Abstract
This study was conducted to evaluate the efficacy of a new topical ectoparasiticidal spot-on containing 4.95 % dinotefuran (w/w), 36.08 % permethrin (w/w) and 0.44 % pyriproxyfen (w/w) (Vectra 3D™, Ceva, Libourne, France) against Portuguese strain of Phlebotomus perniciosus and a French strain of Ctenocephalides canis in dogs. Twelve beagle dogs were exposed for 1 h to 100 P. perniciosus on day 6 for allocation in two groups. One group was treated on day 0, and the other group was the control group. The dogs were exposed for 1 h to 100 P. perniciosus on days 1, 7, 14, 21 and 28. After each sandfly challenge, the same dogs were infested with 100 C. canis. Counts of living fleas were determined 48 h after infestation on days 4, 3, 9, 16, 23 and 30. For sandflies, the anti-feeding effect was 96.9, 99.7, 98.7, 83.5 and 87.0 % on days 1, 7, 14, 21 and 28, respectively. The mortality effect was 97.8, 99.8, 73.7, 27.5 and 39.6 % on days 1, 7, 14, 21 and 28, respectively. At each challenge point, the mortality and anti-feeding effects on sandflies were significantly different between the control and treatment groups (p < 0.05). The adulticidal effect on C. canis remained above 99 % throughout the study period. The results indicate that a combination with dinotefuran, permethrin and pyriproxyfen may be used as an effective part of an overall flea and sandfly control strategy in dogs for monthly use.
Introduction
The phlebotomine sandfly Phlebotomus perniciosus is one of the main vectors of Leishmania infantum, the agent of human and canine leishmaniasis in the Mediterranean Basin and the south of France. Prevention of leishmaniasis in dogs can be achieved using an integrative approach. It could include an effective canine vaccine against L. infantum (Dantas-Torres 2006; Moreno et al. 2012) associated with a topical registered veterinary product (including synthetic pyrethroids, permethrin or deltamethrin) with a highly repellent effect against sandflies (Miró et al. 2008; Solano-Gallego et al. 2009, 2011; Maroli et al. 2010; Gramiccia 2011; Beugnet and Franc 2012). Preventing sandfly bites protects dogs from leishmaniasis and reduces the risk of human infection (Killick-Kendrick 1999; Quinnel and Courtenay 2009; Mazloumi Gavgani et al. 2002). Several products have demonstrated their sandfly anti-feeding effect: a deltamethrin-impregnated collar (Killick-Kendrick et al. 1997; Franc and Bouhsira 2009a; Reithinger et al. 2004 and Reithinger et al. 2001), a permethrin–imidacloprid spot-on (Mencke et al. 2003; Otranto et al. 2007; Miró et al. 2007) and a permethrin–pyriproxyfen spray (Molina et al. 2006). A formulation combining dinotefuran, permethrin and pyriproxyfen (Vectra 3D™) was registered in the USA in 2007 and is indicated for the prevention and the treatment of fleas, ticks and mosquitoes in dogs (Franc et al. 2012). This study was conducted to assess the efficacy of the spot-on formulation on another target: P. perniciosus. The activity on a European strain of Ctenocephalides canis was simultaneously tested. C. canis has a worldwide distribution. Its prevalence in dogs carrying fleas was higher than the prevalence of Ctenocephalides felis in some countries in Europe, such as Albania or Greece (Xhaxhiu et al. 2009; Dobler and Pfeffer 2011). Besides its direct pathogenic role, this flea has been proven to be a vector of various pathogens such as Rickettsia felis, Dipylidium caninum and Acanthocheilonema reconditum (Pantchev et al. 2011; Traversa 2013). To the authors’ knowledge, only a few studies have been addressed to test the efficacy of various insecticides against this flea species (McTier et al. 2000; Cadiergues et al. 2001; Franc and Bouhsira 2009b; Bouhsira et al. 2011). Evaluation of insecticide susceptibilities between two close Aedes species, Aedes aegypti and Aedes albopictus, showed a higher tolerance of A. albopictus than A. aegypti to six larvicides tested (Gómez et al. 2011). By extension, the direct result implementation of the insecticide tests performed for C. felis to C. canis is not a tolerable position and requires strict assessment, especially for registration purposes.
Materials and methods
The study was conducted in the Ecole Nationale Vétérinaire de Toulouse (ENVT) and was a single-centre, randomized, blinded, controlled efficacy study on two groups of six dogs each. Dogs were handled in accordance with the Animal Welfare and Good Clinical Practice, and the study protocol was approved by the ethics Committee of Midi-Pyrenees. All personnel involved with the collection of efficacy data were blinded to the treatment.
Animals
Twelve beagle dogs (seven females and five males with an age range of 5 to 10 years and weighing between 9 and 13.11 kg) were enrolled in the study. They had not been exposed to ectoparasiticides for 3 months prior to treatment and remained in good health throughout the study. They were housed in individual indoor cages in a controlled environment and had a 4-h daily access to a 2 × 4 m concrete run without contact with another dog. Each dog was identified with the number of a subcutaneously implanted microchip. They were fed a commercial dry dog food, ration calculated so as to maintain the animal in a healthy physical state. Water was available ad libitum through automatic lickers. Dogs were maintained and handled with due regard for their welfare and were acclimatized to the caged environment for 13 days prior to treatment. They were observed daily for their general health conditions throughout the trial. No concurrent medication was needed to be given during the study.
Six days prior to treatment, each dog was challenged with 100 unfed adult female P. perniciosus. The number of engorged female sandflies was used for ranking and group allocation. Dogs were ranked in descending order of their individual pre-treatment sandfly engorgement status. They were then introduced into blocks of two animals; each, and within each block, dogs were randomly allocated in two groups: treatment or control group.
Treatment
The six dogs from the control group remained untreated and the six dogs from the treatment group received on day 0 a dinotefuran, permethrin and pyriproxyfen combination spot-on of 1.6 ml (for dogs weighing between 4.1 and 10.0 kg) or 3.6 ml (for dogs weighing between 10.1 and 25.0 kg). For all treated animals, the formulation was applied accordingly to manufacturer’s instructions by parting the hair and applying it directly to the skin: for dogs weighing less than 10.0 kg, the 1.6-ml pipette was used from which one half of the dose was applied between the shoulder blades and the other at the base of the tail. For dogs weighing more than 10.1 kg, a 3.6-ml pipette was applied to three areas: between the shoulder blades, in the middle of the dorsal midline and at the base of the tail. Treatment dosages ranged from 68.27–129.65 mg/kg for permethrin, 9.36–17.78 mg/kg for dinotefuran and 0.83–1.58 mg/kg for pyriproxyfen.
Flea and sandfly maintenance and supply
The P. perniciosus exposure was induced using laboratory reared adult females only. The sandfly strain was obtained from Lisboa, Portugal and was maintained at ENVT under laboratory conditions since 2003.
The C. canis infestation was induced using laboratory reared adult fleas (males and females). This strain was obtained from a wild strain (Montesquieu-Volvestre, Ariège, France) and maintained at ENVT under laboratory conditions since 2008.
Experimental design
The 12 dogs were infested with 100 (±2) P. perniciosus for a total of six times. The day before exposure, sandflies were aspirated from their breeding cage with a vacuum pump and then placed in challenge nets (100 ± 2 female sandflies per net) with cotton soaked with water and sugar. The challenge assessment cages (60 × 40 × 50 cm) were constructed from fine mesh netting mounted on a wooden frame and placed in environmentally controlled rooms. Sandflies were fasted 2 h before exposure to dogs by removing the cottons from cages. Before exposure, dogs were sedated by intramuscular injection of medetomidine (Dexdomitor®, Pfizer Santé animale, Paris, France), ketamine (Clorketam®, Laboratoire Vetoquinol S.A., Lure, France) and diazepam (Valium®, Roche injectable, Neuilly s/ Seine, France) at a dose rate of 4 μg/kg, 9 mg/kg and 0.5 mg/kg, respectively and then placed in individual infestation proof nets containing sandflies. The dosage of the anaesthetic was approximately calculated to immobilize dogs for 1 h. The exposition duration was 60 ± 5 min. During infestations, treated and control dogs were placed into separated infestation rooms where temperature and relative humidity were maintained between 25 and 26 °C and between 58 and 72 %, respectively. Cages and nets were thoroughly cleaned after each sandfly challenge.
After sandfly exposure, dogs were carefully taken out of the net and examined for any dead sandflies (which were counted). Dogs were then replaced in their cage. All living sandflies were aspirated from each challenge net using a vacuum pump, counted and recorded as engorged or unengorged. All dead sandflies were collected, counted and recorded as dead non-engorged or dead engorged. On days 6, 1, 7, 14, 21 and 28, living sandflies recovered from individual animals at the end of the exposure were placed in separate nets and kept in the infestation rooms at room temperature for 1 day. Each individual net was labelled with an animal number and study code. At 1 day post-exposure, dead sandflies of each net were counted and removed. Then, all remaining sandflies were discarded.
Once dogs had regained full consciousness after anaesthesia (i.e. 3 h ± 30 min after the end of exposure to sandflies), they were infested with 100 ± 2 C. canis. Forty-eight hours after infestation, fleas were counted by carefully combing the entire coat of each dog. Each dog was simultaneously combed by two technicians for at least 10 min, using a fine-toothed comb. If no flea was collected during the last minute, the combing was stopped. If not, the dog was combed until no flea was collected during a period of 1 min. Collected fleas were counted, recorded and discarded.
Dogs were infested on days 6, 1, 7, 14, 21 and 28 and combed on days 4, 3, 9, 16, 23 and 30.
Data analysis
Sandfly anti-feeding effect
For each time point after exposure, the anti-feeding rate was evaluated for each group as described below and compared to the control group:
Then, the anti-feeding effect (expressed in percentage) was determined:
Sandfly mortality effect
For each time point after exposure, the mortality rate was evaluated for each group as described below and compared to the control group:
Then, the mortality effect (expressed in percentage) was determined:
Anti-flea efficacy
The data collected were the number of fleas found alive on the dogs after combing. Effect was assessed on a group basis (the total number of fleas in each group of six dogs). Efficacy of the treatment was calculated as follows:
The mean count is calculated using the arithmetic mean respecting the EMEA recommendation. The treated group was compared to the control group. Efficacy evaluations are in accordance with those published in July 12, 2012 in the EMEA/CVMP/EWP/82829/2009-Rev. 2 document of the European Medicines Agency (2012).
Statistical analysis
The non-parametric test of Kruskall Wallis was used to test both sandfly and flea data. Differences were considered significant at a p value lower than 0.05. Analyses were performed with Systat 9 software.
Results
No adverse events related to treatment were observed.
Sandflies and anti-feeding effect
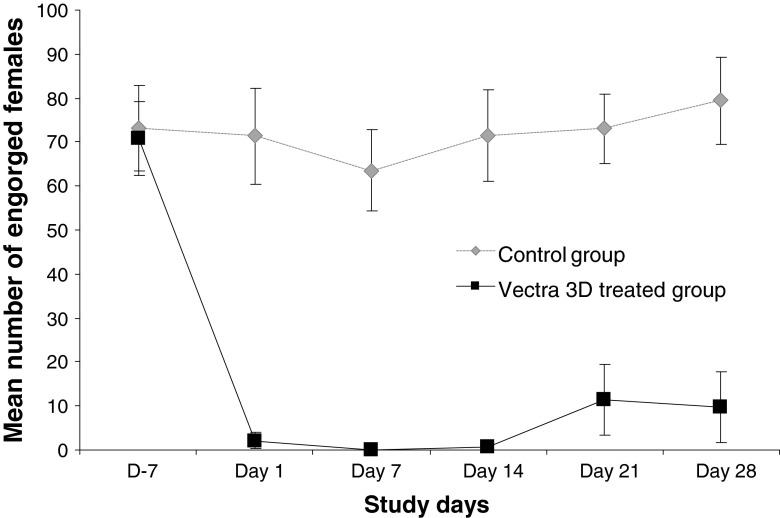
The percentages of engorged females before treatment were 73.40 and 74.28 % for both the treated and the control groups, respectively. Dogs from both groups included in the study demonstrated an equal and high pre-treatment parasite holding ability (i.e. above 50 % of engorged females per dog, Fig. 1). All control dogs maintained an adequate number of engorged females throughout the study. After the treatment, only a few engorged females were found in the treated group during the first 2 weeks. For the six treated dogs, a total of 13, one and five engorged females were observed on days 1, 7 and 14, respectively. In the same conditions, the total number of engorged females was 428, 381 and 429 for the control dogs. The treatment had an anti-feeding effect between 96.89 and 99.70 % during the first 2 weeks and between 83.54 and 86.96 % until the end of the trial.
Fig. 1.
Mean number of engorged Phlebotomus perniciosus females after 1 h exposure to control and treated dogs. Dogs were treated on day 0 with a dinotefuran, permethrin and pyriproxyfen combination spot-on and then weekly challenged with 100 P. perniciosus females
At each challenge point post-treatment (day 1, day 7, day 14, day 21 and day 28), the difference of engorgement status of P. perniciosus females between treated and controlled group was significant (p < 0.05).
Sandflies and mortality effect
Table 1 reports the mortality effect (or insecticidal effect) of the treatment 1 and 24 h after exposure to dogs. The mortality rate observed 24 h after exposure is close to the one obtained after 1 h exposure: the mortality effect of the formulation has not increased within the 24 h post-exposure and was already quite efficient after 1 h exposure. At each challenge point, there was a significant statistical difference (p < 0.05) in the number of dead P. perniciosus after 1 and 24 h of exposure between the treatment and control group.
Table 1.
Mortality and anti-feeding effect of a dinotefuran, permethrin and pyriproxyfen combination against P. perniciosus
| Day 1 | Day 7 | Day 14 | Day 21 | Day 28 | ||
|---|---|---|---|---|---|---|
| Mortality effect | 1 h | 97.6 % | 99.8 % | 72.9 % | 24.1 % | 38.0 % |
| 24 h | 97.8 % | 99.8 % | 73.7 % | 27.5 % | 39.6 % | |
| Anti-feeding effect | 1 h | 96.9 % | 99.7 % | 98.7 % | 83.5 % | 87.0 % |
The spot-on was applied to dogs on day 0, and animals were weekly exposed to 100 P. perniciosus females
Anti-flea efficacy
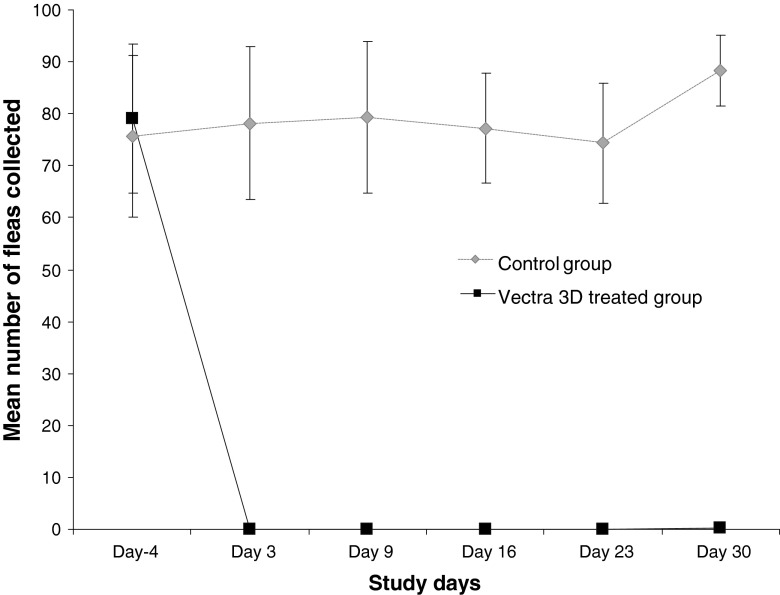
Mean counts (SD) of fleas combed 48 h after each infestation are represented in Fig. 2. On days 3, 9, 16 and 23, no fleas were collected in the treated group: the spot-on provided 100 % adulticidal efficacy. The mean counts obtained in the control group remained between 74.33 and 79.33 for the same period. On day 30, no fleas were observed on four of the six treated dogs and only one flea per dog was combed on the other two dogs: the spot-on provided an efficacy of 99.6 %. Before treatment, no significant statistical difference was observed in the number of combed between groups (p > 0.5). Then at each challenge point, the difference was significant (p < 0.05).
Fig. 2.
Mean numbers of Ctenocephalides canis on treated and control dogs 48 h after each weekly infestation. Dogs were treated on day 0 with a dinotefuran, permethrin and pyriproxyfen combination spot-on and then weekly infested with 100 C. canis. They were combed 48 h after infestation
Discussion
The combination of several products (acaricides, insecticides and insect growth regulators) is available and safe for the integrated control of ectoparasites on domestic dogs (Horak et al. 2012). The development of insecticide resistance is a continuing threat. Simultaneous use of several insecticides exhibiting several mechanisms of action with different molecular targets could be a way to decrease the emerging risk of genetic resistance among pests of domestic animals. In the present study, the efficacy of a spot-on combining dinotefuran, permethrin and pyriproxyfen was experimentally assessed on P. perniciosus and C. canis. Moreover, dinotefuran has been suggested to be used in areas of resistant mosquitoes against common insecticides (Corbel et al. 2004).
Pyriproxyfen has not a repellent or adulticidal activity. We assume that the repellent and insecticidal effects on adults observed here were due to the combination of permethrin and dinotefuran.
The repellent effect provided by the association of pyrethroids and neonicotinoids in the current trial could ensure a rapid and high efficient protection of dogs against P. perniciosus bites, which is interesting in the context of a short stay in an endemic area of canine leishmaniasis. No difference during the first week post-treatment of the anti-feeding effects was noticed between our results and the report of Molina et al. (2012) using a 65 % permethrin spot-on. In this previous study, the values of anti-feeding effect were 99.13 % (day 1) and 93.43 % (day 8). These results were close to the values found here: 96.9 % (day 1) and 99.7 % (day 7). However, the anti-feeding effect obtained with permethrin alone was not as persistent as the combination of dinotefuran and permethrin tested here. The decrease was more dramatically for permethrin alone ranging from 86.8 % at day 15 to 61.03 % at day 29; meanwhile, the repellency was up to 87 % at day 28 in the present study. Another test combining 10 % imidacloprid (w/v)/ and 50 % permethrin (w/v) spot-on presented anti-feeding effects close to those obtained with the currently tested formulation for the first 3 weeks post-treatment (Miró et al. 2007): 97.7 % (day1), 96.3 % (day 7), 96.5 % (day 14), 92.7 % (day 22) and 74 % (day 29). Considering all these results, an added value of dinotefuran was strongly suggested for the anti-feeding lasting effect against P. perniciosus.
The association of permethrin with dinotefuran exhibited a similar insecticidal effect (or mortality effect) against P. perniciosus compared to permethrin alone for the first 2 weeks (Molina et al. 2012) but was more persistent over time. Indeed, the 65 % permethrin solution provided mortality effects of 97.6 % (day 1) and 79.7 % (day 8). Interestingly, the new combination tested here ensured a better and longer insecticidal effect than permethrin in association with imidacloprid (Miró et al. 2007): 53.2 % (day 1), 49.4 % (day 7), 15.1 % (day 14), 13.2 % (day 22) and 2.9 % (day 29). The synergy of action can result to this specific combination as it was demonstrated for imidacloprid and flumethrin against ticks and C. felis and required more investigations (Stanneck et al. 2012). The comparison of long- or medium-term repellent and insecticidal efficacy is often difficult for different topical applications such as collar, spot-on or spray (Horak et al. 2012). Nevertheless, our results suggest a high improved insecticidal activity using the combination of permethrin and pyriproxyfen with the addition of dinotefuran in comparison to the spray associating permethrin and pyriproxyfen at day 7 (29.6 %) to day 28 (0.8 %); meanwhile, values of repellent effect were similar throughout the survey period (Molina et al., 2006). The high mortality effect with high repellent activity was reported by Franc et al. (2012) against Aedes aegypti the day following the treatment without explanation. The lethality or repellency was probably not due to the volatility of permethrin and dinotefuran. Vapour pressures of both compounds are very low: 1.7 × 10−6 Pa at 25 °C for dinotefuran and 7 × 10−5 Pa at 20 °C for permethrin. New surveys with specific designs are necessary to elucidate this issue.
No divergence has been observed for the adulticidal efficacy of the tested formulation between C. canis (100 % efficacy until day 23 and 99.6 % on day 30) and a French strain of C. felis previously tested (100 % efficacy until day 16, 99.7 % on day 23 and 96.2 % on day 30, Bouhsira et al. 2012). These results suggest a similar susceptibility for both Ctenocephalides species to the tested association. The combination of dinotefuran and pyriproxyfen has been tested on cats against C. felis (Murphy et al. 2009). The adulticidal activity of dinotefuran has been found to be more immediate in the first 2 h after the application than imidacloprid. Eventually, Dryden et al. (2011) showed that this combination was efficient in in-home investigations in Florida (USA) for at least 1 month in the control of flea infestations and re-infestations.
To conclude, the tested combination of dinotefuran, permethrin and pyriproxyfen had an immediate repellent effect against P. perniciosus and an adulticidal effect on C. canis that lasted for 4 weeks. This combination can be recommended for use on dogs living or travelling in leishmaniosis endemic areas with an application repeated every 3 to 4 weeks.
Acknowledgments
The authors wish to thank gratefully Martine Roques, Solange Vermot and Sonia Gounaud for their technical assistance.
References
- Beugnet F, Franc M. Insecticide and acaricide molecules and/or combinations to prevent pet infestation by ectoparasites. Trends Parasitol. 2012;28:267–279. doi: 10.1016/j.pt.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Bouhsira E, Yoon SS, Roques M, Manavella C, Vermot S, Cramer LG, Ollagnier C, Franc M. Efficacy of fipronil, amitraz and (S)-methoprene combination spot-on for dogs against adult dog fleas (Ctenocephalides canis, Curtis, 1826) Vet Parasitol. 2011;179:351–353. doi: 10.1016/j.vetpar.2011.03.048. [DOI] [PubMed] [Google Scholar]
- Bouhsira E, Liénard E, Jacquiet P, Warin S, Kaltsatos V, Baduel L, Franc M. Efficacy of permethrin, dinotefuran and pyriproxyfen on adult fleas, flea eggs collection, and flea egg development following transplantation of mature female fleas (Ctenocephalides felis felis) from cats to dogs. Vet Parasitol. 2012;190:541–546. doi: 10.1016/j.vetpar.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Cadiergues MC, Caubet C, Franc M. Comparison of the activity of selamectin, imidacloprid and fipronil for the treatment of dogs infested experimentally with Ctenocephalides canis and Ctenocephalides felis felis. Vet Rec. 2001;149:704–706. [PubMed] [Google Scholar]
- Corbel V, Duchon S, Zaim M, Hougard JM. Dinotefuran: a potential neonicotinoid insecticide against resistant mosquitoes. J Med Entomol. 2004;41:712–717. doi: 10.1603/0022-2585-41.4.712. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. Leishmune vaccine: the newest tool for prevention and control of canine visceral leishmaniosis and its potential as a transmission-blocking vaccine. Vet Parasitol. 2006;141:1–8. doi: 10.1016/j.vetpar.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Dobler G, Pfeffer M. Fleas as parasites of the family Canidae. Parasit Vectors. 2011;4:139. doi: 10.1186/1756-3305-4-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden MW, Payne PA, Vicki S, Riggs B, Davenport J, Kobuszewski D. Efficacy of dinotefuran–pyriproxyfen, dinotefuran–pyriproxyfen–permethrin and fipronil–(S)-methoprene topical spot-on formulations to control flea populations in naturally infested pets and private residences in Tampa, FL. Vet Parasitol. 2011;182:281–286. doi: 10.1016/j.vetpar.2011.05.054. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (2012) Testing and evaluation of the efficacy of antiparasitic substances for the treatment and prevention of tick and flea infestations in dogs and cats. http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/10/WC500004604.pdf. Accessed 28 May 2013
- Franc M, Bouhsira E. Efficacy of a combination of a fipronil-(S)-methoprene spot-on formulation and a deltamethrin impregnated collar in controlling fleas and sandflies on dogs. Vet Ther. 2009;10:71–77. [PubMed] [Google Scholar]
- Franc M, Bouhsira E. Evaluation of speed and duration of efficacy of spinosad tablets for treatment and control of Ctenocephalides canis (Siphonaptera: Pulicidae) infestations in dogs. Parasite. 2009;16:125–128. doi: 10.1051/parasite/2009162125. [DOI] [PubMed] [Google Scholar]
- Franc M, Genchi C, Bouhsira E, Warin S, Kaltsatos V, Baduel L, Genchi M. Efficacy of dinotefuran, permethrin and pyriproxyfen combination spot-on against Aedes aegypti mosquitoes on dogs. Vet Parasitol. 2012;189:333–337. doi: 10.1016/j.vetpar.2012.04.026. [DOI] [PubMed] [Google Scholar]
- Gómez A, Seccacini E, Zerba E, Licastro S (2011) Comparison of the insecticide susceptibilities of laboratory strains of Aedes aegypti and Aedes albopictus. Mem Inst Oswaldo Cruz 106:993–996 [DOI] [PubMed]
- Gramiccia M. Recent advances in leishmaniosis in pet animals: epidemiology, diagnostics and anti-vectorial prophylaxis. Vet Parasitol. 2011;181:23–30. doi: 10.1016/j.vetpar.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Horak IG, Fourie JJ, Stanneck D. Efficacy of slow-release collar formulations of imidacloprid/flumethrin and deltamethrin and of spot-on formulations of fipronil/(s)-methoprene, dinotefuran/pyriproxyfen/permethrin and (s)-methoprene/amitraz/fipronil against Rhipicephalus sanguineus and Ctenocephalides felis felis on dogs. Parasit Vectors. 2012;5:79. doi: 10.1186/1756-3305-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killick-Kendrick R, Killick-Kendrick M, Focheux C, Dereure J, Puech MP, Cadiergues MC. Protection of dogs from bites of phlebotomine sandflies by deltamethrin collars for control of canine leishmaniasis. Med Vet Entomol. 1997;11:105–111. doi: 10.1111/j.1365-2915.1997.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R. The biology and control of phlebotomine sandflies. Clin Dermatol. 1999;17:279–289. doi: 10.1016/S0738-081X(99)00046-2. [DOI] [PubMed] [Google Scholar]
- Maroli M, Gradoni L, Oliva G, Castagnaro M, Crotti A, Lubas G, Paltrinieri S, Roura X, Zini E, Zatelli A. Guidelines for prevention of leishmaniasis in dogs. J Am Vet Med Assoc. 2010;236:1200–1206. doi: 10.2460/javma.236.11.1200. [DOI] [PubMed] [Google Scholar]
- Mazloumi Gavgani AS, Hodjati MH, Mohite H, Davies CR. Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matchedcluster randomised trial. Lancet. 2002;360:374–379. doi: 10.1016/S0140-6736(02)09609-5. [DOI] [PubMed] [Google Scholar]
- McTier TL, Jones RL, Holbert MS, Murphy MG, Watson P, Sun F, Smith DG, Rowan TG, Jernigan AD. Efficacy of selamectin against adult flea infestations (Ctenocephalides felis felis and Ctenocephalides canis) on dogs and cats. Vet Parasitol. 2000;91:187–199. doi: 10.1016/S0304-4017(00)00291-0. [DOI] [PubMed] [Google Scholar]
- Mencke N, Volf P, Volfova V, Stanneck D. Repellent efficacy of a combination containing imidacloprid and permethrin against sandflies (Phlebotomus papatasi) in dogs. Parasitol Res. 2003;90:S108–S111. doi: 10.1007/s00436-003-0905-7. [DOI] [PubMed] [Google Scholar]
- Miró G, Gálvez R, Mateo M, Montoya A, Descalzo MA, Molina R. Evaluation of the efficacy of a topically administered combination of imidacloprid and permethrin against Phlebotomus perniciosus in dog. Vet Parasitol. 2007;143:375–379. doi: 10.1016/j.vetpar.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Miró G, Cardoso L, Pennisi MG, Oliva G, Baneth G. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part two. Trends Parasitol. 2008;24:371–377. doi: 10.1016/j.pt.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Molina R, Miró G, Gálvez R, Nieto J, Descalzo MA. Evaluation of a spray of permethrin and pyriproxyfen for the protection of dogs against Phlebotomus perniciosus. Vet Rec. 2006;159:206–209. doi: 10.1136/vr.159.7.206. [DOI] [PubMed] [Google Scholar]
- Molina R, Espinosa-Góngora C, Gálvez R, Montoya A, Descalzo MA, Jiménez MI, Dado D, Miró G. Efficacy of 65% permethrin applied to dogs as a spot-on against Phlebotomus perniciosus. Vet Parasitol. 2012;187:529–533. doi: 10.1016/j.vetpar.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Moreno J, Vouldoukis I, Martin V, McGahie D, Cuisinier AM, Gueguen S. Use of a LiESP/QA-21 vaccine (CaniLeish) stimulates an appropriate Th1-dominated cell-mediated immune response in dogs. PloS One. 2012;6:e1683. doi: 10.1371/journal.pntd.0001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Ball CA, Gross S. Comparative in vivo adulticidal activity of a topical dinotefuran versus an imidacloprid-based formulation against cat fleas (Ctenocephalides felis) on cats. Vet Ther. 2009;10:9–16. [PubMed] [Google Scholar]
- Otranto D, Paradies P, Lia RP, Latrofa MS, Testini G, Cantacessi C, Mencke N, Galli G, Capelli G, Stanneck D. Efficacy of a combination of 10% imidacloprid/50% permethrin for the prevention of leishmaniasis in kennelled dogs in an endemic area. Vet Parasitol. 2007;144:270–278. doi: 10.1016/j.vetpar.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Pantchev N, Etzold M, Daugschies A, Dyachenko V. Diagnosis of imported canine filarial infections in Germany 2008–2010. Parasitol Res. 2011;109:S61–S76. doi: 10.1007/s00436-011-2403-7. [DOI] [PubMed] [Google Scholar]
- Quinnel RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitol. 2009;136:1915–1934. doi: 10.1017/S0031182009991156. [DOI] [PubMed] [Google Scholar]
- Reithinger R, Teodora U, Davies CR. Topical insecticide treatments to protect dogs from sandfly vectors of leishmaniasis. Emerg Infect Dis. 2001;7:872–876. doi: 10.3201/eid0705.017516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithinger R, Coleman PG, Alexander B, Vieira EP, Assis G, Davies CR. Are insecticide-impreganted dog collars a feasible alternative to dog culling as a strategy for controlling canine visceral leishmaniasis in Brazil? Int J Parasitol. 2004;34:55–62. doi: 10.1016/j.ijpara.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L, Koutinas A, Miró G, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol. 2009;165:1–18. doi: 10.1016/j.vetpar.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86. doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanneck D, Ebbinghaus-Kintscher U, Schoenhense E, Kruedewagen EM, Turberg A, Leisewitz A, Jiritschka W, Krieger KJ. The synergistic action of imidacloprid and flumethrin and their release kinetics from collars applied for ectoparasite control in dogs and cats. Parasit Vectors. 2012;5:73. doi: 10.1186/1756-3305-5-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59. doi: 10.1186/1756-3305-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xhaxhiu D, Kusi I, Rapti D, Visser M, Knaus M, Lindner T, Rehbein S. Ectoparasites of dogs and cats in Albania. Parasitol Res. 2009;105:1577–1587. doi: 10.1007/s00436-009-1591-x. [DOI] [PubMed] [Google Scholar]