Abstract
β-Amyloid (Aβ), a small, fibrillogenic peptide, is known to play an important role in the pathogenesis of Alzheimer’s disease (AD) in the brain. In addition, Aβ accumulates in skeletal muscle cells in individuals with sporadic inclusion body myositis (sIBM), an age-related muscle disease. Because of the socioeconomic burden associated with age-related diseases, particularly AD, there has been considerable emphasis on studying potential therapeutic strategies. The high fat, low carbohydrate ketogenic diet has been used extensively to treat refractory childhood epilepsy and has been studied as a potential treatment for other neurological diseases, including Parkinson’s disease and AD. In this study, we fed young APP/PS1 knock-in mice, which have a whole body knock-in of AD-related genes, a ketogenic diet and determined the effect on Aβ levels in the brain and skeletal muscle, as well motor performance and oxidative stress. Aβ and its precursor, the β-C-terminal fragment of amyloid precursor protein (CTFβ), were unchanged overall in both the brain and quadriceps after 1 month on the ketogenic diet, and there was no effect on nitrotyrosine, a product of oxidative stress. The ketogenic diet improved performance on the Rota-rod apparatus (p=0.007), however. These data indicate that the ketogenic diet may have some efficacy in the treatment of both neurologic and muscle diseases though the underlying mechanisms do not involve amelioration of Aβ pathology.
Keywords: ketogenic diet, Alzheimer’s disease, amyloid, inclusion body myositis
1. Introduction
Alzheimer’s disease (AD), the most common age-related neurodegenerative disease in the developed world, is precipitated initially by the accumulation of β-amyloid (Aβ), a small, fibrillogenic peptide generated from the larger amyloid precursor protein (APP) through the enzymatic activities of two proteases: the β- and γ-secretases. Mutations in both APP and presenilin (PS1 and PS2), the catalytic subunit of γ-secretase, lead to early-onset, familial AD. Aβ oligomerizes, ultimately forming extracellular amyloid plaques, one of the key features of AD pathology. In addition, Aβ oligomers have been shown to trigger the onset of tau pathology, decrease long term potentiation, and induce neurodegeneration (Gotz et al., 2001; Hung et al., 2008; Jo et al., 2011; Lewis et al., 2001; Walsh et al., 2002). Because of its primary role in AD pathogenesis, there has been considerable focus on AD therapeutics targeting Aβ.
Aβ may play a role in other diseases as well. For instance, sporadic inclusion body myositis (sIBM), an age-related degenerative muscle disease, has been associated with intracellular Aβ accumulation (Askanas et al., 1992; Askanas and Engel, 2001). Though a causal role for Aβ in the disease process has yet to be defined, APP is commonly overexpressed in the muscle of sIBM patients (Li et al., 2006; Sarkozi et al., 1993). APP overexpression induces pathological changes in cultured muscle cells, including overexpression of many proteins found in muscle inclusion bodies (Askanas et al., 1996; Wojcik et al., 2006). In addition, overexpression of full-length APP, or its C-terminal fragment (CTFβ), in mouse muscle leads to Aβ deposition, vacuolar changes, lymphocyte infiltration, and, in some cases, muscle weakening (Fukuchi et al., 1998; Jin et al., 1998).
The ketogenic diet has been used for nearly a century to treat refractory childhood epilepsy. This high fat, low carbohydrate diet typically has a 4:1 ratio of fat to combined carbohydrates and protein, allowing for overall calorie maintenance, while supporting the production of ketone bodies, such as β-hydroxybutyrate, acetoacetate, and acetone, by the liver. The ketogenic diet has also been studied as a potential therapy for other neurologic diseases, such as Parkinson’s and Alzheimer’s disease (Henderson, 2008; Kashiwaya et al., 2000; Studzinski et al., 2008; Van der Auwera et al., 2005; Veech, 2004). Indeed, it has been shown to be effective in neuroprotection and Aβ reduction in certain AD animal models. There is some evidence that the ketogenic diet provides its benefits via improvement of mitochondrial function (Studzinski et al., 2008; Veech, 2004). Like the brain, skeletal muscle can use ketone bodies as an energy source and may also benefit from a ketogenic diet, though there has been considerably less focus in this area.
The APP/PS1 knock-in model of AD contains whole-body knock-ins of ‘humanized” APP and PS1 genes containing familial AD mutations. The genes are under endogenous murine promoters, thus eliminating changes due to overexpression of these proteins. These mice have been used extensively to study the pathogenesis of AD in the absence of exogenous overexpression. There are many parallels to human AD in these mice, including the emergence of diffuse and neuritic plaques by 9 months (Murphy et al., 2007). Soluble Aβ is detectable by 3 months and is significantly elevated over wild-type controls (Anantharaman et al., 2006; Chang et al., 2006; Murphy et al., 2007; Siman et al., 2000). Oxidative stress is also elevated in the brains of these mice (Anantharaman et al., 2006; Mohmmad Abdul et al., 2006). In addition, we have previously shown that a short-term (1 month) high-fat, high-calorie Western diet is sufficient to induce an increase in oxidative stress in young APP/PS1 knock-in mice (Studzinski et al., 2009).
In this study, we examined the effect of a high-fat, low-carbohydrate ketogenic diet on APP/PS1 knock-in mice, as well as their wild-type and single knock-in littermates. Since both tissues are associated with Aβ-mediated pathology and these mice have a systemic knock-in of the mutated genes, we sought to determine the effect of the ketogenic diet on both brain and skeletal muscle. To this end, we examined the effect on motor performance, oxidative stress, and Aβ accumulation.
2. Results
Young (1–2 months) APP/PS1 knock-in mice containing both humanized APPΔNL (APPSwe) and PS1P264L mutations, as well as their wild-type and single knock-in (APPΔNL or PS1P264L) littermates were fed a high fat, low carbohydrate “ketogenic” or control diet for 1 month ad libitum. At the endpoint of the study (2–3 months old), mice on the ketogenic diet weighed significantly less than mice fed the control diet (Table 1: p=0.005). Neither the APP nor PS1 mutations had a significant effect on weight (p≥0.15). As expected, blood ketones were elevated in mice fed the ketogenic diet (p≤0.0001), regardless of APP or PS1 genotype (p≥0.5). Blood glucose was unchanged by diet overall (p=0.39). However, mice with the APPΔNL mutation exhibited elevated blood glucose (p=0.04), compared to APPWT mice, regardless of diet. There was a substantial decrease in plasma insulin (>65%) in mice fed the ketogenic diet (p≤0.0001) with no effect of genotype (p≥0.26).
Table 1.
| Genotype
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild-type | APP | PS1 | APP/PS1 | ||||||
|
| |||||||||
| control | KD | control | KD | control | KD | control | KD | ||
| Weight (g) | Baseline | 25±3 | 24±3 | 23±1 | 23±1 | 27±2 | 25±2 | 25±1 | 25±1 |
| 1 montha | 28±2 | 25±2 | 28±1 | 25±1 | 31±2 | 25±2 | 29±1 | 27±1 | |
| Ketones (mM) | Baseline | 0.37±0.07 | 0.3±0.06 | 0.36±0.02 | 0.48±0.09 | 0.46±0.07 | 0.44±0.10 | 0.37±0.03 | 0.42±0.04 |
| 1 montha | 0.37±0.22 | 1.0±0.22 | 0.36±0.10 | 1.09±0.10 | 0.34±0.17 | 0.98±0.17 | 0.36±0.12 | 1.18±0.12 | |
| Glucose (mg/dL) | Baseline | 143±28 | 133±4 | 140±7 | 151±9 | 126±15 | 142±22 | 140±10 | 125±9 |
| 1 month | 89±13 | 106±13 | 104±6 | 111±6 | 98±10 | 91±10 | 109±7 | 115±7 | |
| Insulin (ng/mL) | a | 1.74±0.37 | 0.42±0.37 | 1.82±0.17 | 0.52±0.17 | 1.88±0.29 | 0.64±0.29 | 2.13±0.20 | 0.71±0.20 |
significant diet effect (p ≤ 0.05)
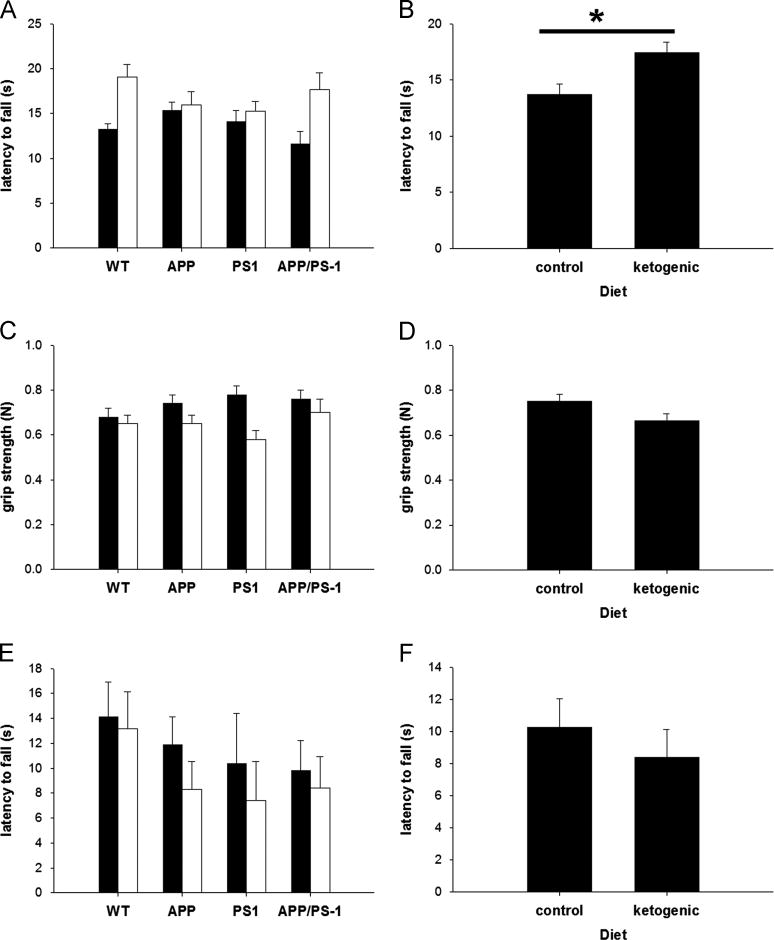
In order to assess the impact of the ketogenic diet on sensimotor function, we performed three different tests for motor performance (Figure 1). Mice fed the ketogenic diet showed improvement in their latency to fall on the Rota-rod apparatus (p=0.007), indicating a benefit of this diet. There was not an improvement in all tests, however. The ketogenic diet led to a reduction in grip strength that approached significance (p=0.057), while there was no effect on latency to fall in the wire suspension test (p=0.45). There was no effect of genotype on any of the motor performance tests used in this study (p≥0.18) and no interaction between diet and genotype for any of the tests (p≥0.31). These data suggest that the ketogenic diet improves only certain aspects of motor performance.
Figure 1. The Ketogenic Diet Improved Rota-rod Performance.
Young (1–2 months old) mice fed the control (filled bars; N=32) or ketogenic (open bars; N=33) diet for 1 month prior to euthanasia were tested for motor performance at the endpoint of the study (2–3 months old). The cohort was a mix of genotypes: wild-type (N=6), APPΔNLh (N=29), PS1P264L (N=10), and APPΔNLh/ PS1P264L (N=20). Latency to fall on the Rota-rod apparatus (A, B) was significantly improved (p=0.007) in animals fed the ketogenic diet. Grip strength (C, D) was marginally adversely affected (p=0.057) by the ketogenic diet. Wire suspension times (E, F) were unaffected by diet (p=0.445). Genotype did not have an effect on motor performance (p>0.18), nor was there an interaction between diet and genotype (p>0.3). For each test, the first panel (A, C, E) shows the breakdown by genotype, and the second panel shows the overall effect of diet (B, D, F).
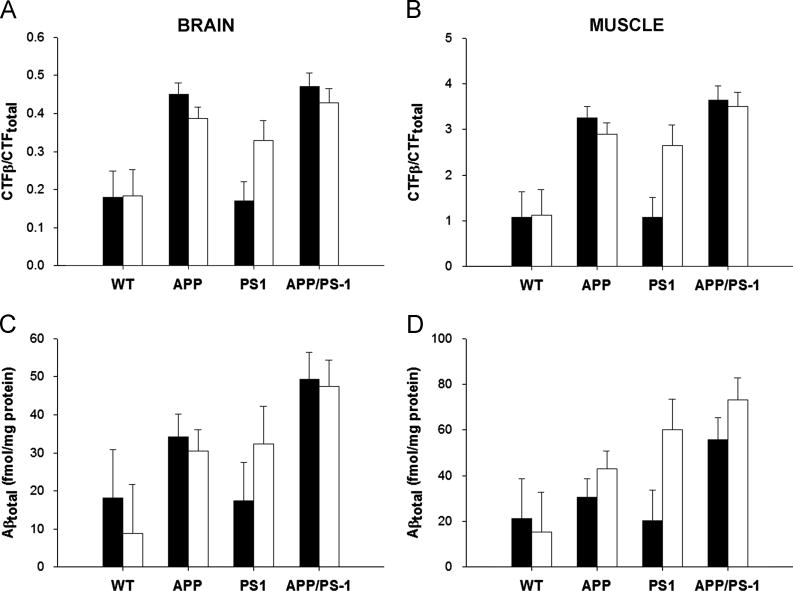
The mouse model used in these studies contains a whole body knock-in of humanized APP and/or PS1, raising the possibility of Aβ accumulation in tissues other than the brain. sIBM, an age-related muscle disease, has also been associated with Aβ accumulation (Askanas et al., 1992; Askanas and Engel, 2001). Therefore, we performed Aβ analyses in both brain and skeletal muscle of the APP/PS1 mice. We measured the levels of AD-related Aβ and its precursor (the C-terminal fragment of amyloid precursor protein (APP); CTFβ) in the brain and quadriceps of mice fed the ketogenic or control diet (Figure 2). As expected, animals containing the APPΔNL mutation had more CTFβ and Aβ in both the brain and skeletal muscle (p≤0.02). The ketogenic diet did not have an overall effect on total Aβ in the brain (p=0.99) of these mice, and Aβ marginally, though insignificantly increased in the muscle (p=0.08). Similarly, there was no overall diet effect on CTFβ in either the brain or muscle (p≥0.33). Interestingly, the ketogenic diet had a modest protective effect on CTFβ in mice containing the APPΔNL mutation (p=0.05 in brain; p=0.07 in muscle), indicating an interaction between diet and the APP genotype. There was no apparent interaction between diet and genotype in the accumulation of Aβ (p≥0.67).
Figure 2. The Ketogenic Diet Did Not Reduce Aβ Levels in Brain or Skeletal Muscle.
The C-terminal fragment of APP (CTFβ; A–B) and Aβ (C–D) levels were measured by ELISA in brain (A, C) and skeletal muscle (B, D) of mice fed the control (filled bars) or ketogenic (open bars) diet. As expected, the presence of the APPΔNLh mutation significantly elevated both CTFs and Aβ over APP wild-type controls (p≤0.02). The ketogenic diet did not affect CTFβ or Aβ in either the brain or muscle when analyzed across all genotypes (p≥0.08). However, there was a modest decrease in CTFβ in both the muscle (p=0.07) and brain (p=0.05) of mice containing the APPΔNL mutation, indicating an interaction between diet and the APP genotype. There was no such interaction for Aβ (p≥0.67).
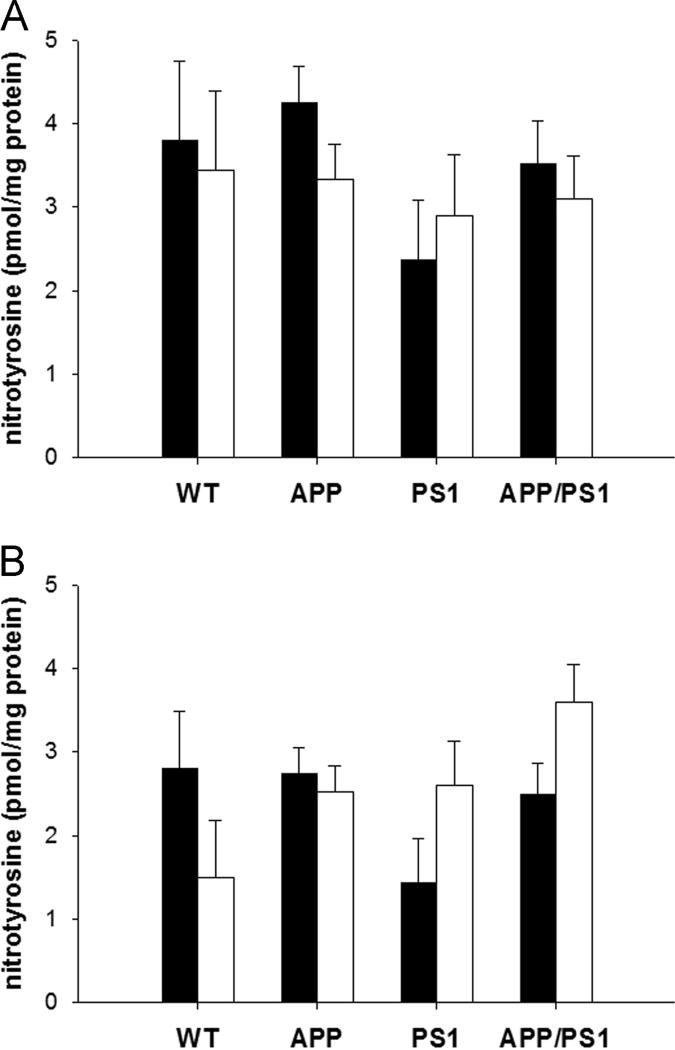
Finally, we determined the effect of diet on oxidative stress by measuring the amount of nitrotyrosine in the brain and muscle of these animals (Figure 3). There was no effect of diet or genotype on nitrotyrosine in the brain (p≥0.14). Mice containing the APPΔNL mutation had significantly more nitrotyrosine in skeletal muscle than their wild-type counterparts (p=0.04), but diet had no effect overall (p=0.6). Interestingly, mice containing the PS1P264L mutation had significantly elevated muscle nitrotyrosine when fed the ketogenic diet (p=0.01), indicating a tissue-specific interaction between diet and the PS1 genotype.
Figure 3. The Ketogenic Diet Did Not Reduce Oxidative Stress.
Nitrotyrosine levels, a measure of oxidative stress, were assayed in brain (A) and skeletal muscle (B) of mice fed the control (filled bars) or ketogenic (open bars) diet for 1 month. Mice containing the APPΔNL mutation had significantly more nitrotyrosine in the skeletal muscle (p=0.04) than their wild-type counterparts, while nitrotyrosine levels in the brain were unaffected by genotype (p≥0.14). There was no overall effect of diet on nitrotyrosine levels in either tissue type (p≥0.56), though there was a significant increase in the muscle of mice containing the PS1P264L mutation (p=0.01), indicating a possible interaction between diet and the PS1 genotype.
3. Discussion
In an effort to determine whether diet induces tissue-specific alterations, we fed young APP/PS1 knock-in mice a high-fat, low-carbohydrate ketogenic diet for 1 month and examined the changes in motor performance, as well as Aβ and nitrotyrosine levels in both brain and skeletal muscle. We showed that mice fed a ketogenic diet exhibited improved performance (via increased latency to fall) on the Rota-rod apparatus (Figure 1), a measure of balance and coordination. This may reflect changes in muscle metabolism in mice fed the ketogenic diet. There was not a universal improvement in motor performance, however: There was a reduction in grip strength and wire suspension performance was unchanged in mice fed the ketogenic diet. The reason for this disparity is unclear, but may reflect the muscle groups affected by the diet (i.e. quadriceps vs. paw flexors).
There was no overall change in Aβ or its precursor (CTFβ) in either the brain or the skeletal muscle (quadriceps) (Figure 2) due to the ketogenic diet. However, in the APPΔNL-containing mice, there was a modest (~10%) diet-induced decrease in the APP CTFβ in both brain and muscle, though no change in Aβ was observed (Figure 2). These data indicate that γ-secretase activity was unchanged in mice fed the ketogenic diet, and that longer treatment could have a greater effect. These results contrast with a previous study in a young (3 months) transgenic AD mouse model overexpressing the London APP mutation (V717I) (Van der Auwera et al., 2005) which demonstrated a small decrease in Aβ in the brain in mice fed the ketogenic diet for 1.5 months. On the other hand, a separate report on the effect of ketosis in aged canines showed that the effect on Aβ was limited to the parietal lobe of the brain- there was no change overall (Studzinski et al., 2008). It is possible that there may have been localized changes in Aβ in our mice that we did not observe. Alternatively, the model systems used may have had a significant impact on the results. For instance, the APP/PS1 knock-in mouse model used in this study does not rely on overexpression of the mutated proteins to achieve Aβ accumulation and pathology, but relies on the nature of the mutations to increase Aβ production. In addition, the APP London mutation has been associated with cerebral amyloid angiopathy in older mice (Van Dorpe et al., 2000), a factor that may also play a role in the efficacy of the ketogenic diet in this model.
The ketogenic diet mimics starvation conditions in which fat stores are mobilized and is thought to work through the improvement of mitochondrial function (Henderson, 2008; Studzinski et al., 2008; Veech, 2004). A secondary effect is likely a decrease in insulin secretion and improvement in insulin resistance due to the reduction in carbohydrate intake. Because of this, the ketogenic diet may also have utility in the treatment of diabetes and its complications. AD has long been associated with metabolic dysregulation and insulin resistance (Akter et al., 2011; Craft and Watson, 2004; Craft, 2007), a fact that may explain why this diet has proved useful in AD models. Young (1–2 months old) mice were used in this study. Though these mice have measurable Aβ at this age, it is unlikely that there is widespread metabolic dysfunction-which may explain the limited effects observed. It is possible that the ketogenic diet would prove more beneficial in older mice already experiencing more substantial pathology. In support of this hypothesis, a recent paper showed that long-term (8 months) feeding of a ketone ester in middle-aged (8.5 months old) improved cognition, as well as Aβ and tau pathology in the 3xTgAD mouse model (Kashiwaya et al., 2012).
In conclusion, AD mice fed the high-fat, low-carbohydrate ketogenic diet weighed less than their counterparts on the control diet, and had substantially reduced plasma insulin (>65%). In addition, these mice displayed improved Rota-rod performance, a measure of motor function, and mice with the APPΔNL mutation showed significantly reduced APP CTFβ in both brain and muscle. On the other hand, there was no overall change in Aβ or nitrotyrosine in either the brain or muscle. Based on this data, it appears that the ketogenic diet may have some limited benefit for both AD and sIBM.
4. Experimental Procedure
4.1. Animals
Mice harboring a whole body knock-in of both APPΔNL (APPswe) and PS1P264L under their endogenous promoters were obtained from a breeding colony at the University of Kentucky. These mice are a cross between two knock-in models previously generated using Cre-lox technology (Reaume et al., 1996; Siman et al., 2000) maintained on a CD-1/129 hybrid background. Heterozygous matings yielded the four different genotypes used in this study: WT/WT (N=6), APPΔNL/WT (APP; N=29), WT/PS1P264L (PS1; N=10)), and APPΔNL/PS1P264L (APP/PS1; N=20), split approximately equally between gender and diet. All mice harboring humanized, mutated APP and/or PS1 were heterozygous for that gene. 1–2 month old mice were housed under a 12 hour light-dark cycle and fed either a ketogenic (79% fat, 8% protein, 1% carbohydrate; BioServ) or control (5% fat, 20% protein, 62% carbohydrate; BioServ) diet ad libitum for 1 month prior to euthanasia. Mice were weighed daily for one week and weekly thereafter. Mice were euthanized by CO2 asphyxiation, followed by decapitation. All animal work was conducted with prior IACUC approval, and was performed in accordance with USDA and PHS guidelines.
4.2. Blood and Plasma Analyses
Blood glucose and ketones were measured at the start and end of the study in non-fasted animals, using the Precision Xtra Advanced Diabetes Management System (Abbott Labs; Abbott Park, IL). Mouse tail veins were lanced after physical restraint, and the blood was spotted on specialized testing strips for each molecule (Abbott Labs). After euthanasia and decapitation, blood was collected in EDTA, centrifuged (1500 × g, 10 min.), and the plasma collected. Plasma insulin was measured by commercially-available, species-specific ELISAs (Linco/Millipore; Billerica, MA), according to package instructions.
4.3. Motor Performance
At the endpoint of this study, motor performance was measured by three different tests. First, coordination and balance were evaluated with a Rota-rod apparatus (Columbus Instruments; Columbus, OH). Mice were placed on a rotating spindle, which accelerated from 0 to 30 rpm over 30 seconds. The latency to fall was recorded by an infrared sensor, with a maximum retention time of 120 seconds. Next, we tested the mice on a wire suspension apparatus- a plastic-coated wire suspended ~45 cm about the bench surface. The mice were allowed to grasp the wire with their forepaws and the latency to fall was recorded. Finally, grip strength was measured using a digital grip strength meter (Columbus Instruments). Mice were allowed to grasp the sensor with their forepaws, then manually pulled back and the force on the sensor recorded. For each test, data was recorded over 5 trials, and the median score used for further analyses.
4.4. Immunoassays
Frozen brain and quadriceps tissue was homogenized in 2% SDS with Complete Protease Inhibitor Cocktail (Amresco; Solon, OH) using an AHS200 PowerMax homogenizer. Insoluble material was then removed by centrifugation (20,800 × g, 30 min., 14°C) and the supernatants frozen until use. Total Aβ was measured by sandwich ELISA as previously described (Murphy et al., 2007). Briefly, SDS extracts were diluted in AC buffer (0.2M sodium phosphate (pH7), 0.4M NaCl, 2 mM EDTA, 0.4% Block Ace (Serotec; Raleigh, NC), 0.4% BSA, 0.05% CHAPS, 0.05% NaN3) for analysis. A standard curve was prepared from recombinant human Aβ1–42 diluted in AC buffer. Standards and samples were measured at least in duplicate. 384-well plates (Immulon 4HBX; Thermo Scientific, Waltham, MA) were coated with 0.5 μg Ab9/well (against human Aβ1–16) and blocked with Synblock (Serotec) for 2 hours. After incubation with the samples and standards, Aβ was detected with biotinylated-4G8 (against Aβ 17–24; Covance, Princeton, NJ), followed by incubation with 0.1 μg/mL neutravidin-HRP (Pierce Technologies; Rockford, IL). The plate was developed with 3′,3′,5′,5′-tetramethylbenzidine (TMB; Kirkeguard and Perry Laboratories; Gaithersburg, MD) and the reaction stopped with 6% o-phosphoric acid. The absorbance at 450 nm was measured with a BioTek multiwell plate reader.
APP C-terminal fragments (CTF; total and CTFβ) were measured by sandwich ELISA as previously described (Holler et al., 2012). Briefly, in order to clear full-length APP, diluted SDS extracts were added to a 96-well plate that had been pre-coated with 1 μg/well antibody 22C11 (against the APP N-terminus; Millipore) and subsequently blocked with Synblock (Serotec). The cleared samples were then transferred to another plate coated with affinity-purified antibody CT20 (raised against the C-terminal 20 amino acids of APP). CTFβ was detected with biotinylated-6E10 (Covance; Emeryville, CA), while total CTFs were detected with biotinylated-4G8 (against Aβ 1–16; Covance). In both cases, the detection antibody was followed by incubation with neutravidin-HRP (Pierce) and development with TMB.
Nitrotyrosine was measured by ELISA as well. Samples were diluted in PBS and incubated overnight in a 384-well microplate (Immulon 4HBX). After blocking with Synblock (Serotec; 2h at room temperature), a biotinylated anti-nitrotyrosine antibody was added (0.2 μg/mL; Cayman Chemical; Ann Arbor, MI) and the plate was incubated overnight. Neutravidin-HRP was added (2 h at room temperature), followed by development with TMB. Samples were compared against a standard curve of nitrotyrosine-BSA (Cayman Chemical).
4.5. Statistics
Data were analyzed using SPSS® for Windows (Hewlett Packard; Houston, TX) using the general linear module (GLM) with the independent variables gender, genotype, and dietary treatment. We also determined whether there were any interactions between diet and genotype. Post-hoc multiple comparisons were performed using Tukey’s test, Dunnett’s test, or similar. We performed correlation analyses using either Pearson’s r or Spearman’s p (for parametric and nonparametric values, respectively.
The ketogenic diet improved Rota-rod performance in young APP/PS1 knock-in mice.
The ketogenic diet did not affect Aβ levels in either the skeletal muscle or brain.
The ketogenic diet did not affect nitrotyrosine levels in skeletal muscle or brain.
Acknowledgments
We would like to thank Dr. Chris Holler and Robin Webb for tissue collection and Dr. Todd Golde for providing Aβ antibodies. Supported by NIH (AG005119, NS058382), The Coins for Alzheimer’s Research Trust and the Alzheimer’s Association (IIRG-10-172905).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? British journal of clinical pharmacology. 2011;71:365–76. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman M, Tangpong J, Keller JN, Murphy MP, Markesbery WR, Kiningham KK, St Clair DK. Beta amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock in mouse model of Alzheimer’s disease. The American journal of pathology. 2006;168:1608–18. doi: 10.2353/ajpath.2006.051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askanas V, Engel WK, Alvarez RB. Light and electron microscopic localization of beta-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol. 1992;141:31–6. [PMC free article] [PubMed] [Google Scholar]
- Askanas V, McFerrin J, Baque S, Alvarez RB, Sarkozi E, Engel WK. Transfer of beta-amyloid precursor protein gene using adenovirus vector causes mitochondrial abnormalities in cultured normal human muscle. Proc Natl Acad Sci U S A. 1996;93:1314–9. doi: 10.1073/pnas.93.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askanas V, Engel WK. Inclusion-body myositis: newest concepts of pathogenesis and relation to aging and Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:1–14. doi: 10.1093/jnen/60.1.1. [DOI] [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. AMPA receptor downscaling at the onset of Alzheimer’s disease pathology in double knockin mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3410–5. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–78. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–52. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- Fukuchi K, Pham D, Hart M, Li L, Lindsey JR. Amyloid-beta deposition in skeletal muscle of transgenic mice: possible model of inclusion body myopathy. Am J Pathol. 1998;153:1687–93. doi: 10.1016/s0002-9440(10)65682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–5. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Henderson ST. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics. 2008;5:470–80. doi: 10.1016/j.nurt.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler CJ, Webb RL, Laux AL, Beckett TL, Niedowicz DM, Ahmed RR, Liu Y, Simmons CR, Dowling AL, Spinelli A, Khurgel M, Estus S, Head E, Hersh LB, Murphy MP. BACE2 expression increases in human neurodegenerative disease. The American journal of pathology. 2012;180:337–50. doi: 10.1016/j.ajpath.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LW, Ciccotosto GD, Giannakis E, Tew DJ, Perez K, Masters CL, Cappai R, Wade JD, Barnham KJ. Amyloid-beta peptide (Abeta) neurotoxicity is modulated by the rate of peptide aggregation: Abeta dimers and trimers correlate with neurotoxicity. J Neurosci. 2008;28:11950–8. doi: 10.1523/JNEUROSCI.3916-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LW, Hearn MG, Ogburn CE, Dang N, Nochlin D, Ladiges WC, Martin GM. Transgenic mice over-expressing the C-99 fragment of betaPP with an alpha-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am J Pathol. 1998;153:1679–86. doi: 10.1016/s0002-9440(10)65681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, Cho K. Abeta(1–42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nature neuroscience. 2011;14:545–7. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci U S A. 2000;97:5440–4. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, Todd King M, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiology of aging. 2012 doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–91. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Li J, Yin C, Okamoto H, Jaffe H, Oldfield EH, Zhuang Z, Vortmeyer AO, Rushing EJ. Proteomic analysis of inclusion body myositis. J Neuropathol Exp Neurol. 2006;65:826–33. doi: 10.1097/01.jnen.0000228204.19915.69. [DOI] [PubMed] [Google Scholar]
- Mohmmad Abdul H, Sultana R, Keller JN, St Clair DK, Markesbery WR, Butterfield DA. Mutations in amyloid precursor protein and presenilin-1 genes increase the basal oxidative stress in murine neuronal cells and lead to increased sensitivity to oxidative stress mediated by amyloid beta-peptide (1–42), HO and kainic acid: implications for Alzheimer’s disease. Journal of neurochemistry. 2006;96:1322–35. doi: 10.1111/j.1471-4159.2005.03647.x. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol Dis. 2007;27:301–11. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Howland DS, Trusko SP, Savage MJ, Lang DM, Greenberg BD, Siman R, Scott RW. Enhanced amyloidogenic processing of the beta-amyloid precursor protein in gene-targeted mice bearing the Swedish familial Alzheimer’s disease mutations and a “humanized” Abeta sequence. J Biol Chem. 1996;271:23380–8. doi: 10.1074/jbc.271.38.23380. [DOI] [PubMed] [Google Scholar]
- Sarkozi E, Askanas V, Johnson SA, Engel WK, Alvarez RB. beta-Amyloid precursor protein mRNA is increased in inclusion-body myositis muscle. Neuroreport. 1993;4:815–8. doi: 10.1097/00001756-199306000-00055. [DOI] [PubMed] [Google Scholar]
- Siman R, Reaume AG, Savage MJ, Trusko S, Lin YG, Scott RW, Flood DG. Presenilin-1 P264L knock-in mutation: differential effects on abeta production, amyloid deposition, and neuronal vulnerability. J Neurosci. 2000;20:8717–26. doi: 10.1523/JNEUROSCI.20-23-08717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski CM, MacKay WA, Beckett TL, Henderson ST, Murphy MP, Sullivan PG, Burnham WM. Induction of ketosis may improve mitochondrial function and decrease steady-state amyloid-beta precursor protein (APP) levels in the aged dog. Brain Res. 2008;1226:209–17. doi: 10.1016/j.brainres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Studzinski CM, Li F, Bruce-Keller AJ, Fernandez-Kim SO, Zhang L, Weidner AM, Markesbery WR, Murphy MP, Keller JN. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP x PS1 knock in mice. J Neurochem. 2009;108:860–6. doi: 10.1111/j.1471-4159.2008.05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera I, Wera S, Van Leuven F, Henderson ST. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr Metab (Lond) 2005;2:28. doi: 10.1186/1743-7075-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dorpe J, Smeijers L, Dewachter I, Nuyens D, Spittaels K, Van Den Haute C, Mercken M, Moechars D, Laenen I, Kuiperi C, Bruynseels K, Tesseur I, Loos R, Vanderstichele H, Checler F, Sciot R, Van Leuven F. Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the london mutant of human APP in neurons. The American journal of pathology. 2000;157:1283–98. doi: 10.1016/S0002-9440(10)64644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–19. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wojcik S, Engel WK, McFerrin J, Paciello O, Askanas V. AbetaPP-overexpression and proteasome inhibition increase alphaB-crystallin in cultured human muscle: relevance to inclusion-body myositis. Neuromuscul Disord. 2006;16:839–44. doi: 10.1016/j.nmd.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]