Abstract
Study Objectives:
Investigate the hypnotic effects of repeated doses of neurokinin-1 receptor antagonist, vestipitant, in primary insomnia.
Design:
Randomized, double-blind, placebo-controlled 28-day parallel-group study.
Setting:
Eleven sleep centers in Germany.
Patients:
One hundred sixty-one patients with primary insomnia.
Interventions:
Patients received vestipitant (15 mg) or placebo for 28 days; 2-night polysomnographic assessment occurred on nights 1/2 and 27/28.
Measurements and Results:
Wake after sleep onset (WASO) was improved on nights 1/2 and 27/28 (ratio, vestipitant versus placebo [95% confidence interval]: 0.76 [0.65, 0.90], P = 0.001 and 0.79 [0.65, 0.96], P = 0.02, respectively), demonstrating maintenance of the effect following repeated dosing. Latency to persistent sleep was shorter with vestipitant on nights 1/2 (P = 0.0006 versus placebo), but not on nights 27/28. Total sleep time (TST) improved with vestipitant (nights 1/2: P < 0.0001, nights 27/28: P = 0.02 versus placebo). Next-day cognitive function tests demonstrated no residual effects of vestipitant (P > 0.05 versus placebo). Adverse events (AEs) occurred in 25% of vestipitant patients versus 22% for placebo. Headache was the most common AE (8% of vestipitant patients versus 9% for placebo).
Conclusions:
Vestipitant improved sleep maintenance in patients with primary insomnia, with no associated next-day cognitive impairment. The effects on wake after sleep onset and total sleep time were maintained following repeated dosing.
Citation:
Ratti E; Carpenter DJ; Zamuner S; Fernandes S; Squassante L; Danker-Hopfe H; Archer G; Robertson J; Alexander R; Trist DG; Merlo-Pich E. Efficacy of vestipitant, a neurokinin-1 receptor antagonist, in primary insomnia. SLEEP 2013;36(12):1823-1830.
Keywords: Cognition, insomnia, NK-1 receptor antagonist, placebo, polysomnography, sleep, substance P receptor, vestipitant
INTRODUCTION
Insomnia is the most common sleep disorder and is characterized by difficulties initiating or maintaining sleep.1,2 The estimated prevalence of insomnia varies greatly due to differences in definitions and assessment methods, though symptoms reportedly occur in up to 50% of the adult population, with general insomnia disorder (insomnia symptoms with distress or impairment) occurring in 10-15%.1–3 The prevalence of primary insomnia, which requires a diagnosis of insomnia symptoms lasting at least 1 mo that cannot be attributable to another sleep disorder, mental disorder, or substance or medication use, is estimated at approximately 6%.3,4 Currently available hypnotic treatments include benzodiazepine receptor agonists, a selective melatonin receptor agonist, and a histamine (H1) receptor antagonist. Early benzodiazepine drugs were approved for short-term use only.5–7 However, studies of the more recently developed compounds, including extended-release formulations of some previously approved products, have demonstrated efficacy and safety over a longer term, including persistent improvements in wake after sleep onset (WASO), which is an important measure used to assess the efficacy of novel treatments.8,9
Along with providing efficacy over the long term, a lack of residual effects is a desirable feature of insomnia treatments. As such, there has been a trend toward development of medications with a short half-life.2,10 Benzodiazepines can be abused,11 particularly by substance abusers,12 and are categorized as schedule IV controlled substances.2 There is consequently a need for primary insomnia therapies that provide long-term benefits but do not have residual effects or abuse liability.
Emerging data suggesting that substance P and neurokinin (NK) receptors are involved in the control of arousal and sleep have resulted in increased interest in this signaling pathway in the field of sleep research. Substance P is released by several brain structures in response to environmental stressors.13 In particular, substance P (acting through the NK1 receptor) is active on the locus coeruleus and raphe nuclei, two structures that are involved in rapid eye movement (REM) sleep regulation and are critical to wakefulness.14–18 In addition, NK1 receptors have been identified in structures such as the thalamus, hypothalamus, and brainstem, areas associated with cortical activation, sleep spindles, electroencephalograph (EEG) synchronization, and the sleep-wake switch.19 The awakening effects of an infusion of substance P were also shown in healthy volunteers, demonstrated by a decreased total sleep time (TST) and increased REM latency.20 Thus, antagonism of NK1 receptors may attenuate substance P-induced arousal, and therefore facilitate sleep. Further evidence in support of this theory comes from studies of depression where NK1 antagonists, such as aprepitant, orvepitant, and casopitant, have shown favorable results on sleep endpoints, including sleep maintenance.21–25
In the current study, the potent NK1 antagonist vestipitant was investigated at a dose of 15 mg,26 which was selected based on the following previous findings. Vestipitant produced transient pharmacologic and sleep EEG changes in healthy volunteers consistent with sleep related improvements, at doses of 15 and 25 mg. This included improved sleep continuity, i.e., reduced WASO, increased TST, and increased time in REM sleep, which were comparable for both doses, suggesting a maximal effect at a dose of 15 mg (GSK study NKD100014, data on file). Thus, the 15-mg dose is the lowest effective dose administered so far in humans. In a positron emission tomography study, a single dose of 15 mg vestipitant exhibited ∼90% NK1 receptor occupancy (GSK, data on file). The hypnotic effects of 15 mg vestipitant were tested in a 2-night crossover study; in that study, acute dosing of vestipitant demonstrated statistically significant improvements in polysomnography (PSG) sleep onset- and sleep maintenance-related parameters in patients with insomnia, without next-day residual effects or other relevant adverse effects (GSK study NKI110334, data on file: NCT00606697). To investigate the maintenance of these effects, the current study assessed the efficacy of a 15-mg dose of vestipitant, following acute dosing over 2 nights, as well as after 4 w of single daily dosing, in patients suffering from primary insomnia. The PSG paradigms used in this study to assess either the acute effects or the chronic effects of vestipitant were developed for insomnia, and their clinical relevance is now established with marketed hypnotics.2,27,28
METHODS
Study Design
This was a randomized, double-blind, placebo-controlled, 28-day parallel group PSG and subject-reported evaluation study to assess the efficacy of bedtime oral doses of vestipitant (15 mg/day) in adult outpatients in whom primary insomnia had been diagnosed (GSK study NKI111364; NCT00992160). The study was conducted across 11 sleep centers in Germany between May and September 2009.
The screening period consisted of an initial clinical visit and a 2-night PSG recording, with a single-blind placebo administration on both days prior to recording. Following the screening period (which included a single-blind placebo run-in at bedtime during the week prior to treatment initiation), eligible patients were randomly assigned via a central Interactive Voice Response System (IVRS) to receive vestipitant (15 mg) or placebo for 4 w (double-blind). This was followed by a 2-w follow-up period with nightly single-blind placebo for the first week. Vestipitant or placebo was taken orally in tablet form for 28 days. On both assessment nights, medication was taken 30 min before lights-out. Patients who missed 3 or more consecutive days of medication were considered noncompliant and were withdrawn from the study. Two-night PSG sessions were conducted at the beginning and at the end of the treatment period, on nights 1/2 and nights 27/28. Lights-out was between 21:00 and 24:00 based on the subjects' usual bedtime; lights-on occurred exactly 8 h after lights-out. Study protocols and all amendments were reviewed and approved by a national (Germany), regional, or investigational center ethics committee or institutional review board, and were carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from each subject prior to screening procedures and subjects were reimbursed for travel only.
Participants
Eligible patients were male and female, 18-64 y of age with a body mass index of < 34 kg/m2 and a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision diagnosis of primary insomnia.29 Patients with physical, psychiatric, and general medical conditions that may contribute to sleep disturbance were excluded. Randomized patients had a PSG TST of 240-420 min on both nights in the screening period, a mean latency to persistent sleep (LPS) of 20 min or more but not less than 15 min on either night, and mean WASO of 60 min or more but not less than 45 min on either night.
Procedures and Study Assessments
PSG assessments were carried out according to the methods of Rechtschaffen and Kales.30 The primary efficacy endpoint was mean WASO on nights 27/28, whereas the primary comparison of interest was between vestipitant and placebo (WASO on nights 27/28). Additionally, the comparison between vestipitant and placebo for the mean WASO on days 1/2 was calculated.
Key secondary efficacy endpoints also measured on nights 1/2 and 27/28 were LPS and TST. Other efficacy endpoints included: objective PSG measures of sleep stages (non-REM sleep time, REM sleep time, stage 1 and stage 2 non-REM sleep time, and slow wave sleep [SWS] time [stages 3 and 4]); subject-reported measures of sleep onset, duration, continuity, and quality using the Post-Sleep Questionnaire (PSQ); and overall insomnia severity using the subject-completed Insomnia Severity Index (ISI).31
Safety endpoints included monitoring of adverse events (AEs), vital signs, and the completion of morning cognition and psychomotor tests (Digit Symbol Substitution Test [DSST], Hopkins Verbal Learning Test-Revised,32 and the Romberg and Heel-to-Toe Gait Tests). Withdrawal symptoms were assessed using the subject-related Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ)33 at the day 14 follow-up visit. A safety visit was performed at day 15 (visit six), and mandatory follow-up safety visits occurred on follow-up days 7 and 14 (visits nine and 10). Patient feedback was collected via the PSQ on night 1 and days 1, 5, 7, and 21-25.
Nocturnal PSG was conducted by trained technologists according to the same instructions and technical specifications at each investigative site. Primary outcomes consisted of established PSG endpoints: WASO, TST, and LPS.9,34,35 Sleep stage data were also collected by PSG.
Subject-reported measures of sleep were measured using the PSQ and collected via IVRS. The PSQ was completed in clinic on the first PSG screening night, on the day 7 follow-up visit, at home for 5 consecutive days during the single-blind run-in period, and for 5 consecutive days prior to the second PSG sessions.
AEs and serious AEs (SAEs) were monitored until follow-up. Other safety analyses included monitoring of vital signs and physical examination; 12-lead electrocardiograph (ECG) and clinical laboratory tests were carried out at screening, on day 15, once each during the second PSG session, and at both follow-up visits.
Statistical Analysis
A sample size of 68 evaluable patients per treatment group (136 total) allowed for a detection of a geometric mean ratio of 0.77 between vestipitant and placebo for WASO log-transformed data, with 80% power using a two-sided t-test at an alpha level of 0.05, and assuming an underlying standard deviation (SD) of 0.54 on the logarithmic scale. Efficacy analyses were carried out in the intent-to-treat (ITT) population, defined as all patients who received at least one dose of study medication and for whom at least one postbaseline efficacy assessment was available.
Adjusted means and 95% confidence intervals were calculated for all endpoints. Endpoints that were not normally distributed (WASO and LPS) were summarized and analyzed on the log normal scale. Therefore, ratios of the treatment difference were presented between vestipitant (15 mg) and placebo. TST was analyzed on the normal scale. Primary statistical comparisons were performed using a mixed-effects model, including terms for baseline, treatment, visit, center groupings, age, sex, treatment by visit, and baseline by visit interactions for each PSG endpoint. No formal noninferiority or equivalence testing was carried out. No adjustments for multiplicity were made.
Summary statistics were produced for other endpoints, including DSST, the Verbal Learning Memory Test, and BWSQ. Verbal Learning Memory Test scores were analyzed using a mixed-effects model. The Romberg Test is scored as ‘negative’ or ‘positive’ and Heel-to-Toe Gait Test results are observations of abnormalities; therefore, statistical analyses were not planned for these endpoints.
RESULTS
Study Population
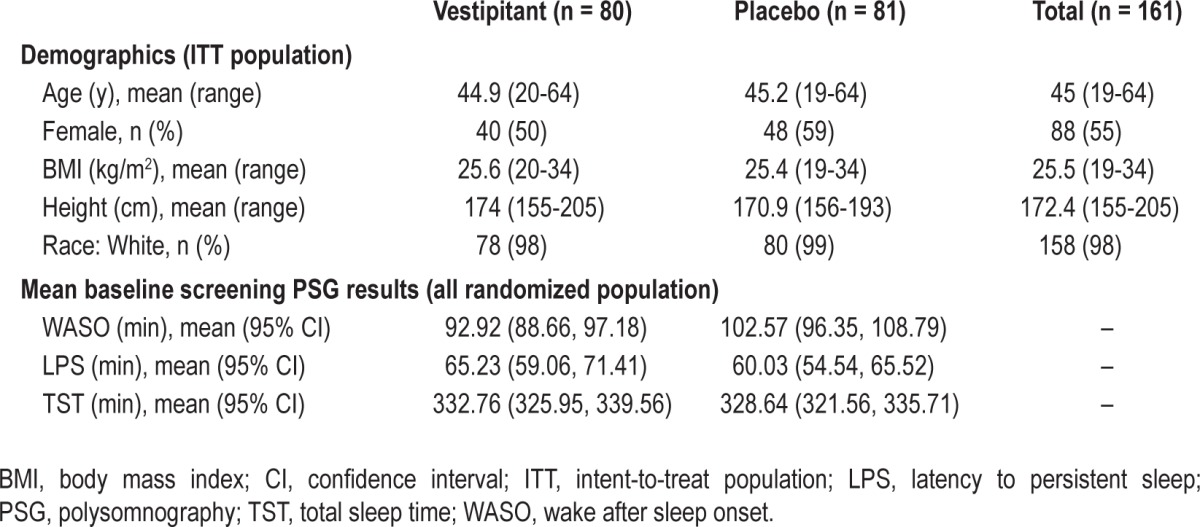
Of the 570 patients screened, 161 patients were randomized to receive study drug (vestipitant, 80; placebo, 81), and 149 (93%) completed the study (Figure 1). The majority of exclusions at this stage were due to patients not meeting the PSG criteria. All 161 patients (100%) received at least one dose of study drug, and so were included in the ITT population. Subject characteristics are presented in Table 1.
Figure 1.
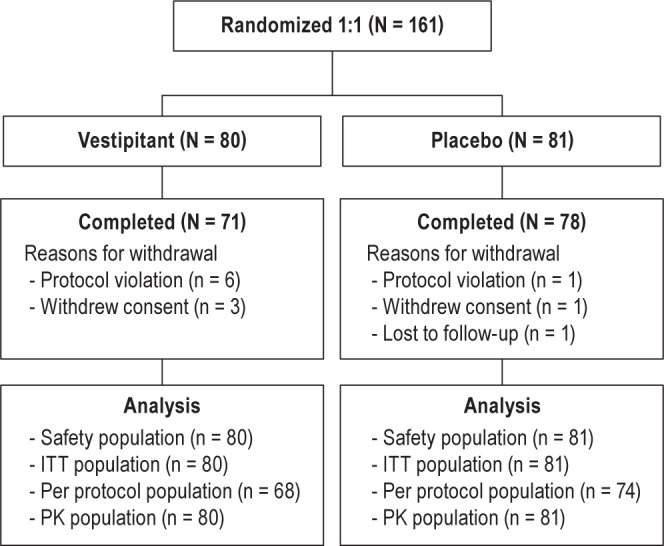
Study design. ITT, intent-to-treat; PK, pharmacokinetic.
Table 1.
Demographic and baseline characteristics
Primary Outcome
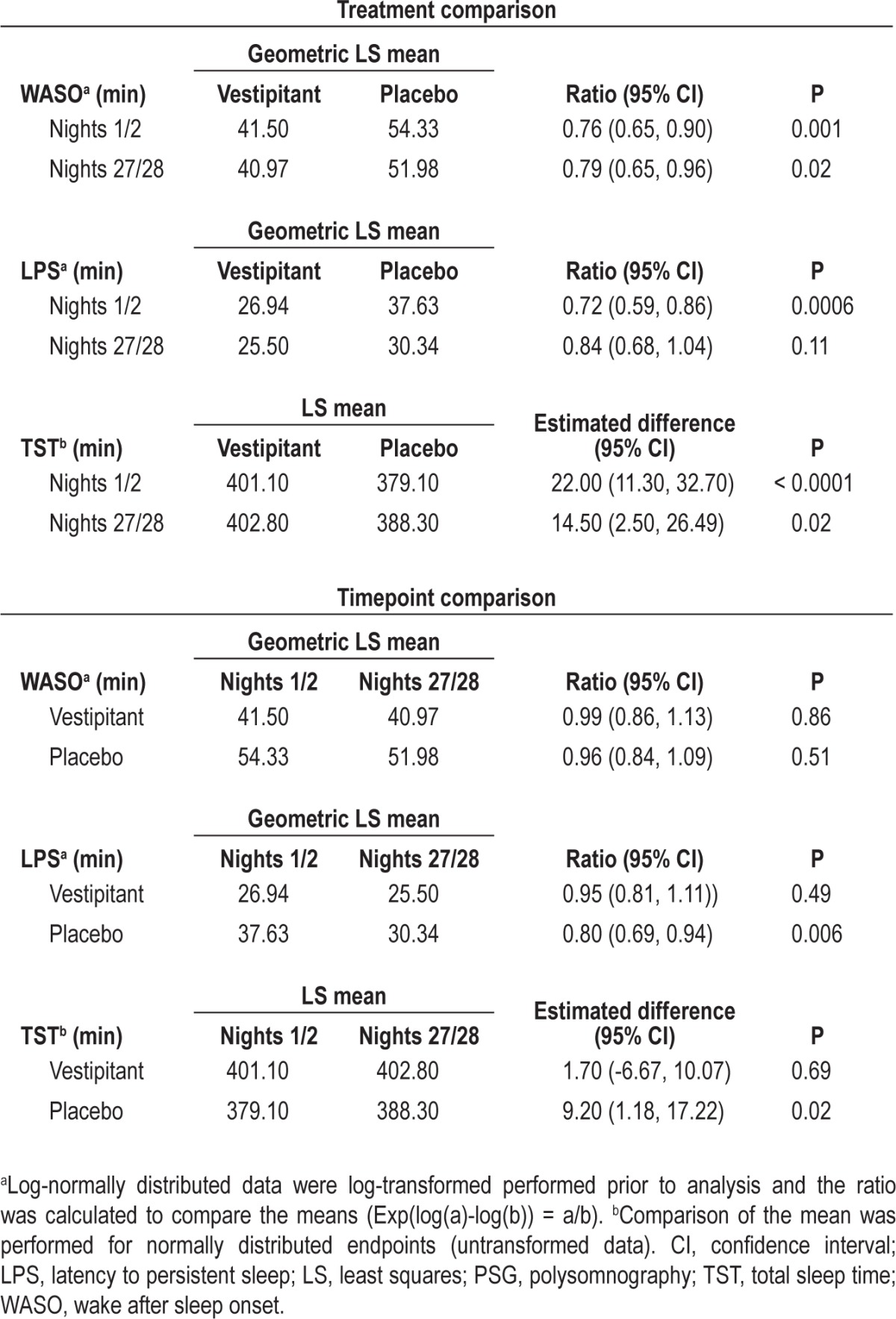
The mean WASO for vestipitant was significantly lower than for placebo on nights 27/28, an effect that was also observed during the first 2 nights (Table 2). Within-treatment group analyses comparing nights 1/2 and nights 27/28 showed no evidence of loss of the sleep maintenance effect of vestipitant (Table 2).
Table 2.
Comparison of PSG measures between treatment groups and timepoints (intent-to-treat population)
Secondary Outcome Measures
On nights 1/2, LPS was significantly shorter for vestipitant compared with placebo (P = 0.0006), but not on nights 27/28 (P = 0.11). There was no significant change in LPS for vestipitant following repeated dosing (P = 0.49); however, there was a significant decrease in LPS in the placebo group (P = 0.006; Table 2). Mean TST on nights 1/2 was significantly higher for vestipitant than placebo (P < 0.0001), an effect that was maintained on nights 27/28 (P = 0.02; Table 2). Post hoc statistical modeling revealed a significant age effect, with a greater reduction of WASO (P < 0.0001) observed in patients younger than 45 y than in those age 45 y or older.
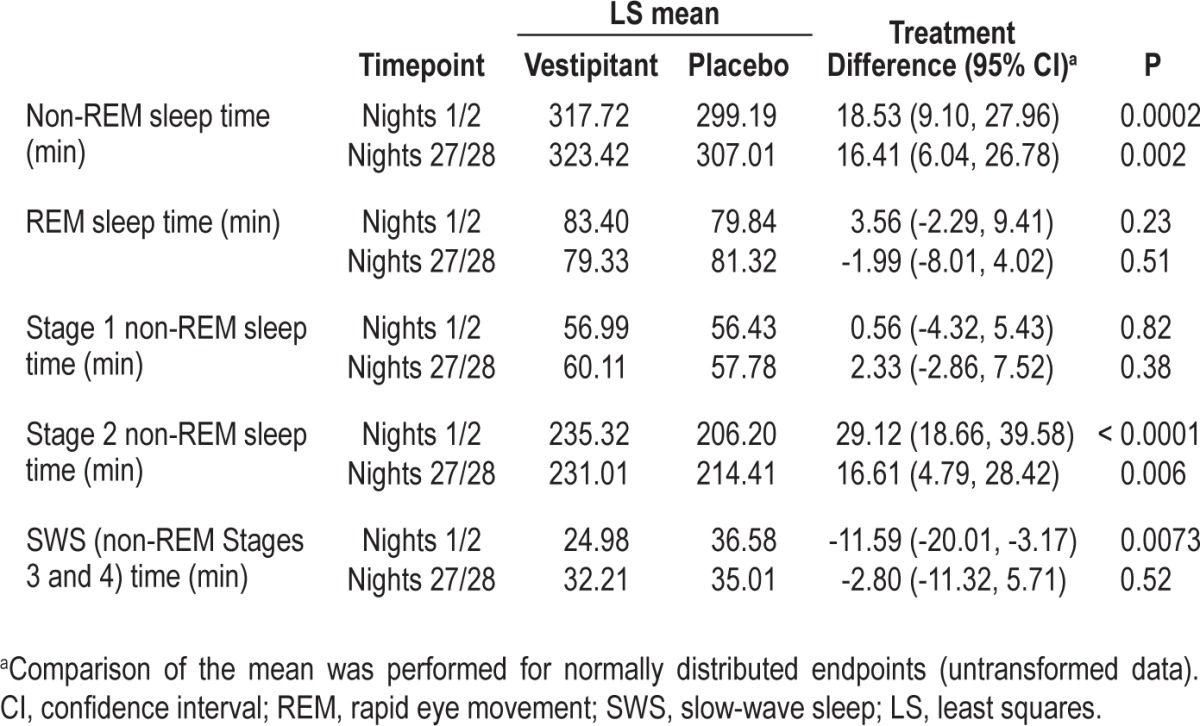
There was an increase in the time spent in non-REM sleep in the vestipitant group versus placebo on both nights 1/2 and nights 27/28 (P = 0.0002 and P = 0002, respectively), but no effect on REM. The amount of stage 1 non-REM sleep time was not different for vestipitant and placebo (Table 3), while the time spent in stage 2 non-REM sleep time in the vestipitant group was significantly greater than in the placebo group on both nights 1/2 and nights 27/28 (P < 0.0001 and P = 0.006, respectively). On nights 1/2, SWS was significantly lower in the vestipitant group compared with placebo (P = 0.007).
Table 3.
Sleep stage data (intent-to-treat population)
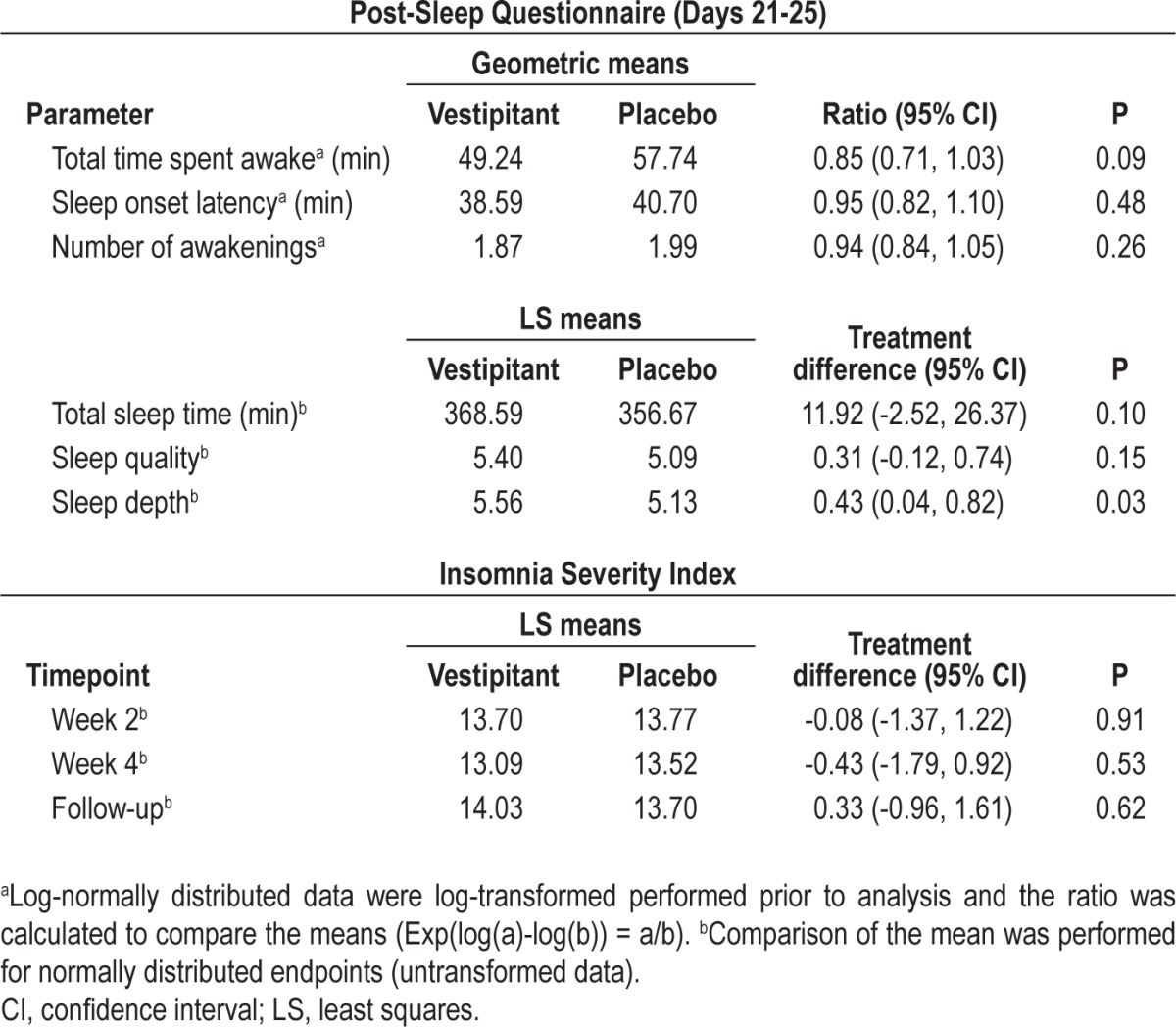
Subjectively assessed PSQ measures are shown in Table 4. The only statistically significant difference was greater ‘sleep depth’ for vestipitant compared with placebo (P = 0.03). There were no statistically significant differences between vestipitant and placebo (all P > 0.05) in the ISI (Table 4).
Table 4.
Subject-reported measures of sleep (intent-to-treat population)
Cognitive and Residual Effects
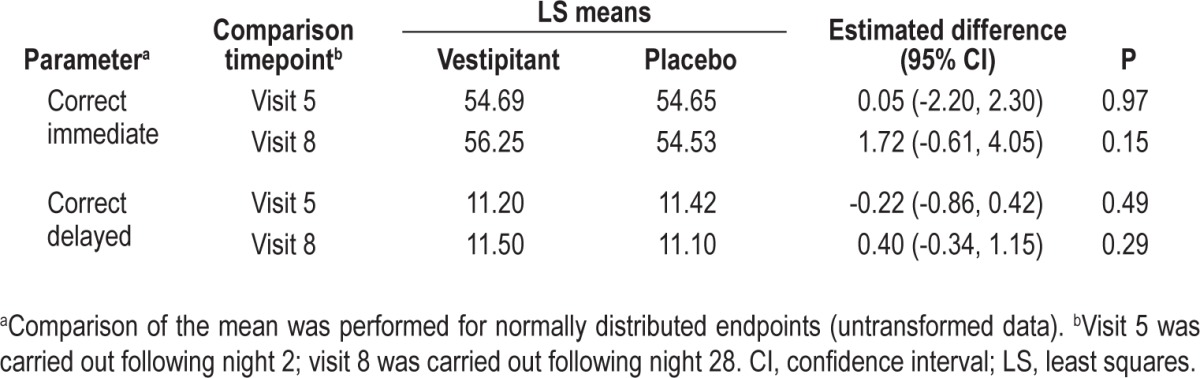
There was no evidence of a difference between vestipitant and placebo in DSST scores (Table 5). In terms of the Romberg Test and the Heel-to-Toe Gait Test, the results for all subjects at all assessment times were considered to be normal, as no loss of postural sense or balance was recorded and no abnormalities in gait or coordination were observed. The results of the verbal learning and memory assessments carried out the morning after treatment did not indicate any statistically significant differences between treatments (Table 6). There was also no evidence of insomnia rebound based on the PSQ data (data not shown), and no evidence of withdrawal symptoms (i.e. no differences between vestipitant and placebo) based on the BWSQ total score (Table 5).
Table 5.
Digit Symbol Substitution Test and Benzodiazepine Withdrawal Symptom Questionnaire (intent-to-treat population)
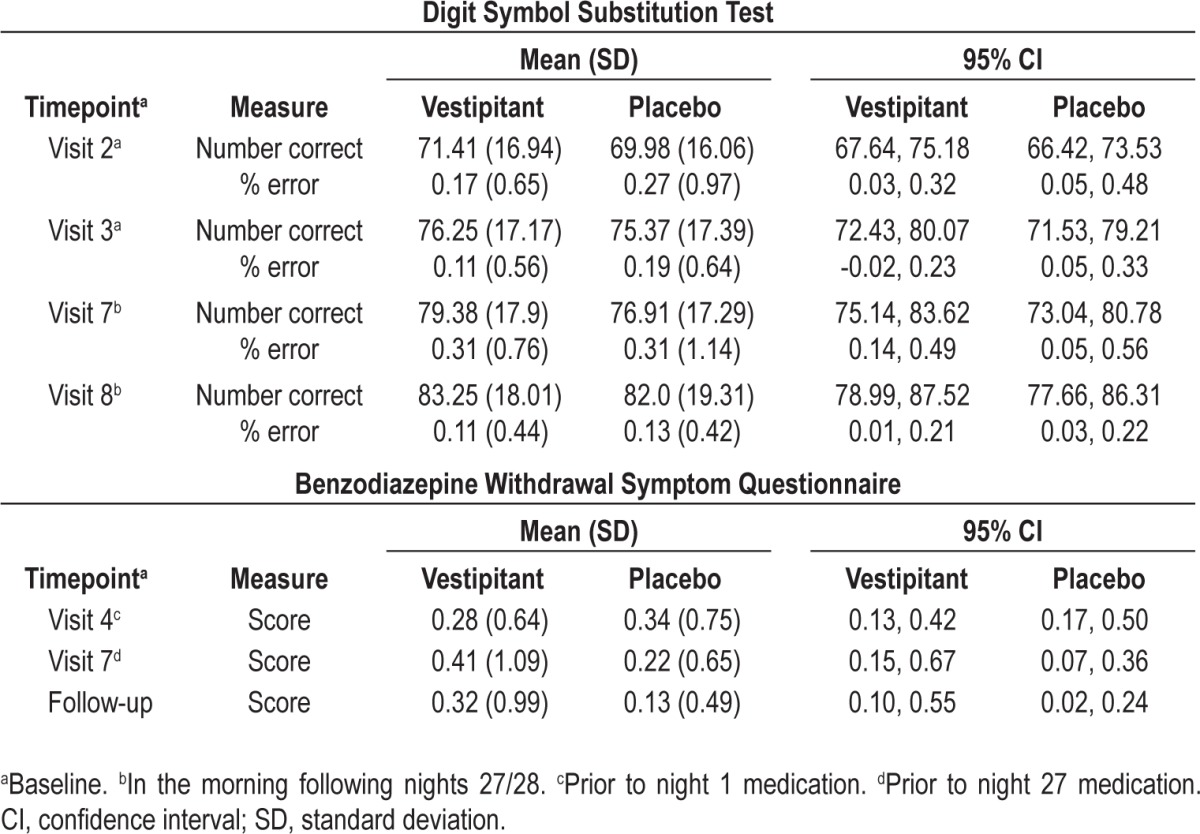
Table 6.
Verbal Learning Memory Test recalls (intent-to-treat population)
Adverse events
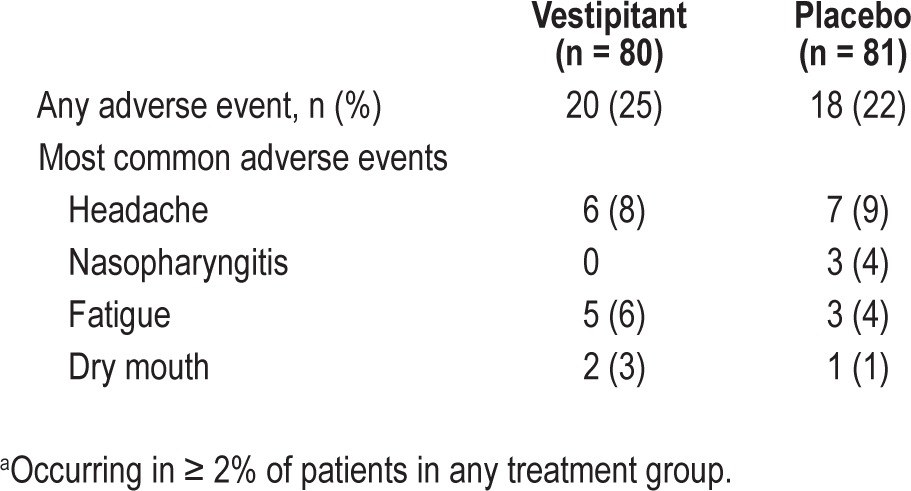
Vestipitant was generally well tolerated. A total of 107 AEs were reported by 38 patients (24%) across both the treatment groups; 58 of these AEs (54%) occurred in the placebo group and 49 (46%) occurred in the vestipitant group. The most commonly occurring AEs in patients receiving vestipitant were headache, fatigue, and dry mouth (Table 7). There were no SAEs and no subjects experienced an AE leading to premature discontinuation of the study medication. No clinically significant changes from baseline in vital signs, hematology, clinical chemistry, urinalyses, or ECGs were reported.
Table 7.
Summary of most common adverse eventsa
DISCUSSION
This was the first study to assess the hypnotic effects of 28 days of vestipitant treatment; previously, only the acute effects had been investigated. Acute vestipitant treatment improved all PSG parameters compared with placebo over the first 2 nights. Importantly, improvements in WASO and TST outcomes persisted following repeated (28-day) dosing. In subject-reported measures, sleep depth was significantly improved compared with placebo. The sleep maintenance effects of vestipitant were not associated with residual next-day cognitive or psycho-motor effects when compared with placebo. Vestipitant also demonstrated an acceptable safety profile in terms of AEs and other safety assessments, and was generally well tolerated in this study.
The improvements in PSG parameters over the first 2 nights confirm the findings of a smaller, previous 2-night study (GSK, data on file: NCT00606697), and the maintenance of this effect over 28 days on WASO and TST endpoints is encouraging. Although vestipitant did not show a statistically significant improvement in LPS on nights 27/28 when compared with placebo, this was most likely due to the improvement observed in the placebo group rather than a loss of effect in the vestipitant group. Notable placebo effects have been observed in other trials of insomnia drugs, on objective and subjective endpoints.36–38 In addition, a higher response (i.e., reduction of WASO) was observed in younger subjects on vestipitant, compared with elderly subjects. Such age effects may also require further investigation.
Analysis of the PSG sleep stage profile indicated an increase in stage 2 sleep, a decrease in deep sleep stage 3-4, and no change in REM sleep, which is consistent with the findings of the previous 2-night study. Interestingly, the first evidence of vestipitant sleep inducing effects associated with an increase in stage 2 sleep was seen in healthy volunteers during phase I trials (GSK, data on file). This may be indicative of a thalamic effect of vestipitant, as stage 2 sleep is characterized by spindles associated with the thalamus.39 It has been suggested that spindle oscillations are generated through synaptic interactions with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and γ-aminobutyric acid (GABA) receptors.40 Such GABAergic and glutamate containing interneurons may be modulated by NK1 receptors, which are present in the cortex and thalamic nuclei.19
Although objective PSG measures showed statistically significant improvement with vestipitant, subjective sleep endpoints were generally not different from placebo. Patient-reported outcomes may be considered to be as important as objective sleep measures,37 and so a lack of improvement in such measures may limit the clinical potential of vestipitant. The lack of effect of vestipitant on self-report sleep measures observed in the current study is in contrast to observations with hypnotic agents acting on GABAA. This could be due to the different cognitive effects produced by NK1 antagonists and GABAA modulators that could affect subjective judgement when assessed the following day. Although the effect sizes of benzodiazepines for self-report sleep measures are generally larger than for PSG measures, this may or may not be the case for NK1 antagonists. The power calculations for the current study were carried out according to the primary endpoint (PSG measures). No such calculation was made with regard to the subjective measures, which were not primary to the protocol. Therefore, any conclusions regarding the self-report endpoints, as well as other nonprimary endpoints, should be made with caution.
An important observation of this study was the lack of residual next-day cognitive or psychomotor effects when compared with placebo. This result is of particular interest because it is in contrast to most other hypnotic agents currently used to treat insomnia, which can induce sedation and reduce memory the morning after dosing.2 For example, the recommended dose of drugs containing zolpidem has recently been lowered, due to risk of next-morning impairment.41
Limitations of the study, with regard to investigating the potential development of tolerance to the sleep maintenance effect of vestipitant, include the lack of a pre-determined noninferiority margin upon which a formal statistical test could have been performed. Because such a margin is defined clinically, and because no such clinical definition exists, a simpler approach was adopted to estimate the effect of vestipitant on WASO over time. Another limitation was that a direct quantitative assessment of rebound was not carried out; PSG measurements could have been made following termination of the drug to test for this phenomenon, which is present with several hypnotic agents.
It is not known how NK1 receptor antagonists might induce and maintain sleep. However, the distribution of NK1 receptors in the brain falls within areas associated with wakefulness promotion (WP) or sleep promotion (SP), including the locus coeruleus (WP), ventral tegmental area (WP), tuberomammillary nucleus (WP), the suprachiasmatic nucleus (SP), and the thalamus (SP).19,42 It is known that activation of NK1 receptors can stimulate the transmission of a number of monoamines such as serotonin (dorsal raphe nucleus), noradrenaline (locus coeruleus), and dopamine (ventral tegmental area),19 and cause phase shifts in the suprachiasmatic nucleus.42,43 Thus, assuming that an agonist such as substance P has a role in sleep/wake cycles then it can be hypothesized that an NK1 antagonist such as vestipitant will reduce wakefulness, and therefore induce and maintain sleep, through reducing noradrenaline transmission from the locus coeruleus to the pedunculopontine tegmental nucleus. Reduction of noradrenaline transmission will also cause direct arousal of the cortex to be reduced and REM sleep disinhibited.44 In addition, the inhibitory effect of noradrenaline on the sleep-inducing ventrolateral preoptic nucleus will be reduced.42 NK1 antagonism has been reported to give an immediate facilitatory effect on serotonin transmission in the raphe, the opposite to that expected.19 Thus, it seems unlikely that NK1 receptor antagonists have a direct effect on 5HT2A receptors in the cortex. However, it could be postulated that raphe NK1 receptor antagonists might have an effect on serotonin-induced phase changes in the suprachiasmatic nucleus by altering serotonin-induced modulation of the pacemaker to light.45 In addition, because substance P causes phase shifts per se,46 similar to light, then an NK1 antagonist would be expected to signal darkness to the circadian clock.42 Data on the effect of dopamine transmission after NK1 antagonism need further study as both facilitation and inhibition have been recorded in separate studies.19
We cannot make firm conclusions on vestipitant mechanisms of action based on the results of the current study, and further studies will be required to assess whether this unique approach offers advantages over other mechanisms. The NK1 receptor mechanism might offer benefits over GABAA augmentation by benzodiazepines by seemingly being more selective. GABAA receptors are widely distributed in the brain. For example, the inhibitory effect of GABA acts at a level that blocks activation of the ventral tegmental area, the locus coeruleus, and the tuberomammillary nucleus.44 This will have a general inhibition on four major pathways of wakefulness. There was a lack of next-day or residual effects following vestipitant dosing both in the current study as well as in an earlier 2-night crossover study (GSK study NKI110334, data on file: NCT00606697). In addition to the lack of residual effects, there was no evidence of reduced hypnotic effect of vestipitant on TST and WASO endpoints over 28 days; both of these features may be due to the lack of accumulation of drug in the blood with repeat administration (GSK, data on file). Overall, these data show that vestipitant, via a novel mechanism of action, can reliably induce and maintain sleep over the entire night in patients with primary insomnia, with no associated next-day cognitive impairment or sedation. Importantly, there was no evidence of reduced hypnotic effect of vestipitant on TST and WASO endpoints, as demonstrated for the first time over 28 days of dosing. Repeated daily vestipitant administration was also generally well tolerated with an acceptable safety profile.
DISCLOSURE STATEMENT
This study was supported by GlaxoSmithKline. Drs. Ratti, Carpenter, Zamuner, Fernandes, and Alexander were GSK employees at the time of the study. Lisa Squassante was a GSK employee at the time of the study and owns GSK shares. Dr. Danker-Hopfe is a shareholder and supervisory board chairman of The Siesta Group Schlafanalyse GmbH. Dr. Archer is a current employee of GSK and was employed by GSK at time of study. Jonathan Robertson is a current employee of GSK. Dr. Trist is presently on the Scientific Advisory Board of Nanomerics Ltd but receives no remuneration. He was a GSK employee at the time of the study but has since retired. He is currently in receipt of a GSK pension and owns GSK shares. Dr. Merlo-Pich was a GSK employee at the time of the study (until 31st December 2011) and is currently employed by F. Hoffmann La Roche R&D.
ACKNOWLEDGMENTS
The authors thank all center staff, participants, and study investigators: Heike Benes, Bettina Bergtholdt, Ingo Fietze, Gabriele Illies, Alen Jambrecina, Reiner Lehmann, Geert Mayer, Susanne Mindt-Pruefert, Christine Paschen, Isabelle Schenkenberger, and Antje Winter. Medical writing and editorial assistance in the development of this manuscript were provided by Louisa Pettinger, PhD, at Fishawack Scientific Communications Ltd.
Current author affiliations: Dr. Carpenter's current affiliation is with Dart NeuroScience, LLC, San Diego, CA; Dr. Zamuner's current affiliation is with Clinical Pharmacology Modelling and Simulation, GlaxoSmithKline, Stockley Park, Uxbridge, UK; Dr. Fernandes' current affiliation is with Clinical Science, Aptuit Verona, Verona, Italy; Dr. Ratti's current affiation is NeRRe Therapeutics Ltd.; Lisa Squassante's current affiliation is with Biostatistics, F.Hoffman-La Roche, Basel, Switzerland; Dr. Archer's and Mr. Robertson's current affiliation is with GlaxoSmithKline (GSK), Quantitative Sciences, Stevenage, UK; Dr. Alexander's current affiliation is with Neuroscience iMed, AstraZeneca, Cambridge, MA; Dr. Merlo-Pich's current affiliation is with CNS Biomarker, Neuroscience DTA, F. Hoffman-La Roche, Basel, Switzerland.
REFERENCES
- 1.Buysse DJ. Insomnia. JAMA. 2013;309:706–16. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 3.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;2:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 5.Neubauer DN. The evolution and development of insomnia pharmacotherapies. J Clin Sleep Med. 2007;3(5 Suppl):S11–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Nowell PD, Mazumdar S, Buysse DJ, Dew MW, Reynolds CF, 3rd, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278:2170–7. [PubMed] [Google Scholar]
- 8.Krystal AD, Erman M, Zammit GK, Soubrane C, Roth T ZOLONG Study Group. Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31:79–90. doi: 10.1093/sleep/31.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 10.Roth T, Roehrs T. Determinants of residual effects of hypnotics. Accid Anal Prev. 1985;17:291–6. doi: 10.1016/0001-4575(85)90029-6. [DOI] [PubMed] [Google Scholar]
- 11.Petitjean S, Ladewig D, Meier CR, Amrein R, Wiesbeck GA. Benzodiazepine prescribing to the Swiss adult population: results from a national survey of community pharmacies. Int Clin Psychopharmacol. 2007;22:292–8. doi: 10.1097/YIC.0b013e328105e0f2. [DOI] [PubMed] [Google Scholar]
- 12.Kan CC, Breteler MH, van der Ven AH, Timmermans MA, Zitman FG. Assessment of benzodiazepine dependence in alcohol and drug dependent outpatients: a research report. Subst Use Misuse. 2001;36:1085–109. doi: 10.1081/ja-100104491. [DOI] [PubMed] [Google Scholar]
- 13.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–72. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 14.Sakai K. Physiological properties and afferent connections of the locus coeruleus and adjacent tegmental neurons involved in the generation of paradoxical sleep in the cat. Prog Brain Res. 1991;88:31–45. doi: 10.1016/s0079-6123(08)63798-x. [DOI] [PubMed] [Google Scholar]
- 15.Kohlmeier KA, Burns J, Reiner PB, Semba K. Substance P in the descending cholinergic projection to REM sleep-induction regions of the rat pontine reticular formation: anatomical and electrophysiological analyses. Eur J Neurosci. 2002;15:176–96. doi: 10.1046/j.0953-816x.2001.01829.x. [DOI] [PubMed] [Google Scholar]
- 16.Ma QP, Bleasdale C. Modulation of brain stem monoamines and gamma-aminobutyric acid by NK1 receptors in rats. Neuroreport. 2002;13:1809–12. doi: 10.1097/00001756-200210070-00024. [DOI] [PubMed] [Google Scholar]
- 17.Carter ME, Brill J, Bonnavion P, et al. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci U S A. 2012;39:E2635–44. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai K. Sleep-waking discharge profiles of dorsal raphe nucleus neurons in mice. Neuroscience. 2011;197:200–24. doi: 10.1016/j.neuroscience.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Ebner K, Sartori SB, Singewald N. Tachykinin receptors as therapeutic targets in stress-related disorders. Pharm Des. 2009;15:1647–74. doi: 10.2174/138161209788168074. [DOI] [PubMed] [Google Scholar]
- 20.Lieb K, Ahlvers K, Dancker K, et al. Effects of the neuropeptide substance P on sleep, mood, and neuroendocrine measures in healthy young men. Neuropsychopharmacology. 2002;27:1041–9. doi: 10.1016/S0893-133X(02)00369-X. [DOI] [PubMed] [Google Scholar]
- 21.Ratti E, Bettica P, Alexander R, et al. Full central neurokin-1 receptor blockade is required for efficacy in depression: evidence from orvepitant clinical studies. J Psychopharmacol. 2013;27:424–34. doi: 10.1177/0269881113480990. [DOI] [PubMed] [Google Scholar]
- 22.Kramer MS, Winokur A, Kelsey J, et al. Demonstration of the efficacy and safety of a novel substance P (NK1) receptor antagonist in major depression. Neuropsychopharmacology. 2003;29:385–92. doi: 10.1038/sj.npp.1300260. [DOI] [PubMed] [Google Scholar]
- 23.Kramer MS, Cutler N, Feighner J, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–5. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 24.Holsboer F. The role of peptides in treatment of psychiatric disorders. J Neural Transm Suppl. 2003:17–34. doi: 10.1007/978-3-7091-6020-6_2. [DOI] [PubMed] [Google Scholar]
- 25.Ratti E, Bellew K, Bettica P, et al. Results from 2 randomized, double-blind, placebo-controlled studies of the novel NK1 receptor antagonist casopitant in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31:727–33. doi: 10.1097/JCP.0b013e31823608ca. [DOI] [PubMed] [Google Scholar]
- 26.Di Fabio R, Griffante C, Alvaro G, et al. Discovery process and pharmacological characterization of 2-(S)-(4-fluoro-2-methylphenyl) piperazine-1-carboxylic acid [1-(R)-(3,5-bis-trifluoromethylphenyl) ethyl]methylamide (vestipitant) as a potent, selective, and orally active NK1 receptor antagonist. J Med Chem. 2009;52:3238–47. doi: 10.1021/jm900023b. [DOI] [PubMed] [Google Scholar]
- 27.Monti JM, Attali P, Monti D, Zipfel A, de la Giclais B, Morselli PL. Zolpidem and rebound insomnia--a double-blind, controlled polysomnographic study in chronic insomniac patients. Pharmacopsychiatry. 1994;27:166–75. doi: 10.1055/s-2007-1014298. [DOI] [PubMed] [Google Scholar]
- 28.Svetnik V, Ma J, Soper KA, et al. Evaluation of automated and semiautomated scoring of polysomnographic recordings from a clinical trial using zolpidem in the treatment of insomnia. Sleep. 2007;30:1562–74. doi: 10.1093/sleep/30.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Washington DC: 2000. Diagnostic and statistical manual of mental disorders, text revision (DSM-IV-TR) [Google Scholar]
- 30.Rechtschaffen A, Kales A. Washington DC: US Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 31.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 32.Lacritz LH, Cullum CM, Weiner MF, Rosenberg RN. Comparison of the hopkins verbal learning test-revised to the California verbal learning test in Alzheimer's disease. Appl Neuropsychol. 2001;8:180–4. doi: 10.1207/S15324826AN0803_8. [DOI] [PubMed] [Google Scholar]
- 33.Tyrer P, Murphy S, Riley P. The Benzodiazepine Withdrawal Symptom Questionnaire. J Affect Disord. 1990;19:53–61. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- 34.Roth T. Measuring treatment efficacy in insomnia. J Clin Psychiatry. 2004;65:8–12. [PubMed] [Google Scholar]
- 35.Roth T, Drake C. Evolution of insomnia: current status and future direction. Sleep Med. 2004;5(Suppl 1):S23–30. doi: 10.1016/s1389-9457(04)90004-4. [DOI] [PubMed] [Google Scholar]
- 36.Bélanger L, Vallières A, Ivers H, Moreau V, Lavigne G, Morin CM. Meta-analysis of sleep changes in control groups of insomnia treatment trials. J Sleep Res. 2007;16:77–84. doi: 10.1111/j.1365-2869.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 37.Huedo-Medina TB, Kirsch I, Middlemass J, Klonizakis M, Siriwardena AN. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCall WV, D'Agostino R, Jr, Dunn A. A meta-analysis of sleep changes associated with placebo in hypnotic clinical trials. Sleep Med. 2003;4:57–62. doi: 10.1016/s1389-9457(02)00242-3. [DOI] [PubMed] [Google Scholar]
- 39.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 40.Destexhe A, Sejnowski TJ. Interactions between membrane conductances underlying thalamocortical slow-wave oscillations. Physiol Rev. 2003;83:1401–53. doi: 10.1152/physrev.00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US Food and Drug Administration. Zolpidem containing products: drug safety communication - FDA requires lower recommended doses. 2013. [Accessed February 2013]. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm334738.htm.
- 42.Szabadi E. Drugs for sleep disorders: mechanisms and therapeutic prospects. Br J Clin Pharmacol. 2006;762:61–6. doi: 10.1111/j.1365-2125.2006.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Challet E, Dugovic C, Turek FW, Van Reeth O. The selective neurokinin 1 receptor antagonist R116301 modulates photic responses of the hamster circadian system. Neuropharmacology. 2001;40:408–15. doi: 10.1016/s0028-3908(00)00165-9. [DOI] [PubMed] [Google Scholar]
- 44.Shibata S, Tsuneyoshi A, Hamada T, Tominaga K, Watanabe S. Effect of substance P on circadian rhythms of firing activity and the 2-deoxyglucose uptake in the rat suprachiasmatic nucleus in vitro. Brain Res. 1992;597:257–63. doi: 10.1016/0006-8993(92)91482-t. [DOI] [PubMed] [Google Scholar]
- 45.Reghunandanan V, Reghunandanan R. Neurotransmitters of the suprachiasmatic nuclei. J Circadian Rhythms. 2006;4:2–22. doi: 10.1186/1740-3391-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim DY, Kang HC, Shin HC, et al. Substance P plays a critical role in photic resetting of the circadian pacemaker in the rat hypothalamus. J Neurosci. 2001;11:4026–31. doi: 10.1523/JNEUROSCI.21-11-04026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


