Abstract
Background
Muscletech Hydroxycut® (Iovate Health Sciences Research, Oakville, Ontario) was a popular weight loss supplement that was recalled by the manufacturer in May 2009 based on reports of hepatotoxicity associated with this supplement.
Objective
To characterize the clinical presentation of Hydroxycut®-associated liver injury and to adjudicate these cases for causal association with Hydroxycut®.
Design
Case series.
Setting
Academic tertiary care hospitals and FDA databases.
Measurements
Assessment of causality and grading of severity of liver injury using methodology developed by the Drug-Induced Liver Injury Network (DILIN) study.
Results
Eight patients who developed liver injury after taking Hydroxycut treated at different medical centers were identified. All were hospitalized and 3 of 8 patients required liver transplantation. Nine other cases with adequate clinical information were obtained from the FDA MedWatch database including one fatal case of acute liver failure. Usual symptoms were jaundice, fatigue, nausea, vomiting and abdominal pain. Most patients exhibited a hepatocellular pattern of injury. Adjudication for causality revealed 8 cases as definite, 5 highly likely, 2 probable and 2 were considered as possible.
Conclusions
Hydroxycut® has been clearly implicated as a cause for severe liver injury that may lead to acute liver failure and death. Weight loss supplements represent a class of dietary supplements that should be regarded as capable of causing severe hepatic toxicity when the usual causes of identified liver injury cannot be otherwise elucidated.
Introduction
Under the Dietary Supplement Health and Education Act of 1994 (DSHEA), dietary supplements do not require prior approval by the Food and Drug Administration (FDA) to be sold on the market. Until recently, regulation of supplements by the FDA was limited to monitoring of safety based on voluntary adverse event reporting, but as of December 22, 2007, dietary supplement manufactures have been required to submit via MedWatch reports of serious adverse event linked to the use of dietary supplements in the U.S. (1). In 2002, a weight-loss supplement called Muscletech Hydroxycut® (Iovate Health Sciences Research, Oakville, Ontario), marketed by the manufacturer as a “fat burner,” began to be heavily advertised for sale in retail chains and over the Internet. Indeed, during 2008, more than 9 million units of Hydroxycut® were sold in the United States (U.S.) (2).
Shortly after its launch, the first report of liver injury from the product appeared (3), followed by 5 additional publications (4-8) that together detailed a total of 11 persons who developed severe liver injury from Hydroxycut®, all of whom recovered. In the past 7 years, the present authors have seen another 8 cases of unusually severe liver injury ascribed to Hydroxcut®. Also, by the end of April 2009, the FDA had received 23 additional reports of severe liver injury attributed to Hydroxycut®, submitted through the voluntary reporting MedWatch system, and more recently, a fatal case was reported to them through MedWatch. Using the Freedom of Information Act (FOIA), we obtained redacted versions of the reports of these 24 cases. On May 1, 2009, the FDA issued a warning to the public to stop using Hydroxycut® products, which was followed by a voluntary recall of all its products by the manufacturer (9).
This report describes 17 thus-far unpublished cases of hepatotoxicity attributed to Hydroxycut®; 8 consist of patients who became ill after ingesting Hydroxycut® and who were seen by the present authors at their respective hospitals between September 2002 and February 2009 (hereafter referred to as ‘clinical center patients’). Added to these are 9 of the 24 cases from the FDA MedWatch database among whom sufficient clinical details were available to permit causality assessment (hereafter referred to as ‘FDA cases’). Each developed acute liver injury that was more severe than that described in previously-reported cases; one patient died as a result of the hepatotoxicity and 3 required liver transplantation. The aim of this report is to characterize the clinical presentation of these 17 cases of Hydroxycut®-associated liver injury.
Patients and Methods
Detailed clinical data were extracted from medical records from 8 previously healthy patients who presented at various academic medical centers in the U.S when they developed severe acute hepatic injury after taking Hydroxycut®. Clinical information on the 24 FDA cases obtained via the FOIA request was reviewed to determine that 9 contained sufficient clinical data for further analysis. To the best of our knowledge, all 17 patients were taking the dietary supplement at the doses suggested by the manufacturer. All available clinical and laboratory data were then abstracted and utilized for further independent in-depth assessment of causality and grading of severity of liver injury using the guidelines developed by the Drug-Induced Liver Injury Network (DILIN) study (10).
Causality assessment and grading of severity were performed independently by 4 of the authors, 2 from academia (W.M.L., T-L.F.), one from the FDA (K.K.) and one from the National Institutes of Health (L.B.S.). Because there is no diagnostic marker for drug-induced liver injury, assessing causality using clinical acumen is challenging, even for experts, since it is an inherently subjective approach. To bring some uniformity to this issue, we utilized the 5 categories developed in the DILIN study to assess the percent likelihood of causality: 1=Definite (>95%), 2=Highly Likely (75-95%), 3=Probable (50-74%), 4= Possible (25-49%), 5=Unlikely (<25%), 6= Insufficient data. Severity of liver injury was graded using the criteria developed and published by the DILIN study (10). The Roussel Uclaf Causality Assessment Method (RUCAM) was initially also employed in assessment of causality. However, this method was abandoned due to significant variability in the scoring and difficulty in reaching consensus, a result that has been previously noted (11,12).
Upon completion of the initial assessment by the 4 reviewers, the results were submitted to the coordinating center (UT Southwestern) where the data were compiled for further analysis. All scores were then re-distributed to the individual reviewers for comparison and deliberation. This was followed by a teleconference where any discrepancies in the DILIN scores were discussed and reconciled, resulting in a final consensus score.
Results
Clinical characteristics and severity of liver disease among the 8 clinical center patients
A summary of the demographic and clinical features of the 8 patients (6 males and 2 females) is shown in Table 1. Their mean age was 30.9 (range 17-54) years; 6 patients were Hispanic, one Caucasian and one Asian. None of the patients had any past medical history of relevance, any alcohol abuse or any parenteral risk factors. Serologies for hepatitis A, B and C, Epstein-Barr virus and cytomegalovirus infection were uniformly negative. Patient weights or BMI data were not available.
Table 1. Clinical characteristics of 8 clinical center cases of Hydroxycut® toxicity.
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Past medical history | None ANA 1:160 |
None | None | None | Obesity ANA 1:20, SMA 1:40 treated with prednisone |
None ANA 1:320 SMA positive |
Obesity ANA initially negative, later became 1:160 Actin antibody 30U/ml |
None |
| Duration of Hydroxycut® use (weeks) |
52 | 8 | 6 | 4 | 104 | 8 | 8 | 4 |
| Peak ALT level | 1,450 | 3,223 | 2,729 | 2,258 | 2,385 | 2,635 | 3,099 | 5,396 |
| Peak AST level | 602 | 1,644 | 1,891 | 1,782 | 2,615 | 1,970 | 3,005 | 4,693 |
| Peak bilirubin level (mg/dL) |
45 | 40.5 | 33.3 | 37.0 | 18.8 | >30 | 11.1 | 33.6 |
| Peak alkaline phosphatase |
226 | 141 | 180 | 167 | 139 | 203 | 144 | 280 |
| Peak prothrombin INR |
6.45 | 4.3 | 1.43 | 1.66 | 1.3 | 1.2 | 1.9 | 3.71 |
| Outcome | Liver transplant on day 17 |
Liver transplant on day 35 |
Recovered | Recovered | Recovered | Recovered | Recovered | Liver transplant on day 40 |
| Symptoms | Nausea | Nausea, vomiting, fatigue and abdominal pain |
Nausea, vomiting, itching and fatigue |
Nausea, vomiting, fatigue and abdominal pain |
Nausea, Vomiting, fatigue and abdominal pain |
Vomiting and fatigue |
Nausea and abdominal pain |
Nausea, vomiting, fatigue |
| Histology | Massive hepatic necrosis |
Sub- massive hepatic necrosis |
Acute hepatitis with cholestasis |
Acute hepatitis with cholestasis |
Massive hepatic necrosis |
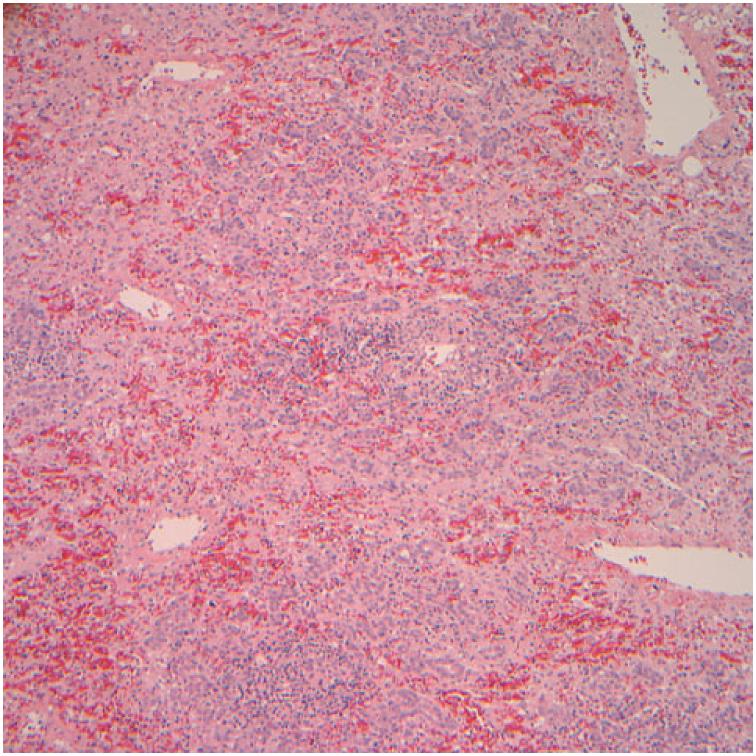
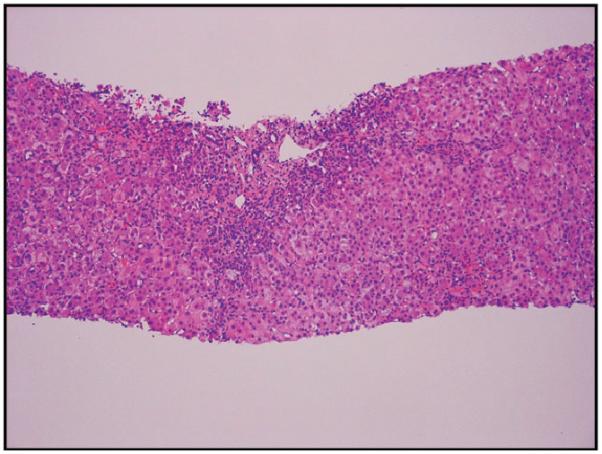
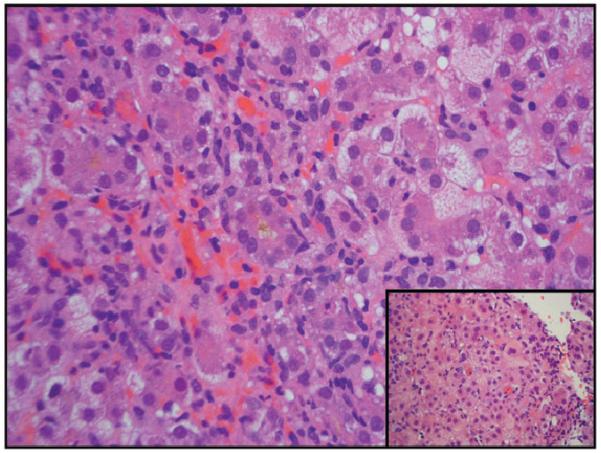
Four patients (3 males/1 female) were positive for anti-nuclear and/or anti-smooth muscle antibodies (patients 1,5,6 and 7) but all four, as well as the four without these autoimmune markers, had normal serum globulin levels. One of the 4 with the autoimmune antibodies progressed to acute liver failure and required liver transplant; the explanted liver did not show features suggestive of autoimmune hepatitis. Two patients with autoimmune markers recovered completely without corticosteroid treatment although the fourth patient was treated briefly with corticosteroids. Thus they did not satisfy these and other requirements for a diagnosis of autoimmune hepatitis using either the International Autoimmune Hepatitis Group criteria (13) or the simplified criteria (14). The latency period, or interval between initiation of ingestion of Hydroxycut® and onset of symptoms, ranged from one to 8 (median 6) weeks, with 2 outliers who had taken the supplement for 52 and 104 weeks, albeit intermittently. Fatigue occurred in 6 of the 8 cases. All patients experienced nausea and/or vomiting. Abdominal pain was present in 4 patients. Jaundice was present in all 8 patients; peak serum bilirubin levels ranged from 11.1 to 45 (mean 31.3) mg/dL. All 8 patients exhibited a hepatocellular pattern of liver injury (R>5)(11). Peak alanine aminotransferase activities (ALT) ranged from 1,450 to 5,396 (mean 2,911) U/L; aspartate aminotransferase activities (AST) ranged from 602 to 4,693 (mean 2,225) U/L, alkaline phosphatase levels ranged from 139 to 280 (mean 185) U/L. No fever, rash or eosinophilia was seen in any of these patients. None developed renal failure. Biopsy tissue was available for 4 of the 8 patients. Two came from the explanted livers, and revealed massive (Figure 1a) or submassive hepatic necrosis. Liver biopsies taken from 2 patients who recovered spontaneously showed severe acute hepatitis with cholestasis (Figure 1b,c).
Figure 1.
a. Liver biopsy for patient 1. Residual hepatic parenchyma with irregularly widened portal areas, mixed inflammatory infiltrates, hepatocellular swelling and spotty hepatocellular dropout. Numerous foci representative of previous bridging necrosis are shown, with bridging of portal areas by metaplastic biliary structures (H&E, 40×).
b,c. Low power view of needle liver biopsy for patient 5. Panlobular hepatic inflammatory infiltrate and cholestasis (H&E, 40×). Higher power view of the same biopsy: a mononuclear, neutrophilic, and eosinophilic portal inflammatory infiltrate is seen with circumferential interface injury and extensive metaplastic ductular proliferation at the edge of the expanded portal tract (H&E, 200×)
All 8 patients had been hospitalized. Three patients had signs of liver failure and required liver transplantation, fulfilling the DILIN criteria for grade 5 severity (death or transplantation). The other 5 included 3 with no signs of liver failure (grade 3) and 2 with prolonged PT/INR (grade 4). All 3 transplanted patients are well, with excellent allograft function and normal liver tests 44 and 16 months after transplantation, respectively, in the 2 patients with long-term follow-up.
Clinical characteristics and severity of liver disease among the nine FDA cases
Data from the 9 cases that had been submitted to the FDA with adequate information for review are summarized in Table 2. Six were females. Their mean and age ranges were similar to those of the 8 clinical center patients. Clinical data were not complete in all 9 MedWatch reports. The median latency was similar to the 8 clinical center cases, as were the peak AST and ALT levels, but the mean peak bilirubin level was significantly lower among these 9 FDA cases, 20.6 vs. 31.3 mg/dL. All 9 FDA cases were hospitalized, jaundiced and had elevated aminotransferase levels. One FDA case was noted to be anti-HCV positive without further confirmatory data. Another case presented with a cholestatic pattern of liver injury; AST 114 U/L, total bilirubin 7.4 mg/dL and alkaline phosphatase 1147 U/L. DILIN severity grade 3 was assigned to the 8 FDA cases who recovered and severity grade 5 to the fatal case.
Table 2. Summary of clinical characteristics of 9 FDA cases of Hydroxycut-associated liver injury.
| Characteristic | n * | Range | |
|---|---|---|---|
| Mean Age (years) | 9 | 30.4 | 23-51 (median 27) |
| Sex | 9 | 3 male/6 female | |
| Latency (weeks) | 6 | 6.5 | 2-13 |
| Abdominal pain | 7 | 6/7 | |
| Nausea/vomiting | 7 | 7/7 | |
| Fatigue | 5 | 4/5 | |
| Jaundice | 9 | 9/9 | |
| Peak ALT (U/L), mean | 8 | 2,538 | 756-3,131 (median 2181) |
| Peak AST (U/L), mean | 9 | 1,671 | 114-2,783 (median 2109) |
| Peak alkaline phosphatase (U/L),mean | 5 | 421 | 176-1,147 (median 257) |
| Peak total bilirubin (mg/dL), mean | 8 | 20.6 | 3-41.4 (median 14.7) |
| Pattern of liver injury | |||
| Hepatocellular (R>5) | 4/5 | ||
| Cholestatic (R<2) | 1/5 |
number of case reports with specific pertinent data
The single fatal case was a 20 year-old Hispanic male with no prior significant medical problems who began taking Hydroxycut® as part of his exercise routine. Thirteen weeks later he developed jaundice, fatigue and nausea. His initial blood test revealed acute hepatitis (ALT 2,323 U/L, AST 1,391 U/L, total bilirubin 10.7 mg/dL, alkaline phosphatase 91 U/L). Serologies for hepatitis viruses A, B and C were negative. Prothrombin time was not obtained at the initial evaluation nor was the history of Hydroxycut® use elicited. During the ensuing three weeks he became increasingly lethargic and jaundiced. On admission, he was encephalopathic and his PT/INR was >13; a history of Hydroxycut® use was obtained. The following day, he underwent exploratory laparotomy for liver transplantation but was found to have intestinal infarction. Liver transplantation was aborted and the patient expired shortly afterward. Autopsy revealed “acute fulminant hepatitis”.
Causality Assessment
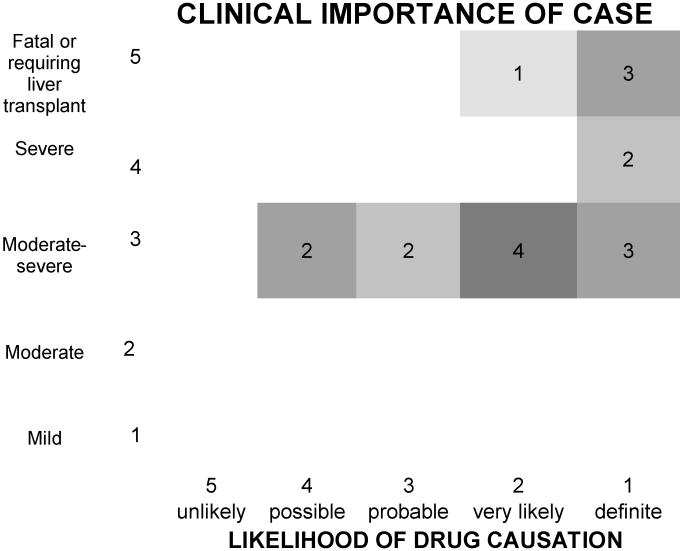
Upon initial evaluation, complete agreement of causality scores was reached among all reviewers in 4 of the 8 clinical center cases and in one of the 9 FDA cases. The scores among the 12 cases without unanimity differed by only one stratum in all but one case, but the differences were reconciled without difficulty in all 17 cases after further discussion. The final causality assignments are shown in Figure 2. Eight cases were considered definite, 5 highly likely, 2 probable and 2 (which included the 2 FDA cases; one patient who was anti-HCV positive and another patient with the cholestatic liver injury) were considered to be possibly related..
Figure 2. Five by five table of likelihood and severity using DILIN criteria9 to adjudicate 17 previously unreported cases of hepatotoxicity due to Hydroxycut®, (modeled after a matrix developed by John Senior, MD, FDA).
Discussion
The 17 cases of Hydroxycut®-associated liver injury presented here were similar to previous reports in having, for the most part, hepatocellular injury. However, they demonstrated a greater degree of severity of injury: all cases were hospitalized, 3 required liver transplantation and there was one fatality due to acute liver failure. Among the 8 clinical center patients, it is notable that 6 of the 8 were Hispanic. It is unclear whether this is due to a genetic predisposition or to the fact that these patients were from Southwestern United States (California, New Mexico and Texas) where Hispanics account for a relatively higher proportion of the population than elsewhere in the United States, or whether the high prevalence of obesity among Hispanic Americans leads to more frequent use of weight-loss supplements. Ethnicity information was lacking for some of the FDA patients. Excluding the 2 outlier patients who were taking Hydroxycut® intermittently for 52 and 104 weeks, respectively, the mean latency period among these cases was 6.4 weeks compared to the 7.8 weeks among the previously reported patients. Also in agreement with the previously reported cases, all patients were symptomatic, the majority describing fatigue, nausea/vomiting and abdominal pain. None of the patients described here had been subjected to re-challenge with the product, although some had taken it intermittently as noted.
With the exception of one of our cases and one previously reported case (5), the predominant pattern of liver injury was severe hepatocellular injury with marked elevation of the aminotransferase levels and minimal abnormalities in alkaline phosphatase levels and histological features in 5 patients showing marked hepatocellular necrosis and injury. It is intriguing that 4 of the 8 persons tested for autoimmune markers were positive for antinuclear antibodies at the time of the acute liver injury. Whether or not this was a consequence of the herbal product, a transient phenomenon associated with the acute injury, or represented identification of previously unrecognized autoimmune hepatitis is not entirely clear. It seems unlikely that pre-existing autoimmune hepatitis was responsible since the cases were mostly males and the findings did not conform to the criteria of the International Autoimmune Hepatitis group (13). Conceivably, Hydroxycut® might have induced an immunological response manifesting as autoimmune-like liver disease (15), such as has been noted previously with other drugs, that include oxyphenisatin, minocycline, and nitrofurantoin (16-18).
The formulation of Hydroxycut® has changed in recent years. The earliest reported cases of acute liver injury related to Hydroxycut® were part of a case series of patients that had developed severe hepatitis after taking various supplements containing ephedra, also called Ma Huang, a plant substance whose natural ingredient is ephedrine (3). In 2004, the sale of supplements containing ephedra was banned by the FDA because of evidence linking ephedra to cardiovascular, neuro-psychiatric and gastrointestinal side effects (19). However, even though Ma Huang was removed from the formulation of Hydroxycut® in 2003, 9 subsequent cases of Ma Huang-free Hydroxycut®-associated hepatotoxicity were reported, all of whom spontaneously recovered (4-8). Despite these reports, Hydroxycut® remained a popular and highly advertised dietary supplement for weight loss that could be purchased in retail stores and over the Internet. On May 1, 2009, following a public warning issued by the FDA regarding the risk of severe liver injury associated with ingestion of Hydroxycut®, its manufacturer recalled all Hydroxycut® products (9).
Assigning causality in cases of dietary supplement-related hepatotoxicity is more complex than that associated with conventional prescription drugs, since individuals using dietary supplements often ingest multiple products and the contents of each product may include many ingredients that are poorly characterized. Doses are not certain, as there is less oversight of these products than of standard prescription medications. Because of these additional challenges, we sought to rigorously adjudicate case histories and to assign causality scores using the methods used in the DILIN study.
The specific ingredient or ingredients in Hydroxycut® responsible for causing the injury remains uncertain. Even with the removal of Ma Huang (ephedra), many formulations still contain other ingredients that may present a health concern such as Garcinia cambogia, Cissus quadrangularis, caffeine and green tea extract.
Garcinia is a tree in Asia and Africa whose fruit extract is used as a supplement ingredient. There are some studies in the published literature that examine its use, but there is no detailed study or evidence that supports the safety of this ingredient. Similarly, Cissus is a plant whose consumption is geographically limited and there is some evidence of its use but no safety studies; rather, there is an article that presents the toxic effects of this plant on goats and sheep (20). Caffeine has been the subject of many published articles, but appropriate consumption levels and long term effects, including adverse effects on the liver, still remain equivocal. The safety of green tea extracts was systematically reviewed by the US Pharmacopeia Dietary Supplements Information Expert Committee (21). In their assessment of French-Spanish reports, Sarma et al. (21) analyzed 13 cases of acute liver injury associated with Exolise® (containing hydro alcoholic extract of green tea) that resulted in acute liver failure, one patient requiring liver transplantation (22), and four other cases of liver injury associated with Tealine® (aqueous extraction of green tea). The clinical presentation, latency, pattern and degree of hepatic biochemical injury of the aforementioned cases of hepatotoxicity related to green tea extracts resemble other cases of Hydroxycut®-associated liver injury (3-8, 22-25).
Toxicity from other constituents contained in Hydroxycut®, some possibly not identified, cannot be ruled out as a cause of the liver injury. Chromium polynicotinate which is found in Hydroxycut®, was reported to cause acute hepatocellular liver injury in a patient who also took various plant extracts including Garcinia cambogia (26) There is even concern for supplement products in general that liver injury may come not from the listed ingredients, but from contaminants such as lead, mercury or arsenic (27-29). There are indeed reports of several other herbals and/or weight loss products purchased via the Internet that are known to have caused liver injury (30). It is also important to keep in mind that the safety of ingredients may vary due to the methods of extraction and preparation and also the possibility of interaction between ingredients that may lead to adverse events.
Hydroxycut® represents the latest dietary supplement in a series of herbal products documented to cause severe liver injury (31-33). Given that MedWatch is a largely voluntary reporting system, we believe that the cases of liver toxicity related to Hydroxycut® described here are a fraction of those that have occurred. To promote the reporting of serious adverse events, the Dietary Supplement and Nonprescription Drug Consumer Protection Act of 2006 requires that, as of December 22, 2007, dietary supplement manufacturers submit, via MedWatch, reports of serious adverse events linked to the use of dietary supplements in the United States. Reports may be submitted to Medwatch online (www.fda.gov/medwatch/report.htm), or, alternatively, a form from the back of the Physicians Desk Reference or obtained from www.fda.gov/medwatch/safety/FDA-3500_fillable.pdf) can be mailed to FDA (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787), faxed to FDA (1-800-FDA-0178), or phoned to FDA (1-800-FDA-1088).
In conclusion, Hydroxycut® has been clearly implicated as a cause for liver injury, largely hepatocellular in nature, and severe enough to have culminated in the need for hospital admission among all 17 identified cases, 4 of whom died or required liver transplantation. The responsible toxic ingredient is not entirely certain, but may be the consequence of the presence in the product of Camellia sinensis.
STUDY HIGHLIGHTS.
- WHAT IS CURRENT KNOWLEDGE
- Hydroxycut®, a weight-loss supplement has been reported to cause non-fatal hepatotoxicity
- WHAT IS NEW HERE
- Application of newly developed Drug Induced Liver Injury Network guidelines for assessment of causality and severity in 17 new cases of Hydroxycut® liver injury
- Report of four cases of severe Hydroxycut® associated acute liver failure that resulted in death or need for liver transplantation
Acknowledgement
The authors thank Professor Eugene Schiff who contributed clinical data on a patient for this report, Dr. Charles Lassman for providing figure 1a, Dr. Thomas Rogers for providing figures 1b and 1c and Dr. Jay Hoofnagle for his thoughtful comments and suggestions.
Financial Support: None
Footnotes
Specific Author Contributions: Tse-Ling Fong, Alejandro Canas-Coto, William Lee, Karl Klontz, Steven Casper and Leonard Seeff were responsible for the study design, data collection, analysis and preparation of the final manuscript. Causality assessment was performed by Tse-Ling Fong, William Lee, Karl Klontz and Leonard Seeff. Tse-Ling Fong, Alejandro Canas-Coto, William Lee, Francisco Durazo, Timothy Davern and Paul Hayashi recruited patients.
Potential competing interests: None
References
- 1. [accessed September 7 2008]; http://www.cfsan.fda.gov/~dms/supplmnt.html.
- 2. [accessed October 7 2009]; http://www.fda.gov/downloads/NewsEvents/PublicHealthFocus/UCM155660.pdf.
- 3.Neff GW, Reddy KR, Durazo FA, et al. Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. J Hepatol. 2004;41:1062–4. doi: 10.1016/j.jhep.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Jones FJ, Andrews AH. Acute liver injury associated with the herbal supplement Hydroxycut® in a soldier deployed to Iraq. Am J Gastroenterol. 2007;102:2357–8. doi: 10.1111/j.1572-0241.2007.01353_10.x. [DOI] [PubMed] [Google Scholar]
- 5.Stevens T, Qadri A, Zein NN. Two patients with acute liver injury associated with use of the herbal weight-loss supplement Hydroxycut®. Ann Intern Med. 2005;142:477–8. doi: 10.7326/0003-4819-142-6-200503150-00026. [DOI] [PubMed] [Google Scholar]
- 6.Shim M, Saab S. Severe hepatotoxicity due to Hydroxycut®: A case report. Dig Dis Sci. 2009;54:406–8. doi: 10.1007/s10620-008-0353-4. [DOI] [PubMed] [Google Scholar]
- 7.Laczek J, Duncan M. Three cases of acute hepatitis in patients taking Hydroxycut® bodybuilding supplement. Am J Gastroenterol. 2008;103(S1):143. [Google Scholar]
- 8.Dara L, Hewett J, Lim JK. Hydroxycut® hepatotoxicity: A case series and review of liver toxicity from herbal weight loss supplements. World J Gastroenterol. 2008;14:6999–7004. doi: 10.3748/wjg.14.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [accessed May 1 2009]; http://www.fda.gov/oc/opacom/hottopics/hydroxycut/consumeradvisory.html.
- 10.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) Prospective study rationale, design and conduct. Drug Safety. 2009;32:56–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danan G, Benichou C. Causality assessment of adverse reactions to drugs-1. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J Clin Epidemiolol. 1993;46:1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 12.Rochon J, Protiva P, Seeff LB, et al. Reliability of the Roussel Uclaf causality assessment method for assessing causality in drug-induced liver injury. Hepatology. 2008;48:1175–1183. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson PJ, McFarlane IG. Meeting report: International autoimmune hepatitis group. Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 14.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 15.Seeff LB. Drug-induced chronic liver disease with emphasis on chronic active hepatitis. Semin Liver Dis. 1981;1:104–115. doi: 10.1055/s-2008-1040723. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds TB, Peters RL, Yamada S. Chronic active lupoid hepatitis caused by a laxative, oxyphenisatin. N Eng J Med. 1971;285:813–820. doi: 10.1056/NEJM197110072851501. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein NS, Bayati N, Silverman AL, Gordon SC. Minocycline as a cause of drug-induced hepatitis: report of four cases and comparison with autoimmune hepatitis. Am J Clin Pathol. 2000;114:91–98. doi: 10.1309/KV2J-VX6Q-L95V-VDE4. [DOI] [PubMed] [Google Scholar]
- 18.Sharp JR, Ishak KG, Zimmerman HJ. Chronic active hepatitis and severe hepatic necrosis associated with nitrofurantoin. Ann Intern Med. 1980;92:14–19. doi: 10.7326/0003-4819-92-1-14. [DOI] [PubMed] [Google Scholar]
- 19. [accessed July 8, 2009];FDA acts to remove ephedra-containing dietary supplements from market. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108379.htm.
- 20.Barakat SE, Adam SE, Maglad MA, Wasfi IA. Effects of Cissus quadrangularis on goats and sheep in Sudan. Rev Elev Med Vet Pays Trop. 1985;38:185–94. [PubMed] [Google Scholar]
- 21.Sarma DM, Barrett ML, Chavez ML, et al. Safety of green tea extracts. Drug Safety. 2008;31:469–484. doi: 10.2165/00002018-200831060-00003. [DOI] [PubMed] [Google Scholar]
- 22.Gloro R, Hourmand-Olivier I, Mosquet B, et al. Fulminant hepatitis during self medication with hydroalcoholic extract of green tea. Eur J Gastroenterol Hepatol. 2005;17:1135–7. doi: 10.1097/00042737-200510000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Bjornsson E, Olsson R. Serious adverse liver reactions associated with herbal weight-loss supplements. J Hepatol. 2007;47:295–302. doi: 10.1016/j.jhep.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis) Ann Intern Med. 2006;144:68–71. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez-Saenz M, Martinez-Sanchez MC. Acute hepatitis associated with the use of green tea infusions. J Hepatol. 2006;44:616–619. doi: 10.1016/j.jhep.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 26.Lanca S, Alves A, Vieira AI, Barata J, de Freitas J, de Carvalho A. Chromium-induced toxic hepatitis. European Journal of Internal Medicine. 2002;13:518–20. doi: 10.1016/s0953-6205(02)00164-4. [DOI] [PubMed] [Google Scholar]
- 27.Ernst E. Heavy metals in traditional Indian remedies. Eur J Clin Pharmacol. 2002;57:891–896. doi: 10.1007/s00228-001-0400-y. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Lead poisoning associated with the use of Ayurvedic medication – five states, 2000-2003. MMWR Morb Mortal Wkly Rep. 2004;53:582–584. [PMC free article] [PubMed] [Google Scholar]
- 29.Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold over the internet. JAMA. 2008;300:915–923. doi: 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi D, Cross TJS, Wong VS. Acute drug induced hepatitis secondary to a weight loss product purchased over the internet. Nutr J. 2007;6:13–15. doi: 10.1186/1475-2891-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Favreau JT, Ryu ML, Braunstein G, et al. Severe hepatotoxicity associated with the dietary supplement LipoKinetix®. Ann Intern Med. 2002;136:590–5. doi: 10.7326/0003-4819-136-8-200204160-00008. [DOI] [PubMed] [Google Scholar]
- 32.Food and Drug Administration Consumer Advisory: Kava-containing dietary supplements may be associated with severe liver injury. U.S. Food and Drug Administration press release; [Accessed 1 June 2009]. Mar 25, 2002. http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm085482.htm. [Google Scholar]
- 33. [Accessed July 8, 2009];FDA advises dietary supplement manufacturers to remove comfrey products from the market. www.fda.gov/Food/Dietary supplements/Alerts/ucm11219.htm.