Abstract
Dipeptidyl peptidase-4 (DPP-4) is a circulating glycoprotein that impairs insulin-stimulated glucose uptake and is linked to obesity and metabolic syndrome. However, the effect of exercise on plasma DPP-4 in adults with metabolic syndrome is unknown. Therefore, we determined the effect of exercise on DPP-4 and its role in explaining exercise-induced improvements in insulin sensitivity. Fourteen obese adults (67.9±1.2yr, BMI: 34.2±1.1kg/m2) with metabolic syndrome (ATP III criteria) underwent a 12-week supervised exercise intervention (60 min/d for 5 d/wk at ~85% HRmax). Plasma DPP-4 was analyzed using an enzyme-linked immunosorbent assay. Insulin sensitivity was measured using the euglycemic-hyperinsulinemic clamp (40mU/m2/min) and estimated by HOMA-IR. Visceral fat (computerized tomography), 2-hour glucose levels (75g oral glucose tolerance), and basal fat oxidation as well as aerobic fitness (indirect calorimetry) were also determined before and after exercise. The intervention reduced visceral fat, lowered blood pressure, glucose and lipids, and increased aerobic fitness (P<0.05). Exercise improved clamp-derived insulin sensitivity by 75% (P<0.001) and decreased HOMA-IR by 15% (P<0.05). Training decreased plasma DPP-4 by 10% (421.8±30.1 vs. 378.3±32.5ng/ml; P<0.04), and the decrease in DPP-4 was associated with clamp-derived insulin sensitivity (r=−0.59; P<0.04), HOMA-IR (r=0.59; P<0.04) and fat oxidation (r=−0.54; P<0.05). Increased fat oxidation also correlated with lower 2-hour glucose levels (r=−0.64; P<0.02). Exercise training reduces plasma DPP-4, which may be linked to elevated insulin sensitivity and fat oxidation. Maintaining low plasma DPP-4 concentrations is a potential mechanism whereby exercise plus weight loss prevents/delays the onset of type 2 diabetes in adults with metabolic syndrome.
Keywords: impaired glucose tolerance, obesity, cytokine, diabetes
1.0 INTRODUCTION
Dipeptidyl peptidase-4 (DPP-4) is an important cytokine that has been implicated in the development of metabolic syndrome and type 2 diabetes [21]. DPP-4 is a glycoprotein membrane bound enzyme that also exists in soluble form and is ubiquitously expressed throughout the body, including: skeletal muscle, kidney, liver, and adipose tissue [27]. DPP-4 was initially implicated in immune and inflammatory processes, but the endocrine role of DPP-4 has gained more attention recently since DPP-4 reduces pancreatic insulin secretion through decreased action of glucagon-like peptide-1 and glucose-dependent insulinotrophic polypeptide [6,9]. Clinically, the use of pharmacological DPP-4 inhibitors improves glycemic control and cardiometabolic health [9,19,25,31,34]. However, our understanding of in vivo DPP-4 in relation to insulin sensitivity is limited, and the effect of DPP-4 on insulin action may be a potential mechanism whereby inflammatory-related cytokines raise cardiometabolic disease risk [2,7,21]. Indeed, recent work suggests that DPP-4 impairs insulin signaling at the level of Akt in skeletal muscle thereby impairing glucose tolerance and raising metabolic syndrome risk [21]. In addition, DPP-4 deficient rodents have increased expression of PPAR-α, which in turn, contributes to higher fat oxidation and insulin sensitivity compared to normal controls [7].
Exercise and weight loss interventions reduce metabolic syndrome criteria in part by reducing cytokines (e.g. TNF-α and leptin) that impair insulin sensitivity [4,16,17,22,35–37]. In fact, work from our lab shows that exercise-induced weight loss reduces cytokines in parallel with improved glucose tolerance and insulin sensitivity [15,18]. Since leptin has been associated with higher DPP-4 concentrations, and leptin is known to decrease following exercise and weight loss interventions, it would seem reasonable to hypothesize that DPP-4 decreases following exercise. Indeed, a study recently reported that a low-fat diet combined with increased physical activity led to a lowering of plasma DPP-4 in overweight children [29]. The generalizability of these findings, however, is limited, as adults with metabolic syndrome were not studied. Moreover, the use of self-reports to characterize physical activity and surrogate measures of insulin sensitivity (fasting glucose and insulin) rather than the use of supervised exercise programs and the euglycemic-clamp to directly measure insulin sensitivity limits our understanding of the role of exercise in regulating DPP-4. Therefore, the objective of this study was to determine the effects of exercise on DPP-4 in adults with metabolic syndrome. We hypothesized that exercise would decrease DPP-4, and that the change in DPP-4 would correlate with increased insulin sensitivity, elevated fat oxidation, and a reduction in cardiometabolic risk factors.
2.0 MATERIALS AND METHODS
2.1 Subjects
Fourteen older (67.9 ± 1.2 yr; Range: 61–77 yrs) obese adults were included in this prospective study, and some of the clamp-derived insulin sensitivity data were reported for a different study [33]. Subjects were recruited from the Cleveland area via newspaper advertisements and community flyers. All subjects met at least 3 of 5 the National Cholesterol Education Program Adult Treatment Panel (ATP) III criteria for metabolic syndrome [13]. Subjects were only included if between the ages of 60–80 years of age, were weight stable (<2 kg in previous 6 months), sedentary (less than < 60 min/week of aerobic exercise and weight training), and free of chronic disease (i.e. hematological, renal, hepatic, cardiovascular). Women were post-menopausal and not on hormone replacement therapy. Subjects were excluded if they smoked or took diabetic or cholesterol lowering medications. Subjects were verbally briefed about the study and signed consent documents approved by the Cleveland Clinic Institutional Review Board.
2.2 Aerobic Fitness
Subjects were screened with a resting electrocardiogram as well as an incremental graded exercise stress test before participation in the intervention to exclude individuals with underlying cardiovascular abnormalities. Maximum oxygen consumption (VO2max) was determined by using a continuous incremental treadmill exercise test (Jaeger Oxygcon Pro; Viasys, Yorba Linda, CA) as previously described [33]. Maximum heart rate (HRmax) obtained during this test was used to prescribe exercise intensity. VO2max was repeated at weeks 4 and 8 to directly measure HRmax so that the appropriate exercise intensity could be maintained throughout training.
2.3 Exercise Training Intervention
Participants underwent a 12-week aerobic exercise intervention. During the first 4 weeks, subjects underwent a fully supervised aerobic exercise program 5 days per week at 60–85% of maximal heart rate (HRmax). Thereafter, the exercise intensity was increased and maintained at 80–85% HRmax for the duration of the intervention. Appropriate exercise intensity was managed using heart rate monitors (Polar Electro, Inc. Woodbury, NY). Exercise adherence was defined as a percent of completed exercise sessions over the intervention. Subjects met weekly with a dietitian to review 3-day diet logs to ensure maintenance of their pre-intervention macronutrient intake, such that weight loss was primarily the result of exercise energy expenditure. Food records were averaged over a 3-day period for analysis before and after the intervention to assess dietary intake.
2.4 Control Period
Metabolic assessments were conducted during a 3-day inpatient stay at the Clinical Research Unit. Resting metabolic rate was determined after subjects rested in the supine position for 30 minutes. Expired air (i.e. VO2 and VCO2) was collected for 20 minutes using a ventilated hood and indirect calorimetry, and the last 10 minutes were used to calculate energy expenditure (Vmax Encore, Viasys, Yorba Linda, CA) [12]. Subjects were then provided weight-maintenance meals (resting metabolic rate×1.2 activity factor; 55% CHO, 30% fat, 15% protein). All post-intervention outcome measures were obtained 16–18 hours after the last exercise session.
2.5 Cardiometabolic Risk Factors
After an overnight fast, height was measured without shoes using a wall-mounted stadiometer and weight was recorded on a digital platform scale with minimal clothing. Dual-x-ray absorptiometry (iDXA; Lunar Prodigy, Madison, WI) was used to quantify total fat and fat free-mass. Computerized tomography (Siemens Medical Solutions, Malvern, PA) was used to determine visceral adipose tissue mass without contrast at the fourth lumbar vertebrae (L4) as previously described [26]. Waist circumference was measured up to 3 times using a plastic Gulick tape measure approximately 2 cm above the umbilicus. Blood pressure was recorded after 10 minutes of rest in the seated position. An intravenous catheter was placed in an antecubital vein. Fasting samples were obtained for DPP-4, TNF-α, leptin, glucose, triglyceride, cholesterol and high-density lipoprotein (HDL) were obtained. A 75 gram oral glucose load was then conducted for assessment of impaired glucose tolerance. An ATP III score was calculated from the sum of risk factors meeting metabolic syndrome criteria before and after the intervention.
2.6 Insulin Sensitivity
After an overnight fast, a 2-hour euglycemic-hyperinsulinemic clamp was performed according to DeFronzo et al. [8]. Prior to insulin infusion, expired air was collected for 20 minutes using a ventilated hood and indirect calorimetry to determine basal substrate oxidation. A constant infusion (40 mU/m2/min) of insulin was then administered via an indwelling catheter placed in an antecubital vein. Glucose was infused at a variable rate to maintain plasma glucose at 90 mg/dl. A retrograde hand catheter was also placed and the hand was warmed to 60°C for collection of arterialized blood. Plasma glucose was collected every 5 minutes to determine the appropriate glucose infusion rate (GIR). Insulin sensitivity was defined as the average GIR divided by the ambient insulin concentrations during the final 30 minutes of the clamp. Expired air was also collected during the last 30 minutes to determine insulin-stimulated substrate oxidation. Non-oxidative glucose disposal was calculated as: GIR – total rates of carbohydrate oxidation. Homeostatic model assessment (HOMA-IR) was also calculated as fasting glucose×fasting insulin/405. Insulin-stimulated suppression of free fatty acids (FFA) was calculated as: [1− (FFAclamp/FFAfast)*100%].
2.7 Biochemical Analysis
Blood samples were centrifuged at 4°C for 10 minutes at 1000 rpm, and then stored at −80°C until subsequent analysis. Plasma DPP-4, TNF-α and leptin was determined using an enzyme-linked immunosorbent assay (ELISA; Roche Modular Diagnostics, Indianapolis, IN). Plasma glucose was determined by a glucose oxidase assay (YSI 2300 STAT Plus, Yellow Springs, OH). Plasma insulin was measured using a radioimmunoassay (Millipore, Billerica, MA). Plasma triglycerides and cholesterol were analyzed using enzymatic methods on an automated platform (Roche Modular Diagnostics, Indianapolis, IN). Plasma FFAs were analyzed by a colorimetric assay (Wako Chemicals, Richmond, VA).
2.8 Statistical Analysis
Pre and post group means were compared using the statistical program R (Leopard build 64-bit, The R Foundation, Vienna, Austria, 2011). All outcomes were assessed using a two-tailed paired t-test. Non-normally distributed data (e.g. HOMA-IR and DPP-4) were log-transformed for statistical analysis to minimize heterogeneity. Pearson's product-moment correlation was used to determine relationships between outcomes. Significance was accepted as P ≤ 0.05. Data are expressed as mean ± standard error of the mean (SEM).
3.0 RESULTS
3.1 Aerobic Fitness and Cardiovascular Risk Factors
Adherence to the exercise program was excellent (94.3 ± 2.7 %) and there were no statistical differences in total caloric or macronutrient intake after the intervention (Table 1). Exercise improved VO2max by 10% (2.1 ± 0.1 vs. 2.3 ± 0.1 L/min; P < 0.05), and elevated basal fat oxidation by approximately 59% (0.54 ± 0.05 vs. 0.79 ± 0.05 mg/kg/min; P < 0.001). The intervention also reduced body weight, blood pressure, triglycerides, total cholesterol and fasting glucose (P < 0.05; Table 2). Overall, the intervention reduced the prevalence of metabolic syndrome in 10 of the 16 adults, and decreased the ATP III score from 3.7 ± 0.2 to 2.5 ± 0.3 (P < 0.001). Plasma TNF-α was unchanged after the intervention, but leptin levels were significantly lower (P<0.02; Table 2).
Table 1.
Caloric and macronutrient intake before and after exercise training.
| Pre | Post | |
|---|---|---|
| Caloric Intake (kcal/d) | 1775.8 ± 148.8 | 1570.3 ± 108.5 |
| Carbohydrate (gram/d) | 237.3 ± 21.9 | 214.0 ± 16.3 |
| Fat (gram/d) | 65.9 ± 6.2 | 55.2 ± 7.3 |
| Protein (gram/d) | 66.1 ± 5.9 | 65.9 ± 3.9 |
Data are mean ± standard error of mean.
Table 2.
Effects of exercise on anthropometrics, fitness and metabolic markers.
| Pre | Post | |
|---|---|---|
| n (m/f) | 3/11 | - |
| Age (years) | 67.9 ± 1.3 | - |
| Body Composition | ||
| Body Weight (kg) | 95.5 ± 3.7 | 87.9 ± 3.5* |
| Body Mass Index (kg/m2) | 34.2 ± 1.1 | 32.6 ± 1.4* |
| Fat Mass (kg) | 38.0 ± 2.4 | 34.0 ± 2.2* |
| Fat Free Mass (kg) | 56.9 ± 2.9 | 54.1 ± 2.8* |
| Visceral Adipose Tissue (cm2) | 212.3 ± 23.9 | 182.2 ± 19.9* |
| Waist Circumference (cm) | 105.7 ± 2.8 | 100.5 ± 2.6* |
| Cardiometabolic | ||
| Systolic Blood pressure (mmHg) | 129.9 ± 3.8 | 118.0 ± 1.9* |
| Diastolic Blood pressure (mmHg) | 79.6 ± 2.5 | 70.7 ± 1.0* |
| Total cholesterol (mg/dl) | 196.4 ± 7.0 | 164.8 ± 7.3* |
| Low-density lipoprotein (mg/dl) | 122.4 ± 5.1 | 105.4 ± 1.9* |
| High-density lipoprotein (mg/dL) | 38.5 ± 2.5 | 38.9 ± 2.3 |
| Free fatty acids (mEq/L) | 0.62 ± 0.05 | 0.63 ± 0.05 |
| Triglycerides (mg/dL) | 183.8 ± 16.1 | 123.0 ± 9.7* |
| TNF-α (pg/ml) | 2.3 ± 0.3 | 2.4 ± 0.3 |
| Leptin (ng/ml) | 24.8 ± 4.6 | 19.5 ± 3.6* |
| Insulin Sensitivity | ||
| Fasting Glucose (mg/dl) | 106.5 ± 1.7 | 99.1 ± 1.9* |
| Fasting Insulin (µU/ml) | 17 ± 1.7 | 11.7 ± 0.9* |
| 2-hour Glucose (mg/dl) | 155.3 ± 8.2 | 144.7 ± 8.1 |
| 2-hour Insulin (µU/ml) | 85.6 ± 10.6 | 47.2 ± 3.9* |
| HOMA-IR† | 4.1 ± 0.4 | 3.2 ± 0.3* |
| GIR/I (mg/kg/min/µU/ml) | 0.03 ± 0.01 | 0.06 ± 0.01* |
| Clamp Insulin (µU/ml) | 85.4 ± 5.5 | 77.1 ± 6.0* |
Data are mean ± standard error of mean. Insulin sensitivity, i.e. GIR/I = glucose infusion rate divided by plasma insulin.
Significant compared to Pre (p < 0.05).
Data log transformed for statistical analysis. To convert mg/dl of glucose to mM, multiple by 0.0555. To convert mg/dl of insulin to pM, multiple by 6.97. To convert mg/dl of cholesterol pan to mM, multiple by 0.259. To convert mEq/L of free fatty acids to mM, multiple by 1. To convert mg/dl of triglycerides to mM, multiple by 0.0113.
3.2 Insulin Sensitivity
Exercise increased clamp-derived insulin sensitivity by 75% (P < 0.001; Table 2) and reduced HOMA-IR by 15% (P < 0.05; Table 2). The intervention did not alter carbohydrate oxidation during the clamp (1.0 ± 0.2 vs. 0.7 ± 0.2 mg/kg/min; P = 0.14), but non-oxidative glucose disposal increased approximately 63% (1.4 ± 0.4 to 3.4 ± 0.4 mg/kg/min; P < 0.001). Although exercise did not lower fasting FFA concentrations (Table 2), it did improve insulin-stimulated FFA suppression (70.8 ± 5.1 vs. 83.5 ± 3.2%, P < 0.05).
3.3 DPP-4 and Correlations
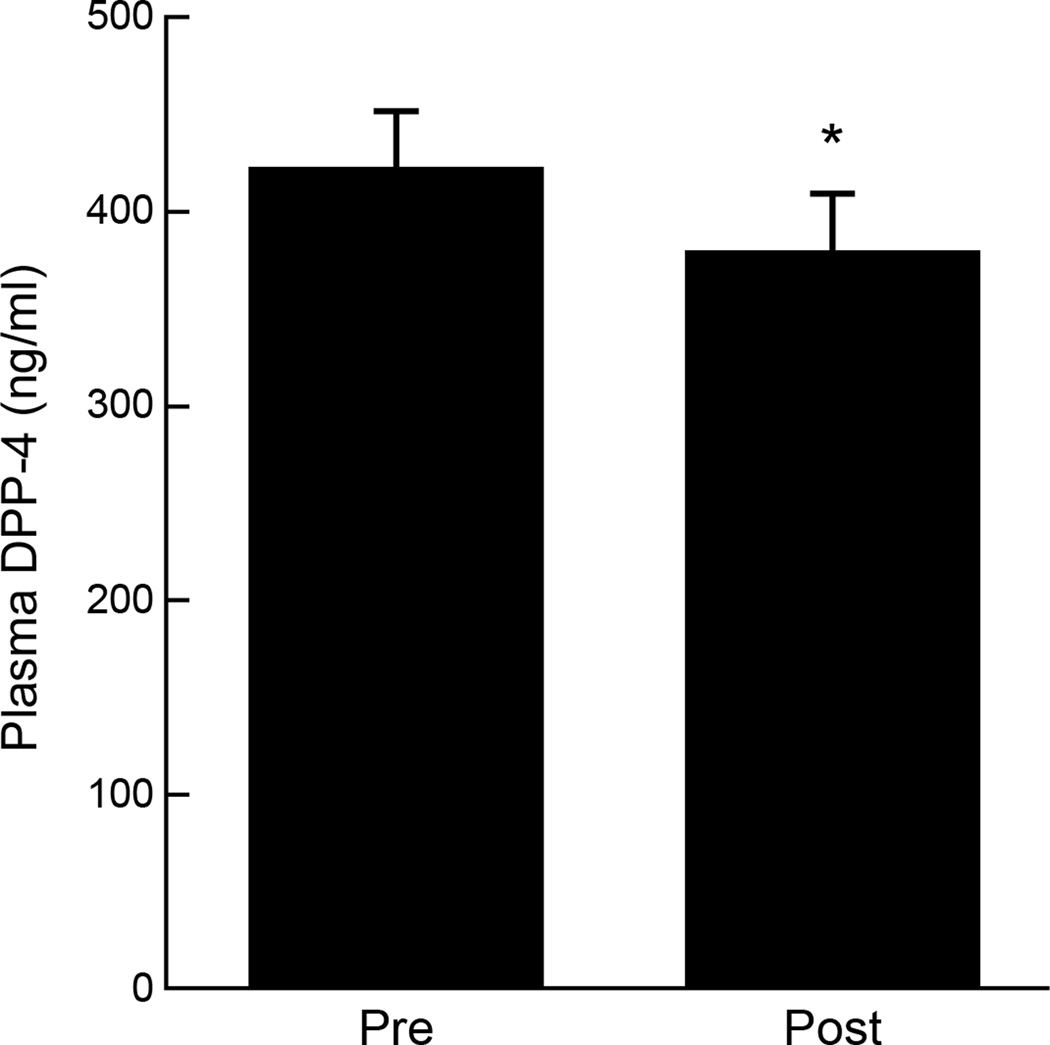
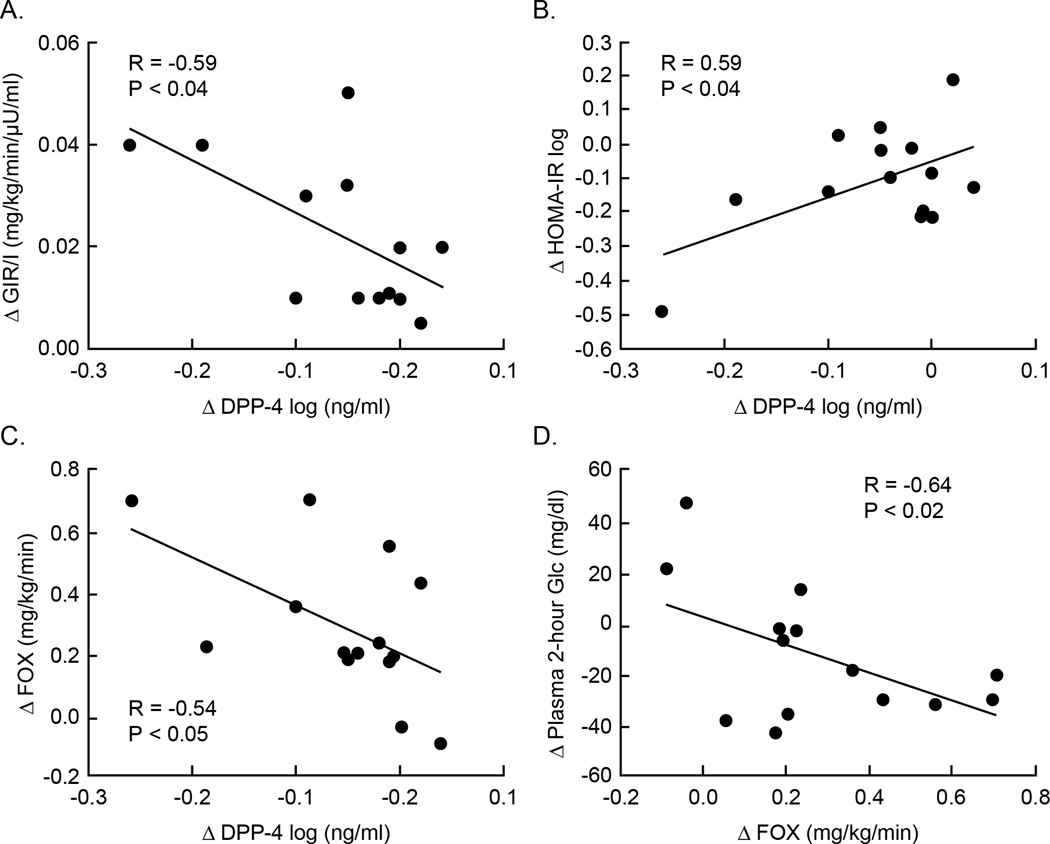
Exercise reduced plasma DPP-4 by approximately 10% (P < 0.05; Figure 1). Baseline DPP-4 correlated with lower aerobic fitness (r = −0.62; P < 0.02). Exercise-induced reductions in plasma DPP-4 correlated with enhanced clamp-derived insulin sensitivity (r = −0.59; P = 0.03; Figure 2a), decreased HOMA-IR (r = 0.59; P < 0.04; Figure 2b) and increased basal fat oxidation (r = −0.54; P < 0.05; Figure 2c). Elevated basal fat oxidation also correlated with reductions in 2-hour glucose concentrations (r = −0.64; P < 0.02; Figure 2d).
Figure 1.
Effect of exercise on plasma DPP-4 concentrations. *Significant compared to Pre (P < 0.04). Data were log transformed for statistical analysis.
Figure 2.
Correlation between the decreased plasma DPP-4 and clamp derived insulin sensitivity (a), HOMA-IR (b), and basal fat oxidation (c) as well as increased basal fat oxidation and reduced 2-hour glucose concentrations (d).
4.0 DISCUSSION
Exercise and weight loss are cornerstone treatments for reducing metabolic syndrome and diabetes risk. However, there are limited data examining the effect of exercise plus weight loss on plasma DPP-4 [29], and the relationship between changes in DPP-4 and the exercise-induced improvement in insulin sensitivity remains unknown in adults with metabolic syndrome who have increased risk for developing type 2 diabetes. The major finding from this study is that exercise training significantly reduced plasma DPP-4 in obese adults with metabolic syndrome (Figure 1). This reduction in DPP-4 was significantly associated with improvements in insulin sensitivity as well as elevated fat oxidation (Figure 2a–c), suggesting that DPP-4 may play a role in the regulation of glucose and lipid metabolism.
This study is the first to examine the effects of exercise training with weight loss on DPP-4. The intervention-mediated reduction in DPP-4 is consistent with prior work reporting that lifestyle modification, including increased physical activity and reduced dietary fat intake, is effective at lowering DPP-4 in overweight children [29]. It is important to recognize that, although this prior work suggests beneficial effects of lifestyle modification on DPP-4, the use of self-reports to characterize physical activity limits our ability to interpret the efficacy of exercise to lower DPP-4. In addition, in previous studies insulin sensitivity was not directly measured by using the euglycemic-clamp but was rather estimated from fasting surrogate measures [21,29]. As a result, our data bridges a knowledge gap and demonstrates that reductions in plasma DPP-4 may be partially responsible for the exercise-induced improvements in insulin sensitivity. Interestingly, the reduction in circulating DPP-4 (~ 40 ng/ml) observed in the current study is consistent with previous work following bariatric surgery in humans, suggesting that therapies decreasing body weight may improve glucose homeostasis through a similar adipocyte endocrine related-mechanism [21]. We recognize that we cannot causally determine if exercise reduced DPP-4 given that our cohort lost approximately 8% body weight. Future work is needed to test the independent effects of exercise on DPP-4 during conditions of energy balance. We also acknowledge that our study has a relatively small sample size, which limits our ability to generalize these findings. However, our findings are consistent with work in children, and suggest that DPP-4 is a potentially important and modifiable glycoprotein that appears to be linked to insulin sensitivity.
The mechanism by which DPP-4 contributes to improved insulin sensitivity after an exercise training plus weight loss intervention has yet to be elucidated. Clamp-derived insulin sensitivity generally reflects skeletal muscle glucose metabolism, as hepatic glucose production is almost completely suppressed [14]. Lamers et al. previously demonstrated in an in vitro study that DPP-4 impairs skeletal muscle insulin signaling at the level of Akt phosphorylation [21]. As such, the association we observed between the increase in clamp-derived insulin sensitivity and the decrease in DPP-4 after exercise training (Figure 2a) suggests that exercise alleviates the deleterious effects of DPP-4 on skeletal muscle glucose uptake. Thus, it is possible that at least some of the exercise-induced improvement in clamp-derived insulin sensitivity is due to reduced exposure of DPP-4 on skeletal muscle insulin signaling, although the effect of exercise on insulin signaling is controversial [11,20]. Fasting surrogates of insulin sensitivity (e.g. HOMA-IR) are primarily reflective of liver glucose metabolism [1]. We observed a reduction in HOMA-IR after the intervention, and this change in HOMA-IR was significantly associated with decreased DPP-4 (Figure 2b). This observation raises the possibility that decreases in DPP-4 may contribute to changes in hepatic insulin sensitivity after exercise. In fact, our finding is consistent with cross-sectional work reporting that DPP-4 is linked to the pathogenesis of non-alcoholic fatty liver disease (NFALD) [3,24]. Collectively, current data suggests that the decrease in DPP-4 following exercise plus weight loss may regulate insulin sensitivity in a multi-organ manner.
A potential link between DPP-4 and hepatic insulin resistance is that decreased DPP-4 is paralleled by changes in the expression of PPAR-α and SREBP-1c, which are important transcription factors that regulate fat oxidation and ectopic lipid storage [7]. One of the novel observations in the current study is that decreased plasma DPP-4 was significantly correlated with increased fat oxidation after exercise with weight loss (Figure 2c). This observation is consistent with recent work showing that DPP-4 inhibition raised fat oxidation, possibly through increased FFA mobilization via sympathetic nervous system activation, in patients with type 2 diabetes [5]. However, it is important to recognize that elevated fat oxidation after DPP-4 treatment is not universal, and decreased FFA flux has been reported [2]. The inconsistency in the literature regarding DPP-4 inhibition and fat oxidation is not readily apparent, but previous exercise studies from our lab suggest that reductions in FFA flux and elevations in fat oxidation after exercise training are important for improving insulin sensitivity [32]. Thus, DPP-4 is a possible link between the increased reliance on fat as an energy source after exercise and the improvement in glucose tolerance (Figure 2d). Given the important connection between lipid metabolism and insulin resistance [30], further work investigating the role of DPP-4 on energy metabolism is warranted.
Elevated DPP-4 is also linked to cardiovascular disease in part because it is associated with poor blood flow and dyslipidemia [21,23,28]. The use of DPP-4 inhibitors has been suggested to induce cardio-protective effects by not only improving insulin sensitivity, but also reducing inflammation [10]. Since higher aerobic fitness is related to a reduction in cytokines known to induce inflammation and accentuate metabolic syndrome risk [17], it would seem reasonable that lower DPP-4 following exercise would play a role in the exercise-induced reduction in metabolic syndrome risk factors. In our intervention, the principle criteria for lowering metabolic syndrome prevalence were reductions in blood pressure, fasting hyperglycemia and triglycerides in 64, 50, and 35% of our cohort. These findings highlight the well-described cardiometabolic benefit older adults may gain by participating in an aerobic exercise plus weight loss intervention. Although higher VO2max was associated with lower DPP-4 concentrations at baseline, we did not observe a significant correlation between the change in DPP-4 and the change in any of the metabolic syndrome risk factors (e.g. blood pressure, fasting hyperglycemia, etc.). As a result, the exercise plus weight loss induced reductions in cardiometabolic risk appear independent of DPP-4 in these adults. Whether DPP-4 has more direct role in modifying macrophage derived inflammatory markers and/or vascular function remains to be determined [10].
In conclusion, exercise training with weight loss decreased DPP-4 concentrations in previously sedentary obese adults with metabolic syndrome. The lower DPP-4 levels were significantly associated with elevated clamp-derived insulin sensitivity and fat oxidation. Although DPP-4 is often considered important for the regulation of pancreatic beta-cell function, our findings suggest that DPP-4 may contribute to the regulation of insulin sensitivity. Taken together, our data highlight that lowering DPP-4 concentrations may be a potential mechanism whereby exercise and weight loss improves glucose regulation and contributes to the prevention of type 2 diabetes.
HIGHLIGHTS.
Exercise with weight loss lowers plasma Dipeptidyl Peptidase-4 (DPP-4) levels.
Lower DPP-4 is linked to elevated insulin sensitivity by the euglycemic-clamp.
Reduced DPP-4 is also associated with improved fat oxidation and glucose tolerance.
Habitual exercise may lower cardiometabolic disease risk by reducing DPP-4.
ACKNOWLEDGEMENTS
S.K.M and J.P.K co-wrote the manuscript and contributed to study design. H.H. was responsible for DPP-4 data analysis. S.K.M. takes responsibility for statistical integrity. S.R.K oversaw medical supervision. S.K.M, A.M., H.H., S.R.K, and J.P.K. collected data and edited the manuscript. We thank the Clinical Research Unit and nursing staff for technical assistance. We also thank the dedicated research assistants and participants for their effort. No competing financial interests exist. This research was supported by the National Institutes of Health RO1 AG12834 (to J.P.K.), National Center for Research Resources, CTSA 1UL1 RR-024989, Cleveland, Ohio. SKM was supported by T32 DK007319.
ABBREVIATIONS
- Akt
protein kinase B
- PPAR-α
Peroxisome proliferator-activated receptor alpha
- TNF-α
Tumor necrosis factor alpha
- SREBP-1c
Sterol retinol-element binding protein one c
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30:89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- 2.Azuma K, Radikova Z, Mancino J, Toledo FG, Thomas E, Kangani C, et al. Measurements of islet function and glucose metabolism with the dipeptidyl peptidase 4 inhibitor vildagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:459–464. doi: 10.1210/jc.2007-1369. [DOI] [PubMed] [Google Scholar]
- 3.Balaban YH, Korkusuz P, Simsek H, Gokcan H, Gedikoglu G, Pinar A, et al. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol. 2007;6:242–250. [PubMed] [Google Scholar]
- 4.Bateman LA, Slentz CA, Willis LH, Shields AT, Piner LW, Bales CW, et al. Comparison of Aerobic Versus Resistance Exercise Training Effects on Metabolic Syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT) Am J Cardiol. 2011;108:838–844. doi: 10.1016/j.amjcard.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boschmann M, Engeli S, Dobberstein K, Budziarek P, Strauss A, Boehnke J, et al. Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94:846–852. doi: 10.1210/jc.2008-1400. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard L, Faucher G, Tchernof A, Deshaies Y, Lebel S, Hould F, et al. Comprehensive genetic analysis of the dipeptidyl peptidase-4 gene and cardiovascular disease risk factors in obese individuals. Acta Diabetol. 2009;46:13–21. doi: 10.1007/s00592-008-0049-4. [DOI] [PubMed] [Google Scholar]
- 7.Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci. 2003;100:6825–6830. doi: 10.1073/pnas.0631828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 9.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30:1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 10.Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol. 2011;55:10–16. doi: 10.1016/j.vph.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Frosig C, Rose AJ, Treebak JT, Kiens B, Richter EA, Wojtaszewski JF. Effects of endurance exercise training on insulin signaling in human skeletal muscle: interactions at the level of phosphatidylinositol 3-kinase, Akt, AS160. Diabetes. 2007;56:2093–2102. doi: 10.2337/db06-1698. [DOI] [PubMed] [Google Scholar]
- 12.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Haus JM, Solomon TPJ, Marchetti CM, Edmison JM, Gonzlez F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab. 2010;95:323–327. doi: 10.1210/jc.2009-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haus JM, Solomon TPJ, Marchetti CM, O'Leary VB, Brooks LM, Gonzalez F, et al. Decreased visfatin after exercise training correlates with improved glucose tolerance. Med Sci Sports Exerc. 2009;41:1255–1260. doi: 10.1249/MSS.0b013e318195bad5. [DOI] [PubMed] [Google Scholar]
- 16.Joseph LJ, Prigeon RL, Blumenthal JB, Ryan AS, Goldberg AP. Weight loss and low-intensity exercise for the treatment of metabolic syndrome in obese postmenopausal women. J Gerontol A Biol Sci Med Sci. 2011;66:1022–1029. doi: 10.1093/gerona/glr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS, Rao DC, Rankinen T, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003;35:1703–1709. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- 18.Kelly KR, Haus JM, Solomon TPJ, Patrick-Melin AJ, Cook M, Rocco M, et al. A low-glycemic index diet and exercise intervention reduces TNF(alpha) in isolated mononuclear cells of older, obese adults. J Nutr. 2011;141:1089–1094. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirino Y, Sato Y, Kamimoto T, Kawazoe K, Minakuchi K. Altered dipeptidyl peptidase-4 activity during the progression of hyperinsulinemic obesity and islet atrophy in spontaneously late-stage type 2 diabetic rats. Am J Physiol Endocrinol Metab. 2011;300:E372–E379. doi: 10.1152/ajpendo.00319.2010. [DOI] [PubMed] [Google Scholar]
- 20.Kirwan JP, del Aguila LF, Hernandez JM, Williamson DL, O'Gorman DJ, Lewis R, et al. Regular exercise enhances insulin activation of IRS-1-associated PI3-kinase in human skeletal muscle. J Appl Physiol. 2000;88:797–803. doi: 10.1152/jappl.2000.88.2.797. [DOI] [PubMed] [Google Scholar]
- 21.Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917–1925. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malin SK, Nightingale J, Choi S, Chipkin SR, Braun B. Metformin modifies the exercise training effects on risk factors for cardiovascular disease in impaired glucose tolerant adults. Obesity. 2012;21:93–100. doi: 10.1002/oby.20235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592–598. doi: 10.1177/0091270008316885. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki M, Kato M, Tanaka K, Tanaka M, Kohjima M, Nakamura K, et al. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep. 2012;5:729–723. doi: 10.3892/mmr.2011.707. [DOI] [PubMed] [Google Scholar]
- 25.Nagakura T, Yasuda N, Yamazaki K, Ikuta H, Yoshikawa S, Asano O, et al. Improved glucose tolerance via enhanced glucose-dependent insulin secretion in dipeptidyl peptidase IV-deficient Fischer rats. Biochem Biophys Res Commun. 2001;284:501–506. doi: 10.1006/bbrc.2001.4999. [DOI] [PubMed] [Google Scholar]
- 26.O'Leary VL, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100:1584–1589. doi: 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raschke S, Eckardt K, Bjorklund-Holven K, Jensen J, Eckel J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS ONE. 2013;8:e62008. doi: 10.1371/journal.pone.0062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3:195–201. doi: 10.1161/CIRCIMAGING.109.899377. [DOI] [PubMed] [Google Scholar]
- 29.Reinehr T, Roth CL, Enriori P, Masur K. Changes of dipeptidyl peptidase IV (DPP-IV) in obese children with weight loss: relationships to peptide YY, pancreatic peptide, and insulin sensitivity. J Pediatr Endocrinol Metab. 2010;23:101–108. doi: 10.1515/jpem.2010.23.1-2.101. [DOI] [PubMed] [Google Scholar]
- 30.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauve M, Ban K, Momen MA, Zhou Y, Henkelman RM, Husain M, et al. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010;59:1063–1073. doi: 10.2337/db09-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon TPJ, Haus JM, Marchetti CM, Stanley WC, Kirwan JP. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2009;297:E552–E559. doi: 10.1152/ajpendo.00220.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon TPJ, Haus JM, Kelly KR, Cook M, Filion J, Rocco M, et al. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92:1359–1368. doi: 10.3945/ajcn.2010.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sudre B, Broqua P, White RB, Ashworth D, Evans DM, Haigh R, et al. Chronic inhibition of circulating dipeptidyl peptidase IV by FE 999011 delays the occurrence of diabetes in male zucker diabetic fatty rats. Diabetes. 2002;51:1461–1469. doi: 10.2337/diabetes.51.5.1461. [DOI] [PubMed] [Google Scholar]
- 35.Villareal DT, Miller BV, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84:1317–1323. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 36.Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults--a randomized clinical trial. J Gerontol A Biol Med Sci. 2009;64:90–95. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You T, Arsenis NC, Disanzo BL, LaMonte MJ. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med. 2013;43:243–246. doi: 10.1007/s40279-013-0023-3. [DOI] [PubMed] [Google Scholar]