Abstract
Study Objectives:
Mild cognitive impairment (MCI) and electroencephalographic (EEG) slowing have been reported as common findings of idiopathic rapid eye movement (REM) sleep behavior disorder (iRBD) and α-synucleinopathies. The objective of this study is to clarify the relation between MCI and physiological markers in iRBD.
Design:
Cross-sectional study.
Setting:
Yoyogi Sleep Disorder Center.
Patients or Participants:
Thirty-one patients with iRBD including 17 younger patients with iRBD (younger than 70 y) and 17 control patients for the younger patients with iRBD.
Interventions:
N/A
Measurements and Results:
Montreal Cognitive Assessment (MoCA) and n-polysomnogram (PSG) were conducted of all participants. In patients with iRBD, the factors associated with MCI were explored among parameters of REM sleep without atonia (RWA), score of Sniffin' Sticks Test (threshold-discrimination-identification [TDI] score), RBD morbidity, and RBD severity evaluated with the Japanese version of the RBD questionnaire (RBDQ-JP). The younger iRBD group showed significantly lower alpha power during wake and lower MoCA score than the age-matched control group. MCI was detected in 13 of 17 patients (76.5%) on MoCA in this group. Among patients wtih iRBD, the MoCA score negatively correlated with age, proportion of slow wave sleep, TDI score, and EEG spectral power. Multiple regression analysis provided the following equation: MoCA score = 50.871–0.116*age -5.307*log (δ power during REM sleep) + 0.086*TDI score (R2 = 0.598, P < 0.01). The standardized partial regression coefficients were -0.558 for age, -0.491 for log (δ power during REM sleep), and 0.357 for TDI score (F = 9.900, P < 0.001).
Conclusions:
Electroencephalographic slowing, especially during rapid eye movement sleep and olfactory dysfunction, was revealed to be associated with cognitive decline in idiopathic rapid eye movement sleep behavior disorder.
Citation:
Sasai T; Matsuura M; Inoue Y. Electroencephalographic findings related with mild cognitive impairment in idiopathic rapid eye movement sleep behavior disorder. SLEEP 2013;36(12):1893-1899.
Keywords: Electroencephalogram, Lewy body disease, mild cognitive impairment, Parkinson disease, REM sleep behavior disorder
INTRODUCTION
Rapid eye movement (REM) sleep behavior disorder (RBD) is characterized by dream-enacting behaviors during REM sleep associated with increased muscle activity in submentalis or limb muscle.1 Several previous reports have described that a certain number of patients with RBD progress to α-synucleinopathies such as Parkinson disease (PD) and dementia with Lewy bodies (DLB).2–5
Mild cognitive impairment (MCI) frequently occurs in PD as a nonmotor symptom of the disorder, even in the early stage of the disease.6 In patients with RBD, some studies have identified MCI such as deficits in attention, executive functions, and visuospatial abilities as a marker of neurodegeneration.7,8
During the past decade, studies using quantitative electroencephalographic (EEG) analysis have revealed EEG slowing as manifesting a cortical dysfunction in patients with idiopathic rapid eye movement sleep behavior disorder (iRBD).9,10 In addition, EEG slowing on polysomnogram (PSG) has been reported as a candidate early marker of the development of MCI in patients with the disorder.11
To date, no report in the relevant literature has presented an assessment in which clinical or EEG variables of RBD are actually associated with MCI in the disorder. This study was conducted to clarify the association between descriptive RBD variables or the EEG spectral power variables and cognitive findings.
METHODS
Patients and Measurements
The Ethical Committee of the Neuropsychiatric Research Institute approved this study. Written informed consent was obtained from all participants. In this study, 31 patients with iRBD were enrolled (67.0 ± 7.5 y, male:female = 24:7). The patient group was divided into two groups by median value of age: (1) younger patients with iRBD (younger than 70 y) and (2) older patients with iRBD (70 y or older). We set the control group age and sex-matched with the younger iRBD group without cognitive complaint and without sleep complaints in whom RBD was excluded completely using PSGs.
All patients with iRBD underwent PSGs and Sniffin' Sticks Testing shortly after the first visit (within 2 w). The control group underwent PSGs without undergoing Sniffin' Sticks Testing. All these patients self-rated the Japanese version of the Epworth Sleepiness Scale12 at the first visit. According to the second edition of the International Classification of Sleep Disorders (ICSD-2),13 diagnoses of RBD were made by at least two sleep disorder expert neurologists or neuropsychiatrists based on results of both PSG findings and through clinical interviews with the patients or their bed partners. Patients with any abnormal neurological finding suggestive of PD, any other neurological or psychiatric disorder, moderate or severe sleep apnea (apnea-hypopnea index ≥ 15), or habitual use of any medication known to modify EEG activity or muscle tone were excluded.
To detect cognitive impairment, the Montreal Cognitive Assessment (MoCA)14,15 and Mini-Mental State Examination (MMSE)16 were administered to all participants before starting treatment. These neuropsychological tests were conducted during 01:00 to 03:00 on the day of Sniffin' Sticks Testing. Sleep disorder expert neurologists and neuropsychiatrists carefully reviewed patients' cognitive status. MCI was diagnosed according to the following criteria: subjective cognitive complaint, cognitive decline on neuropsychological testing, and preserved daily life activities.8 The cognitive decline was judged by the MoCA and MMSE. Simultaneously, the Japanese version of RBD questionnaire (RBDQ-JP)17 was self-checked by all participants to measure the severity of RBD symptoms.
PSG Recording and Scoring
A standard system (Alice 5; Respironics Inc., Murrysville, PA, USA) with video monitoring of patient behavior, diagnostic n-PSG recordings, and measurements including four channels of the scalp EEG (C3/A2, C4/A1, O1/A2, O2/A1), two electrooculographs (EOG), submental electromyograph (EMG), electrocardiograph, nasal/oral airflow measured using both thermistor and nasal pressure sensor, an oximetry sensor for saturation of peripheral oxygen (SpO2) recording, a microphone for snoring sounds, chest/abdominal respiratory effort, and anterior tibialis electromyographs for leg movements (bipolar derivations with two electrodes placed 3 cm apart on the belly of the anterior tibialis muscle of right and left legs) was used.
PSG data were scored according to the current criteria set for scoring of sleep and associated events by the American Academy of Sleep Medicine (AASM)18 with allowance to score slow wave sleep (SWS) activity in the central region. Sleep variables were scored as follows: wake after sleep onset (WASO), stage N1, N2, N3, and REM (% sleep period time), arousal index, and periodic leg movements during sleep (PLMS) index. Because patients with RBD are likely to show a lack of muscle atonia, REM sleep was scored without considering submental EMG atonia.19 The onset and the termination of REM sleep were determined according to the method reported by Lapierre and Montplaisir.19 Tonic EMG activity in submentalis muscle and phasic EMG activity in submentalis as well as anterior tibialis muscles were calculated according to the criteria set by AASM.18
Quantitative EEG Evaluation
The EEG samples during wake were recorded before starting the PSG recording. Technicians instructed the patients to lie awake in bed keeping the eyes closed. The EEG samples during REM sleep were selected from all sections of clear REM sleep between two REMs. The EEG samples during nonrapid eye movement (NREM) sleep were selected from N2 and N3 periods.10 For all PSG data, recording artifacts were eliminated carefully by a board-certified sleep technician. Quantitative EEG evaluation was performed blindly using fast Fourier transform (FFT) on the artifact-free periods during wake, REM sleep, and NREM sleep.11 The FFT on 4-sec epochs with a Hamming window yielding 0.25 Hz of spectral resolution was performed on C3/A2, C4/A1, O1/A2, and O2/A1 derivations using a computer program (CSA play analysis; Norpro Light Systems, Tokyo, Japan).
Statistical Analysis
To test the normality and equality of variances of the outcome parameters, the Shapiro-Wilk test and Levene test were conducted. After checking the normality and equality of variances with a P value greater than 0.05, the following analyses were conducted. Analysis of variance followed by Bonferroni post hoc test was used to compare clinical RBD variables and sleep variables among the iRBD groups (younger and older group) and healthy control patients. The Student t-test was used to compare the EEG spectral power between the younger iRBD group and healthy control patients. To investigate factors associated with deteriorated MoCA score, at first partial correlation analysis controlled by age was conducted on the clinical RBD variables, sleep variables, and EEG power of all patients with iRBD. Then, a multiple regression equation was used to identify an actual influence of the factors with stepwise selection of variables that appeared to be significant on the partial correlation analysis. The multiple regression analysis instead of the analysis of the groups stratified by age was conducted because the correlations between age and the EEG variables or clinical RBD variables were significant but weak. In the multiple regression analysis, the significance level was set as P > 0.05 to verify the significance of the multivariate model. The values of R (multiple correlation coefficient) and R2 (the coefficient of determination) were also expected to be > 0.05 and to be > 0.26 to verify the goodness of fit of the model. Log-transformed EEG powers were used in the multiple regression equation. These statistical analyses were conducted using R version 2.15.1, or SPSS version 17.0 J (IBM, Tokyo, Japan) when possible. Significance was inferred for P < 0.05.
RESULTS
Clinical RBD-Related Variables and PSG Findings
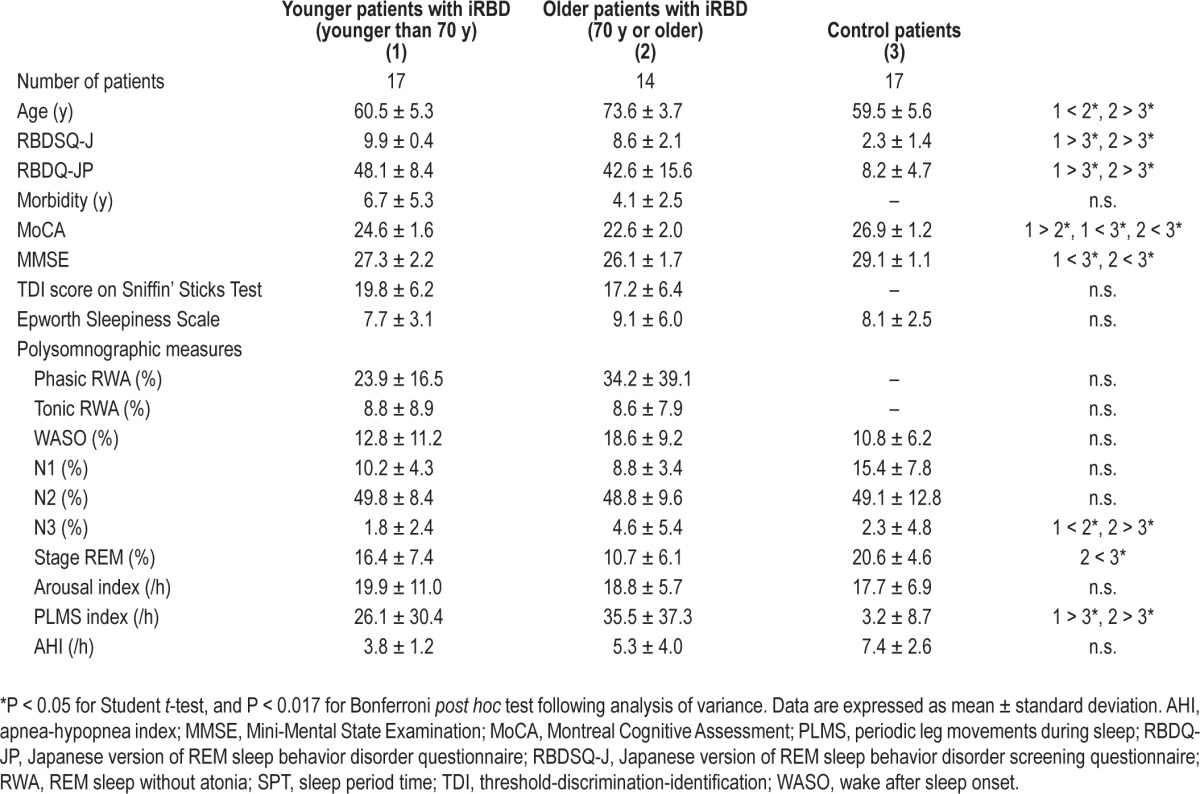
As for clinical RBD-related variables, significant differences were found in RBDSQ-J (F(2, 47) = 100.9, P < 0.01) and RBDQ-JP (F(2, 47) = 75.7, P < 0.01) among the patient groups. Post hoc testing revealed that either the younger iRBD group or older iRBD group showed higher scores of RBDSQ-J and RBDQ-JP than the healthy control patients (P < 0.01).
As for PSG variables, significant differences were found in N3 (F(2, 47) = 2.2, P < 0.01), stage REM (F(2, 47) = 9.8, P < 0.01), and the PLMS index (F(2, 47) = 6.0, P < 0.01) among the three groups. The older iRBD group showed significantly higher proportion of N3 than either the younger iRBD group or the control patients (P < 0.01). In addition, the older iRBD group showed a lower proportion of stage REM compared with the control patients (P < 0.01). Either the younger iRBD group or older iRBD group had a higher PLMS index than the control patients (P < 0.01) (Table 1).
Table 1.
Clinical, polysomnographic and neuropsychological findings
Cognitive Function Measures
Significant differences in the scores of MoCA (F(2, 47) = 28.6, P < 0.01) and MMSE (F(2, 47) = 11.6, P < 0.01) were found among the patient groups (Table 1). The younger iRBD group showed significantly lower scores on MoCA and MMSE than the age-matched control patients (P < 0.05). The older iRBD group showed a significantly lower score than the younger group did only on the MoCA test (P < 0.05).
Figure 1 presents the distribution of the scores of MoCA and MMSE. The MoCA score was lower than the cutoff (25 of 26) for detecting MCI20,21 in 13 of 17 (76.5%) of the younger iRBD group, 13 of 14 (92.9%) of the older iRBD group, and 1 of 17 (5.9%) of control patients. The MMSE score was lower than the cutoff (29 of 30) for detecting MCI21 in 10 of 17 (58.8%) of the younger iRBD group, 13 of 14 (92.9%) of the older iRBD group, and 4 of 17 (23.5%) of control patients.
Figure 1.
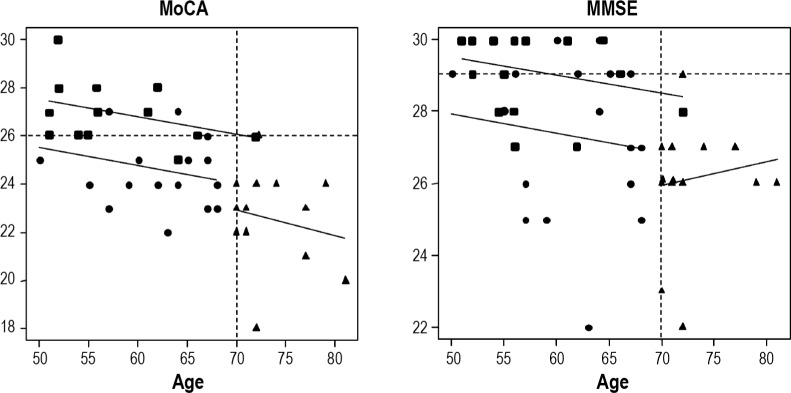
Distribution of the MoCA and MMSE scores among ages. Circles signify the younger RBD group (younger than 70 y), triangles signify the older RBD group (70 y or older), and squares signify the control group for the younger RBD group. MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; RBD, rapid eye movement sleep behavior disorder.
Comparison of EEG Spectral Power Between the Younger iRBD Group and Control Patients
During Wake
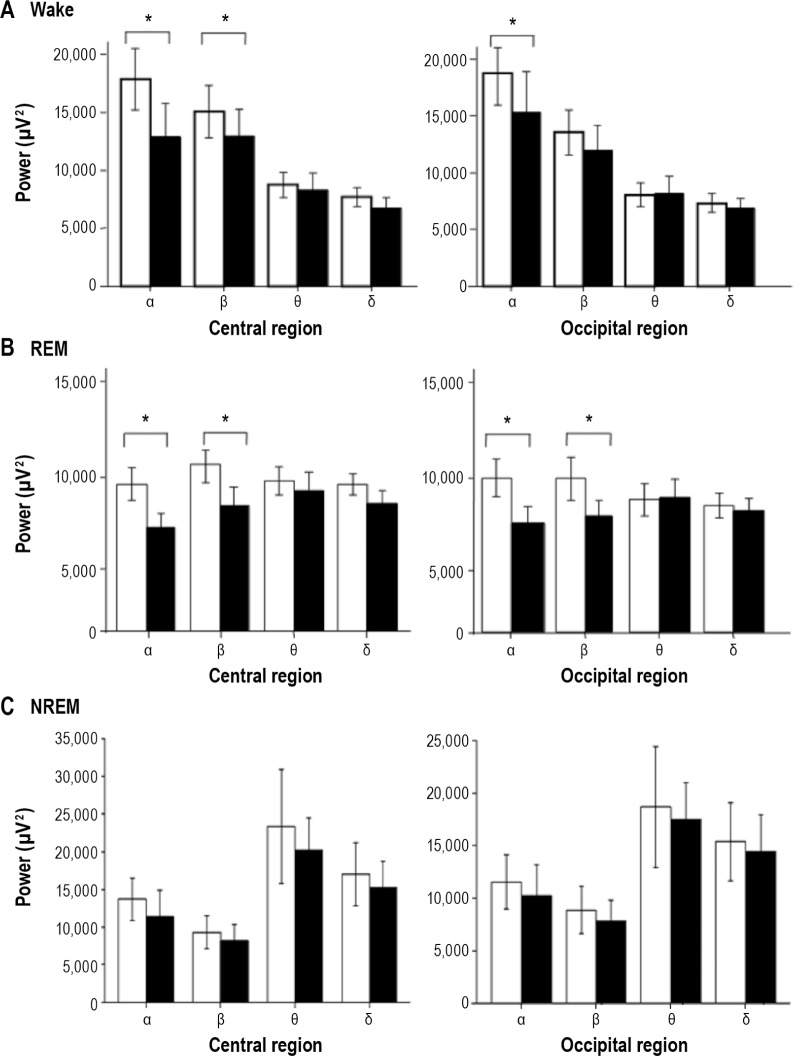
The younger iRBD group showed significantly lower alpha power in central regions (t(32) = -2.488, P < 0.05, Cohen d = 0.77) and occipital regions (t(32) = -2.347, P < 0.05, Cohen d = 0.43) compared with the control patients. This group also showed significantly lower beta power in the central region than that in the control patients (t(32) = -2.265, P < 0.05, Cohen d = 0.51) (Figure 2A).
Figure 2.
Comparison of electroencephalographic spectral power during wake, rapid eye movement sleep, and non-rapid eye movement sleep in patients with idiopathic rapid eye movement sleep behavior disorder and control patients. Black bars show data for the younger rapid eye movement sleep behavior disorder group. White bars show data for the age-matched control group. *P < 0.05, values are expressed as mean ± standard deviation.
During REM Sleep
The younger iRBD group showed significantly lower alpha power (t(32) = -2.476, P < 0.05, Cohen d = 0.58) and beta power (t(32) = -2.354, P < 0.05, Cohen d = 0.45) in the central region than did the control patients. They also showed significantly lower alpha power (t(32) = -2.277, P < 0.05, Cohen d = 0.48) and beta power (t(32) = -2.398, P < 0.05, Cohen d = 0.45) in the occipital region than did the control patients (Figure 2B).
During NREM Sleep
No significant differences in EEG spectral powers in each frequency band were found between the younger iRBD group and the control patients (Figure 2C).
Partial Correlations of the MoCA Score to Clinical RBD-Related Variables or PSG Findings
Among all the patients with iRBD, significant correlation was found between age and each of the following variables: MoCA (r = -0.388, P < 0.05), RBDSQ-J (r = -0.421, P < 0.05), RBDQ-JP (r = -0.388, P < 0.05), beta power in the central region during wake (r = -0.399, P < 0.05), alpha and beta power in the occipital region during wake (r = -0.384, r = -0.425, P < 0.05), and beta power in the central and occipital region during REM sleep (r = -0.391, r = -421, P < 0.05). No EEG variable during NREM sleep showed significant correlation with the MoCA score. To eliminate the influence of age, partial correlation analyses controlled by age were conducted between the MoCA score and the clinical RBD-related variables or EEG spectral power in respective areas during wake, REM sleep, and NREM sleep.
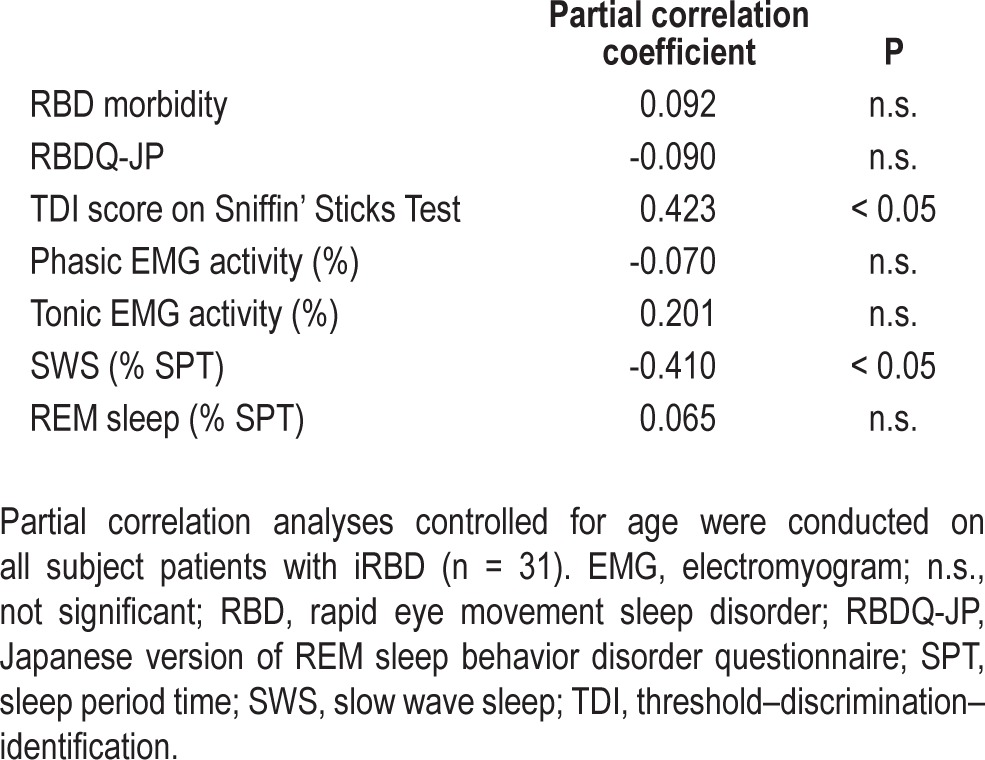
Table 2 shows age-controlled partial correlation coefficients of the MoCA score to the morbidity length of RBD, score of RBDQ-JP, threshold-discrimination-identification (TDI) score on Sniffin' Sticks Test, phasic EMG activity, tonic EMG activity, and proportion of N3 as well as REM sleep. Among these variables, the TDI score was positively correlated with the MoCA score, although the proportion of SWS was correlated negatively with the score (r = -0.410, r = 0.423, P < 0.05).
Table 2.
Partial correlation coefficients of the Montreal Cognitive Assessment score to clinical rapid eye movement sleep disorder-related variables or polysomnographic variables
Partial Correlation Between the MoCA Score and EEG Spectral Powers in Each Frequency Band
During Wake
Age-controlled partial correlation analyses revealed that delta EEG power in the central region showed negative correlation with the MoCA score (r = -0.459, P < 0.05). In the occipital region, beta, theta, and delta EEG powers showed negative correlation with the score (r = -0.495, r = -0.500, r = -0.517, P < 0.05) (Table 3).
Table 3.
Partial correlation between scores of Montreal Cognitive Assessment and electroencephalogram spectral powers in respective frequency bands
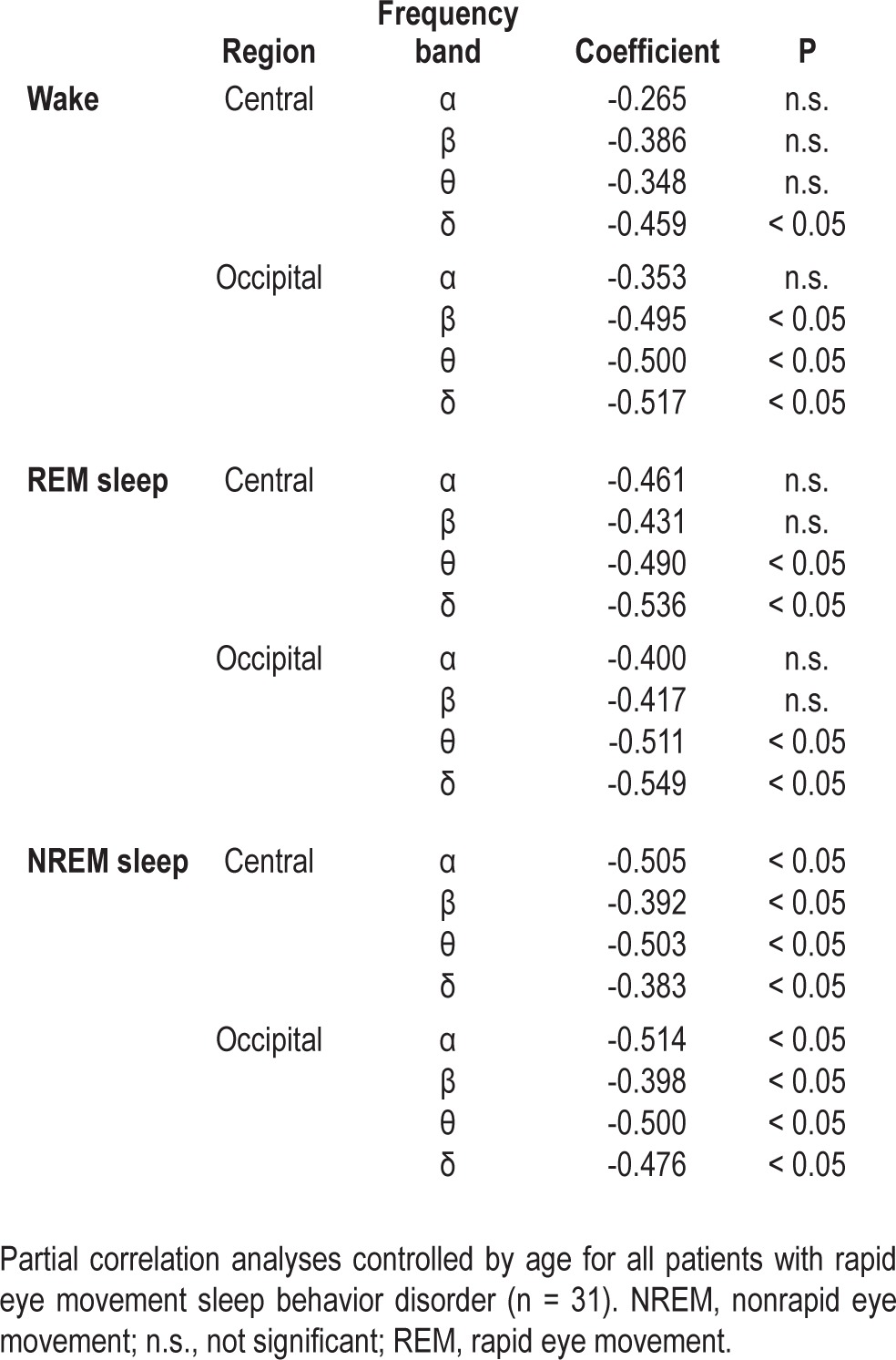
During REM Sleep
Theta and delta power in either the central region or occipital region showed negative correlation to the MoCA score (r = -0.490, r = -0.536, P < 0.05) (Table 3).
During NREM Sleep
All EEG variables during NREM sleep showed significant negative correlation with the MoCA score with the significance level set as P < 0.05; central region: alpha, r = -0.505; beta, r = -0.392; theta, r = -0.503; and delta, r = -0.383; occipital region: alpha, r = -0.514; beta, r = -0.398; theta, r = -0.500; and delta, r = -0.476 (Table 3).
Multiple Regression Analysis
To investigate factors associated with the decreased MoCA score, multiple regression analysis was conducted with the variables, which showed significant partial correlations to the MoCA score. Results indicated the following multiple regression equation: MoCA score = 50.871-0.116*age-5.307*log (δ power during REM sleep in occipital regions) + 0.086*TDI score. For this equation, the R value was 0.773, R2 was 0.598, and the regression coefficients were -0.558 for age, -0.468 for δ power, and 0.357 for TDI score (F = 9.900, P < 0.001).
DISCUSSION
This study revealed MCI in patients with iRBD and the relation between MCI and delta power during REM sleep or olfactory dysfunction in iRBD. The younger iRBD group showed lower scores in both MoCA test and MMSE compared with the age-matched control patients. The older iRBD group showed a lower score only on the MoCA test compared with the younger iRBD group. The distribution of the score of cognitive function tests demonstrated that 76.5% of the younger patients with iRBD and 92.9% of the older patients with the disorder showed the lower MoCA score than the cutoff value for MCI. In contrast, no greater percentage than 58.8% of the younger patients with iRBD showed a lower score of MMSE than the cutoff value for MCI. These results confirmed that the MoCA is more sensitive to MCI even in younger patients with iRBD, as consistent with the previous studies of MCI in patients with PD.20–22
The younger iRBD group showed significantly lower EEG spectral power, especially in fast frequency bands during both wake and REM sleep, compared with age-matched control patients. However, neither EEG power in low-frequency bands nor the percentage of N3 differed between the younger iRBD group and age-matched control patients. In previous studies, the increased percentage of SWS characterized PSG findings of patients with iRBD.23–25 Massicotte-Marquez et al.10 first reported that patients with iRBD had a greater percentage of SWS and delta power during total NREM sleep compared with control patients. However, some previous reports have described negative results for this finding.9,26 Latreille et al.26 have reported that no differences exist in spectral power in delta band or slow wave characteristics during NREM sleep between patients with iRBD and control patients. Moreover, they described that cognitive deficits observed in iRBD patients were associated with neither slow wave characteristics nor delta spectral power.26 Consistent with the study by Latreille et al.,26 the current study showed no significant difference either in the percentage of SWS or in the EEG spectral power in the delta band during NREM sleep in the younger iRBD group, in which patients' ages were similar to their study,26 and the age-matched control patients.
During the past decade, several studies using EEG spectral analysis demonstrated increased EEG power in delta and theta bands during both wake and REM sleep in patients with RBD.9–11,27 Fantini et al.9 reported increased theta power and decreased beta power during wake and REM sleep in frontal, temporal, and occipital regions. This EEG finding has been regarded as representing a modest cognitive decline in the early stages of dementia.28 Iranzo et al.11 reported that patients with RBD who developed MCI shortly later (2.5 ± 1.5 y) had shown increased delta and theta power during wake and REM sleep in central and occipital regions at the baseline. Considering the previous result for increased delta power during NREM sleep in patients with iRBD,10 EEG slowing during NREM sleep as well as that during wake or REM sleep is regarded as related with the cognitive decline. In the current study, the younger iRBD group showed decreased EEG power in alpha or beta bands in central and occipital regions during wake or REM sleep. However, the group showed no increased EEG power in low-frequency bands during each sleep status compared with the age-matched control patients in this study. The reason for this inconsistency was unclear. However, considering that the patients in the younger iRBD group in this study were younger than the iRBD patients investigated in previous studies,9–11 the inconsistency is inferred to result from a difference not only in the sample size or in EEG montage but also in the patients' age. If this is the case, then it is possible that EEG slowing, which might be an early marker of development of MCI associated with RBD,11 is likely to be detectable only in elderly patients with RBD.
To investigate factors associated with an early stage of cognitive impairment in RBD, we conducted partial correlation analysis controlled by age and subsequent multiple regression analysis of all patients with iRBD because the frequency of EEG slowing has been known to increase with normal aging.29,30 Results show that among the clinical RBD-related variables, the MoCA score showed negative correlation with percentage of SWS and positive correlation with TDI score on the Sniffin' Sticks Test. Regarding the values of EEG spectral power, the MoCA score showed negative correlation, especially to theta and delta power during wake and REM sleep, and to EEG powers in all frequency bands during NREM sleep in either central or occipital regions. Reportedly, the quantitative EEG measure is a biomarker associated with cognitive status not only in patients with Alzheimer disease (AD)31 but also in those with PD with dementia or DLB.32,33 In addition, the increased EEG power in delta and theta bands during wake has been reported to differentiate DLB from AD.33 Taking these findings into consideration, the quantitative EEG slowing in the subject iRBD patients might reflect an early stage of cognitive impairment in this disorder.
It is particularly interesting that the multiple regression equation revealed that increased delta power during REM sleep in the occipital region and olfactory dysfunction were associated with MCI in iRBD. As a possible reason for this finding, cholinergic dysfunction could be considered. Acetylcholine is an excitatory neurotransmitter that is well known to be crucial for cognitive processes. In addition, cholinergic activity is widely accepted as promoting REM sleep,34 and is known to be associated with cortical activity.35 In this regard, decreased acetylcholinesterase (AchE) activity in the cerebral cortex (especially in the occipital cortex) is observed even in the early stage of PD, although the decrease is more widespread and profound in PD dementia (PDD) or DLB.36,37 In DLB, increased EEG spectral power has reportedly been observed in theta or delta bands.33 One recent report has described that the presence of RBD symptoms might signal cholinergic system degeneration in the brainstem.38 Moreover, cholinergic denervation of the limbic cortex is a more robust determinant of hyposmia. It can indicate a risk of cognitive impairment in patients with PD.39 Taking these previous results into consideration, the intimate association between the EEG slowing, especially during REM sleep or olfactory dysfunction, and MCI can be explained partially by cholinergic dysfunction occurring in RBD as a prodrome of α-synucleinopathies. However, further research must be undertaken to verify this hypothesis.
This study and its results are burdened by some limitations. First, EEG activities in frontal, temporal, and parietal regions were not evaluated.9 Considering that slow wave activity is likely to appear in the frontal region,40 not only sleep scoring but also spectral analysis should be conducted in the frontal region as well as the central or occipital regions to clarify whether pathological EEG slowing shows electroencephalographic localization. Second, no comprehensive neuropsychological test battery was performed. Third, the sample size was small, and there is an important lack of follow-up data for PSG and neuropsychological tests. Fourth is the lack of control patients for the older iRBD group is a factor. Considering the change over time in the frequency of EEG with normal aging,29,30 the association between degrees of EEG slowing and cognitive impairment compared with that of control patients should be investigated in both younger and older patients with iRBD. Nevertheless, this study revealed physiological findings that reflect an early stage of MCI in idiopathic RBD. In future studies, association between characteristics or overtime changes in the EEG and cognitive dysfunction should be investigated to clarify the process of MCI progression.
In conclusion, patients with iRBD showed MCI and EEG slowing during wake and REM sleep. The EEG slowing especially during REM sleep rather than that during NREM sleep and olfactory dysfunction are thought to reflect a subsequent early change of cognitive function with the disorder.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Sasai reports a Fellowship for Young Clinical Sleep Researchers of Japanese Society of Sleep Research, Charitable Trust Laboratory Medicine Research Foundation of Japan, and a MEXT/JSPS KAKENHI Grant-in-Aid for Young Scientists, No. 24791435. Dr. Inoue reports an Intramural Research Grant (21B-4) for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry (NCNP). Dr. Matsuura reports no disclosure.
REFERENCES
- 1.Gagnon JF, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5:424–32. doi: 10.1016/S1474-4422(06)70441-0. [DOI] [PubMed] [Google Scholar]
- 2.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 3.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeve BF, Saper CB. REM sleep behavior disorder: a possible early marker for synucleinopathies. Neurology. 2006;66:796–7. doi: 10.1212/01.wnl.0000209264.61035.bb. [DOI] [PubMed] [Google Scholar]
- 5.Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75:494–9. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troster AI. Neuropsychological characteristics of dementia with Lewy bodies and Parkinson's disease with dementia: differentiation, early detection, and implications for “mild cognitive impairment” and biomarkers. Neuropsychol Rev. 2008;18:103–19. doi: 10.1007/s11065-008-9055-0. [DOI] [PubMed] [Google Scholar]
- 7.Gagnon JF, Bertrand JA, Genier Marchand D. Cognition in rapid eye movement sleep behavior disorder. Front Neurol. 2012;3:82. doi: 10.3389/fneur.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagnon JF, Vendette M, Postuma RB, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol. 2009;66:39–47. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- 9.Fantini ML, Gagnon JF, Petit D, et al. Slowing of electroencephalogram in rapid eye movement sleep behavior disorder. Ann Neurol. 2003;53:774–80. doi: 10.1002/ana.10547. [DOI] [PubMed] [Google Scholar]
- 10.Massicotte-Marquez J, Carrier J, Decary A, et al. Slow-wave sleep and delta power in rapid eye movement sleep behavior disorder. Ann Neurol. 2005;57:277–82. doi: 10.1002/ana.20373. [DOI] [PubMed] [Google Scholar]
- 11.Iranzo A, Isetta V, Molinuevo JL, et al. Electroencephalographic slowing heralds mild cognitive impairment in idiopathic REM sleep behavior disorder. Sleep Med. 2010;11:534–9. doi: 10.1016/j.sleep.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 13.International classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 14.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov Disord. 2008;23:1043–6. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara Y, Suzuki H, Yasunaga M, et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr Gerontol Int. 2010;10:225–32. doi: 10.1111/j.1447-0594.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- 16.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 17.Sasai T, Matsuura M, Wing YK, Inoue Y. Validation of the Japanese version of the REM sleep behavior disorder questionnaire (RBDQ-JP) Sleep Med. 2012;13:913–8. doi: 10.1016/j.sleep.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 19.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–4. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 20.Gagnon JF, Postuma RB, Joncas S, Desjardins C, Latreille V. The Montreal Cognitive Assessment: a screening tool for mild cognitive impairment in REM sleep behavior disorder. Mov Disord. 2010;25:936–40. doi: 10.1002/mds.23079. [DOI] [PubMed] [Google Scholar]
- 21.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–45. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zadikoff C, Fox SH, Tang-Wai DF, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson's disease. Mov Disord. 2008;23:297–9. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 23.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 24.Schenck CH, Hurwitz TD, Mahowald MW. Symposium: Normal and abnormal REM sleep regulation: REM sleep behaviour disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res. 1993;2:224–31. doi: 10.1111/j.1365-2869.1993.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 25.Oksenberg A, Radwan H, Arons E, Hoffenbach D, Behroozi B. Rapid Eye Movement (REM) sleep behavior disorder: a sleep disturbance affecting mainly older men. Isr J Psychiatry Relat Sci. 2002;39:28–35. [PubMed] [Google Scholar]
- 26.Latreille V, Carrier J, Montplaisir J, Lafortune M, Gagnon JF. Non-rapid eye movement sleep characteristics in idiopathic REM sleep behavior disorder. J Neurol Sci. 2011;310:159–62. doi: 10.1016/j.jns.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Massicotte-Marquez J, Decary A, Gagnon JF, et al. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology. 2008;70:1250–7. doi: 10.1212/01.wnl.0000286943.79593.a6. [DOI] [PubMed] [Google Scholar]
- 28.Prichep LS, John ER, Ferris SH, et al. Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol Aging. 1994;15:85–90. doi: 10.1016/0197-4580(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 29.Tucker DM, Penland JG, Sandstead HH, Milne DB, Heck DG, Klevay LM. Nutrition status and brain function in aging. Am J Clin Nutr. 1990;52:93–102. doi: 10.1093/ajcn/52.1.93. [DOI] [PubMed] [Google Scholar]
- 30.Ehlers CL, Kupfer DJ, Buysse DJ, et al. The Pittsburgh study of normal sleep in young adults: focus on the relationship between waking and sleeping EEG spectral patterns. Electroencephalogr Clin Neurophysiol. 1998;106:199–205. doi: 10.1016/s0013-4694(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 31.Platt B, Drever B, Koss D, et al. Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PLoS One. 2011;6:e27068. doi: 10.1371/journal.pone.0027068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caviness JN, Hentz JG, Evidente VG, et al. Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson's disease. Parkinsonism Relat Disord. 2007;13:348–54. doi: 10.1016/j.parkreldis.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Kai T, Asai Y, Sakuma K, Koeda T, Nakashima K. Quantitative electroencephalogram analysis in dementia with Lewy bodies and Alzheimer's disease. J Neurol Sci. 2005;237:89–95. doi: 10.1016/j.jns.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Platt B, Riedel G. The cholinergic system, EEG and sleep. Behav Brain Res. 2011;221:499–504. doi: 10.1016/j.bbr.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Tamura Y, Chiba S, Takasaki H, Tabata K, Ishimaru Y, Ishimoto T. Biperiden-induced delirium model in rats: a behavioral and electroencephalographic study. Brain Res. 2006;1115:194–9. doi: 10.1016/j.brainres.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 36.Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73:273–8. doi: 10.1212/WNL.0b013e3181ab2b58. [DOI] [PubMed] [Google Scholar]
- 37.Bohnen NI, Kaufer DI, Hendrickson R, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson's disease and parkinsonian dementia. J Neurol. 2006;253:242–7. doi: 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- 38.Kotagal V, Albin RL, Muller ML, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol. 2012;71:560–8. doi: 10.1002/ana.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohnen NI, Muller ML, Kotagal V, et al. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson's disease. Brain. 2010;133:1747–54. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grigg-Damberger MM. The AASM Scoring Manual four years later. J Clin Sleep Med. 2012;8:323–32. doi: 10.5664/jcsm.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]