Abstract
Objectives:
Short sleep duration and poor sleep quality in children have been associated with concentration, problem behavior, and emotional instability, but recently also with disrupted autonomic nervous function, which predicts cardiovascular health. Heart rate variability (HRV) was used as noninvasive indicator of autonomic function to examine the influence of sleep.
Design:
Cross-sectional and longitudinal observational study on the effect of sleep on HRV
Participants:
Belgian children (5-11 years) of the ChiBS study in 2010 (N = 334) and 2011 (N = 293).
Interventions:
N/A.
Methods:
Sleep duration was reported and in a subgroup sleep quality (efficiency, latency, awakenings) was measured with accelerometry. High-frequency (HF) power and autonomic balance (LF/HF) were calculated on supine 5-minute HRV measurements. Stress was measured by emotion and problem behavior questionnaires. Sleep duration and quality were used as HRV predictors in corrected cross-sectional and longitudinal regressions. Stress was tested as mediator (intermediate pathway) or moderator (interaction) in sleep-HRV associations.
Results:
In both cross-sectional and longitudinal analyses, long sleep latency could predict lower HF (parasympathetic activity), while nocturnal awakenings, sleep latency, low sleep efficiency, and low corrected sleep duration were related to higher LF/HF (sympathetic/parasympathetic balance). Parental reported sleep duration was not associated with HRV. The significances remained after correction for stress. Stress was not a mediator, but a moderator (enhancer) in the relationship between sleep quality and HRV.
Conclusions:
Low sleep quality but not parent-reported low sleep duration leads to an unhealthier heart rate variability pattern (sympathetic over parasympathetic dominance). This stresses the importance of good sleep quality for cardiovascular health in children.
Citation:
Michels N; Clays E; De Buyzere M; Vanaelst B; De Henauw S; Sioen I. Children's sleep and autonomic function: low sleep quality has an impact on heart rate variability. SLEEP 2013;36(12):1939-1946.
Keywords: Sleep actigraphy, short-term heart rate variability, sympathetic/parasympathetic balance, stress, children
INTRODUCTION
Insufficient sleep impairs physical and psychological development, which may negatively affect concentration, performance, behavior, emotional well-being, and overall health (promotion of chronic disease).1 The precise biological mechanisms are only partially understood, but the autonomic nervous system could be a potential pathway linking sleep problems with subsequent pathologies such as hypertension, diabetes, and cardiovascular disease.2,3
Heart rate variability (HRV) is commonly used as marker of the autonomic nervous system activity and its two branches because of the sympathetic and vagal parasympathetic innervations of the heart (SA and PA, respectively).4 HRV is defined as the variability of the distance between consecutive R peaks of the electrical heartbeat signal as measured by electrocardiogram. This variability reflects the heart's ability to respond to physiological and environmental stimuli. Subsequently, a reduction of HRV (i.e., reduced PA with or without increased SA) leads to increased morbidity and mortality.5
HRV reduction has also been observed in stress-induced conditions, since stress may disturb the sympathovagal balance.6 Therefore, it is increasingly used as stress biomarker.7 Moreover, sleep deprivation may increase the stress level.8 Because of these possible relations of both sleep and stress with HRV, it is highly relevant to test whether stress is an intermediate pathway or a moderator in the sleep-HRV relationship.
Literature related to sleep effects on HRV in healthy study populations is limited and consists mainly of acute, artificially induced sleep deprivation. Studies on acute and chronic sleep deprivation have shown contradictory effects on both autonomic nervous system branches. A review suggested that low sleep duration and quality can increase sympathetic over para-sympathetic dominance.9 Although acute mild perturbations are reported, adverse outcomes can arise when sleep deprivation becomes chronic.
It is important that this research field should also focus on children, since they are in a period of intense development and at a critical time for acquiring healthy sleep habits.10 Even in children, a trend toward insufficient sleep duration and sleep problems has been reported.10,11 The question remains whether sleep deprivation might influence HRV during childhood, since literature in this age group is lacking. To our knowledge, only one paper has been published on the relation of sleep duration or quality with HRV in children, showing an overall autonomic hypofunction.12 Previous research also suggests that cardiovascular risk factors were increased in a sample of adolescents with high sleep disturbance,13 while in a sample of young children, no influence of sleep quality or quantity was seen on cardiovascular function.14
This paper aimed to examine the cross-sectional association and longitudinal effect of sleep on children's autonomic nervous system, measured by HRV. In doing this, both sleep quality and quantity have been considered. After all, low sleep quality (problems falling asleep or frequent awakenings) decreases sleep duration, and the body may not spend enough time in critical sleep phases even if the total sleep duration remains sufficient.10 Moreover, the possible mediating or moderating effect of stress in this relation was tested.
METHODS
Participants and General Procedures
Participants were recruited from the Belgian ChiBS study (Children's Body composition and Stress), which examined the association between stress and body composition evolution over 2 years (2010-2012). Detailed aims, methods, and population characteristics have been described elsewhere.15
Data used in this paper were collected in 2 survey periods— February-June 2010 and February-June 2011—when the children were between 5 and 11 years old. The study was conducted according to the Declaration of Helsinki, and the project protocol was approved by the Ethics Committee of the Ghent University Hospital. Witten informed consent was obtained from the parents.
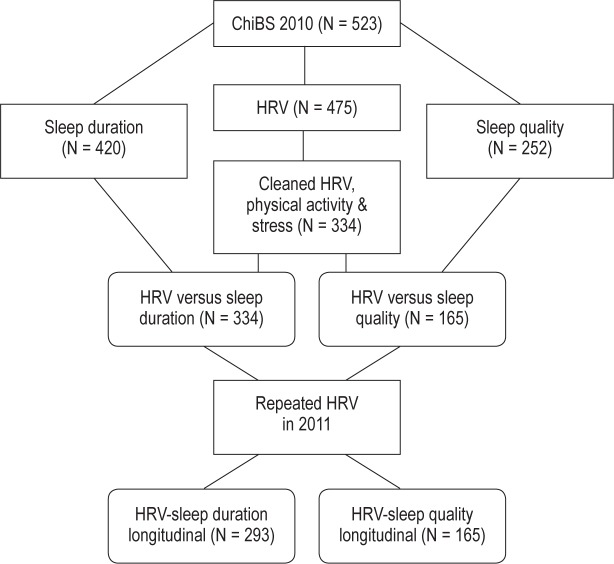
Within the ChiBS study, different measurement modules (HRV, questionnaires, and sleep quality) were optional: parents and children could choose in which modules they wanted to participate. Participation numbers for each of the modules are shown in Figure 1: the numbers were influenced by the high quality data control on HRV, the measurement modules being optional, and the limited availability of sleep quality measuring devices. No difference in sex, parental education, or physical activity of the children was observed between children included in these 2 sets of analyses (334 for sleep duration and 165 for sleep quality) and those excluded; participants were, however, somewhat older (mean age 8.2 and 8.3 versus 7.8).
Figure 1.
Flowchart explaining the participants number. HRV, heart rate variability.
One child with a cardiovascular disease was excluded. Other diseases reported in our sample but not used as exclusion criteria included: allergy/ asthma (N = 80), dermatologic diseases (N = 8), rheumatic/skeletal (N = 6), ADHD (N = 5), urinary diseases (N = 4), digestion (N = 3), and dyslexia (N = 2).
Sleep
Sleep quantity was examined in 2010 with a parental questionnaire as the normal amount of sleep in hours during the week and during the weekend (Friday and Saturday night). A mean parental reported sleep duration per day was calculated as “(5× week + 2× weekend)/7.” Sleep quality was measured in 2010 by an accelerometer (Actigraph) worn on the wrist for 6 nights. Registered data for ≥ 3 nights per child was necessary to convert the data to interpretable sleep quality parameters.16 Raw accelerometer data were converted to sleep parameters with the Actilife software of the accelerometer. Movement for a certain period of time was scored as “awake,” based on the built-in Sadeh scoring algorithm that has previously been validated against polysomnography.17 The following sleep quality parameters were obtained: (1) sleep latency or time between time in bed and sleep onset, (2) minutes scored as wake after sleep onset (WASO), (3) corrected sleep duration or sleep duration without sleep latency and WASO, (4) sleep efficiency or the proportion of actual sleep over the total time spent in bed.
Heart Rate Variability
Inter-beat RR-intervals (RRI) were recorded for 10 min in supine position with an elastic electrode belt (Polar Wearlink 31) in 2010 and 2011. This low-cost device has been validated against an electrocardiogram device in children.18 The heart rate belt was fixed around the chest and measurements started when the signal stabilized. To ensure a non-stressful situation, measurements were done in a quiet, closed room in a familiar building (the local sports park) child by child. Comforting music was played and a blindfold was provided if desired. The child was encouraged to breathe normally and not to speak or move during the measurement. Further data processing was performed with the free professional HRV Analysis Software of the University of Kuopio, Finland.19 Low-frequency (LF) and high-frequency (HF) bands were analyzed between 0.04-0.15 Hz and 0.15-0.4 Hz as default.4 The RR series were detrended using the Smoothness priors method with α = 300, and a cubic interpolation was done at the default rate of 4 Hz. The middle 5 min were manually checked for quality (no large RRI outliers, RRI equidistance, and Gaussian RR distribution), and if necessary, another appropriate 5-min interval was selected. Using spectral autoregression with the forward-backward linear least squares method, the power of LF and HF bands in absolute and normalized units ([nu] LF or HF divided by “total power minus very low frequency power”*100) and the LF/HF ratio were determined. HF reflects the vagal activity (PA) and the LF/HF ratio is assumed to represent the sympathovagal balance. More difficult is the interpretation of LF, since it contains both parasympathetic and sympathetic contributions. As a result, no analyses were performed on LF. Moreover, the HRV assesses fluctuations in autonomic activity but not necessarily in autonomic tone.20
Stress Parameters
Stress arises when the demands of a situation exceed an individual's ability to cope and resolve the problem, resulting in emotional and behavioral disturbances.21 Therefore, stress was measured by negative emotion and behavior questionnaires.
Basic Emotions (Child-Reported)
Children were asked to report on their general feelings of anger, anxiety, sadness, and happiness on a 0- to 10-point Likert-scale (0 “not at all” to 10 “very strong”), analogous to a study of Zimmer-Gembeck.22 For this paper, only the negative emotions anger, anxiety, and sadness were used. These basic emotions are already understandable during infancy, and can as such uncomplicatedly be used in our age group.23
Problem Behavior (Parent-Reported)
Parents were asked to complete the Strengths and Difficulties Questionnaire,24 reporting children's behavioral and emotional problems over the past 6 months. For each of the 20 statements, parents could answer: “not true” (0), “somewhat true” (1), and “certainly true” (2). The following subscales were used for this paper: peer problems, conduct problems, and emotional problems. This questionnaire has shown good validity when compared to the Child Behavior Checklist (CBCL) and the Rutter questionnaire.25
Physical Activity and Socioeconomic Status
Physical activity was studied as parent-reported hours of physical activity per week (sporting in a club and sporting or playing outdoors) and was used as confounder of HRV. Parental education was used as proxy variable for the socioeconomic status using the classification by the International Standard Classification of Education (ISCED). Further categorization was done into 2 groups: low (up to secondary education) and high (tertiary education) status.
Statistical Analysis
Analyses were performed using PASW Statistical Program version 19.0 (SPSS Inc, IBM, IL, USA). The 2-sided level of significance was set at P < 0.05. Logarithmic transformation was used for parameters with a skewed distribution. A stress z-score was calculated as follows: “z-score negative emotions + z-score problem behavior.” Paired sample t-tests were used to analyze the evolution of HRV over the 2 measurement times.
A first set of regressions tested the cross-sectional relation between sleep (duration and quality) and HRV, corrected for age, sex, physical activity, and parental education. A second set of regressions was done with an extra correction for stress to test the sleep-HRV relation independent from stress and to examine the mediating role of stress in this sleep-HRV relation. Mediation is defined as a variable carrying the influence of a predictor to a given outcome, and thus accounting for the observed relationship. According to Baron and Kenny,26 mediation is only present when all 4 conditions are met: (1) the independent variable significantly predicts the dependent variable, (2) the independent variable significantly predicts the mediator, (3) the mediator significantly predicts the dependent variable, and (4) the relationship between the independent variable and the dependent variable is significantly reduced after controlling for the mediator. To test this non-parametrically, the published syntax of Preacher and Hayes was used.27 In addition to the stress z-score sum, the separate concepts “negative emotions” and “problem behavior” were also tested, but results were the same as for the z-score sum.
A third set of regressions tested moderation (i.e., a third variable affecting the direction and/or strength of the relationship between a predictor and outcome variable) by stress in the sleep-HRV relation. This moderation was tested by including an interaction factor.28 In those regressions, continuous parameters were transformed in z-scores and the categorical sex variable was effect coded (-1 and 1). If significant, the moderation effect was further interpreted visually and statistically. Visual representation was done by plotting predicted outcome values (HRV parameters) in relation to the predictor (sleep duration or sleep quality parameter) for 3 representative groups of the moderator (stress parameters): those at the mean, at 1 SD below the mean, and 1 SD above the mean. Statistical interpretation was done by testing the significance of the sleep predictor for 2 groups of the moderator based on a median split. If the predictor was significant in a certain subgroup, the beta and significance values were given.
In a fourth set of regressions corrected for age, sex, physical activity, parental education, and stress, the sleep-HRV relationship was tested using sleep duration data in 3 categories by applying tertiles.
Finally, a longitudinal regression analysis was performed: the impact of baseline sleep (duration or quality) on HRV repeated measures (change between 2010 and 2011) was tested using SPSS mixed models corrected for the same confounders (age, sex, physical activity, parental education, and stress).
RESULTS
Descriptive Data
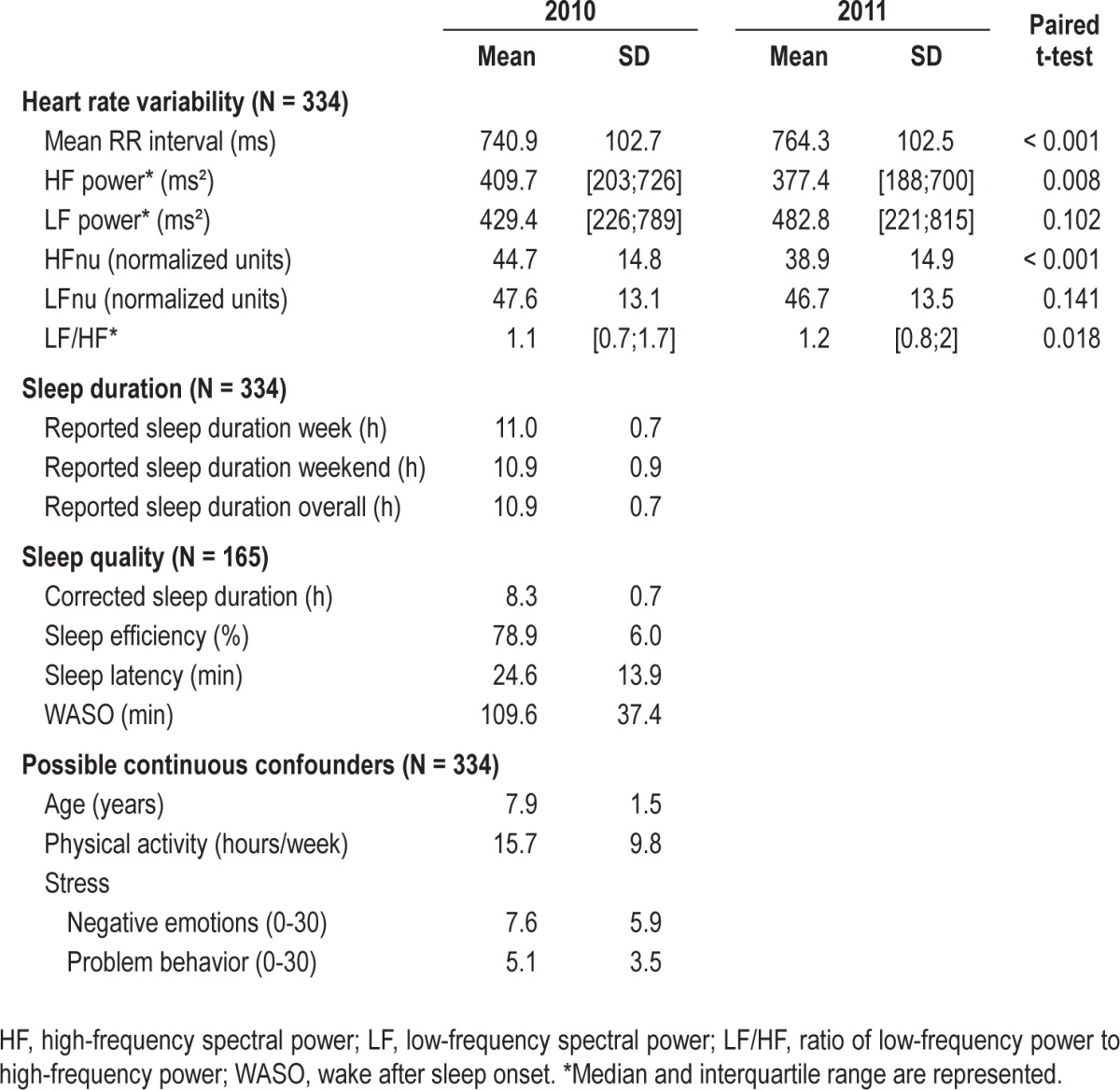
Table 1 shows descriptive data for the HRV, sleep, and possible confounder parameters. Sleep quality data were only available in a subgroup. The sexes were almost evenly distributed (53% boys), and 71% of the parents had a tertiary education. As no difference was found in sleep duration between weekdays and weekend days (P = 0.928) and no week/weekend differences were seen in the performed regression analyses, results are only shown for overall mean sleep duration (no separate analyses for week and weekend). A change in HRV parameters was found between 2010 and 2011. The HF and HFnu were significantly decreased, while mean RR and LF/HF showed an increase.
Table 1.
Descriptive data on baseline and follow-up heart rate variability (HRV), sleep, and possible confounders in the sleep-HRV relation
Cross-Sectional
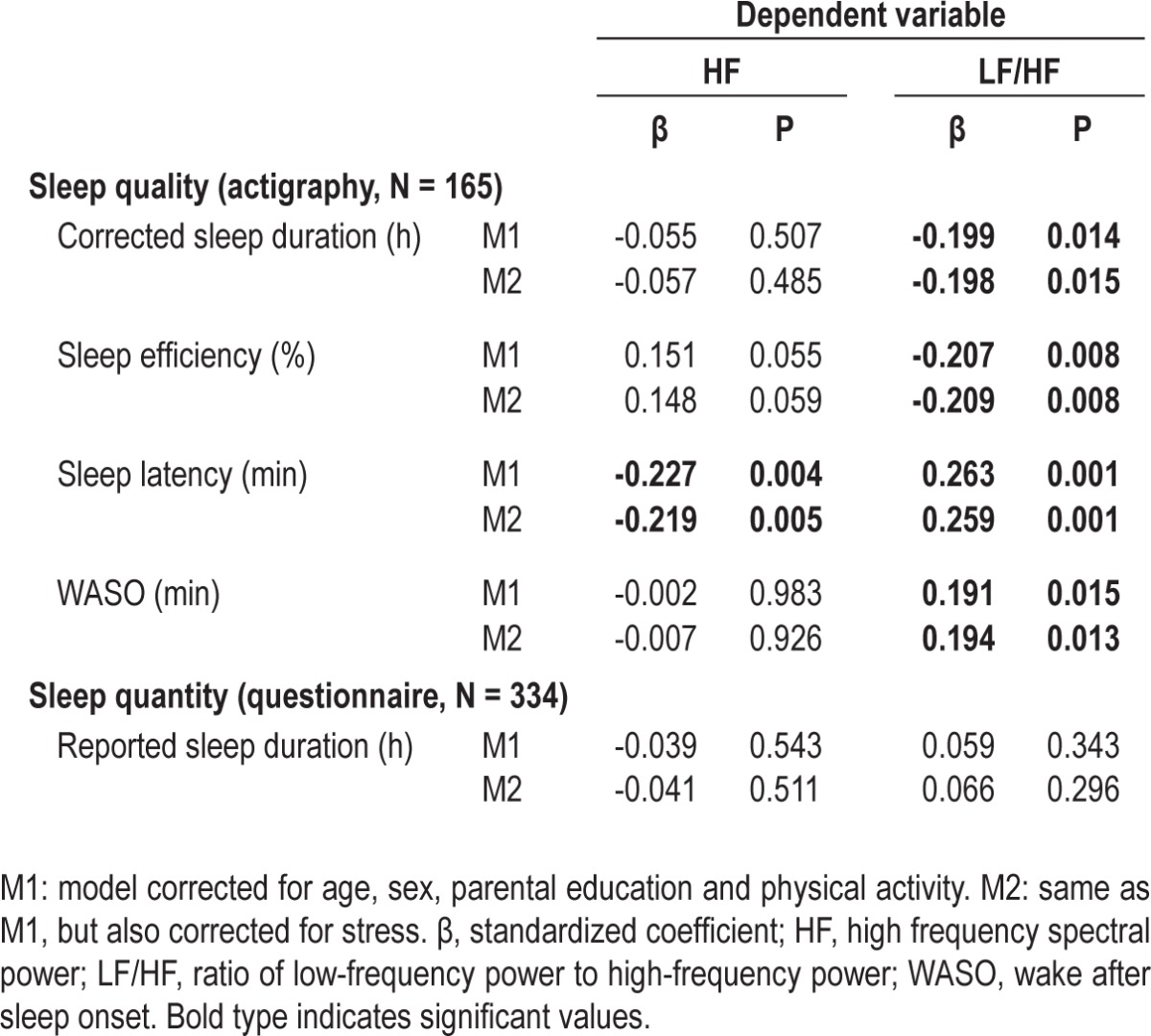
Cross-sectional regression analyses on the sleep (predictor) – HRV (outcome) relation with data collected in 2010 are shown in Table 2. Since sex interaction factors were not significant, analyses were not stratified for sex. Long sleep latency could predict lower HF (parasympathetic activity), while nocturnal awakenings, sleep latency, low sleep efficiency and low corrected sleep duration were related to higher LF/HF (sympathetic/parasympathetic balance). Also, a borderline association was seen between sleep efficiency and higher HF. Parental reported sleep duration was not associated with HRV, neither when using tertiles of parental reported sleep duration (P > 0.05). After correction for stress (the M2 models in Table 2), the significances remained.
Table 2.
Linear regression predicting heart rate variability parameters by sleep data
Longitudinal
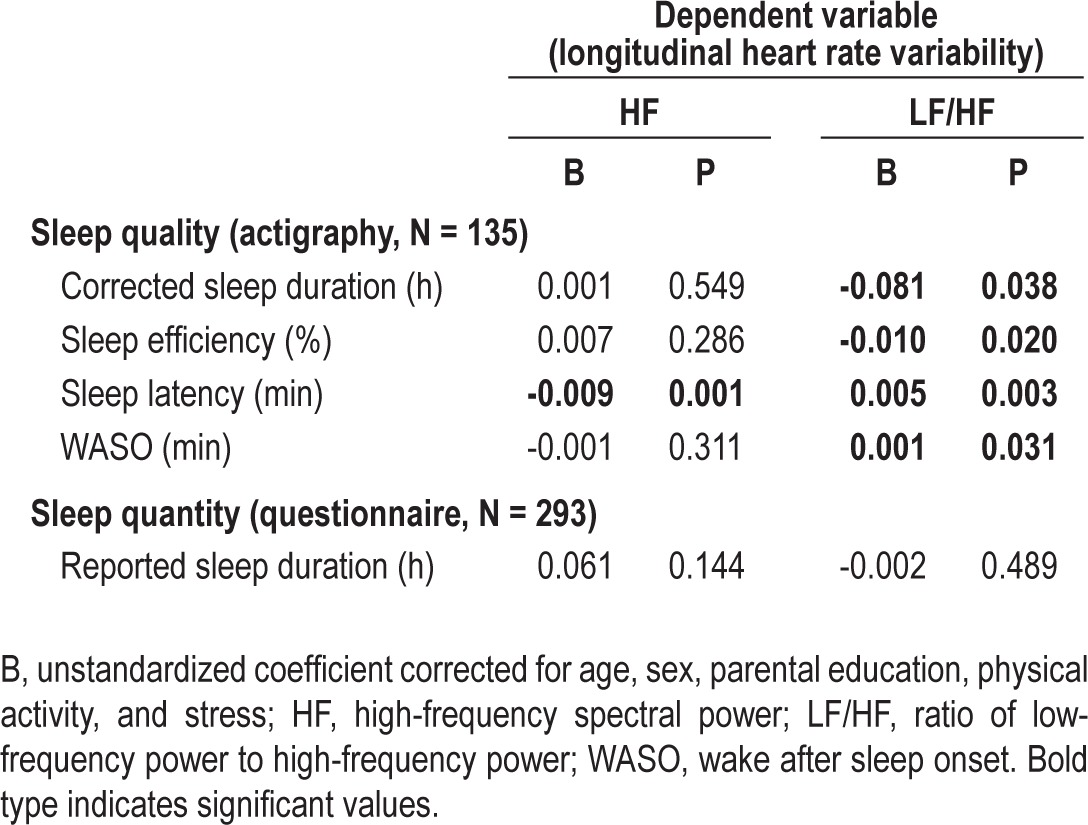
Table 3 shows the longitudinal mixed model analyses, using sleep data collected in 2010 as predictor and the evolution of HRV data collected in 2010 and 2011 as outcome. The same relations were seen as in the cross-sectional analyses: longer WASO, longer latency, less efficiency, and lower corrected sleep duration predicted a higher increase in the LF/HF ratio over one year; while long latency also predicted lower HF. Consequently, low sleep quality could predict an unhealthy longitudinal change in autonomic balance. Nevertheless, reported sleep duration did not predict HRV.
Table 3.
Linear mixed models longitudinal regression predicting heart rate variability parameter change between 2010 and 2011 by sleep data
Mediation and Moderation
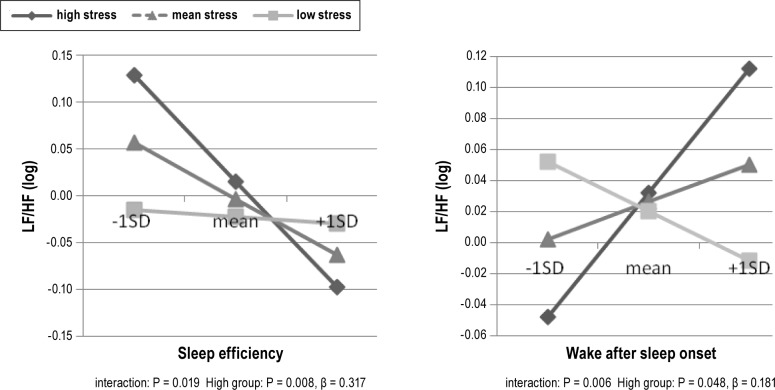
No mediation effect of stress was observed: the sleep-HRV relation was not significantly reduced after correction for stress (zero was included in the 95% intervals of the bootstrapping). Although stress was related to HRV outcome (N. Michels, unpublished data, 2013), stress was not related to sleep outcome (P > 0.05). Nevertheless, stress was a significant moderator in the relation of efficiency and WASO with HRV outcome, as illustrated in Figure 2. Stress was an enhancer since it strengthened the sleep-HRV relation: the relation between sleep quality and HRV was significant only in the high-stressed group (i.e., low sleep efficiency and high WASO were related to a higher LF/HF only in stressed children and not in low-stressed children). The seemingly negative relation of WASO with LF/HF in the low-stressed children was not significant.
Figure 2.
Stress as moderator in the relation between sleep and heart rate variability (HRV). Figures show the sleep-HRV relation for sleep and stress levels equal to the mean, 1 standard deviation above the mean (high) or 1 standard deviation below the mean (low). The significance value of the interaction term for stress in the sleep – HRV relation is given.
DISCUSSION
The results stress the importance of a good sleep quality for general health during childhood. Cross-sectionally, low sleep quality (frequent awakenings, long latency, and low sleep efficiency) was related to a higher sympathovagal balance (LF/HF ratio), partially due to lower PA (measured by the HF parameter), although higher SA might also have been expected. Nevertheless, reported sleep duration was not associated with HRV outcome. Longitudinal results showed the same trend, particularly with a higher sympathovagal balance for those with low sleep quality. Consequently, low sleep quality is related to a more unhealthy change in autonomic balance over one year. Stress was no mediator (i.e., no intermediate pathway) but a moderator (i.e., enhancer) in some aspects of the sleep-HRV relationship.
Sleep Influences HRV
Given the fact that sleep is associated with a decrease in sympathetic activity and catecholamine levels, one might expect sleep deprivation to be associated with an increase in these variables. Indeed, literature suggests that sleep shortage might increase sympathetic activity by higher levels of the catecholamines norepinephrine and epinephrine through activation of the stress system.3,9,29 The literature even suggests that sleep may not only affect hormone levels but also lead to structural changes, such as the amount or sensitivity of hormonal receptors.9 Overall, autonomic nervous system changes could be a pathway linking sleep problems with the common pathophysiology of hypertension, diabetes, and cardiovascular disease.2,3 Moreover, HRV as a marker of the autonomic nervous system has been associated with increased morbidity and mortality (poor sleep quality and quantity are shown as cardiovascular risk factors).5,30 In children, the longitudinal relationship of HRV with cardiovascular disease has not yet been examined, but children's HRV (lower PA and a changed balance) has been associated with cardiovascular risk factors such as systolic blood pressure and obesity.31 Consequently, children's HRV could indeed be associated with later morbidity.
HRV—during both night and daytime—has been used with success in clinical sleep problems such as insomnia, sleep disordered breathing, and periodic limb movements. HRV helps in understanding the health effects and can be used in screening and for monitoring of treatment in children as well as adults.32,33 In addition, it is important to understand the influence of sleep on the autonomic nervous system in a healthy general population. Acute experimental sleep deprivation in healthy adult study subjects has shown conflicting results concerning the effect on both branches of the autonomic system: lower PA and higher SA,34 a higher SA over PA ratio,35 especially PA lowering,36 or even—in contrast—lower SA without changes in PA.37 Although acute mild perturbations are reported, adverse outcomes may arise when sleep deprivation becomes chronic.9 Furthermore, literature on the effects of chronic sleep deprivation on HRV is even more limited: lower PA and SA12 or even no effect38 has been reported. Apart from sleep duration, sleep quality could be important: low sleep quality was associated with lower PA38,39 and a higher SA over PA ratio.38
The question is whether sleep deprivation might already have an influence during childhood, since literature in this age group is very scarce. Previous research suggests that cardiovascular risk factors were increased in a sample of adolescents with high sleep disturbance,13 while in a sample of young children no influence of sleep quality and quantity was seen on cardiovascular function.14 Concerning HRV, overall cardiac autonomic hypofunction was found in 5- and 6-year-olds with short nocturnal sleep (all HRV parameters were decreased except the ratio).12 Also sleep-disrupting diseases such as sleep disordered breathing have been associated with a higher sympathetic over parasympathetic dominance both in healthy and clinical populations.40
Indeed, we have found that sleep already influences HRV in childhood. More specifically, low sleep quality was related to an increased sympathetic over parasympathetic dominance at baseline and after one year. This was partially due to lower PA (measured by the HF parameter), although a higher SA might also be expected. In contrast to another study in children,40 no association with parent-reported sleep duration was found.
Sleep Quality, Not Parent-Reported Sleep Quantity
A recent review suggests that both sleep quality and quantity can influence autonomic function.9 After all, low sleep quality decreases sleep duration, but more importantly, the body may not spend enough time in critical sleep phases.10 Remarkably, sleep quality and not self-reported sleep duration influenced HRV in our sample and also in a previous research in white-collar workers.38 Several reasons could underlie this finding. (1) A previous statement that “specifically the sleep discontinuity and not the amount of experimental sleep loss influences the autonomic system” could be a possible explanation.41 In their experimental sleep deprivation study, catecholamine increase was not associated with the amount of slow wave sleep loss but only with the sleep efficiency and the time interval between arousals. (2) In our population, the absence of a sleep duration effect could be caused by the fact that in the population under study, a higher sleep quality was associated with a shorter parent-reported sleep duration. (N. Michels, unpublished data, 2013) (3) Furthermore, it should be considered that parent-reported sleep duration was quite high in our population; only about 15% of the children slept less than the recommended 10 hours of sleep for this age group.42 Perhaps reported sleep duration only affects HRV in more extreme forms of sleep deprivation. In contrast to the relatively high sleep duration, mean sleep quality was quite low in our population, with sleep efficiency of only 79% and 110 minutes WASO. Poor sleep quality might be defined as a sleep efficiency lower than 90%.43 Also in another child population, a high sleep efficiency of 86.5% was found.44 Although the sleep latency was comparable with that out of the literature,43,44 our WASO was much longer than the 89 minutes found in children 4 to 7 years old.45 (4) Individual differences in the hours of sleep needed may strongly differ making it even harder to find a relation. (5) Moreover, parent-reported sleep duration could be an over- or under-estimation, introducing some error in the data. (6) Finally, the relation between sleep and some adverse outcomes like cardiovascular diseases could be U-shaped: both too low and too high sleep duration may be harmful.30 Nevertheless, also parental reported sleep quantity used as tertiles did not predict HRV in our sample.
The Role of Stress
Apart from the effect of sleep as such, also higher mental and physical demands in the presence of sleep deprivation can influence HRV.9 Indeed, one of the sleep deprivation induced pathways is the activation of stress systems such as the autonomic system and the cortisol system,9,46 linking sleep to stress-related disorders. Consequently, stress could be a mediator or moderator in the sleep-HRV relationship.
To be a mediator, stress should be related to both sleep and HRV. The relationship between stress and sleep appears to be bidirectional: stress can disrupt sleep and low sleep duration/ quality can increase subsequent stress levels.8,47 Experimental sleep deprivation has been shown to lower the psychological threshold for stress perception,48 and poor sleep quality was associated with compromised emotional information processing in 10-year-old children.49 However, in this study, the stress sum score (emotions and problem behavior) was not associated with sleep quantity or quality.
Stress was related to HRV, as we have previously shown that stress questionnaire data and cortisol values were related to children's HRV.50 Since one of the stress pathways is a disrupted sympathovagal balance,6 HRV is increasingly used as stress biomarker.7
In this study, stress was not a mediator in the sleep-HRV relationship. Also in literature, no mediation in a sample of adults was found.39 Nevertheless, stress was a moderator in our study, as some of the sleep-HRV relationships were only significant in highly stressed children. Consequently, stress could act as an enhancer of the unfavorable effect of sleep quality on the autonomic function. In a previous acute sleep deprivation experiment, the HRV change after acute sleep deprivation remained longer when participants had to perform a stress task, although the interaction factor was not significant.34 However, stress-sleep interactions have already been shown in the study of hypertension and obesity.51,52
Strengths and Limitations
Important strengths of this study are: (1) the quality controlled HRV measurements in a quite large child population; (2) the measurement of both sleep duration and quality; (3) the use of an objective measure of sleep quality by using actigraphy since subjective reports were previously not correlated with objective sleep quality45,53; (4) the analyses of stress as mediator or moderator in the sleep-HRV relationship; and (5) the use of longitudinal analyses to examine the predictive power of sleep on HRV over one year.
Nevertheless, some limitations remain. Although actigraphy is a valid objective measure of sleep quality,54 it is not the gold standard because of the accelerometers' low specificity. Polysomnography remains the gold standard, but actigraphy has the advantage of low cost and non-invasiveness. Moreover, sleep quality measurements were performed in a smaller sample of children (165 versus 334 for sleep duration) because of limited availability of accelerometers. Since we only measured basal HRV and not the HRV change during stress, future studies should also test whether HRV stress reactivity depends on sleep deprivation. Also, nocturnal HRV might provide information on autonomic function and sleep quality simultaneously.33 Furthermore, our stress measurements using only short emotion and behavior questionnaires might not fully cover the complex concept of stress that could result in a failure to find a significant mediating role of stress in sleep-HRV. Finally, our results on sleep duration cannot be fully generalized to the overall population, since our sample had quite good sleep duration.
CONCLUSION
In our non-experimental population study, children's low sleep quality but not parent-reported sleep duration was cross-sectionally and longitudinally related to an unhealthier heart rate variability outcome, also after correction for stress. Consequently, sleep quality is an important predictor for the autonomic development already in childhood, even more important than sleep duration. Since this HRV change into a sympathetic over parasympathetic dominance is a pathway to higher morbidity (cardiovascular disease, hypertension, and diabetes), preventive actions should be taken. Prevention should focus on both high sleep quality and stress reduction or coping skills, since stress was sometimes an enhancer in the sleep-HRV relation.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Nathalie Michels is financially supported by the research council of Ghent University (Bijzonder Onderzoeksfonds). Isabelle Sioen and Barbara Vanaelst are financially supported by the Research Foundation – Flanders. The authors want to thank the children and their parents for their voluntary participation.
ABBREVIATIONS
- ChiBS
children's body composition and stress
- HF
high-frequency
- HRV
heart rate variability
- LF
low-frequency
- nu
normalized units
- PA
parasympathetic activity
- RRI
inter-beat RR-intervals
- SA
sympathetic activity
- WASO
wake after sleep onset
REFERENCES
- 1.Institute of Medicine (US) Extent and Health Consequences of Chronic Sleep Loss and Sleep Disorders. In: Colten HR, Altevogt BM, editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington: National Academies Press; 2006. [PubMed] [Google Scholar]
- 2.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24:731–43. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease-a review of the recent literature. Curr Cardiol Rev. 2010;6:54–61. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Task Force of ESC/NASPE. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 5.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–31. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 6.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 7.Marques AH, Silverman MN, Sternberg EM. Evaluation of stress systems by applying noninvasive methodologies: measurements of neuroimmune biomarkers in the sweat, heart rate variability and salivary cortisol. Neuroimmunomodulation. 2010;17:205–8. doi: 10.1159/000258725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akerstedt T. Psychosocial stress and impaired sleep. Scand J Work Environ Health. 2006;32:493–501. [PubMed] [Google Scholar]
- 9.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Mindell J, Owens J. Philadelphia, USA: Lippincott Williams & Wilkins; 2003. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. [Google Scholar]
- 11.National Sleep Foundation. Washington DC: National Sleep Foundation; 2004. Summary findings of the 2004 Sleep America Poll. [Google Scholar]
- 12.Sampei M, Murata K, Dakeishi M, Wood DC. Cardiac autonomic hypofunction in preschool children with short nocturnal sleep. Tohoku J Exp Med. 2006;208:235–42. doi: 10.1620/tjem.208.235. [DOI] [PubMed] [Google Scholar]
- 13.Narang I, Manlhiot C, Davies-Shaw J, et al. Sleep disturbance and cardiovascular risk in adolescents. CMAJ. 2012;184:E913–20. doi: 10.1503/cmaj.111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martikainen S, Pesonen AK, Feldt K, et al. Poor sleep and cardiovascular function in children. Hypertension. 2011;58:16–21. doi: 10.1161/HYPERTENSIONAHA.111.172395. [DOI] [PubMed] [Google Scholar]
- 15.Michels N, Vanaelst B, Vyncke K, et al. Children's Body composition and Stress — the ChiBS study: aims, design, methods, population and participation characteristics. Arch Public Health. 2012;70:17. doi: 10.1186/0778-7367-70-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–41. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 17.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification — an empirical test of methodological issues. Sleep. 1994;17:201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 18.Gamelin FX, Berthoin S, Bosquet L. Validity of the polar S810 heart rate monitor to measure R-R intervals at rest. Med Sci Sports Exerc. 2006;38:887–93. doi: 10.1249/01.mss.0000218135.79476.9c. [DOI] [PubMed] [Google Scholar]
- 19.Niskanen JP, Tarvainen MP, Ranta-Aho PO, Karjalainen PA. Software for advanced HRV analysis. Comput Methods Programs Biomed. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Malik M, Camm AJ. Components of heart rate variability--what they really mean and what we really measure. Am J Cardiol. 1993;72:821–2. doi: 10.1016/0002-9149(93)91070-x. [DOI] [PubMed] [Google Scholar]
- 21.McCance KL, Forshee B, Shelby J. Stress and disease. In: McCance KL, Huether SE, editors. Pathophysiology: the biological basis for disease in adults and children. St Louis: Mosby; 2006. [Google Scholar]
- 22.Zimmer-Gembeck MJ, Lees DC, Bradley GL, Skinner EA. Use of an analogue method to examine children's appraisals of threat and emotion in response to stressful events. Motiv Emotion. 2009;33:136–49. [Google Scholar]
- 23.Flavell JH. Cognitive development: children's knowledge about the mind. Annu Rev Psychol. 1999;50:21–45. doi: 10.1146/annurev.psych.50.1.21. [DOI] [PubMed] [Google Scholar]
- 24.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 25.Goodman R, Scott S. Comparing the Strengths and Difficulties Questionnaire and the Child Behavior Checklist: is small beautiful? J Abnorm Child Psychol. 1999;27:17–24. doi: 10.1023/a:1022658222914. [DOI] [PubMed] [Google Scholar]
- 26.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 27.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–31. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 28.Frazier PA, Tix AP, Barron KE. Testing moderator and mediator effects in counseling psychology research. J Couns Psychol. 2004;51:115–34. [Google Scholar]
- 29.Zhang J, Ma RC, Kong AP, et al. Relationship of sleep quantity and quality with 24-hour urinary catecholamines and salivary awakening cortisol in healthy middle-aged adults. Sleep. 2011;34:225–33. doi: 10.1093/sleep/34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Xie GL, Wang JH, Yang SR. Cardiovascular risk factors significantly correlate with autonomic nervous system activity in children. Can J Cardiol. 2012;28:477–82. doi: 10.1016/j.cjca.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Paruthi S, Chervin RD. Approaches to the assessment of arousals and sleep disturbance in children. Sleep Med. 2010;11:622–7. doi: 10.1016/j.sleep.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Med Rev. 2012;16:47–66. doi: 10.1016/j.smrv.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhong X, Hilton HJ, Gates GJ, et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 2005;98:2024–32. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- 35.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 36.Vaara J, Kyrolainen H, Koivu M, Tulppo M, Finni T. The effect of 60-h sleep deprivation on cardiovascular regulation and body temperature. Eur J Appl Physiol. 2009;105:439–44. doi: 10.1007/s00421-008-0921-5. [DOI] [PubMed] [Google Scholar]
- 37.Holmes AL, Burgess HJ, Dawson D. Effects of sleep pressure on endogenous cardiac autonomic activity and body temperature. J Appl Physiol. 2002;92:2578–84. doi: 10.1152/japplphysiol.01106.2001. [DOI] [PubMed] [Google Scholar]
- 38.Kageyama T, Nishikido N, Kobayashi T, Kurokawa Y, Kaneko T, Kabuto M. Self-reported sleep quality, job stress, and daytime autonomic activities assessed in terms of short-term heart rate variability among male white-collar workers. Ind Health. 1998;36:263–72. doi: 10.2486/indhealth.36.263. [DOI] [PubMed] [Google Scholar]
- 39.Jackowska M, Dockray S, Endrighi R, Hendrickx H, Steptoe A. Sleep problems and heart rate variability over the working day. J Sleep Res. 2012;21:434–40. doi: 10.1111/j.1365-2869.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 40.Liao D, Li X, Rodriguez-Colon SM, et al. Sleep-disordered breathing and cardiac autonomic modulation in children. Sleep Med. 2010;11:484–8. doi: 10.1016/j.sleep.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiemeier H, Pelzer E, Jonck L, Moller HJ, Rao ML. Plasma catecholamines and selective slow wave sleep deprivation. Neuropsychobiology. 2002;45:81–6. doi: 10.1159/000048681. [DOI] [PubMed] [Google Scholar]
- 42.National Sleep Foundation. Children and sleep. Available from: http://www.sleepfoundation.org/article/sleep-topics/children-and-sleep.
- 43.Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000;36:291–301. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- 44.Steenari MR, Vuontela V, Paavonen EJ, Carlson S, Fjallberg M, Aronen ET. Working memory and sleep in 6-to 13-year-old schoolchildren. J Am Acad Child Adolesc Psychiatry. 2003;42:85–92. doi: 10.1097/00004583-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary, and questionnaire for children's sleep patterns. Arch Pediatr Adolesc Med. 2008;162:350–8. doi: 10.1001/archpedi.162.4.350. [DOI] [PubMed] [Google Scholar]
- 46.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 47.Kim EJ, Dimsdale JE. The effect of psychosocial stress on sleep: a review of polysomnographic evidence. Behav Sleep Med. 2007;5:256–78. doi: 10.1080/15402000701557383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minkel JD, Banks S, Htaik O, et al. Sleep deprivation and stressors: Evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12:1015–20. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soffer-Dudek N, Sadeh A, Dahl RE, Rosenblat-Stein S. Poor sleep quality predicts deficient emotion information processing over time in early adolescence. Sleep. 2011;34:1499–508. doi: 10.5665/sleep.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michels N, Sioen I, Clays E, et al. Children's heart rate variability as stress indicator: association with reported stress and cortisol. Biol Psychol. 2013;94:433–40. doi: 10.1016/j.biopsycho.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Bidulescu A, Din-Dzietham R, Coverson DL, et al. Interaction of sleep quality and psychosocial stress on obesity in African Americans: the Cardiovascular Health Epidemiology Study (CHES) BMC Public Health. 2010;10:581. doi: 10.1186/1471-2458-10-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franzen PL, Gianaros PJ, Marsland AL, et al. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosom Med. 2011;73:679–82. doi: 10.1097/PSY.0b013e31822ff440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buckhalt JA, El-Sheikh M, Keller P. Children's sleep and cognitive functioning: race and socioeconomic status as moderators of effects. Child Dev. 2007;78:213–31. doi: 10.1111/j.1467-8624.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- 54.Sadeh A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]