Abstract
There are many age-associated changes in the respiratory and pulmonary immune system. These changes include decreases in the volume of the thoracic cavity, reduced lung volumes, and alterations in the muscles that aid respiration. Muscle function on a cellular level in the aging population is less efficient. The elderly population has less pulmonary reserve, and cough strength is decreased in the elderly population due to anatomic changes and muscle atrophy. Clearance of particles from the lung through the mucociliary elevator is decreased and associated with ciliary dysfunction. Many complex changes in immunity with aging contribute to increased susceptibility to infections including a less robust immune response from both the innate and adaptive immune systems. Considering all of these age-related changes to the lungs, pulmonary disease has significant consequences for the aging population. Chronic lower respiratory tract disease is the third leading cause of death in people aged 65 years and older. With a large and growing aging population, it is critical to understand how the body changes with age and how this impacts the entire respiratory system. Understanding the aging process in the lung is necessary in order to provide optimal care to our aging population. This review focuses on the nonpathologic aging process in the lung, including structural changes, changes in muscle function, and pulmonary immunologic function, with special consideration of obstructive lung disease in the elderly.
Keywords: aging, lung, pulmonary immunology, COPD
Introduction
Pulmonary disease has significant consequences for the aging population. Chronic lower respiratory tract disease, defined as asthma, emphysema, chronic bronchitis, bronchiectasis, and chronic obstructive pulmonary disease (COPD), is the third leading cause of death in people aged 65 years and older.1 According to 2010 census data, 13% of the US population, or 40.3 million people, are older than age 65, which is higher than any previous census. Additionally, the population is aging at an increasingly faster rate each year. Between 2000 and 2010, the population age 65 years and over increased by 15.1% compared to the total US population which only increased by 9.7%.2 With such a large and rapidly growing aging population it is critical to understand how the body changes with age and how this impacts the entire respiratory system. Understanding the aging process in the lungs is necessary in order to provide optimal care to our aging population.
Structural and functional changes with age
The structure of the thoracic cavity, which houses and protects the lungs, is vital for optimal lung function. Changes to the spine, muscles, and ribs over time with aging impact normal lung function. As people normally age, narrowing of the intervertebral disk spaces causes kyphosis or curvature of the spine.3 This curvature decreases the space between the ribs and creates a smaller chest cavity.4 While a small amount of anterior curvature or kyphosis of the thoracic spine is normal, an angle greater than 40°, which is the 95th percentile of normal, is defined as hyperkyphosis.5,6 After age 40, the kyphosis angle begins to increase more rapidly in women than men, from a mean of 43° in women aged 55–60 years, to a mean of 52° in women 76–80 years of age.5 The prevalence and incidence of hyperkyphosis is reported in older adults varying from approximately 20%–40% among both men and women.5,6 In a study of 55 nonsmoking women with variable degrees of thoracic kyphosis Lombardi et al7 found that with increasing vertebral angle there was a significant decline in the fraction of exhaled volume in 1 second (FEV1) and vital capacity (VC) during spirometry testing.7 This effect was most significant once the kyphotic angle was over 55°. Culham et al hypothesized that this effect is not from a decrease in thoracic cavity size alone, but due to the rib space narrowing, which decreases the length of the intercostal muscles.8 The angle of the muscle fibers in relation to the ribs may also affect the efficiency and decrease the movement of the lower ribs during inspiration.8 These changes are structural and based on the origin and insertion of the muscles.
In addition to structural changes, there are changes in intrinsic function of the muscles with age. Overall muscle function in the body decreases by 2% annually as we age.9,10 Aging is associated with reduced inspiratory and expiratory respiratory muscle strength.11 Respiratory muscle decline can lead to an inability to ventilate in the face of increasing demands, such as that seen in respiratory disease. There is also evidence that at the cellular level, the muscles of elderly individuals have less mitochondrial adenosine triphosphate reserves to sustain a sudden increase in metabolic demand.12 If an elderly person becomes ill with pneumonia, and therefore has increased metabolic demands for oxygen in the setting of decreased respiratory muscle strength, decreased cellular energy reserve, and decreased overall muscle function, he or she may not be able to meet those demands. This leads to an increased risk of respiratory failure in older individuals.13
With aging there is a decreased ability to clear mucus from the lungs. Two mechanisms primarily contribute to this decline: 1) reduced cough strength and 2) alterations in the body’s ability to clear particles in the airways. First, cough plays a vital role in clearing mucus from the airways. Coughing is a maneuver that requires generation of a high forced expiratory flow. During a cough maneuver, inspiratory muscles contract to allow the lungs to take in a large tidal volume necessary to augment a sustained high expiratory flow.14 Next, the expiratory muscles contract to build high positive intrapleural and intraairway pressures for the development of peak expiratory flow rates.15 Finally, when the glottis is opened, the cough occurs, and the mucus is expelled from the airway into the mouth. Any decrease in the strength of the respiratory muscles will greatly impact an individual’s ability to generate the force required for an effective cough.16 Aging is associated with both inspiratory and expiratory respiratory muscle strength reduction.11 Polkey et al showed a 13% decrease in transdiaphragmatic pressure gradients, a surrogate for diaphragm strength, in older subjects (ages 67–81) as compared to younger subjects (ages 21–40).17 Tolep et al compared the maximum transdiaphragmatic pressure (Pdimax) obtained during voluntary maximal inspiratory efforts in nine young (19–28 years) and ten elderly (65–75 years) subjects and found that the average Pdimax of the elderly subjects (128 ± 9 cm H2O) was significantly lower than the average Pdimax of the younger subjects (171 ± 8 cm H2O).18 More specifically, there is age-related atrophy of muscle fibers, termed sarcopenia, which may also explain the reduced respiratory strength in the elderly. The decrease in muscle fiber strength can be as high as 20% by age 70.18–21 There are complex changes involving the mitochondria, muscle fiber disorganization, age-related muscle fiber transitions, and metabolic shifts in the aging muscle that can also explain the reduction in muscle strength.22
The mucociliary elevator refers to the action of ciliated cells along the upper and lower airway to beat in synchrony, trapping and clearing mucus and foreign particles out of the lungs.23 The upper airway nasal mucociliary cells work to remove large particles before they enter the smaller airways, and the lower airway mucociliary cells remove fine particles from the airway over a longer period of time.24 There are alterations in both the clearance of large and small particles with aging. De Oliveira-Maul et al used the clearance of saccharin that was inhaled through the nares of healthy subjects to measure large airway nasal mucociliary function. They demonstrated that in people over age 40, there was a delayed nasal mucociliary clearance time of saccharin compared to subjects under 40 years of age.25 Using radiolabeled particles that can travel past the upper airway and enter into the smaller airways in healthy nonsmoking subjects, Svartengren et al evaluated clearance of the labeled particles in different age groups ranging from age 19–81 years. They found that age alone was negatively associated with airway clearance of radiolabeled particles at 1, 2, 7, 14, and 21 days.26 The association between age and decreased clearance by the mucociliary elevator may be due to beat frequency of the cilia. The studies of beat frequency in cilia are confounded by the presence of cigarette smoking, which has a large impact on beat frequency. Age has not been a statistically significant predictor of decreased beat frequency.27 The impact of structure and functional changes created by the aging process is summed up in Figure 1.
Figure 1.
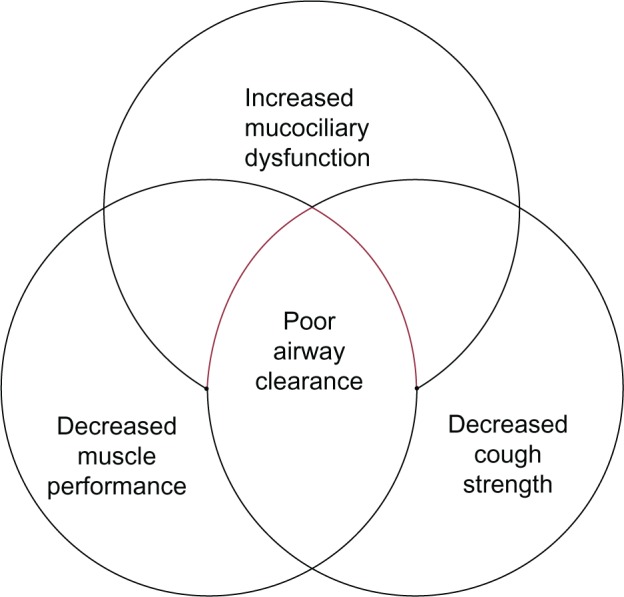
The physiologic changes in aging which place elderly patients at risk for poor airway clearance.
Aging and inflamm-aging
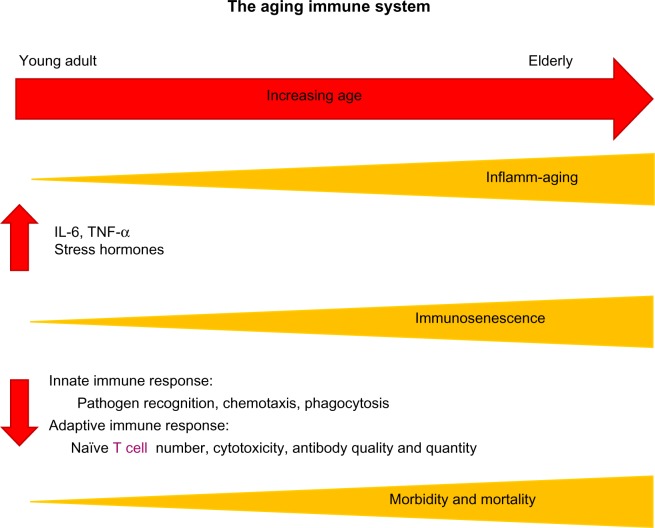
In addition to these age-related structural changes in the lung, advanced age contributes to systemic immune dysfunction. Of particular interest is the basal activation of the innate immune system in aged individuals in the absence of an immunologic threat.28 This phenomenon, referred to as “inflamm-aging”, is marked by elevated levels of tissue and circulating proinflammatory cytokines in aged subjects.28 Specifically, increased levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) have been observed in aging rodent and human studies.29–32 Theoretically, heightened levels of these cytokines in the absence of an immunologic threat or infectious target may be a contributory factor to reduced elasticity and destruction of the delicate lung parenchyma with advanced age. Related to inflamm-aging is the blunted immune response, known as “immunosenescence”, following a pathogenic threat or tissue injury.28 Multiple studies have established reduced levels of mediators such as TNF-α, IL-6, interferon-γ, nitric oxide, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1α after different types of antigenic stimulation in aged animals.33–38 This basal level of inflammation, for example, elevated levels of IL-6, has been suggested to contribute to this subsequent immunosenescence following an immune challenge.28,39 Using a model of IL-6 knockout mice, Gomez et al demonstrated restoration of cytokine production of IL-1β, IL-12, and TNF-α following lipopolysaccharide (LPS) challenge in aged IL-6 knockout animals to levels comparable to young wild-type.40 These data suggest that the basal elevation of circulating IL-6 observed in aged wild-type animals prior to stimulation may contribute to the inability to upregulate cytokine production in the presence of an infectious threat as represented by LPS.40 In addition to these cytokine alterations with aging, more recent data demonstrate a role for microRNAs in inflamm-aging and cellular senescence.41 Specifically, microRNA 146a has been associated with a “senescent associated proinflammatory status” in the setting of vascular remodeling.42 Together, these data support the relationship between inflamm-aging and immunosenescence, suggesting that a disruption in the balance of pro- and anti-inflammatory mediators results in a baseline proinflammatory environment with advanced age that subsequently dampens an appropriate innate and adaptive immune response (Figure 2).
Figure 2.
Increasing age leads to elevated basal levels of inflammation (inflamm-aging) and increased immunosenescence, which are associated with changes in both innate and adaptive immune responses, contributing to the heightened morbidity and mortality seen in the elderly.
Abbreviations: IL, interleukin; TNF, tumor necrosis factor.
In addition to this reduced activation, there are data that support a shift in the temporal response to injury with aging, such that this initial immunosenescence over time results in a protracted immune response and chronic inflammation.43,44 These studies will be discussed in depth later in the context of pulmonary inflammation with aging; however, it is important to consider that this imbalance of immune mediators, delayed immune activation, and protracted course of inflammation may result in increased morbidity and mortality in aging individuals following infection, environmental exposures, or systemic injury45–48
Age and pulmonary immunity
The lung has immunologic defenses that are both complex and resourceful, utilizing both an innate and adaptive immune response to inhaled antigens. Innate immunity is the critical first line of defense for the lungs. Adaptive immunity (acquired immunity) is antigen-specific and is required to ward off encapsulated bacteria, viruses, and intracellular pathogens. This form of immunity relies on immunologic memory and lymphocyte production of antibodies to nonself threats. Some important changes in the innate immunologic response occur with aging. Toll like receptors (TLRs) are key molecules in recognition and initiation of the innate immune response. In the context of aging, there are conflicting data regarding the impact of age on murine and human expression of TLRs or downstream signaling mediators.38,49–51 While some of these murine studies on monocytes and macrophages report reduced expression of one or several TLRs,34,49 another report demonstrates alterations in downstream TLR signaling involving p36.51 Reduction in p38 signaling is supported by studies in human monocytes from aged subjects, where dampened p38 signaling was associated with diminished phosphorylation of p38.37 Additionally, these authors observed a reduction in TLR1 but no changes in TLR2 expression. While the data are divergent on how aging impacts TLR expression, the data do suggest that alteration in the TLR pathways plays a role in an age-related aberrant initiation of the innate immune response and may contribute to an inability in rapidly recognizing and eradicating a pathogen. In studies of cigarette smoke exposure, elevated expression and nuclear translocation of nuclear factor-kβ murine neutrophil chemokines, CXCL1 and CXCL2, were observed in aged mice.52 This was accompanied by a protracted neutrophilia in the lung parenchyma.52 Moreover, following exposure to environmental toxins such as diesel exhaust, the increased pulmonary neutrophilia in lung parenchyma led to congestion and delayed clearance in aged animals as compared to young mice.53 These data suggest that inhaled pollutants cause a prolonged, aberrant pulmonary immune response, which may translate into increased tissue damage, playing a part in environmental, age-related pathology like COPD. Pulmonary infection with Francisella tularensis in aged rodents demonstrated delayed production of neutrophil chemokines in conjunction with an attenuated neutrophil recruitment at early times points,43 supporting the concept of an aberrant initial immune response with age. At later time points, inoculation of LPS into the respiratory tract of aged mice was associated with subsequent heightened levels of chemokines CXCL1 and CXCL2, IL-1β, and lingering pulmonary neutrophilia at 72 hours in aged animals as compared to young.44 Considering the delicate lung alveolar architecture and the highly hydrolytic enzymatic degranulation products of activated neutrophils, this may contribute to excessive tissue damage and reduced lung function over time.
In addition to age-related perturbations in recruitment following inhalational injury or infectious insult, McLachlan et al examined cytotoxic activity of monocytes in older patients compared to younger patients in response to LPS exposure, and found that older patients display less reactive oxygen species (ROS).54 Cytotoxicity generated by the production of ROS and reactive nitrogen intermediates (RNI) is a key function of M1, or proinflammatory macrophages, responsible for activating the Type 1 helper T cells (Th1) pathway. Alteration in macrophage polarization marked by reduced ROS and RNI is reported with advanced age.55 Supporting the study by McLachlan et al, alveolar macrophages from aged rats had reduced basal and LPS-activated levels of ROS and RNI.56 Our lab and others have also demonstrated that aging is associated with lower levels of inducible nitric oxide synthase (iNOS), an enzyme that regulates production of ROS and RNI under control of the interferon-γ receptor.57,58 In conjunction with these changes in macrophage phenotype and cytotoxicity, others found that neutrophils from older individuals (≥85 years) produced less superoxide.54,59 These changes in reactivity have implications for compromising host defenses with age.
In addition to changes in innate system functioning with age, there are changes seen in adaptive immunity with age. In order to activate B- and T cells, dendritic cells (DCs) must migrate from sites of tissue injury and infection to local lymph nodes. Several studies demonstrated DCs from aged mice show poor migration and homing. DC migration in response to chemokine ligand-21, a key DC chemokine that is localized in lymph nodes and binds chemokine receptor type 7 and presents on DC cell membranes, was reduced in aged mice as compared to young.60 Interestingly, following respiratory infection in the lungs of aged mice with either mouse hepatitis virus-1, respiratory syncytial virus, influenza A virus, or severe acute respiratory syndrome coronavirus, elevated expression of prostaglandin D2 correlated with reduced homing of lung DCs to regional lymph nodes and T cell activation.61 Functional antagonism of prostaglandin D2 resulted in upregulation of chemokine receptor type 7, the critical receptor for DC migration, and improved DC homing to draining lymph nodes, subsequently improving T cell activation and survival after viral infection.61
There are many adaptive immune functions that are inefficient with age. The thymus is primarily responsible for producing naïve T cells and is replaced by fatty tissue by age 60 years.62 This leads to an increase in memory T cells relative to naïve T cells.63 Both naïve CD4+ and CD8+ T cells are reduced in aged animals and humans relative to their memory T cells counterparts.64–66 In regard to CD8+ T cells, it has been suggested that repeated or latent cytomegalovirus infection may results in expansion of CD8+ memory cells, again diminishing the naïve CD8+ T cell pool.67 Moreover, the proliferative aptitude of CD4+ T cells from aged donors appears to be reduced following T cell receptor engagement with high-dose anti-CD3 antibody in comparison to young controls,68 suggesting a weaker novel pathogen-specific immune response. Meyer et al looked at the ratio of CD4+ to CD8+ T cells in bronchoalveolar lavage fluid in young versus old normal volunteers and found an increase in CD4+/CD8+ ratio as a function of age, suggesting there are fewer naïve cells available to be converted to memory cells in the face of a novel infection.69,70 Recently, forkhead box N1, a transcription factor known to play a role in embryonic thymus development, has been shown to play a critical role in preventing thymic involution and preservation of naïve T cell subsets with aging.71 Overexpression of forkhead box N1 was demonstrated to increase early thymic progenitors, decrease splenic CD4+ memory T cells, and increase splenic naïve CD4+ and CD8+ T cells.71 These data suggest a possible potential target to increase the number of naïve T cells in aged individuals, increasing the ability of the elderly to respond to novel antigens. The antibody-secreting capacity of B cells is reduced with age, perhaps leading to a less robust immunologic response.72
Aging and respiratory disease development
The prevalence of COPD is two to three times higher in people over age 60.73,74 It is projected that from 1990 to 2020, COPD will move from the sixth to the third leading cause of death worldwide.75 The Rotterdam study found that of healthy 55-year-olds without COPD, one in six women and one in four men will develop COPD later in life, with the risk for developing COPD over the coming 40 years being 24% and 16%, respectively.76 Cigarette smoking is the greatest risk factor for developing COPD in genetically susceptible individuals. COPD is characterized by airway and lung inflammation, mucociliary dysfunction, alveolar destruction, and airway fibrosis.77 The increased burden of COPD seen in the elderly population may be due to age-associated changes in the structure and function of the lung, increasing the pathogenetic susceptibility to COPD. These changes, described in elderly lifelong nonsmokers, are characterized by airspace dilatation resulting from loss of supporting tissue without alveolar wall destruction, similar to changes seen with COPD.77 The Global initiative for chronic Obstructive Lung Disease (GOLD) criteria, accepted by the American Thoracic Society and the European Respiratory Society, is the standard for the diagnosis and classification of COPD, and is assessed by measuring the ratio of FEV1 to the forced vital capacity (FVC).78 FEV1 peaks between ages 20–36 years, and then begins to decline as we age.79 The annual rate of decline after the age of 25 is 20 mL per year and further declines to a loss of 38 mL per year after age 65.79 FVC begins to decline later in life than FEV1 and at a slower pace. Because of the unparalleled rate of decline, use of the FEV1/FVC ratio alone to diagnose COPD will over represent a COPD diagnosis when no such pathology may exist.80 To complicate this matter, Ohar et al found that COPD is underdiagnosed in the United States, arguing that this is due to underutilization of spirometry as a screening test for COPD.81 Therefore, it is recommended to practitioners that a combination of spirometry and symptoms typical of COPD be utilized in the diagnosis of COPD in the elderly.
COPD is often associated with multiple comorbidities which can effect overall severity of disease. These include osteoporosis, mental illness, malnutrition, risk of cardiovascular disease, and skeletal muscle dysfunction.82 Low body mass index is commonly seen in patients with COPD and has been shown to be inversely related to mortality in COPD.83,84 Anxiety and depression are prevalent comorbidities and have also been shown to be related to negative outcomes in COPD. It is estimated that 40% of individuals with COPD have depression, compared to a prevalence of 15% in the general population.85 Cognitive impairments are common and associated with COPD in the elderly. It is estimated that anywhere between 42%–70% of aged persons with COPD have concomitant dementia or neurocognitive impairment.86–88 This may be related to hypoxemia and hypercapnia associated with COPD.89 It may also be due to the common occurrence of cardiovascular disease in elderly with COPD.90 These conditions certainly impact the ability to tolerate and comply with prescribed COPD therapies.
Inhaled bronchodilator therapy is the mainstay of treatment for the management of COPD. Treatment options are varied and include metered dose inhaler, dry powder inhaler, or nebulized formulations. There are many factors which may impact effective treatment use in the elderly COPD population including arthritis, weakness, poor manual dexterity, cognitive impairments, and visual limitations.91 Careful consideration regarding treatment recommendations must be made in the aging COPD population.
Summary
There are many age-associated changes in the respiratory and pulmonary system. The size of the thoracic cavity decreases, limiting lung volumes and altering the muscles that aid in respiration. Muscle function on a cellular level is less efficient and has decreased reserve. Cough strength is reduced in the elderly population due to anatomic changes and muscle atrophy. Clearance of particles from the lung through the mucociliary elevator is negatively impacted and associated with ciliary dysfunction. There are many complex changes in immunity with aging that increase susceptibility to infections, including a less robust immune response from both the innate and adaptive immune systems. Finally, COPD has the highest prevalence in the elderly and deserves special consideration in regard to treatment in this fragile population. Additional research is needed to improve our understanding of the determinants of lung aging and the effects on lung immunity.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Miniño AM. Death in the United States, 2011. NCHS Data Brief. 2013;115:1–8. [PubMed] [Google Scholar]
- 2.Werner CA.Census 2010 Brief C2010BR-09: The Older Population: 2010 Washington, DC: US Department of Commerce; 2011Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdfAccessed May 28, 2013 [Google Scholar]
- 3.Bartynski WS, Heller MT, Grahovac SZ, Rothfus WE, Kurs-Lasky M. Severe thoracic kyphosis in the older patient in the absence of vertebral fracture: association of extreme curve with age. AJNR Am J Neuroradiol. 2005;26(8):2077–2085. [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1(3):253–260. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ensrud KE, Black DM, Harris F, Ettinger B, Cummings SR. Correlates of kyphosis in older women. The Fracture Intervention Trial Research Group. J Am Geriatr Soc. 1997;45(6):682–687. doi: 10.1111/j.1532-5415.1997.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 6.Fon GT, Pitt MJ, Thies AC., Jr Thoracic kyphosis: range in normal subjects. AJR Am J Roentgenol. 1980;134(5):979–983. doi: 10.2214/ajr.134.5.979. [DOI] [PubMed] [Google Scholar]
- 7.Lombardi I, Jr, Oliveira LM, Mayer AF, Jardim JR, Natour J. Evaluation of pulmonary function and quality of life in women with osteoporosis. Osteoporosis Int. 2005;16(10):1247–1253. doi: 10.1007/s00198-005-1834-3. [DOI] [PubMed] [Google Scholar]
- 8.Culham EG, Jimenez HA, King CE. Thoracic kyphosis, rib mobility, and lung volumes in normal women and women with osteoporosis. Spine. 1994;19(11):1250–1255. doi: 10.1097/00007632-199405310-00010. [DOI] [PubMed] [Google Scholar]
- 9.Arora NS, Rochester DF. Effect of body weight and muscularity on human diaphragm muscle mass, thickness, and area. J Appl Physiol. 1982;52(1):64–70. doi: 10.1152/jappl.1982.52.1.64. [DOI] [PubMed] [Google Scholar]
- 10.Brown M, Hasser EM. Complexity of age-related change in skeletal muscle. J Gerontol A Biol Sci Med Sci. 1996;51(2):B117–B123. doi: 10.1093/gerona/51a.2.b117. [DOI] [PubMed] [Google Scholar]
- 11.Freitas FS, Ibiapina CC, Alvim CG, Britto RR, Parreira VF. Relationship between cough strength and functional level in elderly. Rev Bras Fisioter. 2010;14(6):470–476. [PubMed] [Google Scholar]
- 12.Desler C, Hansen TL, Frederiksen JB, Marcker ML, Singh KK, Juel Rasmussen L. Is there a link between mitochondrial reserve respiratory capacity and aging? J Aging Res. 2012;2012 doi: 10.1155/2012/192503. Article ID 192503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sevransky JE, Haponik EF. Respiratory failure in elderly patients. Clin Geriatr Med. 2003;19(1):205–224. doi: 10.1016/s0749-0690(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 14.McCool FD. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl 1):48S–53S. doi: 10.1378/chest.129.1_suppl.48S. [DOI] [PubMed] [Google Scholar]
- 15.Hegland KW, Troche MS, Davenport PW. Cough expired volume and airflow rates during sequential induced cough. Front Physiol. 2013;4:167. doi: 10.3389/fphys.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Davenport P, Sapienza C. Effect of expiratory muscle strength training on elderly cough function. Arch Gerontol Geriatr. 2009;48(3):361–366. doi: 10.1016/j.archger.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Polkey MI, Harris ML, Hughes PD, et al. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med. 1997;155(5):1560–1564. doi: 10.1164/ajrccm.155.5.9154857. [DOI] [PubMed] [Google Scholar]
- 18.Tolep K, Higgins N, Muza S, Criner G, Kelsen SG. Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med. 1995;152(2):677–682. doi: 10.1164/ajrccm.152.2.7633725. [DOI] [PubMed] [Google Scholar]
- 19.Chen HI, Kuo CS. Relationship between respiratory muscle function and age, sex, and other factors. J Appl Physiol. 1989;66(2):943–948. doi: 10.1152/jappl.1989.66.2.943. [DOI] [PubMed] [Google Scholar]
- 20.Berry JK, Vitalo CA, Larson JL, Patel M, Kim MJ. Respiratory muscle strength in older adults. Nurs Res. 1996;45(3):154–159. doi: 10.1097/00006199-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Faulkner JA, Brooks SV, Zerba E. Skeletal muscle weakness and fatigue in old age: underlying mechanisms. Annu Rev Gerontol Geriatr. 1990;10:147–166. doi: 10.1007/978-3-662-38445-9_9. [DOI] [PubMed] [Google Scholar]
- 22.Mobasheri A, Mendes AF. Physiology and pathophysiology of musculoskeletal aging: current research trends and future priorities. Front Physiol. 2013;4:73. doi: 10.3389/fphys.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanner A. Clinical aspects of mucociliary transport. Am Rev Respir Dis. 1977;116(1):73–125. doi: 10.1164/arrd.1977.116.1.73. [DOI] [PubMed] [Google Scholar]
- 24.Chilvers MA, O’Callaghan C. Local mucociliary defence mechanisms. Paediatr Respir Rev. 2000;1(1):27–34. doi: 10.1053/prrv.2000.0009. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira-Maul JP, de Carvalho HB, Miyuki Goto D, et al. Aging, diabetes, and hypertension are associated with decreased nasal mucociliary clearance. Chest. 2013;143(4):1091–1097. doi: 10.1378/chest.12-1183. [DOI] [PubMed] [Google Scholar]
- 26.Svartengren M, Falk R, Philipson K. Long-term clearance from small airways decreases with age. Eur Respir J. 2005;26(4):609–615. doi: 10.1183/09031936.05.00002105. [DOI] [PubMed] [Google Scholar]
- 27.Agius AM, Smallman LA, Pahor AL. Age, smoking and nasal ciliary beat frequency. Clin Otolaryngol Allied Sci. 1998;23(3):227–230. doi: 10.1046/j.1365-2273.1998.00141.x. [DOI] [PubMed] [Google Scholar]
- 28.Panda A, Arjona A, Sapey E, et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30(7):325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovacs EJ, Grabowski KA, Duffner LA, Plackett TP, Gregory MS. Survival and cell mediated immunity after burn injury in aged mice. J Am Aging Assoc. 2002;25(1):3–9. doi: 10.1007/s11357-002-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez CR, Nomellini V, Baila H, Oshima K, Kovacs EJ. Comparison of the effects of aging and IL-6 on the hepatic inflammatory response in two models of systemic injury: scald injury versus i.p. LPS administration. Shock. 2009;31(2):178–184. doi: 10.1097/SHK.0b013e318180feb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gómez CR, Acuña-Castillo C, Nishimura S, et al. Serum from aged F344 rats conditions the activation of young macrophages. Mech Ageing Dev. 2006;127(3):257–263. doi: 10.1016/j.mad.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 33.Ren Z, Gay R, Thomas A, et al. Effect of age on susceptibility to Salmonella Typhimurium infection in C57BL/6 mice. J Med Microbiol. 2009;58(Pt 12):1559–1567. doi: 10.1099/jmm.0.013250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murciano C, Yáñez A, O’Connor JE, Gozalbo D, Gil ML. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol Med Microbiol. 2008;53(2):214–221. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 35.Shaik-Dasthagirisaheb YB, Kantarci A, Gibson FC. Immune response of macrophages from young and aged mice to the oral pathogenic bacterium Porphyromonas gingivalis. Immun Ageing. 2010;7:15. doi: 10.1186/1742-4933-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murciano C, Villamón E, Yáñez A, O’Connor JE, Gozalbo D, Gil ML. Impaired immune response to Candida albicans in aged mice. J Med Microbiol. 2006;55(Pt 12):1649–1656. doi: 10.1099/jmm.0.46740-0. [DOI] [PubMed] [Google Scholar]
- 37.van Duin D, Mohanty S, Thomas V, et al. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178(2):970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 38.Chelvarajan RL, Liu Y, Popa D, et al. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79(6):1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- 39.Gomez CR, Goral J, Ramirez L, Kopf M, Kovacs EJ. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006;25(6):581–585. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- 40.Gomez CR, Karavitis J, Palmer JL, et al. Interleukin-6 contributes to age-related alteration of cytokine production by macrophages. Mediators Inflamm. 2010;2010:475139. doi: 10.1155/2010/475139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olivieri F, Rippo MR, Monsurro V, et al. MicroRNAs linking inflamm-aging, cellular senescence and cancer. Ageing Res Rev. 2013 May 17; doi: 10.1016/j.arr.2013.05.001. Epub. [DOI] [PubMed] [Google Scholar]
- 42.Olivieri F, Lazzarini R, Recchioni R, et al. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age (Dordr) 2013;35(4):1157–1172. doi: 10.1007/s11357-012-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mares CA, Ojeda SS, Li Q, Morris EG, Coalson JJ, Teale JM. Aged mice display an altered pulmonary host response to Francisella tularensis live vaccine strain (LVS) infections. Exp Gerontol. 2010;45(2):91–96. doi: 10.1016/j.exger.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito Y, Betsuyaku T, Nasuhara Y, Nishimura M. Lipopolysaccharide-induced neutrophilic inflammation in the lungs differs with age. Exp Lung Res. 2007;33(7):375–384. doi: 10.1080/01902140701634843. [DOI] [PubMed] [Google Scholar]
- 45.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg Jv, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003;115(4):278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 46.Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 47.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203(6):865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 48.Butcher SK, Killampalli V, Chahal H, Kaya Alpar E, Lord JM. Effect of age on susceptibility to post-traumatic infection in the elderly. Biochem Soc Trans. 2003;31(2):449–451. doi: 10.1042/bst0310449. [DOI] [PubMed] [Google Scholar]
- 49.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169(9):4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 50.Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol. 2005;77(4):503–512. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- 51.Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126(12):1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Moriyama C, Betsuyaku T, Ito Y, et al. Aging enhances susceptibility to cigarette smoke-induced inflammation through bronchiolar chemokines. Am J Respir Cell Mol Biol. 2010;42(3):304–311. doi: 10.1165/rcmb.2009-0025OC. [DOI] [PubMed] [Google Scholar]
- 53.Sunil VR, Patel KJ, Mainelis G, et al. Pulmonary effects of inhaled diesel exhaust in aged mice. Toxicol Appl Pharmacol. 2009;241(3):283–293. doi: 10.1016/j.taap.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLachlan JA, Serkin CD, Morrey KM, Bakouche O. Antitumoral properties of aged human monocytes. J Immunol. 1995;154(2):832–843. [PubMed] [Google Scholar]
- 55.Dace DS, Apte RS. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11(1):177–185. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- 56.Tasat DR, Mancuso R, O’Connor S, Molinari B. Age-dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophages. Aging cell. 2003;2(3):159–164. doi: 10.1046/j.1474-9728.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 57.Kissin E, Tomasi M, McCartney-Francis N, Gibbs CL, Smith PD. Age-related decline in murine macrophage production of nitric oxide. J Infect Dis. 1997;175(4):1004–1007. doi: 10.1086/513959. [DOI] [PubMed] [Google Scholar]
- 58.Ding A, Hwang S, Schwab R. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol. 1994;153(5):2146–2152. [PubMed] [Google Scholar]
- 59.Polignano A, Tortorella C, Venezia A, Jirillo E, Antonaci S. Age-associated changes of neutrophil responsiveness in a human healthy elderly population. Cytobios. 1994;80(322):145–153. [PubMed] [Google Scholar]
- 60.Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008;68(15):6341–6349. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121(12):4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211(2):144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Timm JA, Thoman ML. Maturation of CD4+ lymphocytes in the aged microenvironment results in a memory-enriched population. J Immunol. 1999;162(2):711–717. [PubMed] [Google Scholar]
- 64.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T-cells in normal elderly humans: the T-cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179(2):609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong MS, Dan JM, Choi JY, Kang I. Age-associated changes in the frequency of naïve, memory and effector CD8+ T-cells. Mech Ageing Dev. 2004;125(9):615–618. doi: 10.1016/j.mad.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Provinciali M, Moresi R, Donnini A, Lisa RM. Reference values for CD4+ and CD8+ T lymphocytes with naïve or memory phenotype and their association with mortality in the elderly. Gerontology. 2009;55(3):314–321. doi: 10.1159/000199451. [DOI] [PubMed] [Google Scholar]
- 67.Khan N, Shariff N, Cobbold M, et al. Cytomegalovirus seropositivity drives the CD8 T-cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169(4):1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 68.Mirza N, Pollock K, Hoelzinger DB, Dominguez AL, Lustgarten J. Comparative kinetic analyses of gene profiles of naive CD4+ and CD8+ T-cells from young and old animals reveal novel age-related alterations. Aging Cell. 2011;10(5):853–867. doi: 10.1111/j.1474-9726.2011.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franceschi C, Bonafè M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18(16):1717–1720. doi: 10.1016/s0264-410x(99)00513-7. [DOI] [PubMed] [Google Scholar]
- 70.Meyer KC, Ershler W, Rosenthal NS, Lu XG, Peterson K. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med. 1996;153(3):1072–1079. doi: 10.1164/ajrccm.153.3.8630547. [DOI] [PubMed] [Google Scholar]
- 71.Zook EC, Krishack PA, Zhang S, et al. Overexpression of Foxn1 attenuates age-associated thymic involution and prevents the expansion of peripheral CD4 memory T-cells. Blood. 2011;118(22):5723–5731. doi: 10.1182/blood-2011-03-342097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song H, Price PW, Cerny J. Age-related changes in antibody repertoire: contribution from T-cells. Immunol Rev. 1997;160:55–62. doi: 10.1111/j.1600-065x.1997.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 73.Buist AS, McBurnie MA, Vollmer WM, et al. BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 74.Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the Nippon COPD Epidemiology study. Respirology. 2004;9(4):458–465. doi: 10.1111/j.1440-1843.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 75.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 76.van Durme YM, Verhamme KM, Stijnen T, et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest. 2009;135(2):368–377. doi: 10.1378/chest.08-0684. [DOI] [PubMed] [Google Scholar]
- 77.Sharma G, Hanania NA, Shim YM. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6(7):573–580. doi: 10.1513/pats.200904-022RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 79.Brandstetter RD, Kazemi H. Aging and the respiratory system. Med Clin North Am. 1983;67(2):419–431. doi: 10.1016/s0025-7125(16)31212-3. [DOI] [PubMed] [Google Scholar]
- 80.Dyer C. The interaction of ageing and lung disease. Chron Respir Dis. 2012;9(1):63–67. doi: 10.1177/1479972311433766. [DOI] [PubMed] [Google Scholar]
- 81.Ohar JA, Sadeghnejad A, Meyers DA, Donohue JF, Bleecker ER. Do symptoms predict COPD in smokers? Chest. 2010;137(6):1345–1353. doi: 10.1378/chest.09-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agusti A, Soriano JB. COPD as a systemic disease. COPD. 2008;5(2):133–138. doi: 10.1080/15412550801941349. [DOI] [PubMed] [Google Scholar]
- 83.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 84.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 85.Norwood RJ. A review of etiologies of depression in COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(4):485–491. [PMC free article] [PubMed] [Google Scholar]
- 86.Petty TL, Bliss PL. Ambulatory oxygen therapy, exercise, and survival with advanced chronic obstructive pulmonary disease (the Nocturnal Oxygen Therapy Trial revisited) Respir Care. 2000;45(2):204–211. discussion 211–213. [PubMed] [Google Scholar]
- 87.Grant I, Heaton RK, McSweeny AJ, Adams KM, Timms RM. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1982;142(8):1470–1476. [PubMed] [Google Scholar]
- 88.Hung WW, Wisnivesky JP, Siu AL, Ross JS. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(2):134–137. doi: 10.1164/rccm.200902-0276OC. [DOI] [PubMed] [Google Scholar]
- 89.Kirkil G, Tug T, Ozel E, Bulut S, Tekatas A, Muz MH. The evaluation of cognitive functions with P300 test for chronic obstructive pulmonary disease patients in attack and stable period. Clin Neurol Neurosurg. 2007;109(7):553–560. doi: 10.1016/j.clineuro.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 90.Fillit H, Nash DT, Rundek T, Zuckerman A. Cardiovascular risk factors and dementia. Am J Geriatr Pharmacother. 2008;6(2):100–118. doi: 10.1016/j.amjopharm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 91.Armitage JM, Williams SJ. Inhaler technique in the elderly. Age Ageing. 1988;17(4):275–278. doi: 10.1093/ageing/17.4.275. [DOI] [PubMed] [Google Scholar]