Abstract
Objectives:
Studies investigating the relationship between nonapnea sleep disorders and the risk of acute coronary syndrome (ACS) are scant. This study evaluated whether the risk of ACS is associated with sleep disorders other than sleep apnea in Taiwan.
Methods:
This longitudinal nationwide population-based cohort study investigated the incidence and risk of ACS in 49,099 cases of nonapnea sleep disorders newly diagnosed from January 1997 to December 2001. In total, 98,198 control participants without sleep disorders were randomly selected, frequency matched by age and sex from the general population. The follow-up period started from the date of entering the study cohort to the date of an ACS event, censoring, or December 31, 2010. We conducted Cox proportional hazard regression analysis to estimate the effects of nonapnea sleep disorders on ACS risk.
Results:
The nonapnea sleep disorder cohort had an adjusted hazard ratio (HR; 95% confidence interval [CI] = 1.29-1.60) of subsequent ACS 1.43-fold higher than that of the cohort without sleep disorders. The highest crude effect of nonapnea sleep disorders on ACS incidence was detected among young adults. However, by adjusting for probable risk factors, the HR of ACS increased with age. Compared with women, men had an adjusted HR of 1.57 (95% CI = 1.42-1.75). Hypertension, diabetes mellitus (DM), and hyperlipidemia were also significant factors associated with the increased risk of ACS.
Conclusion:
This nationwide population-based cohort study provides evidence that patients with nonapnea sleep disorders are at higher risk of developing acute coronary syndrome, which increases with age.
Citation:
Chung WS; Lin CL; Chen YF; Chiang JY; Sung FC; Chang YJ; Kao CH. Sleep disorders and increased risk of subsequent acute coronary syndrome in individuals without sleep apnea: a nationwide population-based cohort study. SLEEP 2013;36(12):1963-1968.
Keywords: Acute coronary syndrome (ACS), nationwide population-based cohort study, nonapnea sleep disorder
INTRODUCTION
Insomnia is a common complaint in the general population.1–3 Insomnia or sleep disturbance is the most prevalent sleep disorder. Insomnia is defined as difficulty in falling asleep, remaining asleep, and early final awakening. The association is clear between sleep disorders and comorbidities such as prior psychiatric disorders, circulatory diseases, and gastrointestinal diseases.3 Conversely, insomnia may predispose patients to cardiovascular and cerebrovascular risk factors. More than 50% of stroke patients have sleep disordered breathing, mostly in the form of obstructive sleep apnea, which is recognized as an independent risk factor for cardiovascular diseases (CVDs) and cerebrovascular diseases (CVAs).4,5 However, studies investigating the possibility that nonapnea sleep disorders may increase acute coronary syndromes (ACSs) are scant. Vgontzas et al.6 showed that insomnia with short sleep duration is associated with increased risk of hypertension in a cross-sectional study. The longitudinal study by Fernandez-Mendoza et al.7 further demonstrated that the risk of incident hypertension was significantly increased in insomnia with objective short sleep duration. Recent studies have also shown insomnia to be associated with atherosclerosis risk.8
ACS types, including unstable angina and myocardial infarction with or without ST-segment elevation, are life-threatening disorders that retain high morbidity and mortality despite advances in treatment.9 Grandner et al.10 showed the association between sleep disorders and increased risk of myocardial infarction. However, this study did not objectively assess sleep apnea and, therefore, could not control for its contribution to the increased risk of myocardial infarction. We evaluate sleep disorders other than sleep apnea in association with the development of ACS in Taiwan. The study results are from a nationwide population-based cohort study assessing the possibilities of increased risk of ACS among patients with nonapnea sleep disorders. The original data were derived from Taiwan's National Health Insurance Research Database (NHIRD).
MATERIALS AND METHODS
Data Sources
The National Health Insurance (NHI) program in Taiwan is a universal insurance system established by the Bureau of National Health Insurance in the Department of Health and was implemented in 1995. The insurance program provides health-care to 99% of the 23.74 million people in Taiwan, and has a contract with 97% of Taiwan hospitals and clinics.11 The National Health Research Institute (NHRI) possesses the registration and claims data of 1 million persons, systematically selected from all insurants. The NHRI in Taiwan manages the NHI data bank. The NHI dataset includes information on medical facility registries, details of inpatient orders, ambulatory care, dental services, prescribed drugs, physicians providing services, and contains registration files with encrypted identification. Diagnoses are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). This cohort study was approved by the Ethics Review Board of China Medical University (CMU-REC-101-012).
Study Participants
We selected patients with newly identified sleep disorders other than sleep apnea as the nonapnea sleep disorders cohort, including nonorganic sleep disorders and sleep disturbances diagnosed by physicians (ICD-9-CM codes 307.4 and 780.5) from January 1997 to December 2001. The date of nonapnea sleep disorder diagnosis was used as the index date. We excluded patients who had sleep apnea syndrome (ICD-9-CM codes 780.51, 780.53, and 780.57) and patients with a history of ACS before the index date. Nonapnea sleep disorders were classified into insomnia (ICD-9-CM code 780.52), sleep disturbance (ICD-9-CM code 780.5), unspecified sleep disturbance (ICD-9-CM code 780.50), unspecified hypersomnia (ICD-9-CM code 780.54), unspecified disruptions of 24-h sleep-wake cycle (ICD-9-CM code 780.55), dysfunctions associated with sleep stages or arousal from sleep (ICD-9-CM code 780.56), unspecified sleep related movement disorder (ICD-9-CM code 780.58), and other (ICD-9-CM code 780.59), and other sleep disorders including specific disorders of sleep of nonorganic origin (ICD-9-CM code 307.4). The comparison cohort comprised randomly selected patients without a history of sleep disorders, sleep apnea, and ACS, frequency matched by sex, age, and index date. Two participants were selected for the comparison cohort for each nonapnea sleep disorder participant.
Outcome Measures
The person-y of follow-up were estimated for study patients from the index date to ACS diagnoses, including acute myocardial infarction and unstable angina (ICD-9-CM codes 410 and 411.1), censoring caused by death during hospitalization, loss to follow-up, withdrawal from the insurance system, or the end of December 31, 2010. Comorbidities included in our study were hypertension (ICD-9-CM codes 401-405), DM (ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272), CVA (ICD-9-CM codes 430-438), depression (ICD-9-CM codes 296.2, 296.3, 300.4, and 311), and chronic obstructive pulmonary disease (COPD; ICD-9-CM codes 490-496).
Statistical Analysis
All statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). The proportionate distribution of demographic characteristics and comorbidities between the cohorts of nonapnea sleep disorders and nonsleep disorders were compared and tested using the χ2 test. The mean age between both cohorts was measured and tested using the t test. We assessed the overall, sex- and age-specific incidences of ACS for both nonapnea sleep disorder and nonsleep disorder cohorts, and used Cox proportional hazard regression analysis to estimate the hazard ratios (HRs) with 95% confidence intervals (CIs) of ACS development in the nonapnea sleep disorder cohort, and compared with the nonsleep disorder cohort. The Cox proportion hazard regression models were used to assess the effects of nonapnea sleep disorders on the risk of ACS after adjusting for variables that the χ2 analyses showed to have a significant association. We also measured the incidence associated with a specific comorbidity for both cohorts, and the HR for those with the comorbidity compared with those without the comorbidity. To assess the difference in the ACS-free rates between the two cohorts, we applied Kaplan-Meier analysis and the log-rank test.
RESULTS
The prevalence of nonapnea sleep disorder patients from 1997 to 2001 was 0.47% in 1997, 0.93% in 1998, 2.2% in 1999, 3.37% in 2000, and 4.1% in 2001. During 1997-2001, we identified 49,099 patients for the nonapnea sleep disorder cohort and 98,198 patients for the nonsleep disorder cohort. Almost half of the patients were 41-65 y of age, and 63.7% were women. The distribution of age and sex was similar between the two cohorts. The nonapnea sleep disorder cohort was more prevalent with hypertension, DM, hyperlipidemia, CVA, COPD, and heart failure than the nonsleep disorder cohort (P < 0.0001) (Table 1).
Table 1.
Comparison of demographics and comorbidity between patients with nonapnea sleep disorders and patients without sleep disorders
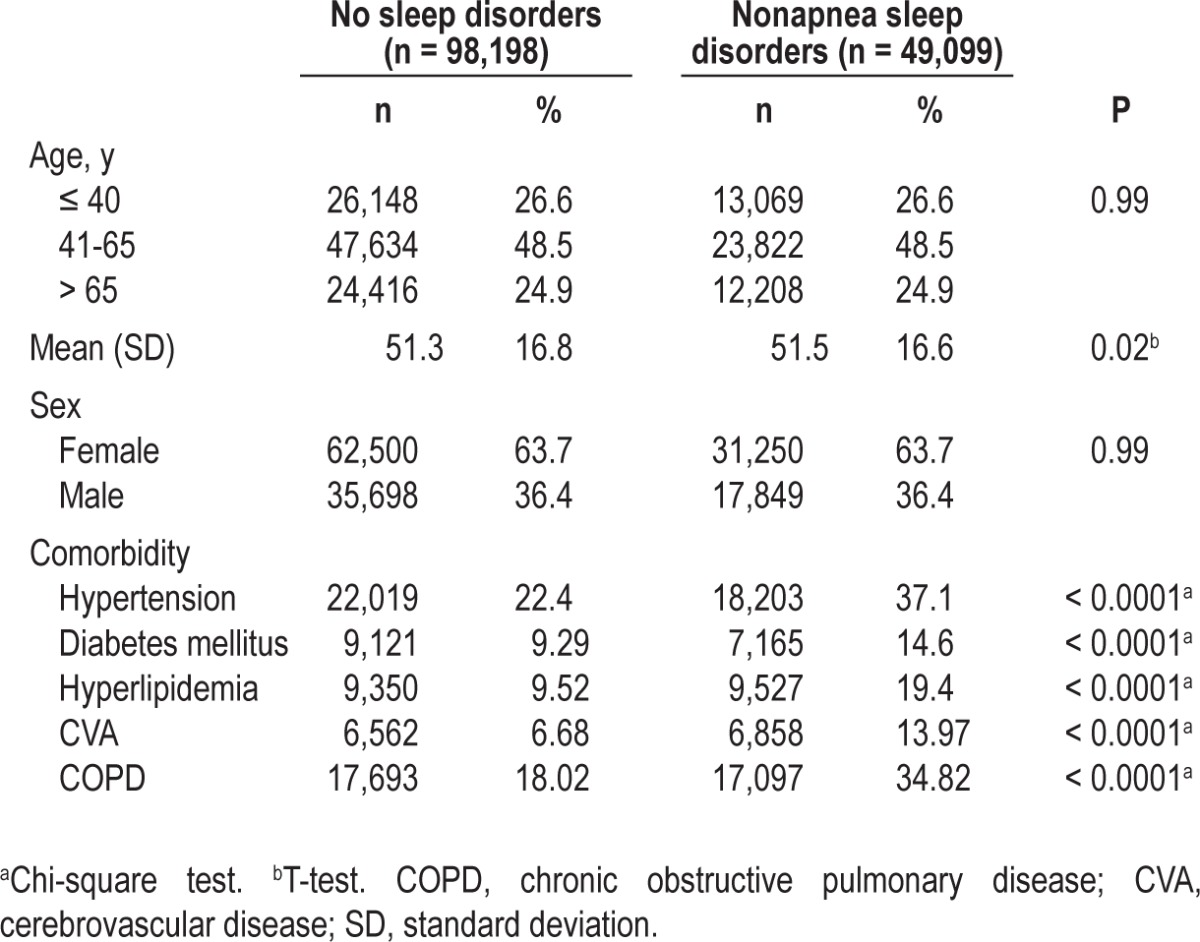
During the follow-up period, the overall incidence of ACS was almost twofold higher in patients with nonapnea sleep disorders than in patients with nonsleep disorders (14.6 versus 7.66 per 10,000 person-y) (Table 2). After adjusting for the covariates, the risk of developing ACS remained significant for patients with nonapnea sleep disorders (HR = 1.43; 95% CI = 1.29-1.60). Men had a higher incidence of ACS in both cohorts and a 57% increase of ACS risk, compared with women. The incidence of ACS increased with age in both cohorts. The age-specific crude relative risk of ACS for patients with nonapnea sleep disorders was higher in young adults than in middle-aged and older adults (age 40 y or younger, crude HR = 2.91, 95% CI = 1.63-5.19; 41-65 y of age, crude HR = 2.06, 95% CI = 1.77-2.40; older than 65 y, crude HR = 1.69, 95% CI = 1.46-1.95, respectively). However, the adjusted HR was 5.02 (95% CI = 3.37-6.76) for middle-aged adults and 8.90 (95% CI = 6.56-12.10) for older adults compared with young adults (age 40 y or younger). The incidence of ACS increased with increased numbers of comorbidities in both cohorts. After adjusting for sex and age, patients with more than three comorbidities had a 5.09-fold risk of developing ACS compared with those without comorbidities (Table 2).
Table 2.
Acute coronary syndrome incidence and hazard ratios of acute coronary syndrome for nonapnea sleep disorders cohort compared to cohort without sleep disorders by sex, age, and comorbidity number
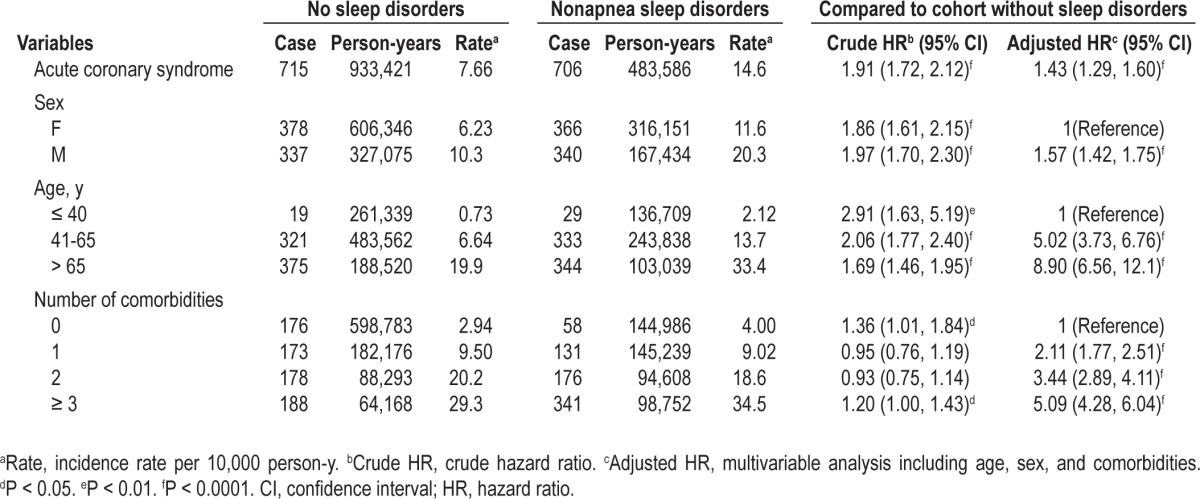
However, the effect of nonapnea sleep disorders on the risk of ACS development decreased if only one comorbidity was considered (Table 3). The multivariable Cox model showed that hypertension, DM, and hyperlipidemia were also significant factors associated with the increased risk of ACS. We also observed the interactions between nonapnea sleep disorders and certain comorbidities including DM, CVA, and hyperlipidemia. We further examined the association between ACS risk and different subgroups of nonapnea sleep disorder, and we determined the adjusted HRs to be 1.28 for insomnia, 1.51 for sleep disturbance, and 1.71 for other sleep disorders (Table 4).
Table 3.
Comparison of incidence and hazard ratios of acute coronary syndrome by individual comorbidity between cohort with nonapnea sleep disorders and cohort without sleep disorders
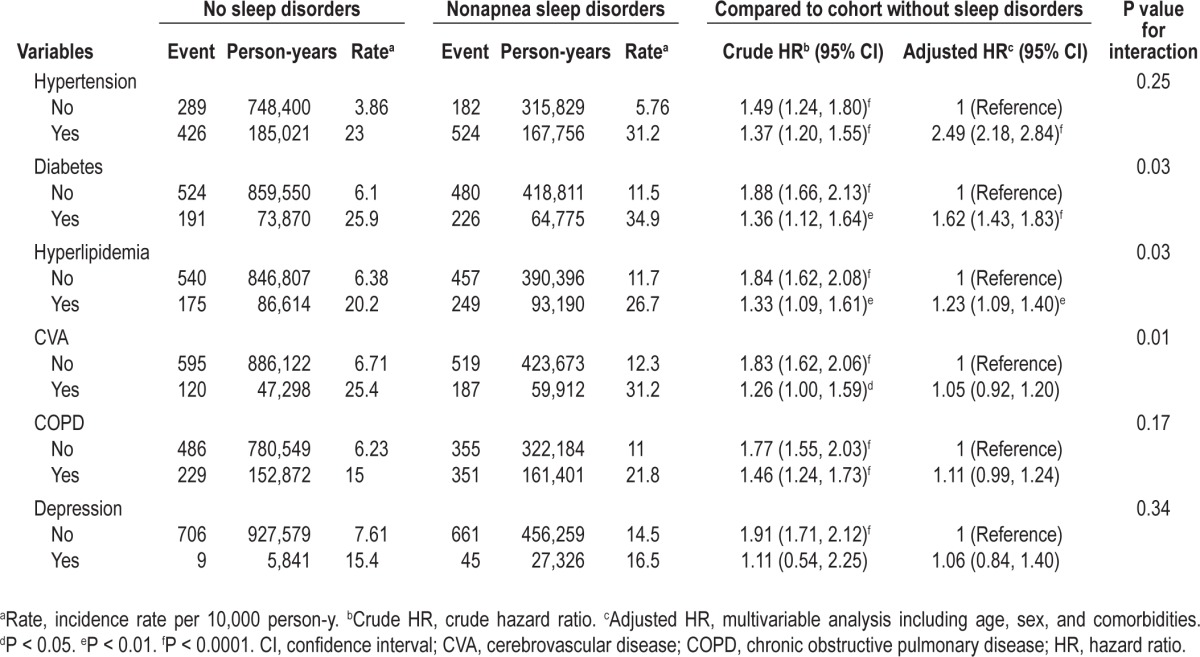
Table 4.
Incidence and hazard ratios of acute coronary syndrome in patients with different types of nonapnea sleep disorders
Figure 1 shows the Kaplan-Meier curves of freedom from ACS in both cohorts. A significant difference exists in ACS occurrence between the nonapnea sleep disorders cohort and the nonsleep disorders cohort (log-rank test, P < 0.0001).
Figure 1.
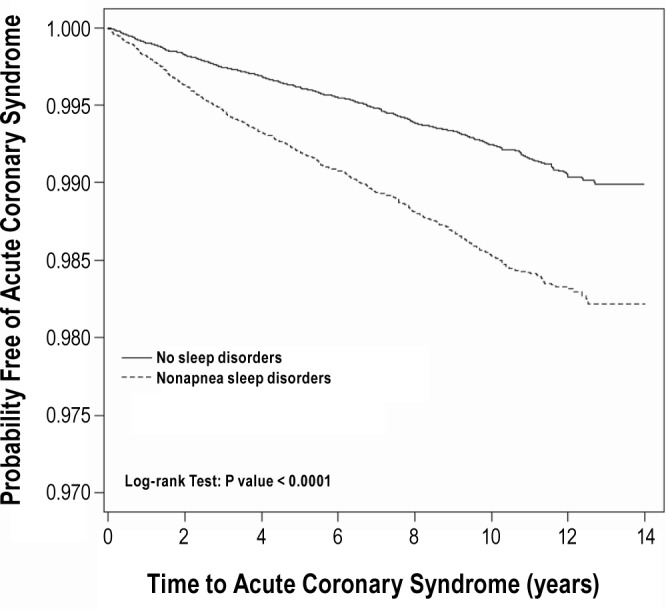
Probability free of ACS for patients with nonapnea sleep disorders (dashed line) or without sleep disorders (solid line).
DISCUSSION
ACS includes life-threatening diseases that possess high morbidity and mortality despite advances in treatment.9 The ACS risk factors are similar to those for stroke, and include older age, hypertension, hyperlipidemia, DM, cigarette smoking, and lack of physical activity. Obstructive sleep apnea is associated with an increased risk of CVD and stroke, and is also strongly associated with obesity, insulin resistance, dyslipidemia, and hypertension.12,13 Screening for and treating obstructive sleep apnea in patients with coronary artery disease who may undergo percutaneous coronary intervention may result in a decreased risk of cardiac death.14 A previous study, by Grandner et al.,10 demonstrated an association between sleep disorders and increased risk of myocardial infarction. However, this study did not objectively assess sleep apnea and, therefore, could not control for its contribution to the increased risk of myocardial infarction.
Several cohort studies have observed that insomnia symptoms are associated with a moderately increased risk of CVD.15–17 Nonetheless, these studies did not exclude participants with obstructive sleep apnea, which may accentuate the association. Studies addressing the association between nonapnea sleep disorders and CVD are scant. According to our research, this study is the first to provide epidemiologic data for the Asian population to address the association between nonapnea sleep disorders and ACS. The yearly prevalence rate of nonapnea sleep disorders in this study increased from 0.47% in 1997 to 4.1% in 2001, which may explain why the participants sought medical treatment for their sleep disorders in increasingly greater numbers. However, the prevalence was lower than in previous studies conducted in other countries, ranging from 10-40%.16,18–21 Patients with insomnia have been observed to rarely visit a physician to discuss their sleep problems, and four of 10 patients self-medicated with nonprescription medication or with alcohol.22 Most patients with nonapnea sleep disorder were middle-aged adults, signifying a possible association with psychological stress.23 A significant association was observed in our study between nonapnea sleep disorders and comorbidities such as hypertension, DM, hyperlipidemia, CVA, COPD, and heart failure, a finding that is consistent with those of previous studies.7,8,24,25
Most patients in the nonapnea sleep disorders cohort in our study were women. Numerous studies have observed a female preponderance of insomnia; the meta-analysis of Zhang and Wing26 and other studies27,28 also confirmed a high female insomnia risk. After adjusting for age and other comorbidi-ties, we observed that men with nonapnea sleep disorders had a higher risk of developing ACS than did women, which is consistent with findings of previous studies.29,30 However, it is not supported by the study by Laugsand et al.,16 who reported that women have a slightly higher risk of acute myocardial infarction than men because of difficulties in initiating sleep, and cumulative insomnia symptoms. In addition, the agespecific crude relative risk showed that the effect of nonapnea sleep disorders on ACS risk was higher among young adults than middle-aged and older adults. However, the risk of ACS increased with age after adjusting for sex and comorbidities. This might be because older adults have a higher proportion of comorbidities interacting with nonapnea sleep disorders, leading to development of ACS. This epiphenomenon can be explained by ACS risk having increased when the patients had more comorbidities in addition to nonapnea sleep disorders. Conversely, the effect of nonapnea sleep disorders on the risk of ACS development decreased if only one comorbidity was considered. This phenomenon can be explained by effect modification. We observed the interactions between nonapnea sleep disorders and certain comorbidities including DM, CVA, and hyperlipidemia. Comorbidities such as hypertension, DM, and hyperlipidemia also increased the risk of ACS in our study. Patients with nonapnea sleep disorder with more than three comorbidities had a 5.09-fold risk of developing ACS compared to those without comorbidities.
The nonapnea sleep disorders cohort in the current study had a 1.43-fold increased risk of subsequent ACS compared with the nonsleep disorders cohort after controlling for age, sex, and comorbidities. The results were relatively robust in different multivariable models. Studies have found inconsistent cardiovascular effects of insomnia by using questionnaires or other methods,6,31–35 which differs from our study population based on physician diagnoses. We further examined the subgroup effects of nonapnea sleep disorders on ACS risk and determined the adjusted HRs to be 1.28 for insomnia, 1.51 for sleep disturbance, and 1.71 for other sleep disorders. Although there are several potential mechanisms, the nature of the association between nonapnea sleep disorders and ACS remains unclear.36,37 A recent study by Fernandez-Mendoza et al.7 showed that insomnia with short sleep duration increased the risk of incident hypertension. Objective short sleep duration in insomnia may serve as a useful predictor of the biological severity of incident hypertension.7 Our study also showed that a significantly higher proportion of hypertension and hyperlipidemia existed in the nonapnea sleep disorder cohort than in the nonsleep disorder cohort. Nakazaki et al.8 observed that insomnia and short sleep duration were associated with atherosclerosis risk leading to CVD. Atherosclerosis and incident hypertension may predispose patients to sudden reduced or occluded blood flow of the coronary arteries, which may explain why the patients in our study with nonapnea sleep disorders were shown to have a higher risk of developing ACS. We conducted further mediation analysis and found that the inclusion of hypertension and hyperlipidemia in the multivariable models attenuated the effect of nonapnea sleep disorders on the risk of ACS (the adjusted HR decreased from 1.59 to 1.41 after adding hypertension to the multivariable model that included sex, age, DM, CVA, COPD, and depression, to 1.55 after adding hyperlipidemia, and to 1.43 after controlling for hypertension and hyperlipidemia together).
To evaluate the contribution of more specific sleep disorders, the sleep disorder diagnoses associated with ACS incidence were further analyzed. Results showed that 40.2% of sleep disorders had been diagnosed as insomnia, 37.2% as sleep disturbance, and 22.6% as others. The incidence rates of ACS were 14.3, 13.5, and 17.0 per 10,000 person-y, with adjusted HRs of 1.28 (95% CI = 1.10-1.47), 1.52 (95% CI = 1.31-1.76), and 1.61 (95% CI = 1.36-1.90), respectively.
However, our study has limitations. First, the NHIRD does not provide detailed patient information such as smoking habits, alcohol consumption, body mass index, physical activity, socioeconomic status, and family history, which all are potential confounding factors in this study. However, these factors may be randomly distributed in these two large cohorts. Second, the evidence derived from a retrospective cohort study is of lower methodological quality than that from randomized trials because a retrospective cohort study design is subject to certain biases related to confounder adjustments. Third, the lack of data on objective sleep measurement or other mental health conditions highly comorbid with sleep disorders and a history of medication is a critical limitation. In addition, a possible survival effect may be another limitation. Despite these limitations, the strength of this study is in providing a nationwide population-based cohort longitudinal study for the Asian population regarding the link between nonapnea sleep disorders and the risk of subsequent ACS events. Our findings may benefit from further analyses regarding specific sleep disorders contributing to ACS incidence in future studies. In summary, we found that patients with nonapnea sleep disorders are at a higher risk of developing ACS, and this risk increases with age. Because the number of patients with nonapnea sleep disorders is increasing, enhancing sleep disorder management may be vital for ACS prevention.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported partly by China Medical University Hospital (Grant Number 1MS1), Taichung Hospital (Grant Number 10202), and Taiwan Department of Health Clinical Trial and Research Center for Excellence (Grant Numbers DOH102-TD-B-111-004 and DOH102-TD-C111-005). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Yen-Jung Chang and Chia-Hung Kao contributed equally to this work. The authors thank the National Health Research Institute in Taiwan for providing the insurance claims data.
SUPPLEMENTAL MATERIAL
Hazard ratios for risk factors of acute coronary syndromes
REFERENCES
- 1.Su TP, Huang SR, Chou P. Prevalence and risk factors of insomnia in community-dwelling Chinese elderly: a Taiwanese urban area survey. Aust N Z J Psychiatry. 2004;38:706–13. doi: 10.1080/j.1440-1614.2004.01444.x. [DOI] [PubMed] [Google Scholar]
- 2.Cho YW, Shin WC, Yun CH, Hong SB, Kim J, Earley CJ. Epidemiology of insomnia in korean adults: prevalence and associated factors. J Clin Neurol. 2009;5:20–3. doi: 10.3988/jcn.2009.5.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallander MA, Johansson S, Ruigomez A, Garcia Rodriguez LA, Jones R. Morbidity associated with sleep disorders in primary care: a longitudinal cohort study. Prim Care Companion J Clin Psychiatry. 2007;9:338–45. doi: 10.4088/pcc.v09n0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassetti CL. Sleep and stroke. Semin Neurol. 2005;25:19–32. doi: 10.1055/s-2005-867073. [DOI] [PubMed] [Google Scholar]
- 5.Portela PC, Fumado JC, Garcia HQ, Borrego FR. Sleep-disordered breathing and acute stroke. Cerebrovasc Dis. 2009;27:104–10. doi: 10.1159/000200447. [DOI] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the penn state cohort. Hypertension. 2012;60:929–35. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakazaki C, Noda A, Koike Y, Yamada S, Murohara T, Ozaki N. Association of insomnia and short sleep duration with atherosclerosis risk in the elderly. Am J Hypertens. 2012;25:1149–55. doi: 10.1038/ajh.2012.107. [DOI] [PubMed] [Google Scholar]
- 9.Kolansky DM. Acute coronary syndromes: morbidity, mortality, and pharmacoeconomic burden. Am J Manag Care. 2009;15:S36–41. [PubMed] [Google Scholar]
- 10.Grandner MA, Jackson NJ, Pak VM, Gehrman PR. Sleep disturbance is associated with cardiovascular and metabolic disorders. J Sleep Res. 2012;21:427–33. doi: 10.1111/j.1365-2869.2011.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng TM. Taiwan's National Health Insurance system: high value for the dollar. In: Okma KGH, Crivelli L, editors. Six countries, six reform models: the healthcare reform experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan World Scientific; 2009. New Jersey: 2009. pp. 71–204. [Google Scholar]
- 12.Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003;41:1429–37. doi: 10.1016/s0735-1097(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 13.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 14.Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:1310–4. doi: 10.1016/j.jacc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Chien KL, Chen PC, Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–84. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124:2073–81. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 17.Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30:1121–7. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 19.Ohayon MM, Hong SC. Prevalence of insomnia and associated factors in South Korea. J Psychosom Res. 2002;53:593–600. doi: 10.1016/s0022-3999(02)00449-x. [DOI] [PubMed] [Google Scholar]
- 20.Üstün TB, Privett M, Lecrubier Y, et al. Form, frequency and burden of sleep problems in general health care: a report from the WHO collaborative study on psychological problems in general health care. Eur Psychiatry. 1996;11:S5–10. [Google Scholar]
- 21.Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M. Prevalence of insomnia in a survey of 12,778 adults in France. J Sleep Res. 2000;9:35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 22.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22(Suppl 2):S347–53. [PubMed] [Google Scholar]
- 23.Medinger F, Varghese R. Psychological growth and the impact of stress in middle age. Int J Aging Hum Dev. 1981;13:247–63. doi: 10.2190/YLBV-TJEX-YYTG-6BEY. [DOI] [PubMed] [Google Scholar]
- 24.Plantinga L, Rao MN, Schillinger D. Prevalence of self-reported sleep problems among people with diabetes in the United States, 2005-2008. Prev Chronic Dis. 2012;9:E76. [PMC free article] [PubMed] [Google Scholar]
- 25.Budhiraja R, Parthasarathy S, Budhiraja P, Habib MP, Wendel C, Quan SF. Insomnia in patients with COPD. Sleep. 2012;35:369–75. doi: 10.5665/sleep.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 27.Li RH, Wing YK, Ho SC, Fong SY. Gender differences in insomnia--a study in the Hong Kong Chinese population. J Psychosom Res. 2002;53:601–9. doi: 10.1016/s0022-3999(02)00437-3. [DOI] [PubMed] [Google Scholar]
- 28.Henderson S, Jorm AF, Scott LR, Mackinnon AJ, Christensen H, Korten AE. Insomnia in the elderly: its prevalence and correlates in the general population. Med J Aust. 1995;162:22–4. doi: 10.5694/j.1326-5377.1995.tb138406.x. [DOI] [PubMed] [Google Scholar]
- 29.Sinha SS, Tremmel JA. Sex differences in acute coronary syndrome. SIS year book. 2007:1–8. [Google Scholar]
- 30.Ostadal P, Ostadal B. Women and the management of acute coronary syndrome. Can J Physiol Pharmacol. 2012;90:1151–9. doi: 10.1139/y2012-033. [DOI] [PubMed] [Google Scholar]
- 31.Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3:489–94. [PMC free article] [PubMed] [Google Scholar]
- 32.Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A, Kales A. Insomnia in central Pennsylvania. J Psychosom Res. 2002;53:589–92. doi: 10.1016/s0022-3999(02)00450-6. [DOI] [PubMed] [Google Scholar]
- 33.Bonnet MH, Arand DL. Cardiovascular implications of poor sleep. Sleep Med Clin. 2007;2:529–38. [Google Scholar]
- 34.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men—a 10-year prospective population based study. Sleep. 2001;24:425–30. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 35.Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J Occup Health. 2003;45:344–50. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 36.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22:960–8. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hazard ratios for risk factors of acute coronary syndromes


