Three articles have appeared in 2013 with important information concerning the potential role of peptides in sleep-wake state regulation. They come with new data emerging from new technical approaches, one in humans examining release of hypocretin/orexin (Hcrt/Orx) and melanin concentrating hormone (MCH) using microdialysis, the other two in transgenic mice examining the effect of selective stimulation of MCH neurons using optogenetics. Collectively, they support the notion that these peptides can selectively modulate different waking behaviors and/or sleep-wake states.
The article by Blouin et al.1 with Siegel as senior author claims in the title that “human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction.” This formidable study measured levels of Hcrt/Orx in dialysates of extracellular fluid from the amygdala in epileptic patients under study for temporal lobe resection. Most simply, the data show higher release of Hcrt/Orx with several waking behaviors as compared to sleeping, and conversely higher release of MCH with sleeping as compared to most waking behaviors. The data are further interpreted to conclude that maximal release of Hcrt/Orx occurs with particular waking behaviors or emotions, notably social interaction and positive emotion, and minimal release with other waking conditions or behaviors, notably pain, along with eating. Conversely, maximal release of MCH occurs during waking with eating. Nonetheless, the results appear to largely corroborate what was found in rodent studies, first showing by c-Fos expression that Hcrt/Orx neurons are maximally active in association with waking behaviors, whereas MCH neurons are maximally active in association with sleep.2,3 Electrophysiological studies subsequently confirmed that Hcrt/Orx neurons discharged maximally during active waking, whereas MCH neurons discharged maximally during sleep, particularly REM or paradoxical sleep (PS).4–6 Their respective discharge rates were inversely correlated with postural muscle tone recorded from EMG of the neck muscles (Figure 1).6 However, whether Hcrt/Orx neurons are selectively active with positively rewarding situations or positive emotions, as suggested in the current article along with previous rodent studies5,7,8 or in association with fear or other conditions of arousal and stress, as suggested by other rodent studies9,10 remains to be established. This point is obviously important when considering whether narcolepsy with cataplexy is normally prevented by the discharge of Hcrt/Orx neurons and release of their peptide predominantly in association with positive emotions. With regard to MCH, the present study suggests that MCH neurons might discharge during waking, notably during and after eating. This finding would conform to certain rodent studies involving effects of MCH administration as well as gene expression in MCH neurons in association with eating.11 Thus the apparent role of MCH neurons in promoting sleep would appear to be consistent between the current human and rodent studies, yet the precise role of those neurons in sleep and potentially in eating remains to be further examined.
Figure 1.
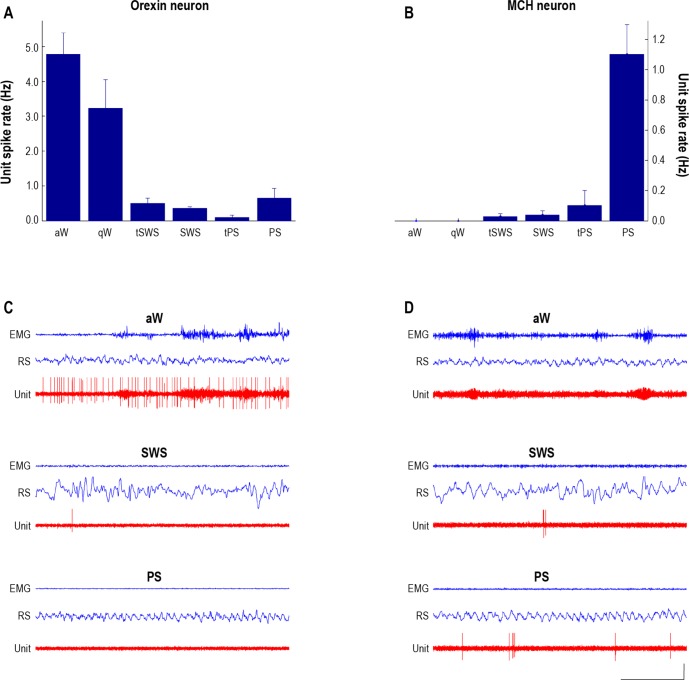
Discharge profiles of identified Orx and MCH neurons. Bar graphs showing the average spike rates (spikes per sec in Hz) of neurobiotin-labeled immunohistochemically identified Orx (A) and MCH (B) neurons along with (5 sec) periods of unit recordings with EEG and EMG (C and D, respectively). A and C: The Orx+ unit (C32u10) discharged maximally during active wake (aW) (average rate of 4.85 Hz). From aW, it decreased firing during quiet wake (qW), more significantly during transition to slow wave sleep (tSWS), to fire minimally during slow wave sleep (SWS), transition to paradoxical sleep (tPS) and paradoxical sleep (PS). Although the Orx unit was silent for long periods of PS (as illustrated), including periods of phasic twitching, it increased its firing with intervening periods of increased postural muscle tone and within several seconds prior to awakening (not shown). During maximal firing, it tended to fire in trains of spikes (with a mode interspike interval of 93.02 ms and corresponding instantaneous firing frequency of 10.75 Hz). B and D: The MCH+ unit (C134u01) discharged maximally during PS (1.1 Hz). It was silent during both aW and qW, fired at very low rates during tSWS, SWS, and tPS and at its highest (albeit still low) rate during PS. During maximal firing, it tended to fire very phasically with doublets or clusters of spikes (a mode interspike interval of 17.01 ms and corresponding instantaneous firing frequency of 58.75 Hz). The discharge rates of the Orx and MCH units were respectively (and reciprocally) positively and negatively correlated with EMG amplitude across the sleep-waking cycle. Calibrations in C and D: horizontal, 1 sec; vertical, 1 mV (EEG, EMG), 2 mV (unit). Adapted with permission from Lee4 and Hassani.6
One of the problems in specifying the role of Hcrt/Orx and MCH neurons is that they lie intermingled with other neurons in the hypothalamus where they represent < 10% of the neurons. Assessment of their activity in correlation with behavior and sleep-wake states has thus necessitated selective recording and identification employing the juxtacellular labeling technique in head-fixed rats.4,6 Fully assessing their role in behavior and states further necessitates their selective manipulation using a causative, in addition to correlative, approach. This approach has recently been made possible by the development and application of the optogenetic technique, entailing insertion of a gene for a light sensitive channel selectively into the Hcrt/Orx or MCH neurons. First applied by Adamantidis et al.12 with de Lecea as senior author, it was demonstrated that selective activation of Orx neurons in transgenic mice increased the probability of awakening from sleep. In two recent articles, the optogenetic approach has now been applied to study the effect of selective activation of MCH neurons upon sleep-wake states.
The article by Konadhode et al.13 with Shiromani as senior author claims in the title that “Optogenetic stimulation of MCH neurons increases sleep.” Most impressively, they show that optic stimulation of the MCH neurons can increase NREM and REM sleep during the night when the mice are normally awake the majority of the time and when arousal systems, including Hcrt/Orx neurons would be active. Moreover, they show an increase in the amplitude of delta EEG activity, which serves as an index for intensity and depth of slow wave sleep (SWS). The authors conclude the MCH neurons can promote sleep, including both NREM with slow waves and REM sleep.
The article by Jego et al. with Adamantidis as senior author14 claims in the title, “Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus.” Employing an impressive array of channel opsins for both the activation and silencing of the MCH neurons, they show that optic stimulation of the MCH neurons during NREM sleep results in increased transitions to REM sleep and that stimulation during REM sleep results in increased duration of REM sleep prior to awakening. They also show that inactivation of the MCH neurons decreases the frequency of theta activity during REM sleep. They then go on to show similar effects on REM sleep duration by stimulation of the terminal fibers of the opsin expressing neurons in the tuberomammillary nucleus (TMN), as well as the medial septum, where the MCH neurons project. They conclude that through their various projections and influence upon arousal systems, MCH neurons can promote REM sleep.
Jego et al. go much further to consider how MCH neurons act upon their target neurons, examining in vitro the projection from the MCH neurons to nearby histamine neurons in the TMN. They show that optic stimulation of the MCH neurons produces inhibitory postsynaptic currents (IPSCs) in the TMN neurons, which are both fast and blocked by bicucul-line, an antagonist of GABAA receptors and thus undoubtedly elicited by release of GABA. What of the MCH effect? The authors found in another double transgenic and knockout (KO) mouse lacking MCH1 receptors that the postsynaptic effect of optic stimulation is still present and not reduced in amplitude. However, the frequency of IPSCs on the TMN neurons is reduced, suggesting there is normally a presynaptic facilitation of GABA release by MCH.
Collectively, these studies raise several interesting points and questions.
First, do MCH neurons play a role in waking behavior, notably eating? Whereas the release of MCH appears to be somewhat higher during eating in Siegel's study, stimulation of MCH neurons was not reported to elicit eating in the predominantly awake animals at night in Shiromani's study, nor in the predominantly sleeping animals during the day in Adamantidis's study. Perhaps the increased MCH release occurring during and more particularly after eating in Siegel's study is associated with a drowsy state leading to sleep. In unit recording studies in rats in our lab, no units which discharged during waking with cortical fast activity (n = 49) were found to be MCH+ neurons, and conversely all MCH+ neurons (n = 7) discharged only during sleep (Figure 1).6,15
Second, do MCH neurons promote NREM sleep with slow waves? From Shiromani's results, it would appear that MCH neurons have this capacity. The increase in NREM sleep and delta power during the night, when the mice are normally awake the majority of the time, provides a strong argument for this role. Perhaps the Adamantidis study did not report such effects because the stimulation was done during the day, when the mice are already sleeping the majority of the time and for which time no effect of sleep augmentation was reported in the Shiromani study. In unit recording studies in our lab, the MCH neurons were found to discharge at a very low rate during SWS (Figure 1).6 If the total population of MCH neurons (∼6000 in the rat) fires in this manner during SWS, they could presumably produce an increase in MCH release through the brain, which might be adequate to promote SWS.
Third, do MCH neurons promote REM sleep? From both Adamantidis's and Shiromani's studies, there is strong evidence to indicate that MCH neurons can promote REM sleep. These results and conclusions are supported by recording studies in our lab showing that MCH neurons fire at their maximal rate during PS (Figure 1).6 Collectively, the results indicate that MCH neurons promote sleep and the full sleep cycle progressing through SWS into PS.
Fourth, do MCH neurons act upon target neurons through release of GABA alone? Adamantidis showed that stimulation of the MCH neurons produced inhibitory currents in the hista-mine neurons by release of GABA onto GABAA receptors and independent of MCH receptors. And it has been shown in our lab that MCH varicosities contain the vesicular GABA transporter (VGAT) and form GABAergic (gephyrin+) synapses on their target noradrenaline (NA) neurons in the locus coeruleus (LC).16 However, only a small proportion of the MCH varicosities contain VGAT and form synapses (< 10%), indicating that the vast majority of the MCH varicosities would likely only release MCH and act in a nonsynaptic manner on receptors located on target neurons or their afferent inputs. Indeed, postsynaptic as well as presynaptic effects of MCH have been demonstrated on certain neurons in vitro,17,18 and MCH has been shown to antagonize the excitatory effect of Hcrt/Orx on the NA LC neurons.19 In addition, MCH suppresses glutamate release from presynaptic terminals on hypothalamic neurons, including the Hcrt/Orx neurons.17,20 Since the first demonstration of the potential role of MCH in sleep-wake regulation was by intracerebroventricular administration of the peptide, which produced increased SWS and PS,3 MCH neurons must thus be presumed to promote sleep by these multiple actions of the peptide, in addition to the actions of the co-transmitter, GABA.
Fifth, how can MCH neurons promote both NREM sleep with slow waves (0.5-4 Hz) and REM sleep with rhythmic theta activity (6.5-9 Hz)? Whereas the Shiromani group showed increased delta activity during NREM sleep with MCH stimulation, the Adamantidis group showed decreased rhythmic theta activity during REM sleep with MCH inactivation, suggesting that the MCH neurons would normally stimulate rhythmic theta activity during REM sleep. These results could perhaps be interpreted in a coherent manner when assuming first that MCH or MCH neurons can dampen the activity of arousal neurons, including the Orx neurons, to generally facilitate sleep, including both SWS and PS.20 In addition, according to evidence that MCH acts to suppress glutamate release from presynaptic terminals,17,20 it could facilitate slow waves in the cortex by increased disfacilitation.21 The MCH neurons could also facilitate rhythmic theta activity if they facilitate GABA release, as Adamantidis's results suggest, which is critical in theta generation.22
Sixth, how do MCH and Hcrt/Orx neurons fire to release their peptide and/or amino acid neurotransmitter? In our in vivo recordings, we found that both Hcrt/Orx and MCH neurons tended to fire in a phasic manner, instead of continuously, the Hcrt/Orx particularly with movement and increased muscle tone, the MCH neurons with absence of movement and muscle tone (Figure 1). The Hcrt/Orx neurons tended to fire in trains of spikes, and the MCH neurons in highly spaced doublets or clusters of spikes. From analysis of interspike intervals, the average instantaneous firing frequency of the Hcrt/Orx neurons was found to be around 10 Hz (median 9 Hz, range 7 to 26 Hz, n = 6) and that of the MCH neurons around 20 Hz (median 18 Hz, range 15 to 59 Hz, n = 5). From classical studies, it appears that bursts of spikes are necessary for release of peptides, whereas single spikes are sufficient for releasing GABA or glutamate, which would be co-released from some of the Hcrt/Orx or MCH varicosities, respectively.16,23 So, it is likely that these neurons can release glutamate or GABA with single spikes but Hcrt/Orx or MCH, respectively, only with trains or bursts of spikes. Interestingly, in both of the optogenetic studies, no effect was obtained with stimulation frequencies of 1 Hz but only with frequencies of 10 or 20 Hz for both the Hcrt/Orx and MCH neurons.12–14 One would thus imagine that the peptide is being released at these higher frequencies and reciprocally that the peptide release measured in humans1 depends upon this type of phasic discharge by the Hcrt/Orx and MCH neurons during the behavior or states in which their clear-cut maximal release occurs.
Collectively, these new findings lead to the suggestion that pharmacological manipulation of peptide transmission might be effectively applied to the treatment of sleep disorders such as narcolepsy and insomnia.
CITATION
Jones BE; Hassani OK. The role of Hcrt/Orx and MCH neurons in sleep-wake state regulation. SLEEP 2013;36(12):1769-1772.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Blouin AM, Fried I, Wilson CL, et al. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat Commun. 2013;4:1547. doi: 10.1038/ncomms2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–16. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- 3.Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–22. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGregor R, Wu MF, Barber G, Ramanathan L, Siegel JM. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J Neurosci. 2011;31:15455–67. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 9.Boutrel B, Kenny PJ, Specio SE, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–73. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furlong TM, Vianna DM, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 2009;30:1603–14. doi: 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- 11.Georgescu D, Sears RM, Hommel JD, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–40. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konadhode RR, Pelluru D, Blanco-Centurion C, et al. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci. 2013;33:10257–63. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jego S, Glasgow SD, Herrera CG, et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013 Aug 22; doi: 10.1038/nn.3522. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassani OK, Henny P, Lee MG, Jones BE. GABAergic neurons intermingled with orexin and MCH neurons in the lateral hypothalamus discharge maximally during sleep. Eur J Neurosci. 2010;32:448–57. doi: 10.1111/j.1460-9568.2010.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Cid-Pellitero E, Jones BE. Immunohistochemical evidence for synaptic release of GABA from melanin-concentrating hormone containing varicosities in the locus coeruleus. Neuroscience. 2012;223:269–76. doi: 10.1016/j.neuroscience.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 17.Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol. 2001;533:237–52. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu M, Dumalska I, Morozova E, van den Pol A, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci U S A. 2009;106:17217–22. doi: 10.1073/pnas.0908200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisetti A, Cvetkovic V, Bayer L, Jones BE, Serafin M, Muhlethaler M. Melanin concentrating hormone antagonises the hypocretin/orexin-induced depolarization of neurons in the locus coeruleus and ventral tuberomammillary nuclei. Abstr Soc Neurosci. 2009 277.6/EE18. [Google Scholar]
- 20.Rao Y, Lu M, Ge F, et al. Regulation of synaptic efficacy in hypocretin/ orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28:9101–10. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci U S A. 2001;98:1924–9. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 23.Henny P, Brischoux F, Mainville L, Stroh T, Jones BE. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience. 2010;169:1150–7. doi: 10.1016/j.neuroscience.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]