Abstract
Objective:
To evaluate circulating adipocyte and epidermal fatty acid-binding protein (FABP4 and FABP5) concentrations in patients with obstructive sleep apnea (OSA), as well as the effects of continuous positive airway pressure (CPAP) treatment.
Methods:
Our cross-sectional study included 125 patients. After polysomnography, 58 participants met the criteria for CPAP treatment and were included in a closed cohort study of 8 weeks of CPAP treatment. General anthropometric and biochemical data and circulating FABP4 and FABP5 levels were determined in all patients at baseline and after CPAP treatment in those receiving this therapy.
Results Circulating FABP4 but not FABP5 levels were higher in patients with OSA (P = 0.003). FABP4 but not FABP5 values were associated with parameters of OSA severity independently of age, gender, adiposity and insulin resistance (P < 0.05). FABP4 but not FABP5 concentrations were determinants of OSA presence (OR: 1.11, P = 0.010) and severity (OR: 1.06, P = 0.020). After CPAP treatment, FABP4 levels decreased in the more severe patients (P = 0.019), while FABP5 levels increased in all patients (P < 0.001).
Conclusions FABP4 is directly associated with obstructive sleep apnea severity and did not change with continuous positive airway pressure treatment, while FABP5 was not associated with obstructive sleep apnea severity and increased with continuous positive airway pressure treatment. FABP4 and FABP5 have different associations with obstructive sleep apnea. FABP4 but not FABP5 could be considered a marker of metabolic alterations in obstructive sleep apnea patients.
Citation:
Català R; Cabré A; Hernández-Flix S; Ferré R; Sangenís S; Plana N; Texidó A; Masana L. Circulating FABP4 and FABP5 levels are differently linked to OSA severity and treatment. SLEEP 2013;36(12):1831-1837.
Keywords: Adipocyte fatty acid-binding protein, continuous positive airway pressure, epidermal fatty acid-binding protein, obstructive sleep apnea, therapy
INTRODUCTION
Obstructive sleep apnea (OSA) is a very prevalent disease in adult population, and its prevalence increases with age.1,2 OSA is a risk factor for cardiovascular disease, particularly when accompanied by marked nocturnal hypoxemia.1,2 The relationship between OSA and cardiovascular risk is complicated by confounding factors involved in the two entities such as obesity, age, and gender. It has been recently reported that, in severe OSA patients, treatment with continuous positive airway pressure (CPAP), in addition to improving daytime symptoms (excessive sleepiness) and quality of life,3 has a considerable impact on metabolic alterations, including decreased cholesterol levels and improved insulin resistance, which reduce cardiovascular risk.4,5
The association between OSA and cardiometabolic alterations is likely due to common pathophysiological pathways. Recently there has been active research focusing on molecules linking OSA with metabolic alterations. Among these studies, the fatty acid binding proteins (FABP) are good candidates as markers of OSA and metabolic alterations.
FABPs are a family of metabolically related, low molecular weight proteins acting as cytoplasm fatty acid transporters. The adipocyte FABP (FABP4) is one of the better known FABP subtypes. Data from animal studies have suggested that increased FABP4 levels are associated with dyslipidemia and with the development of insulin resistance.6,7 Interestingly, FABP4 is also expressed in macrophages, particularly during intracellular lipid accumulation, as in the foam cell formation process.8 In FABP4 knock out animals, the epidermal FABP (FABP5) is overexpressed in adipocytes, replacing, in part, the FABP4 intracellular function.6
Albeit mainly intracellular, these proteins are in part released into plasma, and circulating levels can be measured. The role of circulating FABP4 as a metabolic derangement biomarker has been widely established by our group and other groups.10–14 FABP4 plasma levels are associated with obesity, type 2 diabetes, and metabolic syndrome.10–12 Interestingly, the increase in plasma FABP4 levels precedes the abovementioned metabolic alterations13 and thus has been suggested as a biomarker of metabolic risk.14 FABP4 has also been associated with vascular disease because FABP4 levels are associated with the coronary atherosclerosis burden.15 Circulating FABP4 correlates with endothelial dysfunction and a causal role, beyond its role as a biomarker, in vascular damage has been suggested.16 Circulating FABP4 levels have been studied in OSA patients, and an association with OSA presence and severity has been reported.17,18 Moreover, CPAP treatment seems to decrease FABP4 levels.19 Recently, Bhushan and colleagues reported that childhood obesity and OSA are associated with higher plasma FABP4 levels, which increase cardiometabolic risk.20 The clinical significance of circulating FABP5 is less well known. It has a significant direct correlation to FABP4 and has been suggested to have a role similar to that of FABP4 as a biomarker of cardiometabolic risk. Moreover, its association to carotid intima-media thickness has been reported, although its impact as a cardiovascular risk marker is not clearly established.21,22 Both FABP4 and FABP5 are also expressed in alveolar macrophages and endothelial peribronchial cells, although only high FABP4 levels have been associated with asthma and bronchopulmonary dysplasia.23 There are no data on the correlation between FABP5 and OSA.
We hypothesized that, because of the close metabolic relationship between FABP4 and FABP5, both adipokines would be associated with OSA severity and associated metabolic disorders.
Taking into consideration the complimentary metabolic role of FABP4 and FABP5 at the cellular level, we aimed to analyze the association of these two molecules with OSA and its treatment.
MATERIALS AND METHODS
Design and Study Participants
This study included 2 parts: a cross-sectional study and a closed-cohort intervention study. We included 125 men and women, aged 18-75 years, with suspected OSA who were referred to the sleep disorders unit of our hospital. OSA was assessed by overnight polysomnography (PSG). Exclusion criteria included ventilatory failure, previous cardiovascular disease, other major chronic medical illness, psychiatric disorders, and severe alcoholism. All participants received dietary counseling and sleep hygiene advice at the beginning of the study.
Sleep Study
PSG was performed using a computerized diagnostic system (eXea PSG, BITMED, Zaragoza, Spain). Manual scoring of sleep and respiratory events were performed according to standard criteria. Sleep parameters included the apnea/hypopnea index (AHI score), duration of desaturation, arousal index, oxygen desaturation index (ODI), and lowest and mean oxygen saturation (SpO2). After PSG, OSA severity was quantified as follows AHI < 5/h (non OSA), 5/h ≥ AHI < 15/h (mild), 15/h ≥ AHI < 30/h (moderate), and AHI ≥ 30/h (severe).
Clinical Measurements
Anthropometric parameters and blood pressure were measured. Forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), and the FEV1/FVC ratio were determined at baseline by simple spirometry. The Epworth Sleepiness Scale score was calculated at baseline (ESS0) and at the end of intervention period.24
Body fat was measured according to the ultrasound image review consensus (MyLab 50 X-Vision ultrasonograph, Esaote, Italy).25 Briefly, the thicknesses of subcutaneous and visceral adipose tissue (SAT and VAT, respectively) were measured by placing the probe (7.5-12 MHz) perpendicular to the skin on the epigastrium. The SAT was defined as the distance between the anterior surface of the linea alba and the fat-skin barrier. The VAT extends from the anterior surface of the liver (left lobe) to the posterior surface of linea alba. The preaortic intraabdominal fat (PIF) was the distance between the anterior aortic wall and the posterior surface of the rectus abdominis muscle, measured 1-5 cm above the umbilicus at the xipho-umbilical line.
CPAP Intervention
Following confirmation of OSA, all severe and moderate OSA with daytime sleepiness (ESS0 > 10) or associated cardiovascular risk factors were treated with CPAP for 8 weeks, and effective pressure was verified initially (Goodknight 420G CPAP, Covidien-Nellcor and Puritan Bennett, Boulder, USA). Adherence was defined as follows: CPAP used at least 4 h/night during 75% of the study nights. Withdrawal criteria included worsening of the clinical symptoms, non-adherence, or voluntary departure from the study.
Laboratory Analysis
Blood samples were drawn after an overnight fast, and serum and plasma samples were obtained. Lipid profiles and glucose, HbA1c, creatinine and γ-glutamyl transpeptidase (GGT) levels were measured immediately, using standard enzymatic and colorimetric techniques adapted to a Modular P-800 autoanalyzer (Roche Diagnostics, Barcelona, Spain). FABP4, FABP5 and insulin levels were determined with commercial ELISA kits (BioVendor–Laboratory Medicine, Brno, Czech Republic; and Mercodia AB, Uppsala, Sweden) in serum samples stored at -80°C. All samples were analyzed individually and simultaneously. Insulin resistance was calculated using the homeostasis model assessment method.26 Biochemical assessments were performed at baseline and at the end of the intervention period.
Statistical Analysis
Two-sided P < 0.05 obtained with SPSS version 19.0 (Chicago, USA) was considered statistically significant. Statistics are detailed in the supplemental material.
RESULTS
Anthropometry and Biochemical Baseline Characteristics and Their Correlations to FABP4 and FABP5
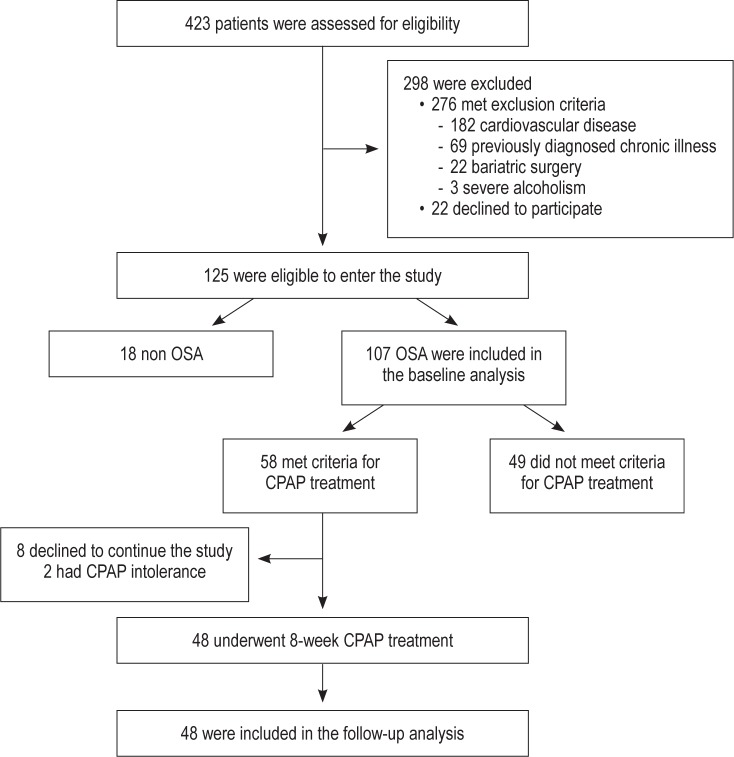
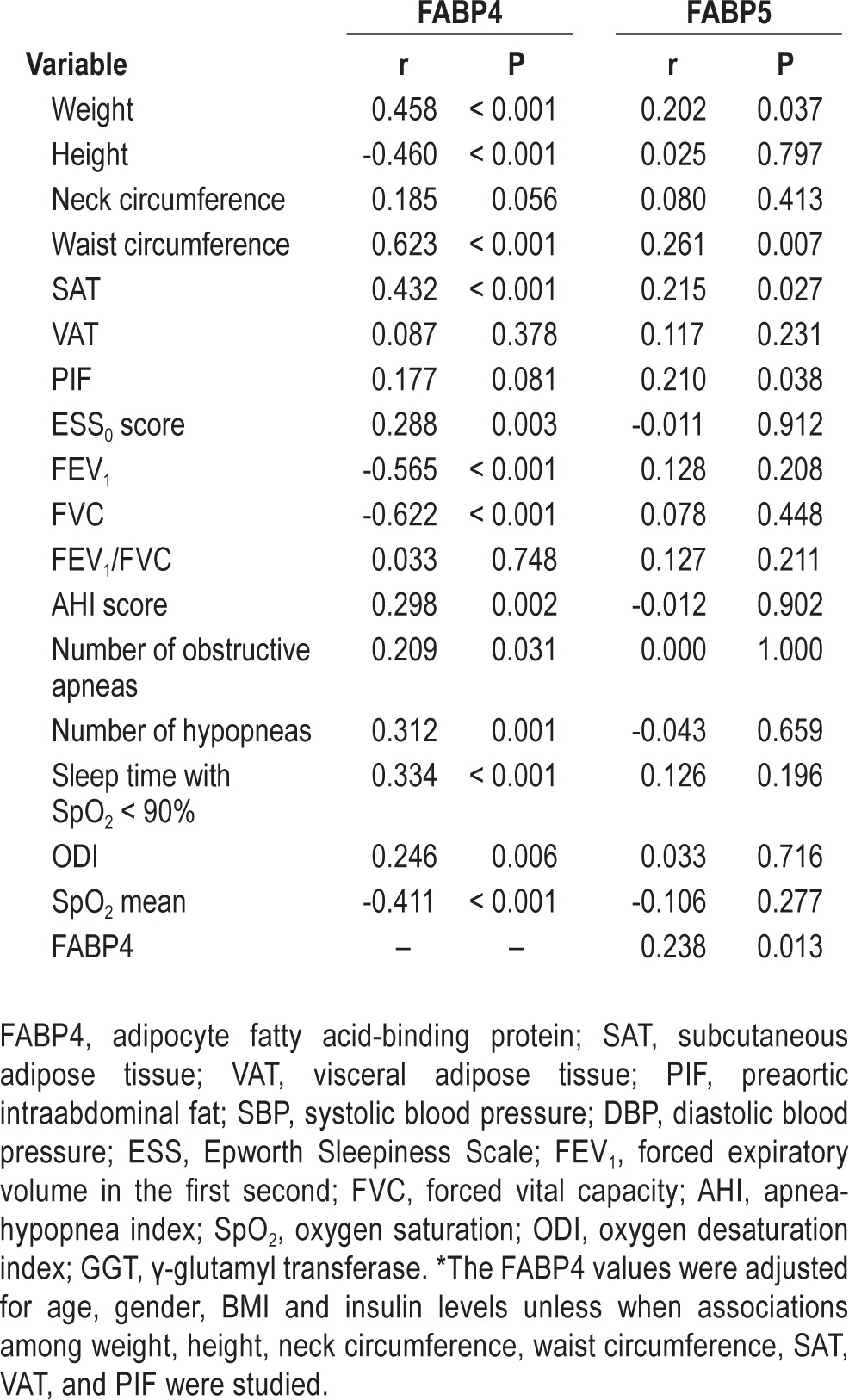
The 125 participants were distributed into 4 categories according the AHI score obtained by PSG: non-OSA (n = 18), mild OSA (n = 33), moderate OSA (n = 24), and severe OSA (n = 50). Among OSA patients, 49 subjects did not meet criteria for CPAP treatment (including mild OSA patients and those moderate OSA patients without cardiovascular risk factors or ESS0 < 10). CPAP treatment was prescribed to a total of 58 participants, 8 of whom were diagnosed with moderate OSA with cardiovascular risk factors or ESS0 > 10, and to all 50 participants with severe OSA criteria. Of these participants, 2 discontinued CPAP treatment and were excluded from the follow-up study, and 8 participants declined to undergo the follow-up study (Figure 1). Finally, 48 patients underwent follow-up; among them, 7 had moderate OSA, and 41 had severe OSA. The baseline characteristics of the study participants, distributed according to the above-mentioned OSA categories, are summarized in Table 1 and in Table S1. We observed a positive association between baseline circulating FABP4 levels and anthropometric variables and glucose metabolism variables independent of age, gender, BMI, and insulin levels (Table 2). Although FABP5 levels were also associated with weight and waist circumference, their associations were weaker (Table 2). FABP4 levels were more strongly correlated with SAT than FABP5; however, only FABP5 was significantly associated with PIF (Table 2).
Figure 1.
Flow-chart of the study design. OSA, obstructive sleep apnea; CPAP, continuous positive airway pressure.
Table 1.
Baseline characteristics of the study population
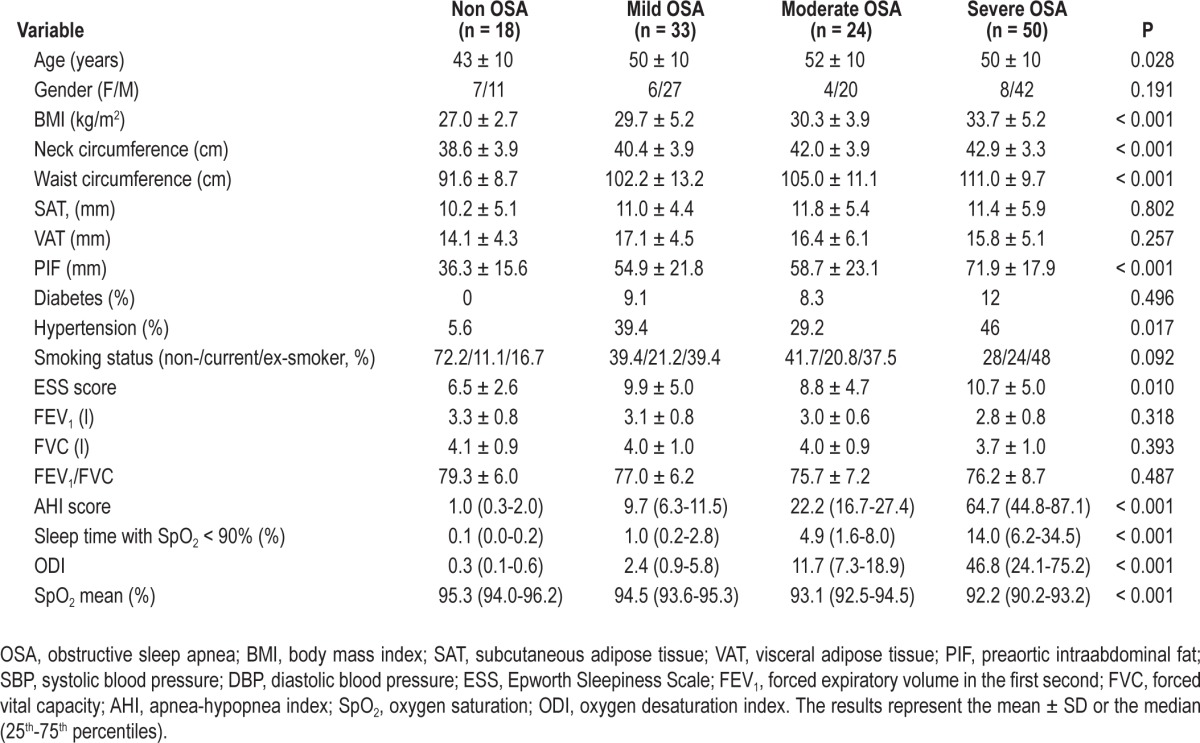
Table 2.
Correlations of the adjusted FABP4* and FABP5 levels with anthropometic, biochemical and polysomnographic variables at baseline
Association of Circulating FABP4 and FABP5 with OSA Parameters
Baseline circulating FABP4 levels were higher in OSA patients compared to non-OSA patients (25.8 ± 9.7 μg/L vs. 19.1 ± 7.9 μg/L, P = 0.003) independent of age, gender, BMI, and insulin levels. Higher baseline levels of FABP4 were observed with increasing OSA severity (overall P = 0.017; Figure 2). FABP5 levels were similar in the non-OSA and OSA groups (P = 0.919) regardless of OSA severity (P = 0.641; Figure 2).
Figure 2.
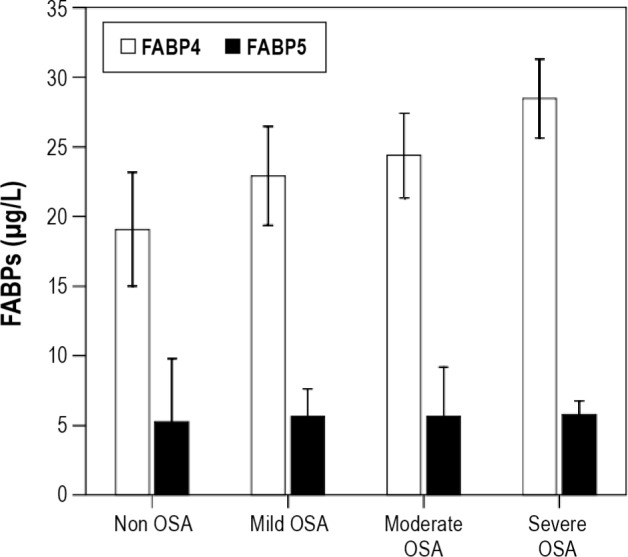
Comparison of circulating FABP4 (white bars) and FABP5 (black bars) levels in the serum of the study participants at baseline according to OSA severity. FABP4, adipocyte fatty acid-binding protein; FABP5, epidermal fatty acid-binding protein; OSA, obstructive sleep apnea. The results represent the mean and SD for FABP4 levels or the median and 25th-75th percentiles for FABP5 levels. The FABP4 values were adjusted for age, gender, BMI and insulin levels.
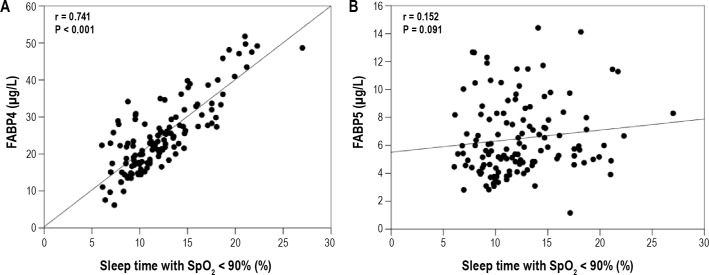
Moreover, baseline circulating FABP4, but not FABP5, levels were associated with specific OSA parameters independent of confounding variables (Table 2). Briefly, FABP4 at baseline was positively correlated with the individual perception of daytime somnolence (ESS0) (r = 0.288, P = 0.003) and the verification of OSA alterations by PSG variables such as the AHI score (r = 0.298, P = 0.002), the number of obstructive apnea and hypopnea episodes (r = 0.209, P = 0.031 and r = 0.312, P = 0.001, respectively), ODI (r = 0.246, P = 0.006), and sleep time with SpO2 levels < 90% (r = 0.334, P < 0.001) and 85% (r = 0.317, P = 0.001; Table 2). The association between baseline circulating FABP4 levels and sleep time with SpO2 levels < 90% was increased after adjusting for of age, gender, and BMI on both variables (r = 0.741, P < 0.001; Figure 3). Baseline circulating FABP4 levels were also inversely correlated with SpO2 mean levels (r = -0.411, P < 0.001), FEV1 (r = -0.565, P < 0.001), and FVC (r = -0.622, P < 0.001). None of these parameters were correlated with FABP5 plasma concentrations (Table 2 and Figure 3). Moreover, a binary logistic regression analysis revealed that each increment of 1 unit in circulating FABP4 accounted for a 10.8% increased risk of having OSA (P = 0.010). Within OSA patients, an increment of 1 unit of circulating FABP4 levels accounted for a 6.2% increased risk of having severe OSA vs. mild OSA (P = 0.020). Both observations were independent of the age, gender, BMI, and insulin values of the patients.
Figure 3.
Association of sleep time with SpO2 levels < 90% with circulating FABP4 or FABP5 levels in the study population at baseline. FABP4, adipocyte fatty acid-binding protein; FABP5, epidermal fatty acid-binding protein; SpO2, oxygen saturation. The FABP4 values were adjusted for age, gender, BMI, and insulin levels and the SpO2 values were adjusted for age, gender and BMI.
Effect of CPAP Intervention on Circulating FABP4 and FABP5
At the end of the intervention study (8 weeks), adherence to CPAP treatment was achieved by 83% of the participants (n = 48) with a median of 6.0 (5.0-7.0) h per night and a mean CPAP pressure of 8.9 ± 2.0 cm H2O
All the participants from the follow-up study showed improvement in their daytime sleepiness reported by the ESS score, and only 1 of those patients (2%) had an ESS score > 10 at the end of the 8-week CPAP treatment period.
Anthropometry parameters (weight and BMI) changed significantly after CPAP treatment (94.1 ± 11.6 kg pre-CPAP vs. 92.7 ± 11.5 kg post-CPAP, P = 0.013, and 32.9 ± 4.5 kg/m2 pre-CPAP vs. 32.5 ± 4.5 kg/m2 post-CPAP, P = 0.010, respectively). However, no changes were observed regarding glucose metabolism (data not shown).
The mean FABP4 concentration showed a decreasing trend following CPAP treatment (28.4 ± 9.1 μg/L pre-CPAP vs. 27.1 ± 3.8 μg/L post-CPAP, P = 0.442; Figure 4), and this reduction became significant when only the most severe patients (those with a higher AHI score and sleep time with SpO2 levels < 90%, n = 14) were taken into consideration (30.7 ± 9.3 μg/L pre-CPAP vs. 27.7 ± 4.4 μg/L post-CPAP, P = 0.026). Those patients allocated to received CPAP despite not presenting daytime sleepiness (ESS0 ≤ 10, n = 20), did not show statistically significant differences in their FABP4 levels in response to CPAP treatment (26.0 ± 8.5 μg/L pre-CPAP vs. 26.9 ± 4.0 μg/L post-CPAP, P = 0.218). The FABP5 plasma concentrations after CPAP were increased (5.7 [4.4-7.5 μg/L] pre-CPAP vs. 7.4 [6.3-9.4] μg/L post-CPAP, P < 0.001; Figure 4).
Figure 4.
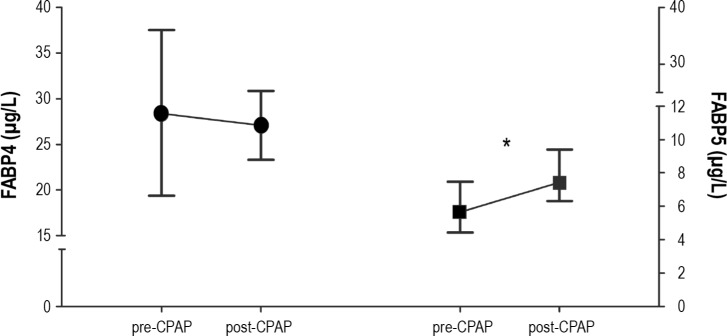
Effect of CPAP treatment on circulating FABP4 (circles) and FABP5 (squares) levels in OSA patients. CPAP, continuous positive airway pressure; FABP4, adipocyte fatty acid-binding protein; FABP5, epidermal fatty acid-binding protein; OSA, obstructive sleep apnea. The results represent mean and the SD for FABP4 levels or the median and 25th-75th percentiles for FABP5 levels. The FABP4 values were adjusted for age, gender, BMI, and insulin levels. *P < 0.001.
DISCUSSION
OSA is strongly associated with metabolic syndrome alterations such as hypertension, dyslipidemia, insulin resistance, obesity, and increased cardiovascular risk,27 and FABPs are emerging cardiometabolic risk biomarkers. Our main result is that FABP4 and FABP5 show different biological behaviors in the context of OSA. While FABP4 is correlated with OSA presence and severity and its levels decrease with treatment only in the most severe patients, FABP5 is not associated with OSA parameters and shows increased expression after CPAP.
OSA shares common pathological pathways with that of metabolic syndrome components, particularly obesity and its associated lipid and glucose metabolism alterations, as well as with subclinical inflammatory and prooxidant status.28 The circulating FABPs released from the adipose tissue, both from adipocytes and macrophages, have been related to metabolic alterations, with stronger evidence for FABP4. We observed that OSA patients have increased FABP4 values. This observation is in accordance with a previous publication in the Chinese population.19 Moreover, our data extend this observation by showing that FABP4 plasma levels increase significantly with OSA severity. The amount of circulating FABP4 is strongly linked to the quantity of adipose tissue, mainly the SAT.29 However after adjusting the data for BMI and waist circumference the correlation between both sleep time with SpO2 levels < 90% and FABP4 remained highly significant. Because FABP4 exhibits higher expression in SAT, the impact of fat on FABP4 is mainly observed in women; however, our population was 80% men. These observations suggest that the OSA and FABP4 correlation is highly fat independent. The association between FABP5 and corporal adiposity is different. In this cohort of OSA patients, FABP5 showed a weak association with SAT but a strong correlation with PIF. Therefore a different fat distribution could explain some of the differences between FABPs. Another possibility to take into consideration is that OSA itself could contribute to FABP4 secretion. Both FABP4 and FABP5 are expressed in lung tissues, mainly in alveolar macrophages.23 FABP4 is also synthesized in peribronchial endothelial cells.23 Therefore, a direct pulmonary effect of hypo-oxygenation in OSA patients leading to increased FABP4, but not FABP5, production cannot be ruled out. Moreover, the FABP changes were independent of BMI supporting a direct effect associated with OSA alterations. The impact of sleep abnormalities, such as apnea and hypoxia, on the sympathetic system could be implicated in the observed metabolic alterations. Surprisingly, CPAP treatment had different effects on FABP4 and FABP5 levels. It was expected that the plasma concentrations of both FABP would decrease after improving oxygenation; however, only FABP4 decreased significantly in these more severe patients, which is in accordance with that previously published data by Sovova et al.19 Moreover, the FABP4 levels of those patients that fulfilled the criteria for CPAP treatment but who did not present clinical daytime sleepiness were not altered after the 8-week CPAP treatment. Barbé et al. have previously demonstrated that CPAP prescription in OSA patients without daytime sleepiness does not reduce the incidence of cardiovascular events.30
Regarding FABP5, CPAP treatment produced an enhancement of its circulating levels. This phenomenon may be explained by recent observations showing that FABP5, but not FABP4, gene expression in adipose tissue is inhibited by hypoxia.31 Accordingly, improving oxygenation could lead to higher FABP5 production in fat. Moreover, FABP5 concentrations were correlated with PIF; and when FABP5 concentrations were adjusted according to PIF, there was a significant inverse correlation between OSA severity and FABP5 levels. The impact of CPAP on body adiposity has been addressed in some studies, with inconsistent results. Our data are in accordance with those of other studies32 suggesting an effect on body weight.
In our population, there was a significant correlation between baseline circulating FABP4 and sleep time with SpO2 levels below 90%. In vitro studies have shown that oxidative products promote FABP4 upregulation.8 The repeated episodes of deoxygenation and reoxygenation in OSA, which induce oxidative stress, contribute to inflammation and end-organ injury, thereby leading to the increased cardiovascular risk associated with OSA.27 It has been reported that circulating FABP4 directly interferes with myocardial cell function by reducing their contractibility,33 and some preliminary data suggest that FABP4 could alter endothelium function16; therefore, FABP4 might contribute to the increased vascular risk observed in OSA.
Limitations of the Study
We did not perform a second PSG after CPAP treatment; thus, we could not establish a correlation between FABP changes and clinical evolution. The only clinical measure of the effect of CPAP treatment in OSA patients used in this study was EES, which is also commonly used in clinical practice, and its evolution was associated with changes in FABP4 expression. The sample size, particularly in the intervention study, was rather small, complicating the statistical significance of any changes. Fat distribution was studied by ultrasonography, providing only a partial assessment of total fat distribution. BMI differences between the OSA and non-OSA patients hindered interpretation of the results.
CONCLUSIONS
Increases in FABP4 expression are associated with OSA presence and severity, and FABP4 levels decrease following CPAP treatment in the most severe patients, suggesting that FABP4 is associated with alterations that occur during OSA. Although metabolically complimentary to FABP4, FABP5 levels are not correlated with OSA, most likely because of the different roles that these proteins play at the pulmonary level and their different associations with body fat distribution.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by Catalan Respiratory Society (SOCAP, grant 2009), Spanish Respiratory Society (SEPAR, grant 2009) and CIBER in Diabetes and Associated Metabolic Disorders (ISCIII, Ministerio de Economía y Competitividad, Madrid, Spain). They had no role in the design, execution or publication of this study. Dr. Masana has participated in the RECIPE study supported by DANONE and he is a recipient of lecture fees from MSD and Kowa. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Drs. Català and Cabré contributed equally to this study. The authors are grateful to Rosa Tomàs for her assistance with initiating the project and to Maria Porto for her technical contributions to the study. The study protocol was approved by our hospital's ethics committee, and all subjects gave written informed consent. Author contributions: Conception and design: Drs. Català, Cabré, Texidó, Masana; Patients recruitment and clinical management: Drs. Català, Hernández-Flix, Ferré, Sangenís, Plana, Texidó, Masana; Analysis and interpretation: Drs. Català, Cabré, Texidó, Masana; Drafting the manuscript: Drs. Català, Cabré, Hernández-Flix, Texidó, Masana.
SUPPLEMENTAL MATERIAL
METHODS
Sleep Studies
An oronasal thermistor was used to identify the absence of flow rate during respiratory apnea events. The measurement of nasal cannula pressure was used for the coding of hypopnea. If the signals were lost, alternative measurements included the following: for apnea, nasal cannula, and hypopnea by the thermistor. An oximeter, based on a 4-beat exponential average (PureLight technology of NONIN MEDICAL, Inc., Plymouth, Minnesota, USA), was used. Respiratory movement belts were based on inductive technology. Manual scoring of sleep and respiratory events was performed by trained personnel, according to the standard criteria.1,2 Apnea was defined as an absence of airflow ≥ 10 seconds, and hypopnea was defined as an airflow reduction (> 50%) lasting ≥ 10 seconds, with > 3% decrease in oxygen desaturation. Obstructive sleep apnea was defined as an apneahypopnea index (AHI) score ≥ 5 events per hour. We included a manual overview of ODI events. ODI was assessed at 3% desaturation. CPAP titration was performed using an auto-CPAP device, following a validated protocol.3
Laboratory Analysis
Blood was collected in EDTA, citrate and serum tubes at 08:00 after a 10-h overnight fast. The blood tubes were centrifuged at 3000 rpm for 10 minutes at 4°C within 1 h, and plasma and serum were obtained. Lipids, GGT, HbA1c, and creatinine were analyzed immediately; and plasma and serum aliquots were stored at -80°C until the FABP4, FABP5 and insulin analyses were performed.
Statistical Analysis
The normality distribution was assessed using the Kolmogorov-Smirnov test. A log-transformation was performed before the analyses when the variables exhibited a skewed distribution. The data are presented as the means ± SDs or the median (25th-75th percentiles) as appropriate for continuous variables (normally or non-normally distributed, respectively) and as frequencies for categorical variables. The Mann-Whitney U or χ2 test was used to compare continuous or categorical variables between groups. Spearman correlation tests were used to analyze bivariate associations. The effects of confounding variables on FABP4 expression (variables strongly correlated with FABP4 levels) were excluded by adjusting FABP4 levels for age, sex, BMI, and insulin levels, using a regression lineal model. The CPAP effect was analyzed using Wilcoxon signed-rank test. Logistic regression analyses were conducted to evaluate the associations of FABP4 and FABP5 with the presence and severity of OSA. Tertiles of the AHI score (1 to 3) and sleep time with SpO2 levels < 90% (1 to 3) were calculated in the patients on CPAP treatment. Both variables were fused into a single variable (2 to 6) to identify the most severe patients, who were defined as the patients with higher scores (5-6).
RESULTS
Circulating FABP4 levels, but not FABP5 levels, were significantly correlated with age, sex, BMI and insulin levels (Table S2).
We studied SAT, VAT, and PIF because FABP4 is closely associated with total fat amounts, particularly SAT, which was confirmed in this group of patients. VAT and SAT, assessed by ultrasonography, were measured in the xiphoid region and were not correlated with the total fat amount but provide an idea of relative proportions. PIF was more clearly associated with total visceral fat. In our study, BMI was positively correlated with SAT (r = 0.378, P < 0.001), VAT (r = 0.320, P < 0.001) and PIF (r = 0.675, P < 0.001).
REFERENCES
- 1.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 2.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st. ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 3.Masa JF, Jiménez A, Durán J, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004;170:1218–24. doi: 10.1164/rccm.200312-1787OC. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Durán J, Esnaola S, Ramón R, Iztueta A. Obstructive sleep apneahypopnea andrelated clinical features in a population-based sample subjects aged 30 to 70 years. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Siccoli MM, Pepperell JC, Kohler M, Craig Se, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2008;31:1551–8. doi: 10.1093/sleep/31.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Börgel J, Sanner BM, Bittlinsky A, et al. Obstructive sleep apnea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J. 2006;27:121–7. doi: 10.1183/09031936.06.00131304. [DOI] [PubMed] [Google Scholar]
- 5.Dorkova Z, Petrascova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134:686–92. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 6.Scheja L, Makowski L, Uysal KT, et al. Altered insulin secretion associated with reduced lipolytic efficiency in aP2−/− mice. Diabetes. 1999;48:1987–94. doi: 10.2337/diabetes.48.10.1987. [DOI] [PubMed] [Google Scholar]
- 7.Coe NR, Simpson MA, Bernlohr DA. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J Lipid Res. 1999;40:967–72. [PubMed] [Google Scholar]
- 8.Fu Y, Luo N, Lopes-Virella MF, Garvey WT. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis. 2002;165:259–69. doi: 10.1016/s0021-9150(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 9.Furuhashi M, Ishimura S, Ota H, Miura T. Lipid chaperones and metabolic inflammation. Int J Inflam. 2011;2011:642612. doi: 10.4061/2011/642612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabré A, Lázaro I, Girona J, et al. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis. 2007;195:150–8. doi: 10.1016/j.atherosclerosis.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Tso AW, Xu A, Sham PC, et al. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care. 2007;30:2667–72. doi: 10.2337/dc07-0413. [DOI] [PubMed] [Google Scholar]
- 12.Xu A, Tso AW, Cheung BM, et al. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 2007;115:1537–43. doi: 10.1161/CIRCULATIONAHA.106.647503. [DOI] [PubMed] [Google Scholar]
- 13.Cabré A, Babio N, Lázaro I, et al. FABP4 predicts atherogenic dyslipidemia development. The PREDIMED study. Atherosclerosis. 2012;222:229–34. doi: 10.1016/j.atherosclerosis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 14.von Eynatten M, Breitling LP, Roos M, Baumann M, Rothenbacher D, Brenner H. Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler Thromb Vasc Biol. 2012;32:2327–35. doi: 10.1161/ATVBAHA.112.248609. [DOI] [PubMed] [Google Scholar]
- 15.Miyoshi T, Onoue G, Hirohata A, et al. Serum adipocyte fatty acid-binding protein is independently associated with coronary atherosclerotic burden measured by intravascular ultrasound. Atherosclerosis. 2010;211:164–9. doi: 10.1016/j.atherosclerosis.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Aragonès G, Saavedra P, Heras M, Cabré A, Girona J, Masana L. Fatty acid-binding protein 4 impairs the insulin-dependent nitric oxide pathway in vascular endothelial cells. Cardiovasc Diabetol. 2012;11:72. doi: 10.1186/1475-2840-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam DC, Xu A, Lam KS, et al. Serum adipocyte-fatty acid binding protein level is elevated in severe OSA and correlates with insulin resistance. Eur Respir J. 2009;33:346–51. doi: 10.1183/09031936.50075408. [DOI] [PubMed] [Google Scholar]
- 18.Balc MM, Arslan U, Frat H, et al. Serum levels of adipocyte fatty Acid-binding protein are independently associated with left ventricular mass and myocardial performance index in obstructive sleep apnea syndrome. J Invest Med. 2012;60:1020–6. doi: 10.2310/JIM.0b013e31826868f2. [DOI] [PubMed] [Google Scholar]
- 19.Sovova E, Hobzova M, Stejskal D, Sova M, Kolek V, Zapletalova J. Treatment of obstructive sleep apnea with continuous positive airway pressure decreases adipocyte fatty acid-binding protein levels. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012;156:58–62. doi: 10.5507/bp.2011.066. [DOI] [PubMed] [Google Scholar]
- 20.Bhushan B, Khalyfa A, Spruyt K, et al. Fatty-acid banding protein 4 gene polymorphisms and plasma levels in children with obstructive sleep apnea. Sleep Med. 2011;12:666–71. doi: 10.1016/j.sleep.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeung DCY, Wang Y, Xu A, et al. Epidermal fatty-acid-binding protein: a new circulating biomarker associated with cardiometabolic risk factors and carotid atherosclerosis. Eur Heart J. 2008;29:2156–63. doi: 10.1093/eurheartj/ehn295. [DOI] [PubMed] [Google Scholar]
- 22.Hong J, Gu W, Zhang Y, et al. Different association of circulating levels of adipocyte and epidermal fatty acid-binding proteins with metabolic syndrome and coronary atherosclerosis in Chinese adults. Atherosclerosis. 2011;217:194–200. doi: 10.1016/j.atherosclerosis.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Ghelfi E, Karaaslan C, Berkelhamer S, Akar S, Kozakewich H, Cataltepe S. Fatty acid—binding proteins and peribronchial angiogenesis in bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2011;45:550–6. doi: 10.1165/rcmb.2010-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiner E, Arriero JM, Signes-Costa J, Marco J, Fuentes I. Validation of the Spanish version of the Epworth Sleepiness Scale in patients with a sleep apnea syndrome. Arch Bronconeumol. 1999;35:422–7. doi: 10.1016/s0300-2896(15)30037-5. [DOI] [PubMed] [Google Scholar]
- 25.Vlachos IS, Hatziioannou A, Perelas A, Perrea DN. Sonographic assessment of regional adiposity. AJR Am J Roentgenol. 2007;189:1545–53. doi: 10.2214/AJR.07.2366. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation and much more. Am J Respir Crit Care Med. 2008;177:369–75. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 29.Fisher RM, Eriksson P, Hoffstedt J, et al. Fatty acid binding protein expression in different adipose tissue depots from lean and obese individuals. Diabetologia. 2001;44:1268–73. doi: 10.1007/s001250100643. [DOI] [PubMed] [Google Scholar]
- 30.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. for the Spanish Sleep and Breathing Network. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 31.Mazzatti D, Lim FL, O'Hara A, Wood IS, Trayhurn P. A microarray analysis of the hypoxia-induced modulation of gene expression in human adipocytes. Arch Physiol Biochem. 2012;118:112–20. doi: 10.3109/13813455.2012.654611. [DOI] [PubMed] [Google Scholar]
- 32.Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9:679–87. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 33.Lamounier-Zepter V, Look C, Alvarez J, et al. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res. 2009;105:326–34. doi: 10.1161/CIRCRESAHA.109.200501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.