611. Published Online First: 7 December 2011. doi:10.1098/rsif.2011.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakhkar NJ, Lee I-H, Kim H-W, et al. Bone formation controlled by biologically relevant inorganic ions: Role and controlled delivery from phosphate-based glasses. Adv Drug Deliv Rev. 2013;65:405–20. doi: 10.1016/j.addr.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Habibovic P, Barralet JE. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2011;7:3013–26. doi: 10.1016/j.actbio.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto N, Yoshida K, Hashimoto K, et al. Thermal stability of β-tricalcium phosphate doped with monovalent metal ions. Mater Res Bull. 2009;44:1889–94. [Google Scholar]
- 19.Lehmann SW, Lee J. Lithium-associated hypercalcemia and hyperparathyroidism in the elderly: What do we know? J Affect Disord. doi: 10.1016/j.jad.2012.08.028. Published Online First: September 2012. doi:10.1016/j.jad.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Marti JL, Yang CS, Carling T, et al. Surgical approach and outcomes in patients with lithium-associated hyperparathyroidism. Ann Surg Oncol. 2012;19:3465–71. doi: 10.1245/s10434-012-2367-6. [DOI] [PubMed] [Google Scholar]
- 21.Zamani A, Omrani GR, Nasab MM. Lithium's effect on bone mineral density. Bone. 2009;44:331–4. doi: 10.1016/j.bone.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Chalecka-Franaszek E, Chuang D-M. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci U S A. 1999;96:8745–50. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedgepeth CM, Conrad LJ, Zhang J, et al. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Whetstone HC, Lin AC, et al. Beta-Catenin Signaling Plays a Disparate Role in Different Phases of Fracture Repair: Implications for Therapy to Improve Bone Healing. Plos Med. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monroe DG, McGee-Lawrence ME, Oursler MJ, et al. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milat F, Ng KW. Is Wnt signalling the final common pathway leading to bone formation? Mol Cell Endocrinol. 2009;310:52–62. doi: 10.1016/j.mce.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 28.Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112:3491–501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang R, Oyajobi BO, Harris SE, et al. Wnt/β-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone. 2013;52:145–56. doi: 10.1016/j.bone.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, de Groot K, van Blitterswijk C, et al. Electrolytic deposition of lithium into calcium phosphate coatings. Dent Mater. 2009;25:353–9. doi: 10.1016/j.dental.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Fielding GA, Roy M, Bose S, et al. Antibacterial and biological characteristics of plasma sprayed silver and strontium doped hydroxyapatite coatings. Acta Biomater. 2012 doi: 10.1016/j.actbio.2012.04.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi M. Role of nutritional zinc in the prevention of osteoporosis. Mol Cell Biochem. 2009;338:241–54. doi: 10.1007/s11010-009-0358-0. [DOI] [PubMed] [Google Scholar]
- 33.Hadley KB, Newman SM, Hunt JR. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J Nutr Biochem. 2010;21:297–303. doi: 10.1016/j.jnutbio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi M, Weitzmann MN. Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol Cell Biochem. 2011;355:179–86. doi: 10.1007/s11010-011-0852-z. [DOI] [PubMed] [Google Scholar]
- 35.Ito A, Kawamura H, Otsuka M, et al. Zinc-releasing calcium phosphate for stimulating bone formation. Mater Sci Eng C. 2002;22:21–5. [Google Scholar]
- 36.Rude RK, Gruber HE, Norton HJ, et al. Dietary magnesium reduction to 25% of nutrient requirement disrupts bone and mineral metabolism in the rat. Bone. 2005;37:211–9. doi: 10.1016/j.bone.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Maier JA, Bernardini D, Rayssiguier Y, et al. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim Biophys Acta Bba - Mol Basis Dis. 2004;1689:6–12. doi: 10.1016/j.bbadis.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Cooke JP, Losordo DW. Nitric Oxide and Angiogenesis. Circulation. 2002;105:2133–5. doi: 10.1161/01.cir.0000014928.45119.73. [DOI] [PubMed] [Google Scholar]
- 39.Zou L. Lasting Controversy on Ranibizumab and Bevacizumab. Theranostics. 2011;1:395–402. doi: 10.7150/thno/v01p0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stendig-Lindberg G, Koeller W, Bauer A, et al. Experimentally induced prolonged magnesium deficiency causes osteoporosis in the rat. Eur J Intern Med. 2004;15:97–107. doi: 10.1016/j.ejim.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Xue W, Dahlquist K, Banerjee A, et al. Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants. J Mater Sci Mater Med. 2008;19:2669–77. doi: 10.1007/s10856-008-3395-4. [DOI] [PubMed] [Google Scholar]
- 42.Landi E, Logroscino G, Proietti L, et al. Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo behaviour. J Mater Sci Mater Med. 2008;19:239–47. doi: 10.1007/s10856-006-0032-y. [DOI] [PubMed] [Google Scholar]
- 43.Coulombe J, Faure H, Robin B, et al. In vitro effects of strontium ranelate on the extracellular calcium-sensing receptor. Biochem Biophys Res Commun. 2004;323:1184–90. doi: 10.1016/j.bbrc.2004.08.209. [DOI] [PubMed] [Google Scholar]
- 44.Brown EM. Is the calcium receptor a molecular target for the actions of strontium on bone? Osteoporos Int. 2003;14:25–34. doi: 10.1007/s00198-002-1343-6. [DOI] [PubMed] [Google Scholar]
- 45.Tat SK, Pelletier JP, Mineau F, et al. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone. 2011 doi: 10.1016/j.bone.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Kostenuik PJ. Osteoprotegerin A Physiological and Pharmacological Inhibitor of Bone Resorption. Curr Pharm Des. 2001;7:613–35. doi: 10.2174/1381612013397807. others. [DOI] [PubMed] [Google Scholar]
- 47.Saidak Z, Marie PJ. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol Ther. 2012;136:216–26. doi: 10.1016/j.pharmthera.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–68. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 49.Ni G, Chiu K, Lu W, et al. Strontium-containing hydroxyapatite bioactive bone cement in revision hip arthroplasty. Biomaterials. 2006;27:4348–55. doi: 10.1016/j.biomaterials.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 50.Chen YW, Feng T, Shi GQ, et al. Interaction of endothelial cells with biodegradable strontium-doped calcium polyphosphate for bone tissue engineering. Appl Surf Sci. 2008;255:331–5. [Google Scholar]
- 51.Tian M, Chen F, Song W, et al. In vivo study of porous strontium-doped calcium polyphosphate scaffolds for bone substitute applications. J Mater Sci Mater Med. 2009;20:1505–12. doi: 10.1007/s10856-009-3713-5. [DOI] [PubMed] [Google Scholar]
- 52.Barralet J, Gbureck U, Habibovic P, et al. Angiogenesis in Calcium Phosphate Scaffolds by Inorganic Copper Ion Release. Tissue Eng Part A. 2009;15:1601–9. doi: 10.1089/ten.tea.2007.0370. [DOI] [PubMed] [Google Scholar]
- 53.Ewald A, Käppel C, Vorndran E, et al. The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J Biomed Mater Res A. 2012;100A:2392–400. doi: 10.1002/jbm.a.34184. [DOI] [PubMed] [Google Scholar]
- 54.Gérard C, Bordeleau L-J, Barralet J, et al. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials. 2010;31:824–31. doi: 10.1016/j.biomaterials.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Wu C, Zhou Y, Xu M, et al. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34:422–33. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 56.Stähli C, Muja N, Nazhat SN. Controlled Copper Ion Release from Phosphate-Based Glasses Improves Human Umbilical Vein Endothelial Cell Survival in a Reduced Nutrient Environment. Tissue Eng Part A. 2013;19:548–57. doi: 10.1089/ten.tea.2012.0223. [DOI] [PubMed] [Google Scholar]
- 57.Sen CK, Khanna S, Venojarvi M, et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am J Physiol - Heart Circ Physiol. 2002;282:H1821–H1827. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
- 58.Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189:147–63. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 59.Jomova K, Baros S, Valko M. Redox active metal-induced oxidative stress in biological systems. Transit Met Chem. 2012;37:127–34. [Google Scholar]
- 60.Simonsen LO, Harbak H, Bennekou P. Cobalt metabolism and toxicology—A brief update. Sci Total Environ. 2012;432:210–5. doi: 10.1016/j.scitotenv.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Patntirapong S, Habibovic P, Hauschka PV. Effects of soluble cobalt and cobalt incorporated into calcium phosphate layers on osteoclast differentiation and activation. Biomaterials. 2009;30:548–55. doi: 10.1016/j.biomaterials.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 62.Wu C, Zhou Y, Fan W, et al. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 2012;33:2076–85. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 63.Fan W, Crawford R, Xiao Y. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials. 2010;31:3580–9. doi: 10.1016/j.biomaterials.2010.01.083. [DOI] [PubMed] [Google Scholar]
- 64.Ignjatović N, Ajduković Z, Savić V, et al. Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones. J Mater Sci Mater Med. 2013;24:343–54. doi: 10.1007/s10856-012-4793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pacary E, Legros H, Valable S, et al. Synergistic effects of CoCl2 and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J Cell Sci. 2006;119:2667–78. doi: 10.1242/jcs.03004. [DOI] [PubMed] [Google Scholar]
- 66.Kim KS, Rajagopal V, Gonsalves C, et al. A Novel Role of Hypoxia-Inducible Factor in Cobalt Chloride- and Hypoxia-Mediated Expression of IL-8 Chemokine in Human Endothelial Cells. J Immunol. 2006;177:7211–24. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- 67.Dzondo-Gadet M, Mayap-Nzietchueng R, Hess K, et al. Action of boron at the molecular level: effects on transcription and translation in an acellular system. Biol Trace Elem Res. 2002;85:23–33. doi: 10.1385/BTER:85:1:23. [DOI] [PubMed] [Google Scholar]
- 68.Studer D, Millan C, Öztürk E, et al. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cell Mater. 2012;24:118–135. doi: 10.22203/ecm.v024a09. discussion 135. [DOI] [PubMed] [Google Scholar]
- 69.Gorustovich AA, Steimetz T, Nielsen FH, et al. A histomorphometric study of alveolar bone modelling and remodelling in mice fed a boron-deficient diet. Arch Oral Biol. 2008;53:677–82. doi: 10.1016/j.archoralbio.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 70.Hakki SS, Dundar N, Kayis SA, et al. Boron enhances strength and alters mineral composition of bone in rabbits fed a high energy diet. J Trace Elem Med Biol. doi: 10.1016/j.jtemb.2012.07.001. Published Online First: August 2012. doi:10.1016/j.jtemb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Jugdaohsingh R, Calomme MR, Robinson K, et al. Increased longitudinal growth in rats on a silicon-depleted diet. Bone. 2008;43:596–606. doi: 10.1016/j.bone.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nielsen FH. Micronutrients in Parenteral Nutrition: Boron, Silicon, and Fluoride. Gastroenterology. 2009;137:S55–S60. doi: 10.1053/j.gastro.2009.07.072. [DOI] [PubMed] [Google Scholar]
- 73.Pietak AM, Reid JW, Stott MJ, et al. Silicon substitution in the calcium phosphate bioceramics. Biomaterials. 2007;28:4023–32. doi: 10.1016/j.biomaterials.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Carlisle EM. Biochemical and morphological changes associated with long bone abnormalities in silicon deficiency. J Nutr. 1980;110:1046–56. doi: 10.1093/jn/110.5.1046. [DOI] [PubMed] [Google Scholar]
- 75.Bergmann C, Lindner M, Zhang W, et al. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J Eur Ceram Soc. 2010;30:2563–7. [Google Scholar]
- 76.Kanazawa T, Umegaki T, Yamashita K, et al. Effects of additives on sintering and some properties of calcium phosphates with various Ca/P ratios. J Mater Sci. 1991;26:417–22. [Google Scholar]
- 77.Hing KA, Revell PA, Smith N, et al. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials. 2006;27:5014–26. doi: 10.1016/j.biomaterials.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 78.Patel N, Best SM, Bonfield W, et al. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J Mater Sci Mater Med. 2002;13:1199–206. doi: 10.1023/a:1021114710076. [DOI] [PubMed] [Google Scholar]
- 79.Jones JR, Tsigkou O, Coates EE, et al. Extracellular matrix formation and mineralization on a phosphate-free porous bioactive glass scaffold using primary human osteoblast (HOB) cells. Biomaterials. 2007;28:1653–63. doi: 10.1016/j.biomaterials.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 80.Xynos ID, Edgar AJ, Buttery LD, et al. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Commun. 2000;276:461–5. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 81.Gomez-Vega JM, Saiz E, Tomsia AP, et al. Bioactive glass coatings with hydroxyapatite and Bioglass particles on Ti-based implants. 1. Processing. Biomaterials. 2000;21:105–11. doi: 10.1016/s0142-9612(99)00131-3. [DOI] [PubMed] [Google Scholar]
- 82.Reffitt D, Ogston N, Jugdaohsingh R, et al. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32:127–35. doi: 10.1016/s8756-3282(02)00950-x. [DOI] [PubMed] [Google Scholar]
- 83.Li H, Chang J. Bioactive Silicate Materials Stimulate Angiogenesis in Fibroblast and Endothelial Cell Co-culture System through Paracrine Effect. Acta Biomater. doi: 10.1016/j.actbio.2013.02.014. Published Online First: February 2013. doi:10.1016/j.actbio.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 84.Wang C, Lin K, Chang J, et al. Osteogenesis and angiogenesis induced by porous β-CaSiO3/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials. 2013;34:64–77. doi: 10.1016/j.biomaterials.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 85.Zhaojun W, Lin W, Zhenyong W, et al. Effects of manganese deficiency on serum hormones and biochemical markers of bone metabolism in chicks. J Bone Miner Metab. doi: 10.1007/s00774-012-0417-6. Published Online First: 14 February 2013. doi:10.1007/s00774-012-0417-6. [DOI] [PubMed] [Google Scholar]
- 86.Lüthen F, Bulnheim U, Müller PD, et al. Influence of manganese ions on cellular behavior of human osteoblasts in vitro. Biomol Eng. 2007;24:531–6. doi: 10.1016/j.bioeng.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Lewiecki EM, Miller PD. Skeletal Effects of Primary Hyperparathyroidism: Bone Mineral Density and Fracture Risk. J Clin Densitom. 2013;16:28–32. doi: 10.1016/j.jocd.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Filaire E, Toumi H. Reactive oxygen species and exercise on bone metabolism: Friend or enemy? Joint Bone Spine. 2012;79:341–6. doi: 10.1016/j.jbspin.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 89.Wauquier F, Leotoing L, Coxam V, et al. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009;15:468–77. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Paluszkiewicz C, Ślósarczyk A, Pijocha D, et al. Synthesis, structural properties and thermal stability of Mn-doped hydroxyapatite. J Mol Struct. 2010;976:301–9. [Google Scholar]
- 91.Sopyan I, Ramesh S, Nawawi NA, et al. Effects of manganese doping on properties of sol–gel derived biphasic calcium phosphate ceramics. Ceram Int. 2011;37:3703–15. [Google Scholar]
- 92.Bracci B, Torricelli P, Panzavolta S, et al. Effect of Mg(2+), Sr(2+), and Mn(2+) on the chemico-physical and in vitro biological properties of calcium phosphate biomimetic coatings. J Inorg Biochem. 2009;103:1666–74. doi: 10.1016/j.jinorgbio.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 93.Bose S, Tarafder S, Banerjee SS, et al. Understanding in vivo response and mechanical property variation in MgO, SrO and SiO2 doped β-TCP. Bone. 2011;48:1282–90. doi: 10.1016/j.bone.2011.03.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roy M, Fielding GA, Bandyopadhyay A, et al. Effects of zinc and strontium substitution in tricalcium phosphate on osteoclast differentiation and resorption. Biomater Sci. 2012;1:74–82. doi: 10.1039/C2BM00012A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fielding G, Bose S. SiO2 and ZnO Dopants in 3D Printed TCP Scaffolds Enhances Osteogenesis and Angiogenesis in vivo. Acta Biomater. doi: 10.1016/j.actbio.2013.07.009. Minor revised version under review, June 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tarafder S, Bodhak S, Bandyopadhyay A, et al. Effect of electrical polarization and composition of biphasic calcium phosphates on early stage osteoblast interactions. J Biomed Mater Res B Appl Biomater. 2011;97:306–14. doi: 10.1002/jbm.b.31816. [DOI] [PubMed] [Google Scholar]
- 97.Bodhak S, Bose S, Bandyopadhyay A. Influence of MgO, SrO, and ZnO Dopants on Electro-Thermal Polarization Behavior and In Vitro Biological Properties of Hydroxyapatite Ceramics. J Am Ceram Soc. 2011;94:1281–8. [Google Scholar]
- 98.Zreiqat H, Ramaswamy Y, Wu C, et al. The incorporation of strontium and zinc into a calcium–silicon ceramic for bone tissue engineering. Biomaterials. 2010;31:3175–84. doi: 10.1016/j.biomaterials.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 99.Bose S, Tarafder S, Banerjee SS, et al. Understanding in vivo response and mechanical property variation in MgO, SrO and SiO2 doped β-TCP. Bone. 2011;48:1282–90. doi: 10.1016/j.bone.2011.03.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laurencin D, Almora-Barrios N, de Leeuw NH, et al. Magnesium incorporation into hydroxyapatite. Biomaterials. 2011;32:1826–37. doi: 10.1016/j.biomaterials.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 101.Bohner M. Silicon-substituted calcium phosphates – A critical view. Biomaterials. 2009;30:6403–6. doi: 10.1016/j.biomaterials.2009.08.007. [DOI] [PubMed] [Google Scholar]