Abstract
Objectives
To determine whether urinary excretion of C-type natriuretic peptide (CNP) is elevated in acute decompensated heart failure (ADHF) and predicts adverse outcomes.
Background
Urinary CNP has been detected in heart failure patients but its clinical significance and prognostic utility in ADHF, compared to established kidney injury biomarkers, is unknown.
Methods
We measured 24-hour urinary excretion and concurrent plasma concentrations of CNP22, CNP53, and NT-CNP53 in 58 ADHF patients and 20 controls. Urinary KIM-1, NGAL, and plasma NT-proBNP were also measured. Mortality and all-cause rehospitalization/death were assessed over a mean (SD) follow up of 1.5 (0.9) years.
Results
ADHF patients had higher urinary excretion of all three CNP molecular forms than controls. Plasma CNP22 and CNP53 were elevated in ADHF but showed limited correlation with urinary excretion, suggesting that mainly renal-derived CNP appears in urine. Plasma NT-proBNP and urinary KIM-1 were also elevated in ADHF (p<0.0001); urinary NGAL was similar to controls. At 6 months, event-free survival in ADHF patients was 86% for mortality and 59% for all-cause rehospitalization/death. On Cox regression analysis urinary NT-CNP53 was the only predictor of mortality (HR 1.7, 95%CI 1.1-2.4, p=0.01) and all-cause rehospitalization/death (HR 1.8, 95%CI 1.3-2.4, p=0.0004), even after adjustment. Integrated discrimination analysis suggested urinary NT-CNP53 combined with plasma NT-proBNP improved prediction of adverse outcomes.
Conclusions
This is the first study demonstrating the clinical utility of urinary CNP molecular forms. In ADHF, urinary NT-CNP53 correlates with prognosis, better predicted outcomes than urinary NGAL and KIM-1, and improved the prognostic value of plasma NT-proBNP.
Keywords: Biomarkers, C-type Natriuretic Peptide, Acute Decompensated Heart Failure, Outcomes
Introduction
Acute decompensated heart failure (ADHF) is the leading cause of hospitalization in patients over 65 years of age and continues to confer a disturbingly high mortality rate (1). Accurate risk stratification is important in the effort to improve outcomes in ADHF, as an aid to clinical decision making and appropriate patient selection for clinical trials. To this end, N-terminal pro-B-type natriuretic peptide (NT-proBNP), a circulating marker of ventricular stretch, remodeling and neurohumoral activation, is strongly associated with prognosis in ADHF (2) and has been widely integrated into routine clinical evaluation. Nevertheless, ADHF pathophysiology is complex and there is growing recognition that optimal risk assessment of ADHF patients may require a multimarker approach (3,4).
Renal dysfunction is a prevalent and independent predictor of adverse outcomes in patients with ADHF (5,6) and a candidate for inclusion in any multimarker strategy. However, conventional creatinine-based estimates of glomerular function or urine albumin excretion fail to incorporate the potential prognostic impact of renal tubular injury, as recently demonstrated in HF patients (7-9). Conversely, the novel urinary biomarker C-type natriuretic peptide (CNP) is produced in the kidney as well as the endothelium, has been localized to renal tubules (10,11), and as part of the natriuretic peptide family may provide greater information concerning renal integrity and the cardio-renal interaction in ADHF.
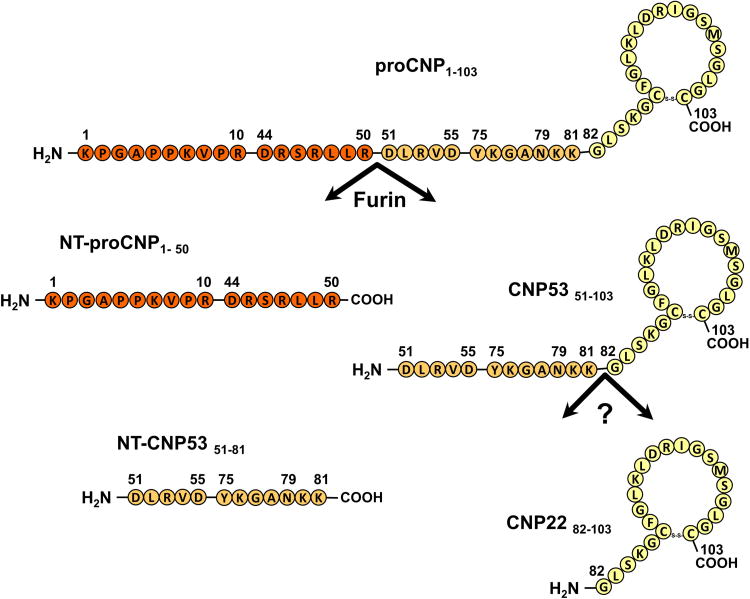
CNP is synthesized as the precursor 103 amino acid (AA) protein, proCNP (AA 1-103), which is then cleaved into NT-proCNP (AA 1-50) and CNP53 (AA 51-103) by the intracellular endoprotease furin (Fig 1)(12). Additional downstream processing cleaves CNP53, giving rise to the biologically active mature form CNP22 (AA 82-103) and inactive form NT-CNP53 (51-81)(12,13). While urinary CNP22 excretion has been shown to be elevated in stable HF (14), the clinical utility of other CNP molecular forms has not been studied. Furthermore, the prognostic significance of urinary CNP excretion in ADHF is unknown. We tested the hypotheses that excretion of NT-CNP53 which, like NT-proBNP, may have a longer half-life and be more resistant to degradation than its biologically active mature form CNP22, would: (i) be associated with prognosis in patients with ADHF, (ii) provide greater prognostic information than contemporary urinary biomarkers of tubular injury (kidney injury molecule 1, KIM-1; neutrophil gelatinase-associated lipocalin, NGAL), and (iii) be of incremental predictive value to plasma NT-proBNP in the risk stratification of patients with ADHF.
Figure 1. Biosynthesis and processing of C-type natriuretic peptide (CNP).
Precursor protein, proCNP, is cleaved into NT-proCNP and CNP53 by the intracellular endoprotease furin. Additional downstream processing cleaves CNP53 into the biologically active mature form CNP22, and an inactive form, NT-CNP53.
Methods
Patient population
Sixty ADHF patients and 20 healthy subjects were recruited. ADHF patients hospitalized at St Mary's Hospital, Mayo Clinic, Rochester, MN, were prospectively identified and enrolled from an ongoing register of consecutive admissions between August 2009 and August 2010. Patients with a clinical diagnosis of HF consistent with Framingham criteria were included (15). Patients with an incomplete or incorrect urine collection for adequate urinary biomarker analysis were excluded (n=2), leaving a total of 58 consecutive ADHF patients providing consent. These patients underwent baseline history assessment, physical examination, and transthoracic echocardiography as part of routine clinical care. Twenty-four hour urine collection and plasma samples for assessment of CNP molecular forms, urinary KIM-1, NGAL and plasma NT-proBNP were obtained upon admission. Urine samples were collected on ice with acetic acid (30 ml of 1:1 acetic acid; 17.4 M). After the timed urine collection (mean 22.9±4 hours), total volume was recorded and samples aliquoted and stored at -80°C until analysis. Results are expressed using the Modification of Diet in Renal Disease (MDRD) estimated GFR (eGFR) (16).
Control subjects were recruited from a population (Rochester, MN) of volunteers; all were non-smokers and had no history of cardiovascular (CV) or systemic disease. Twenty-four hour urine collection and plasma samples were obtained upon enrollment. All participants provided written informed consent for participation and the study was approved by the Mayo Clinic Institutional Review Board.
Urine biomarker assays
CNP22 (AA 82-103)
Urinary CNP22 was determined using a non-equilibrium radioimmunoassay (RIA) [Phoenix Pharmaceutical, Burlingame, CA], using an antibody that detects human CNP22 as previously described (11). The range of the standard curve was 0.5-128pg, with a lower limit of detection of 0.5pg. Inter- and intra-assay variability was 11% and 5% respectively. Recovery was 85%. Cross-reactivity was 0% with ANP, BNP, endothelin, NT-CNP53, and 59% with CNP53.
CNP53 (AA 51-103) and NT-CNP53 (AA 51-81)
Urinary CNP53 and NT-CNP53 were determined using a similar non-equilibrium RIA [Phoenix Pharmaceutical, Burlingame, CA], using antibodies that detect human CNP53 (CNP53, AA51-103), and the first 29 amino acids of CNP53 starting from the amino-terminal only when separated from the ring structure (NT-CNP53, AA51-81). For CNP53 and NT-CNP53 the range of the standard curve was 0.5-128 pg. Inter- and intra-assay variability for CNP53 was 8% and 7% respectively. Recovery was 81±4%. Cross reactivity was 100% with CNP22, 0% with NT-CNP53, ANP, and BNP. Inter- and intra-assay variability for NT-CNP53 was 10% and 6% respectively. Recovery was 82±5.2%. Cross-reactivity was 0% with ANP, BNP, CNP22, CNP53 and endothelin.
NGAL and KIM-1
Urine concentrations of NGAL and KIM-1 were measured by enzyme-linked immunoassay as per instructions (Quantikine® ELISA, R&D Systems). The minimum detectable dose for NGAL was 0.012ng/ml, and for KIM-1 0.009ng/ml. Intra- and inter-assay coefficients of variation were <5% and <8% respectively. NGAL is recognized to form complexes with MMP9; recombinant human MMP-9/NGAL complex demonstrated 0.3% cross-reactivity in the assay used. There was no significant cross-reactivity or interference in the KIM assay.
Urine biomarker excretion
Mean urine flow (ml/h) was determined from total urine volume (ml) and urine collection time (hours). Urine biomarker excretion was calculated as the product of urine biomarker concentration (pg/ml or ng/ml) and urine flow rate (ml/h) and adjusted for urinary creatinine excretion (ng/gCr).
Plasma biomarker assays
Blood was drawn into EDTA tubes and centrifuged at 4°C, 2500rpm, for 10 minutes. 1ml plasma was aliquoted and stored at -80°C until assayed. Plasma concentrations of CNP molecular forms were determined using a non-equilibrium RIA [Phoenix Pharmaceutical, Burlingame, CA]; using anti-human CNP antibodies (17). Plasma NT-proBNP was measured by electrochemiluminescence immunoassay (18). The lower limit of detection for NT-proBNP was 5pg/ml; inter-assay and intra-assay variability was 3.1% and 2.5% respectively. There was no cross-reactivity with CNP forms.
Statistical analysis
All urinary biomarkers demonstrated a non-Gaussian distribution therefore values are presented as median (25th, 75th percentile). For comparisons between ADHF and control subjects, non-parametric Wilcoxon rank-sum tests were used. Spearman's rank correlation was used to ascertain relationships between continuous variables. Biomarker excretion data was normalized by natural logarithmic transformation prior to Cox regression analysis to detect independent predictors of: i) mortality, and ii) time to first non-elective all-cause rehospitalization/death. Mortality and rehospitalization were ascertained from institutional records including local primary care data. Patients were otherwise censored at time of last known follow-up. Survival rates were calculated using the Kaplan-Meier method. C-statistics were used to compare the discriminatory ability of biomarkers. Confidence intervals were calculated for c-statistics using an approximate jackknife method of calculating standard errors. The integrated discrimination index (IDI) (19) was utilized to evaluate the improvement in predictive accuracy using the combination of urinary NT-CNP53 CNP and plasma NT-proBNP over the use of NT-proBNP alone, for study outcomes. Probability values are 2-sided; p<0.05 was considered significant. Data were analyzed using JMP software version 9.0 (SAS Institute, Inc, Cary, NC) and SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
Clinical characteristics of the study population are shown in Table 1. ADHF Patients were older than controls, 40% were female, and mean (SD) left ventricular ejection fraction (LVEF) was 38.4% (18.9). Twenty-two (38%) ADHF patients presented with dyspnea alone; 4 (7%) presented with edema alone; and 24 (41%) with combined dyspnea and peripheral edema. Fifty-five percent presented in NYHA Class III. Plasma NT-proBNP was significantly elevated in ADHF patients and eGFR reduced compared to controls (Table 1). Urinary creatinine concentration was lower in ADHF than controls, consistent with instigation or escalation of diuretic therapy during ADHF management (Table 1).
Table 1. Clinical characteristics.
| Variable | Control (n = 20) | ADHF (n = 58) | p-value |
|---|---|---|---|
|
| |||
| Age*, y | 53.5 (6.1) | 70.1 (10.4) | <0.0001 |
| Male gender, n (%) | 10 (50) | 35 (59) | 0.50 |
| Ischemic etiology, n (%) | - | 19 (33) | - |
| Co-morbidity | |||
| Hypertension, n (%) | - | 36 (62) | - |
| Diabetes, n (%) | - | 25 (43) | - |
| Thyroid disease, n (%) | - | 11 (19) | - |
| Atrial fibrillation, n (%) | - | 38 (66) | - |
| Previous CVA, n (%) | - | 7 (12) | - |
| CRT, n (%) | - | 14 (24) | - |
| Medications on admission | |||
| ACEI or ARB, n (%) | - | 38 (66) | - |
| Beta-blocker, n (%) | - | 44 (76) | - |
| Loop diuretic, n (%) | - | 49 (84) | - |
| Aldosterone antagonist, n (%) | - | 12 (21) | - |
| Mean LVEF*, % | - | 38.0 (18.9) | - |
| Serum creatinine*, mg/dL | 0.7 (0.18) | 1.2 (0.8) | <0.0001 |
| eGFR*, ml/min/1.73m2 | 115.9 (21.1) | 60.5 (30.3) | <0.0001 |
| Plasma biomarkers (pg/ml)† | |||
| NT-proBNP | 37.8 (21.9, 67.3) | 2461 (1222, 6994) | <0.0001 |
| CNP22 | 6.4 (4.3, 18.8) | 11.7 (8.3, 19.6) | 0.005 |
| CNP53 | 3.8 (3.6, 4.3) | 5.8 (5.0, 7.6) | 0.0001 |
| NT-CNP53 | 6.5 (5.4, 7.7) | 6.1 (5.3, 6.9) | 0.56 |
| Urine variables | |||
| Urine volume* (ml) | 1878.0 (653.7) | 1824.8 (1129.3) | 0.80 |
| Urine collection time* (h) | 24.0 (0) | 22.9 (4.0) | 0.05 |
| Urinary creatinine* (mg/dL) | 75.5 (38.1) | 55.3 (37.8) | 0.04 |
| Urine protein/creatinine ratio (mg/mg)† | 0.02 (0.01, 0.02) | 0.03 (0.02, 0.08) | 0.0007 |
| Urine biomarker excretion (ng/gCr)† | |||
| KIM-1 | 475.0 (198.9, 604.9) | 1354.0 (876.5, 2101.5) | <0.0001 |
| NGAL | 298.8 (225.2, 458.3) | 350.2 (137.2, 1405.7) | 0.94 |
| CNP22 | 7.2 (6.7, 9.6) | 14.0 (8.1, 27.0) | 0.0003 |
| CNP53 | 64.7 (21.6, 109.1) | 115.2 (63.1, 227.8) | 0.02 |
| NT-CNP53 | 19.4 (13.3, 29.6) | 35.8 (20.0, 72.6) | 0.0015 |
Values expressed as mean (SD)
Values expressed as median (25th, 75th percentile).
CNP22, C-type natriuretic peptide-22; CNP53, C-type natriuretic peptide-53; CRT, cardiac resynchronization therapy; CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; KIM-1, kidney injury molecule 1; LVEF, left ventricular ejection fraction; NGAL, neutrophil gelatinase-associated lipocalin; NT-CNP53, N-terminal fragment of C-type natriuretic peptide -53; NT-proBNP, N-terminal pro-B type natriuretic peptide.
Acute decompensated heart failure and urinary biomarker excretion
Median urinary excretion of KIM-1 and all three CNP molecular forms was significantly higher in ADHF than controls, as was the urinary total protein/creatinine ratio (Table 1). Urinary NGAL excretion was unchanged (p=0.94). KIM-1 demonstrated a weak non-significant association with eGFR (Spearman's ρ= -0.19; p=0.1), but there was no significant relationship between any urinary biomarker and NYHA class (III or IV) at presentation, nor any significant trends associated with LVEF (off inotropes).
Correlations between excretion rates of urinary CNP and other measured biomarkers were assessed (Supplemental Table 1). Moderate correlations were observed between urinary CNP molecular forms but only urinary CNP22 displayed any, albeit modest, correlation with its concentration in the plasma (ρ=0.28, p=0.04). Urinary CNP22 and CNP53 were weakly associated with plasma NT-proBNP (ρ=0.45, p=0.0003; ρ=0.33, p=0.01 respectively); urinary NT-CNP53 was not. Urinary CNP22 (ρ=0.68, p=0.0001) and urinary KIM-1 (ρ=0.78, p<0.0001) demonstrated marked correlations with urinary total protein/creatinine ratio which was not evident with the other urinary biomarkers.
Medications on admission for ADHF patients are shown in Table 1. On exploratory analysis, urinary NGAL was higher in the context of ACEI or ARB use [median (25th, 75th percentile): 444.0 (219.2, 2144.1)ng/gCr] versus no ACEI or ARB use [177.3 (106.3, 333.6)ng/gCr; p=0.03] and urinary NT-CNP53 was lower in ADHF patients admitted on loop diuretics compared to those without [34.0 (17.6, 61.3) vs. 60.4 (43.6, 246.1)ng/gCr; p=0.01].
Plasma concentrations of C-type natriuretic peptide
Plasma CNP22 and CNP53 were elevated in ADHF compared to controls, whereas plasma NT-CNP53 was unchanged (Table 1). Plasma CNP22 demonstrated limited association to its concurrent urine excretion (ρ=0.28, p=0.04), and a weakly positive trend with urine CNP53 (ρ=0.24, p=0.07) and NT-CNP53 excretion (ρ=0.26, p=0.05) (Supplemental Table 1). By contrast, neither plasma CNP53 nor plasma NT-CNP53 displayed any relationship to urinary excretion of any CNP molecular form.
Clinical outcomes
Of the 58 ADHF patients studied, there were 18 deaths and 18 additional rehospitalizations (13 CV rehospitalizations) over a mean (SD) follow-up of 1.5 (0.9) years. Two patients were admitted for elective cardiac resynchronization therapy; these were not included as events in the final analysis. At 6 months, the overall event-free survival rate (95%CI) was 85.8% (77.1-95.4) for mortality and 58.9% (47.2-73.4) for rehospitalization/death.
Univariate cox regression analysis of baseline factors showed age to be the only variable associated with study outcomes (mortality: HR 1.08, 95%CI 1.02-1.15, p=0.01; rehospitalization/death: HR 1.04, 95%CI 1.00-1.09, p=0.04). Sex, NYHA Class, LVEF, and comorbidities: hypertension and diabetes were not significantly associated with either outcome. Among urinary and plasma biomarkers assessed, Cox regression analysis revealed only urinary NT-CNP53 excretion to be significantly predictive of mortality and all-cause rehospitalization/death (Table 2). This association persisted after adjusting for age, urinary protein/creatinine ratio, and plasma NT-proBNP (Table 2).
Table 2. Predictive value of urinary NT-CNP53 excretion and plasma NT-proBNP for clinical outcome in ADHF patients.
Univariate and adjusted Cox proportional hazard analysis.
| Model | Outcome | |||
|---|---|---|---|---|
|
| ||||
| Death | All-cause rehospitalization / death | |||
| HR (95% CI) | p-value | HR (95%CI) | p-value | |
|
| ||||
| Urinary NT-CNP53* | ||||
| Unadjusted | 1.67 (1.14-2.37) | 0.01 | 1.78 (1.30-2.39) | 0.0004 |
| Model 1 | 1.54 (1.05-2.22) | 0.03 | 1.75 (1.28-2.36) | 0.0007 |
| Model 2 | 1.60 (1.06-2.38) | 0.03 | 1.74 (1.26-2.36) | 0.001 |
| Model 3 | 1.67 (1.08-2.57) | 0.02 | 1.79 (1.28-2.47) | 0.0009 |
|
| ||||
| Plasma NT-proBNP* | ||||
| Unadjusted | 1.28 (0.85-1.93) | 0.24 | 1.24 (0.94-1.65) | 0.13 |
| Model 1 | 1.35 (0.89-2.04) | 0.16 | 1.26 (0.95-1.67) | 0.11 |
| Model 2 | 1.30 (0.85-1.98) | 0.21 | 1.22 (0.91-1.63) | 0.17 |
Ln transformed data (hazard ratio are per 1 log unit increase)
Model 1: Adjusted for age
Model 2: Adjusted for age and urine protein/creatinine ratio
Model 3: Adjusted for age, urine protein/creatinine ratio, and plasma NT-proBNP
NT-CNP53, N-terminal fragment of C-type natriuretic peptide -53; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
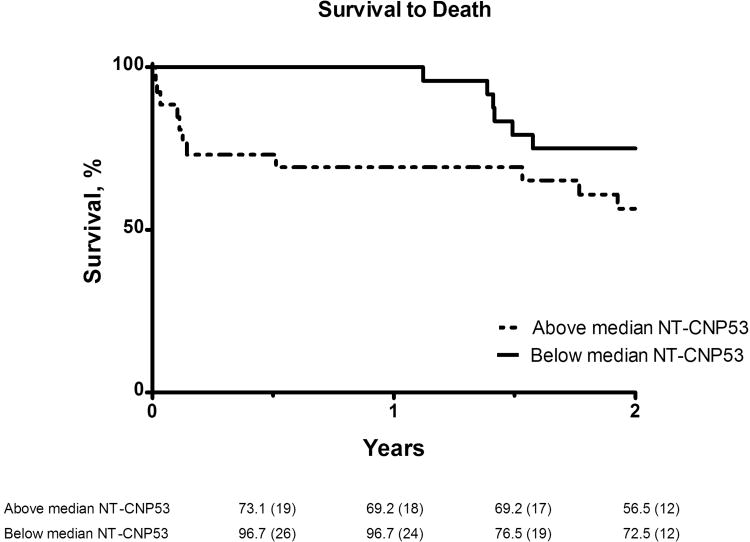
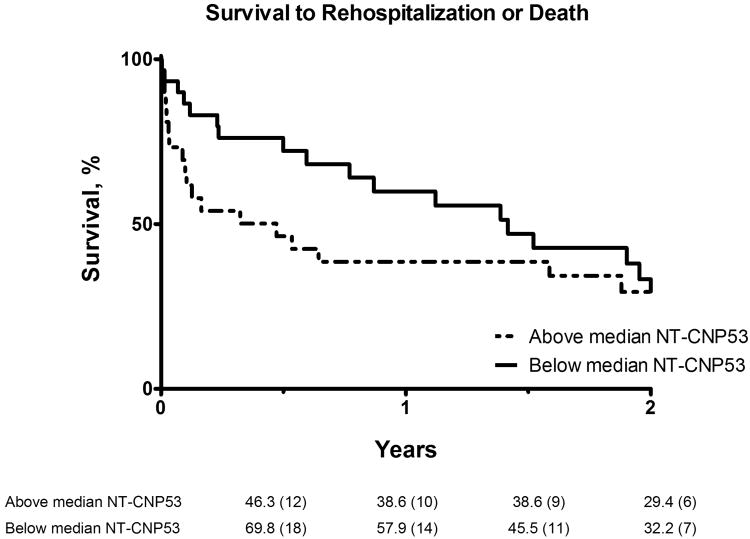
Six-month Kaplan-Meier event-free survival rates were lower in patients with urinary NT-CNP53 excretion above the median compared to those below [mortality rate (95%CI): 73.1% (57.9-92.3) vs. 96.7% (90.5-100); rehospitalization/death rate (95%CI): 46.3% (30.6-70.0) vs. 69.8% (55.1-88.5); Fig 2a-b]. Moreover, on analysis of the c-statistic for the occurrence of all-cause mortality, the addition of urinary NT-CNP53 to plasma NT-proBNP provided incremental predictive value compared to plasma NT-proBNP alone (Table 3). Examination of the integrated discrimination index (IDI) provided further evidence that this combination improved prediction of adverse outcomes in this cohort (Table 3). No other urinary or plasma biomarker in this study demonstrated significant predictive value.
Figure 2. Kaplan-Meier survival analysis for (a) mortality, (b) all-cause rehospitalization/death.
Event-free survival curves shown for patients with urinary NT-CNP53 excretion above and including (dashed line), and below (solid line) the median value. Numbers given below each figure are survival rates (%) and number of patients at risk (in parentheses) at each 6-month interval.
Table 3. Measures of predictive accuracy.
| Model | C-index (95% CI) | Integrated discrimination improvement, % (SE) | p-value |
|---|---|---|---|
|
| |||
| Death | |||
| NT-proBNP† | 0.57 (0.43-0.71) | - | - |
| NT-CNP53‡ | 0.66 (0.53-0.78) | - | - |
| NT-proBNP†and NT-CNP53‡ | 0.69 (0.56-0.82) | 30 (11)* | 0.004* |
|
| |||
| Death / rehospitalization | |||
| NT-proBNP† | 0.56 (0.46-0.66) | - | - |
| NT-CNP53‡ | 0.67 (0.59-0.76) | - | - |
| NT-proBNP†and NT-CNP53‡ | 0.69 (0.61-0.78) | 17 (5.0)* | 0.001* |
Plasma NT-proBNP
Urinary NT-CNP53
compared to NT-proBNP alone
NT-proBNP, N-terminal pro-B type natriuretic peptide; NT-CNP53, N-terminal fragment of C-type natriuretic peptide -53; SE, standard error.
Discussion
This is the first study to investigate the prognostic value of urinary CNP in patients with ADHF. The major finding was that increased urinary excretion of NT-CNP53 was associated with increased mortality and also predicted the composite outcome of all-cause rehospitalization/death in patients with ADHF, independent of age, renal glomerular function and plasma NT-proBNP. Furthermore elevated NT-CNP53 excretion offered incremental predictive value to plasma NT-proBNP and was the only urinary biomarker among those investigated to demonstrate prognostic significance. These findings support accumulating evidence for CNP's involvement in HF pathophysiology and more specifically that its detection in urine may contribute to risk stratification in ADHF patients.
Urinary C-type natriuretic peptide in heart failure
CNP is predominantly produced in the kidney (11,14,20) and endothelium (17), but has also been detected in other tissues (21-23). Despite limited diuretic or natriuretic activity relative to that of atrial (ANP) and BNP, CNP acts as a venodilator (24) and modestly elevated plasma levels have been detected in HF patients, though markedly less than elevations of ANP and BNP (25). Urinary CNP most likely derives from a combination of glomerular filtration, trans-tubular reuptake and secretion from post-glomerular blood, as well as tubular secretion of locally produced CNP. On account of its low circulating levels, susceptibility to rapid removal by the clearance receptor (NPR-C), and degradation by neutral endopeptidase, the detection of CNP in urine is predominantly thought to reflect local renal production (14). The lack of significant correlation between urinary excretion rates and plasma concentrations of CNP peptides in the present study supports this hypothesis. In addition, the detection of three distinct CNP molecular forms in the urine of both ADHF patients and controls, suggests local renal processing of CNP occurs too.
Few studies have described urinary CNP excretion in HF patients and these initial studies, confined to the biologically active mature form CNP22, have yielded conflicting results (14,26-28). Compared with healthy controls, increased urinary CNP excretion was observed by Mattingly et al. (n=6) and Ng et al. (n=34) in stable HF and ADHF patients respectively (14,26), but not by Bentzen et al. (n=11) or Kalra et al. (n=16) (27,28). This disparity may be due to differentially timed collections of CNP22 which is especially prone to rapid degradation. Urinary excretion of other molecular forms of CNP has not previously been examined in HF. Though understanding of CNP biology continues to evolve, it is currently thought to serve as a renoprotective peptide, activated by humoral mechanisms and/or hypoxia in the setting of renal injury, to preserve glomerular function and suppress pro-fibrotic processes (11).
Urinary C-type natriuretic peptide and prognosis in heart failure
Renal dysfunction portends a poor prognosis in ADHF (5,6). In this study over 50% of ADHF patients exhibited a GFR below 60ml/min (CKD stage 2 and above), consistent with previous reports (5,6). While reduced renal perfusion is a major determinant, recent studies have highlighted an independent association between elevated urinary excretion of tubulospecific proteins such as KIM-1 and NGAL, and adverse outcomes in HF patients (7-9). These biomarkers of structural tubular damage are postulated to reflect a decline in renal function or adverse remodeling preceding a reduction in GFR and/or highlight an additional insult such as renal hypoxia. In the human kidney, CNP has been localized to renal tubules (10) and thus its excretion in urine is proposed to be an additional marker of tubular integrity. However, only patients with clinically stable HF have been investigated, thus providing a rationale for the present examination in ADHF.
Herein we observed a greater than two-fold increase in KIM-1 excretion among ADHF patients compared to controls, but in contrast to prior reports (9), urinary NGAL excretion was unchanged and neither KIM-1 nor NGAL were significantly associated with clinical outcomes. However, all three forms of urinary CNP were elevated in ADHF and excretion of NT-CNP53, in particular, outperformed both KIM-1 and NGAL as an indicator of mortality and the combined endpoint of all-cause rehospitalization/death.
Currently the mechanism of NT-CNP53 generation and its relationship to HF prognosis remains unclear. Conceivably, tubular dysfunction sufficient to elevate NT-CNP53 may exceed the capacity of renal homeostatic mechanisms, already maximally employed in ADHF, thereby conferring a poorer prognosis. Since natriuretic peptides are counter-regulatory in HF, CNP-induced vasorelaxation may counter-regulate increases in peripheral vascular resistance. A net local excess of NT-CNP53, relative to the biologically active form CNP22 could portend a maladaptive neurohumoral response, corroborating the lack of prognostic significance observed for urinary CNP22 or plasma CNP forms in this study. Clearly further studies are needed to address these key questions and delineate mechanisms of CNP activation in ADHF.
Urinary C-type natriuretic peptide in a multimarker risk prediction strategy for heart failure
There is increasing interest in the use of multimodal biomarkers for risk prediction in HF. This study suggests that additional utilization of urinary NT-CNP53 may offer a more precise estimation of renal dysfunction in ADHF, including detection of tubular dysfunction and/or renal parenchymal injury or remodeling. Furthermore, urinary NT-CNP53 provided incremental predictive value to established prognostic markers in ADHF: age, renal glomerular function (eGFR, urine protein/creatinine ratio), and plasma NT-proBNP, supporting its use as a complementary index of disease severity within a multimarker approach to ADHF assessment. Specifically the combination of plasma NT-proBNP and urinary NT-CNP53 provided the optimal prognostic information in this ADHF cohort. Though both are biologically inactive, these two differentially localized natriuretic peptides are thought to reflect distinct pathological insults: NT-proBNP resulting from myocardial pressure-volume overload, and NT-CNP53, a marker of renal injury and/or dysfunction. The absence of a statistical correlation between them supports this notion, and highlights their combined value for an integrated cardiorenal risk prediction strategy in ADHF.
Limitations
This was a small study but follow up was 100%. A larger study including stratification by HF etiology and new versus recurrent presentation is now warranted to confirm the current findings. Heterogeneous ADHF management strategies and polypharmacy may have masked associations pertaining to urinary biomarkers. On exploratory analysis, no marked correlations were observed between medications on presentation and urine biomarker levels, but further investigation is needed to accurately define pharmacodynamic effects on CNP excretion. Ascertainment of clinical outcomes was restricted to our institution and local services, as the majority of patients admitted to our HF service exclusively utilize institutional or locally provided clinical care. Inclusion criteria were deliberately broad to reflect the spectrum and high risk of HF patients presenting for hospital admission
Conclusion
In this proof-of-concept study, elevated urinary excretion of NT-CNP53 was significantly associated with adverse outcomes in ADHF patients, independent of GFR and with incremental prognostic value to plasma NT-proBNP. These findings highlight the importance of the cardiorenal interaction in determining clinical outcomes in ADHF and support a potential role for a dual natriuretic peptide multimarker approach which combines plasma NT-proBNP and urinary NT-CNP53 for risk stratification in this population.
Supplementary Material
Acknowledgments
Funding: Financial Support: This work was supported by grants from the National Institutes of Health [RO1 HL36634 and PO1 HL76611], the Mayo Clinic Center for Clinical and Translational Research Grant [#TL1RR024152] and the Mayo Foundation.
None
Abbreviations
- ADHF
Acute decompensated heart failure
- CKD
Chronic kidney disease
- CNP
C-type natriuretic peptide
- GFR
Glomerular filtration rate
- KIM-1
Kidney injury molecule 1
- NGAL
Neutrophil gelatinase-associated lipocalin
- NT-proBNP
N-terminal pro-B type natriuretic peptide
Footnotes
Conflict of Interest: Dr. J.C. Burnett Jr., Dr. S. J. Sangaralingham, D. M. Heublein and Mayo Foundation have filed a patent for the use of urinary C-type natriuretic peptide.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 2.Bettencourt P, Azevedo A, Pimenta J, Frioes F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–74. doi: 10.1161/01.CIR.0000144310.04433.BE. [DOI] [PubMed] [Google Scholar]
- 3.Lee DS, Vasan RS. Novel markers for heart failure diagnosis and prognosis. Current opinion in cardiology. 2005;20:201–10. doi: 10.1097/01.hco.0000161832.04952.6a. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–59. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 5.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–96. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 7.Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96:1297–302. doi: 10.1136/hrt.2010.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jungbauer CG, Birner C, Jung B, et al. Kidney injury molecule-1 and N-acetyl-beta-D-glucosaminidase in chronic heart failure: possible biomarkers of cardiorenal syndrome. European journal of heart failure. 2011;13:1104–10. doi: 10.1093/eurjhf/hfr102. [DOI] [PubMed] [Google Scholar]
- 9.Collins SP, Hart KW, Lindsell CJ, et al. Elevated urinary neutrophil gelatinase-associated lipocalcin after acute heart failure treatment is associated with worsening renal function and adverse events. European journal of heart failure. 2012 doi: 10.1093/eurjhf/hfs087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Totsune K, Takahashi K, Murakami O, et al. Natriuretic peptides in the human kidney. Hypertension. 1994;24:758–62. doi: 10.1161/01.hyp.24.6.758. [DOI] [PubMed] [Google Scholar]
- 11.Sangaralingham SJ, Heublein DM, Grande JP, et al. Urinary C-type natriuretic peptide excretion: a potential novel biomarker for renal fibrosis during aging. American journal of physiology Renal physiology. 2011;301:F943–52. doi: 10.1152/ajprenal.00170.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Wu F, Pan J, Morser J, Wu Q. Furin-mediated processing of Pro-C-type natriuretic peptide. J Biol Chem. 2003;278:25847–52. doi: 10.1074/jbc.M301223200. [DOI] [PubMed] [Google Scholar]
- 13.Kojima M, Minamino N, Kangawa K, Matsuo H. Cloning and sequence analysis of a cDNA encoding a precursor for rat C-type natriuretic peptide (CNP) FEBS Lett. 1990;276:209–13. doi: 10.1016/0014-5793(90)80544-s. [DOI] [PubMed] [Google Scholar]
- 14.Mattingly MT, Brandt RR, Heublein DM, Wei CM, Nir A, Burnett JC., Jr Presence of C-type natriuretic peptide in human kidney and urine. Kidney Int. 1994;46:744–7. doi: 10.1038/ki.1994.329. [DOI] [PubMed] [Google Scholar]
- 15.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC., Jr Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. The American journal of physiology. 1992;263:H1318–21. doi: 10.1152/ajpheart.1992.263.4.H1318. [DOI] [PubMed] [Google Scholar]
- 18.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. Journal of the American College of Cardiology. 2006;47:345–53. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 20.Cataliotti A, Giordano M, De Pascale E, et al. CNP production in the kidney and effects of protein intake restriction in nephrotic syndrome. Am J Physiol Renal Physiol. 2002;283:F464–72. doi: 10.1152/ajprenal.00372.2001. [DOI] [PubMed] [Google Scholar]
- 21.Totsune K, Takahashi K, Ohneda M, Itoi K, Murakami O, Mouri T. C-type natriuretic peptide in the human central nervous system: distribution and molecular form. Peptides. 1994;15:37–40. doi: 10.1016/0196-9781(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara H, Sakaguchi H, Itakura M, et al. Autocrine regulation of rat chondrocyte proliferation by natriuretic peptide C and its receptor, natriuretic peptide receptor-B. J Biol Chem. 1994;269:10729–33. [PubMed] [Google Scholar]
- 23.Kalra PR, Clague JR, Bolger AP, et al. Myocardial production of C-type natriuretic peptide in chronic heart failure. Circulation. 2003;107:571–3. doi: 10.1161/01.cir.0000047280.15244.eb. [DOI] [PubMed] [Google Scholar]
- 24.Stingo AJ, Clavell AL, Aarhus LL, Burnett JC., Jr Cardiovascular and renal actions of C-type natriuretic peptide. The American journal of physiology. 1992;262:H308–12. doi: 10.1152/ajpheart.1992.262.1.H308. [DOI] [PubMed] [Google Scholar]
- 25.Del Ry S, Passino C, Maltinti M, Emdin M, Giannessi D. C-type natriuretic peptide plasma levels increase in patients with chronic heart failure as a function of clinical severity. Eur J Heart Fail. 2005;7:1145–8. doi: 10.1016/j.ejheart.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Ng LL, Geeranavar S, Jennings SC, Loke I, O'Brien RJ. Diagnosis of heart failure using urinary natriuretic peptides. Clin Sci (Lond) 2004;106:129–33. doi: 10.1042/CS20030234. [DOI] [PubMed] [Google Scholar]
- 27.Bentzen H, Pedersen RS, Nyvad O, Pedersen EB. Effect of exercise on natriuretic peptides in plasma and urine in chronic heart failure. Int J Cardiol. 2004;93:121–30. doi: 10.1016/S0167-5273(03)00156-6. [DOI] [PubMed] [Google Scholar]
- 28.Kalra PR, Clague JR, Coats AJ, Anker SD, Poole-Wilson PA, Struthers AD. C-type natriuretic peptide production by the human kidney is blunted in chronic heart failure. Clin Sci (Lond) 2010;118:71–7. doi: 10.1042/CS20090092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.