Abstract
MicroRNAs (miRNAs) are a family of small RNAs which are ∼20 nucleotides in length and are non-translated. To date more than 700 miRNAs have been identified and their involvement in many essential cellular processes is now apparent. By binding with target mRNAs, miRNAs are able to regulate both mRNA stability and mRNA translational efficiency. Integrins are a family of transmembrane proteins that both regulate cell-matrix interactions and serve as receptors that mediate intracellular signaling and a variety of cellular processes, including inflammatory responses, immunoresponses, and tumorogenesis. Integrin expression may also be regulated by miRNAs which can also modulate integrin signaling and function.
Integrins are heterodimer adhesion proteins comprised of an α and a β subunit. Cumulatively, there are 18 α subunits and 8 β subunits that can combine to form 24 distinct αβ receptor complexes. Additionally, each integrin can be classfied into one of four groups based on its extracellular binding ligand: collagen, laminin, RGD (Arg-Gly-Asp) or leukocyte-specific receptors. Collagen ligand integrins include integrins α1 and α2 subunits, known to be regulated by specific miRNAs. Amongst the laminin ligand integrins, there are no integrin α subunits known to be regulated by miRNA. As for the RGD ligand integrins, integrin α5 is the only α subunit found to be regulated by miRNAs (miR-31, miR-17-92 cluster, and miR-148b). Finally, amongst the α subunits that comprise the leukocyte-specific receptor ligand integrins, integrins αD, αL, αM, αX have been reported regulation by different miRNAs. As for the integrin β subunits, regulation by miRNAs has been reported for all but β6 and β7 to date. However, computational predictions suggest that numerous miRNA potentially regulate a variety of target integrins. These predictions will undoubtedly guide future investigations of mechanisms underlying integrin expression mechanism and may ultimately yield new therapeutic tools.
Keywords: integrin, microRNA, messenger RNA, gene expression
Introduction
MicroRNA (miRNA) are non-coding RNAs which are typically ∼20 nucleotides in length. miRNA is transcribed by RNA polymerase II from individual miRNA genes, from coding gene introns, or from polycistronic transcripts that encode multiple related miRNAs. So far more than 700 human miRNAs have been identified. Most miRNAs are associated with the RNA-induced silencing complex (RISC), and destabilize or inhibit target mRNA translation via binding to the 3’ untranslated terminus (3’UTR). miRNAs provide precise post-transcriptional control of target gene mRNA. In some cases of abnormally functioning miRNA, the target mRNA is transcribed to abnormally high levels, which can results in a disease state. The large number and variable function of miRNAs identified to date indicates that miRNA are involved in a vast array of cellular processes, including development, growth, and tumorogenesis (1,2).
Integrins are a family of heterodimeric adhesion proteins involved in a variety of dynamic cellular processes, including cellular adhesion, migration, phagocytosis, growth and development, and tumor metastasis (3). The known 18 α subunits and 8 β subunits can combine to form 24 distinct αβ integrin complexes, accounting for a highly diverse family of adhesion molecules, both structurally and functionally. The α subunit is comprised of a seven-bladed β-propeller as well as calf-1 and calf-2 domains which together serve as a support for the integrin head. Notably, some integrin α subunits also contain an I domain which interacts with collagens. In addition, the α subunit contains a transmembrane domain and a short cytoplasmic domain, about which is little known. The integrin β subunits contain a plexin-sempahorin-integrin domain (PSI); a hybrid domain; a βI domain; and four cysteine-rich epidermal growth factor repeats (I-EGF). Similar to the α subunit, the β subunit also contains a transmembrane domain and typically a short cytoplasmic domain. However, integrin β4 has a uniquely long cytoplasmic tail of over 1,000 amino acids which is believed to play an important role in outside-in signaling (4). In all cases, the surface of the β-propeller of subunit α associates with the hybrid domain of subunit β and forms a stable complex (5).
As different integrin αβ complexes have different extracellular ligands, they can be divided into four main groups based on their ligands: collagen, laminin, RGD (Arg-Gly-Asp) or leukocyte-specific receptors. The collagen ligand group includes integrin α1β1, α2β1, α10β1 and α11β1. The laminin ligand integrins includes integrin α3β1, α6β1, α7β1 and α6β4. The RGD ligand integrins include integrin α5β1, α8β1, αVβ3, αVβ5, αVβ6, αVβ8 and αIIbβ3. Finally, the leukocyte-specific receptor group includes integrin complexes specifically expressed on the surface of neutrophils, such as integrin α4β1, integrin α9β1, integrin α4β7, integrin αEβ7. Also in this group and expressed on neutrophils, integrin β2 associates with various integrin α subunits to form integrins αDβ2, αLβ2, αMβ2, αXβ2 (Figures 1 and 2). While numerous integrins are now known to be regulated by miRNAs this review will focus primarily on three particular subunits, integrins β1, β2, and αV, which have been best characterized in this regard and are representative of the growing literature as a whole.
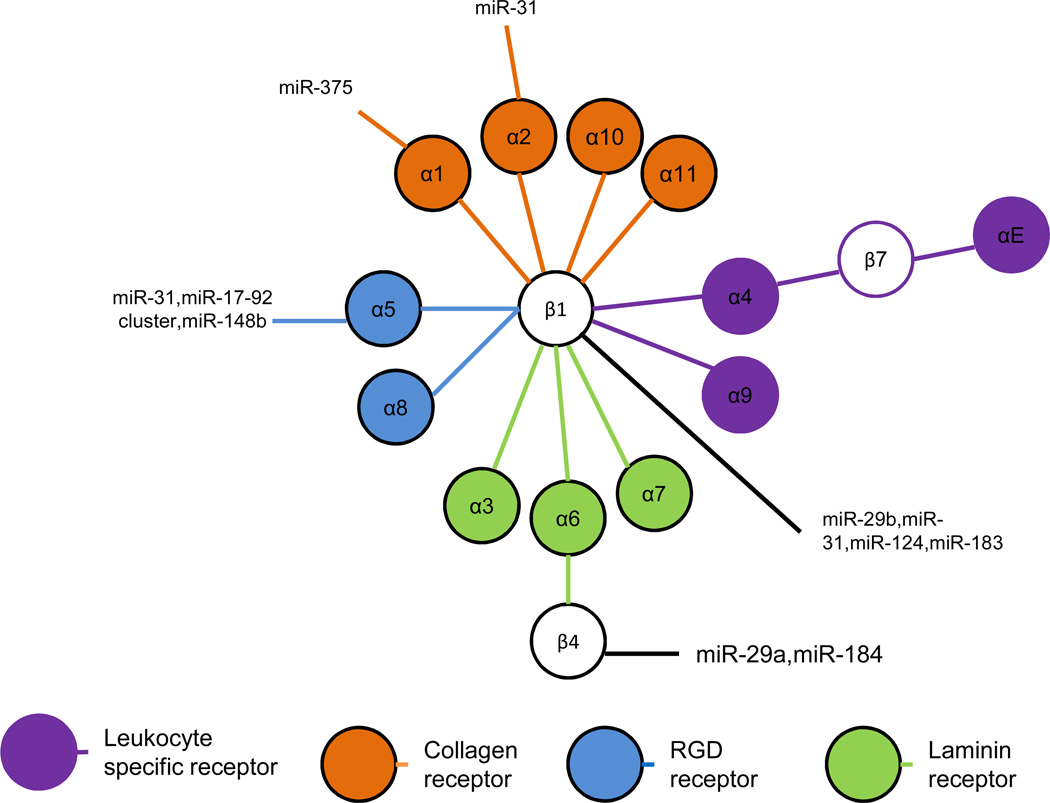
Figure 1. MicroRNAs regulate integrin β1 and associated α subunits.
Integrin β1 and its α subunits form multiple heterodimers. Based on the different extracellular ligands, integrin β1 heterodimers can be classified into four groups: collagen ligand, RGD ligand, laminin ligand and leukocyte-specific expressing receptors. In addition to associating with integrin β1, α6 also associates with integrin β4. Similarly, α4 also associates with β7 while integrin β7 also associates with αE. Integrin β1 and integrin β4 are regulated by distinct microRNAs. The associated subunits α1, α2 and α5 have also been confirmed to be regulated by specific miRNAs as shown.
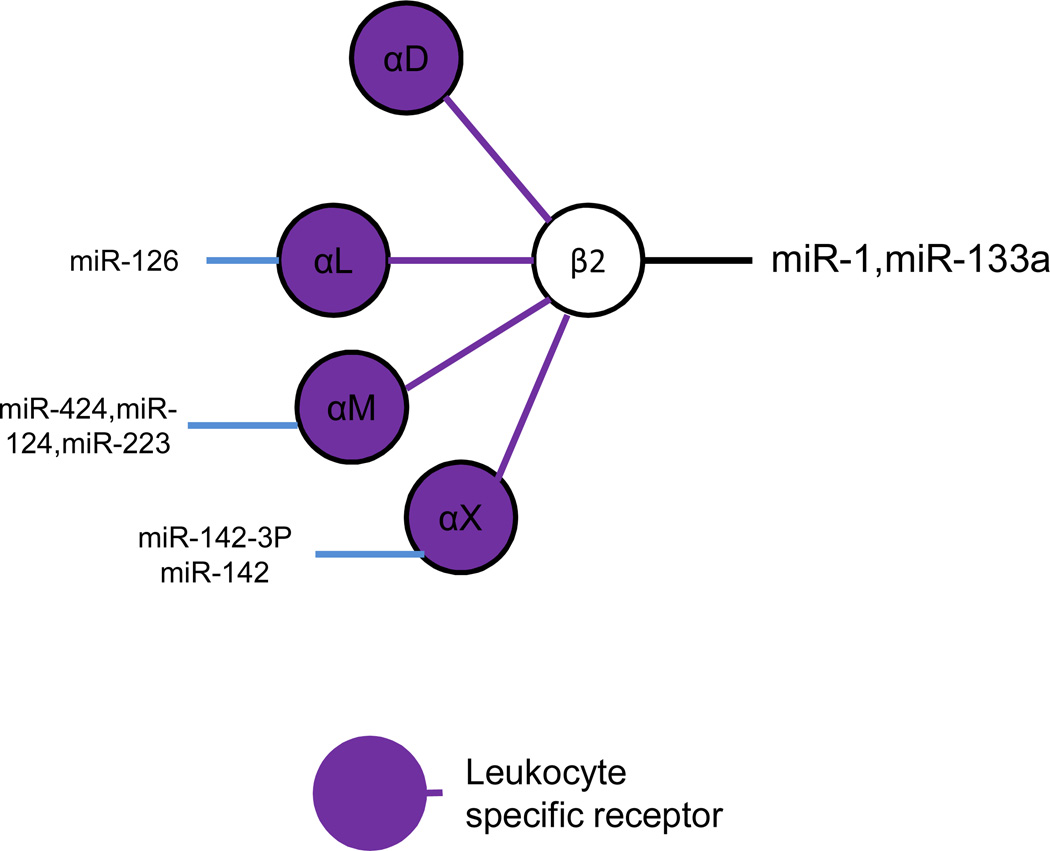
Figure 2. MicroRNAs regulate integrin β2 and associated α subunits.
Integrin β2 associates with αD, αL,αM, and αX, and is exclusively expresse on the surface of leukocytes. To date, integrins β2, αD, αM, and αX, but not αL, have been confirmed to be regulated by miRNAs.
Integrin β1 and associated α subunits
Integrin β1 is expressed in a variety of cell types and plays an important role in many cellular processes, including epithelial, neural and skeletal differentiation and development. Moreover, integrin β1 associates with different ligands in various tissues, suggesting that it has variable cell-specific functions. For example, integrins α1β1, α2β1, α10β1 and α11β1 associate with collagen and integrins α3β1, α6β1, and α7β1 interact with laminin. Separately, integrins α5β1 and α8β1 associate with RGD extracellular matrix proteins, such as fibronectin, vitronectin and fibrinogen, while integrins α4β1 and α9β1 are expressed as leukocyte-specific receptors.
Like most integrins, integrin β1 is also regulated by multiple specific and non-specific miRNAs. For example, overexpression of miR-31 downregulates integrin α2, α5, αV, and β3, and indirectly effects decreased integrin β1 expression in breast cancer cells. Consequently, miR-31-mediated inhibition of integrin β1 expression results in decreased cancer cell invasion and metastasis (6). In addition, miR-29b has been reported to inhibit collagen I and collagen II expression, and also reduce expression of integrin β1 in hepatic stellate cells (7). Moreover, miR-29 inhibited transformation of hepatic stellate cells into myofibroblast-like cells, implicating targeting of miR-29 as a potential therapeutic strategy for liver fibrosis (8,9). In addition to downregulating collagens and integrin β1, miR-29 also showed protective effects on salt-induced hypertension and renal injury in a rat model (10). In an unrelated study, miR-124 levels were found to be significantly lower in glioblastoma patients compared to healthy controls while integrin β1 was expressed at significantly higher levels in patients relative to controls (11). Overexpression of miR-124 in a glioblastoma cell line inhibited tumor migration and invasion, accompanied by decreased expression of integrin β1 (11). Similarly, endogenous miR-124 levels were found to be lower in oral squamous cell carcinomas, whereas overexpression of miR-124 attenuated integrin β1 expression and reduced the adherence and motility of squamous carcinoma cells (12). Finally, miR-124 has been found to regulate neuronal differentiation through the downregulation of laminin-1 and integrin β1 (13).
In separate reports, miR-183 has been shown to be involved in H2O2-induced neural senescence by regulating the expression of integrin β1 and kinesin 2 (14) while transfection of either miR-338b or miR-451 resulted in reduced expression of integrin β1, accompanied by epithelial polarization and integrin β1 translocation to the basolateral membrane in an epithelial differentiation model (15). Cumulatively, evidence suggests that miRNA regulation of integrin β1 is involved in cellular differentiation as well as carcinogenesis, metastasis and tumor cell invasion, and that different miRNAs play both time-specific and signal pathway-specific roles in these functions.
Among the collagen receptor integrins, integrin α1 is a heterodimer subunit associated with integrin β1. Regulation of integrin α1 by miRNA is evidenced by inhibited differentiation of neurites in mice by miR-375 accompanied by reduced integrin α1 expression (16). This finding highlights the importance of integrin α1 in murine neuronal development and supports the regulation of integrin α1β1 by miR-375.
Integrin α2 has been reported to be downregulated by miR-31. Indirectly, miR-31 also inhibits integrin β1 expression In human cancer cells (3). Consequently, miR-31 significantly inhibited tumor cell spreading in a ligand-dependent manner. In addition to integrin α2, miR-31 also directly downregulates integrins α5, αV, and β3 in human cancer cells (6). Significantly, miR-31 did not affect the expression of integrin β6, a heterodimer partner of αV, in breast cancer MDA-MB-231 cells (6). These studies indicate that miR-31 specifically targets several integrin subunits to regulate key aspects of cancer cell invasion and metatases. To date, however, there are no reports of miRNA regulation of integrins α10β1 or α11β1.
Among the α subunits in the RGD receptor group, integrin β1 forms heterodimer complexes with integrin α5 and integrin α8. As noted above, integrin α5 has been reported to be downregulated by miR-31 but also by miR-17∼92 cluster, a combination of pre-miRNAs and mature RNAs (17,18). Downregulation of integrin α5 by miR-31 inhibits tumor cell spreading, invasion and metastasis in a variety of tumor cell models (6,19,20). In contrast to integrin α5, however, miRNA regulation of integrin α8 has not yet been described.
In the laminin ligand group, the α subunits associating with integrin β1 are α3, α6, and α7. To date, no miRNA has been reported to regulate integrin α3, α6, or α7 expression. In this group, integrin α6 also associates with integrin β4, characterized by it atypically long cytosolic tail, and which has been shown to be involved in many critical cellular processes, including tumorogenesis and metastasis (21). Recent studies have shown that miR-29a downregulates integrin β4 expression and facilitates tumor cell invasion (22). In addition, miR-184 also downregulates integrin β4 and specific miR-184 mutations effecting integrin β4 targeting have been linked to family keratoconus (23). Further, polymorphisms in the 3’-UTR of integrin β4 associated with imperfect binding of miRNA correlate with poor outcomes in breast cancer patients (24).
Integrin β1 also forms complexes with α4 and α9 and expresses specifically on neutrophils as leukocyte-specific receptor ligands. However, specific miRNAs that regulate either integrin α4 or integrin α9 have yet to be identified. Notably, the other leuckocyte-specific receptor integrins include the integrin β7 subunit, expressed specifically on leukocytes, which forms a heterodimer with α4 and αE. Similar to the β1 integrins from the same subclass, no miRNA has been reported to regulate either integrin β7, α4 or αE expression.
Integrin β2 and associated α subunits
Integrin β2 associates with several α integrins (D, L, M, X) which are expressed as leukocyte-specific receptors. Integrin β2 plays an important role in neutrophil functions, such as rolling and migration to sites of inflammation. In one mouse study, silencing of integrin β2 with siRNA resulted in neutrophilia, splenomegaly and significant defects in neutrophil trafficking, but had little effect on T cell function (25). Integrin β2 (CD18) has also been shown to be overexpressed on the surface of neutrophils in patients with myeloproliferative disorders in whom miR-133a and miR-1 were also found to be downregulated (26). This study suggests a potential regulatory relationship between integrin β2 and both miR-133a and miR-1 in myeloproliferative disorders.
Integrin αL (CD11a) is regulated by miR-126 and has been linked to the progression of systemic lupus erythematosus (SLE) (27). Overexpression of miR-126 in CD4+ T cells from healthy donors caused the demethylation and upregulation of integrin αL and CD70, thereby causing T cell and B cell hyperactivity. In contrast, inhibition of miR-126 in CD4+ T cell from patients with SLE decreased T cell and B cell activity (27).
Integrin αM (also known as CD11b or MAC-1), is regulated by miR-124, miR-223, and miR-424. miR-124 indirectly regulates integrin αM and has been shown to influence macrophage migration into the central neuronal system as well as microglial cell differentiation and activity in neural systems. These effects of miR-124 occur via the inhibition of transcription factor C/EBP-α resulting in the downregulation of PU.1 which induces the expression of integrin αM (28). Separately, by targeting myocyte enhancer factor 2, miR-223 markedly inhibited bone marrow cell differentiation into CD11b+ Gr11+ myeloid-derived suppressor cells (MDSC) (19). Moreover, by interfering with integrin αM expression on the surface of MDSC, miR-223 further impaired tumor growth in an in vivo model. As for miR-424, evidence suggests that it is able to regulate integrin αM expression associated with monoblast maturation (29).
Integrin αX (CD11c) is known to be regulated by both miR-142 and mIR-142-3P. Cloning miR-142 into the dengue virus-2 strain 3-UTR and in vivo use of this virus restricted infection of CD11b+, CD11c+ and CD45+ cells, resulting in a loss of viral replication (27). This work highlighted the importance of hematopoietic cells for Dengue virus replication and indicated that CD11c is a critical target of miR-142. By regulating interleukin-6 expression, miR-142-3P plays a critical role in the immune response of integrin αX (CD11c) positive dendritic cells (30). miR-142-3P likely controls the immune responses and differentiation of hematopoietic-derived cells, such as dendritic cells and T lymphocytes, in which miR-142-3P is highly expressed. In contrast, there are relatively low levels of miR-142-3P in non-hematopoietic cell line, such as endothelial cells and fibroblasts (30). To date, no miRNA has been reported to regulate integrin αD (also known as CD11D).
Integrin αV and associated β subunits
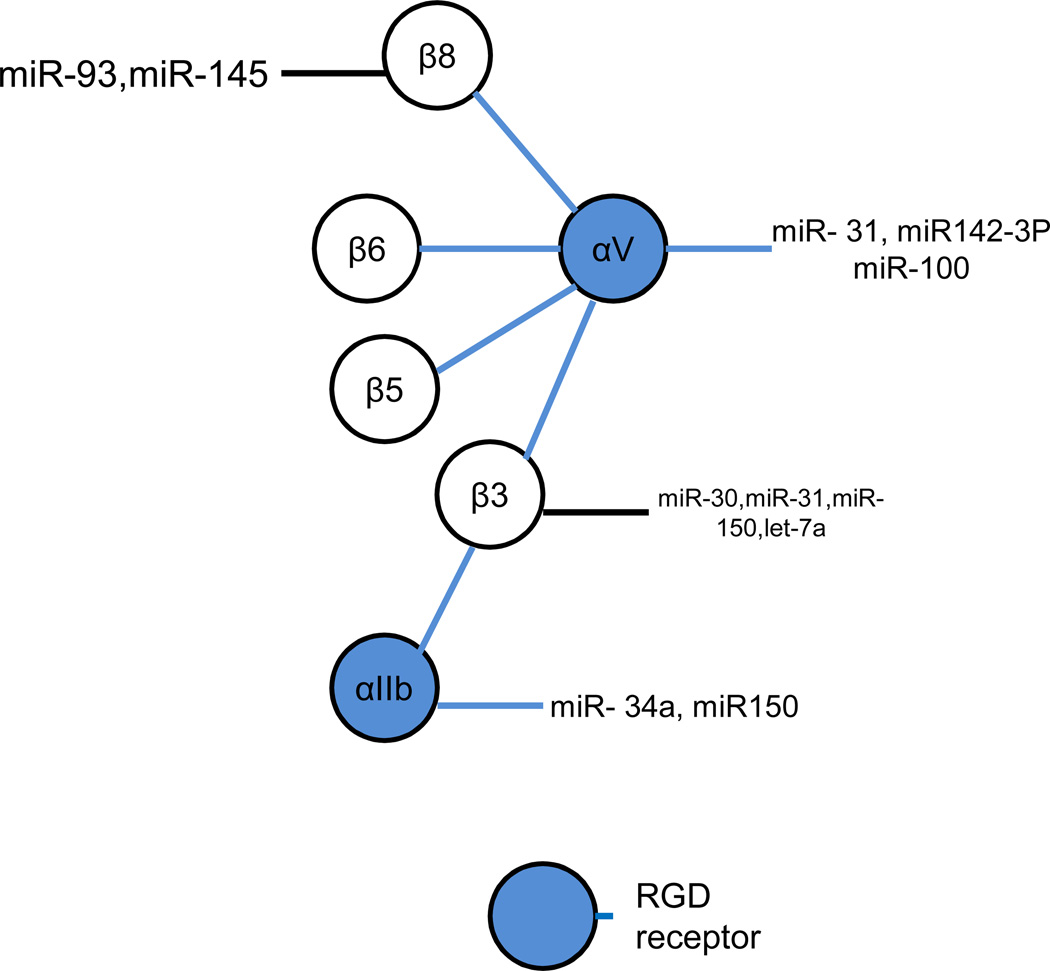
Integrin αV is a one of the few integrin α subunits that associates with multiple β units, of which each complex belongs to the RGD ligand group. Integrin αV is regulated by miR-142-3P and miR-31 and there is evidence that it is regulated by miR-100 as well. Serum miR-142-3p levels in patients with systemic sclerosis are significantly higher than in patients with scleroderma spectrum disorder, SLE, dermatomyositis, or healthy controls (23). Overexpression of integrin αvβ5 and integrin αvβ3 on the surface of the fibroblast cells from systemic sclerosis patients correlates with circulating miR-124-3P levels. As noted earlier, miR-31 is involved in cancer cell invasion and metastasis via the regulation of expression of several integrins on cancer cells including integrin αV (6). Recently, increased expression of integrin αV was reported in intestinal tissue characterized by inflammatory neovascularization in a murine model of graft-versushost disease (31). Expression analysis identified miR-100 as markedly downregulated in this model and inactivation of miR-100 resulted in increased neovascularization and inflammation consistent with worse disease. These findings suggest miR-100 regulation of integrin αV although a direct link was not confirmed.
Integrin β3 is known to be regulated by several different miRNAs. For example, in addition to regulation by miR-31 (3), there is evidence of integrin β3 regulation by miR-30 as levels of miR-30 are significantly reduced while integrin β3 expression is significantly increased in breast tumor initiating cells (BT-IC) (34). Moreover, overexpression of miR-30 in BT-IC induces silencing of integrin β3 and apoptosis. There is also evidence of integrin β3 regulation by let-7 family miRNA as let-7 miRNA levels are also reduced in BT-IC (34) while miR-let-7a is absent and expression of integrin β3 is increased in malignant melanoma cells (32). In addition, transfection of melanoma cells with let-7a pre-miRNA results in the downregulation of integrin β3 as well as reduced invasive potential of transfected cells, an observation which could ultimately lead to novel therapeutic strategies as the metastatic potential of melanomas cells has previously been linked to integrin β3 (33). Finally, miR-150 has been shown to regulate megakaryocyte-erythrocyte progenitor (MEP) cell development and expression of integrin β3 on the surface of differentiated cells (34).
Of note, integrin β3 also associates with integrin αIIb (CD41), whose expression is also regulated by miR-150 as well as miR-34a and. Specifically, by targeting transcription factor MYB, miR-150 drives MEP cell differentiation toward megakaryocytes which is accompanied by increased expression of integrin αIIb, (34). In addition, overexpression of miR-34a promotes megakaryocyte differentiation and is also associated with increased integrin αIIb expression (35).
Although no miRNA has been reported to regulate integrin β5, one study reported silencing of integrin β5 increased miR-125b expression and suppressed mesenchymal stem cell differentiation, suggesting integrin β5 regulation of miRNA-125b (36). As of this writing there are no reports of miRNA regulation of integrin β6.
Integrin β8 is regulated by miR-145 and miR-93. miR-145 plays a central role in corneal epithelium formation from progenitor cells and the maintenance of epithelial integrity (17). Transfection with lentivirus containing the mature miR-145 sequence gave rise to defective epithelium. qPCR and luciferase reporter assays showed that miR-145 suppressed integrin β8 expression in both human corneal epithelial cells and primary corneal epithelial progenitor cells (37). Nude mice injected with miR-93-transfected U87 astrocytoma cells showed increased tumor growth compared to mice injected with mock transfected cells (9). Co-culture of miR-93-transfected U87 cells with endothelial cells facilitated endothelial cell spreading, growth, migration and tube formation compared to controls. Computational analysis suggested that miR-93 targets integrin β8. This link was further supported by evidence that silencing of integrin β8 mimicks effects of miR-93 on tumor cell angiogenesis and survival, suggesting that the effects of miR-93 on tumor cell growth may be due to reduced intergrin β8 expression .
As noted, integrins β5, β6 and β8 have been shown to be indirectly downregulated by miR-31 in a tumor cell model (6). Although miR-31 directly targets the 3’-UTR of integrins α2, α5, αV, and β3, the downregulation of integrins β5, β6 and β8 may be mediated by the reduction of integrin heterodimer stability, or through targeting of upstream regulators.
Computational Prediction of Integrin-Regulating miRNAs
In contrast to the limited number of miRNAs which have been proven to regulate specific integrins, the majority of computationally-predicted integrin-regulatory miRNAs have not yet been investigated. miRNAs which possess conserved targeting sequences specific for different integrins, identified with publically available TargetScan software (wwww.targetscan.org, Whitehead Institute for Biomedical Research, MIT, Cambridge, MA), are listed in Tables 1 and 2. Among the predicted miRNAs, several have already been confirmed as integrin-regulatory miRNAs. However, not all miRNAs that have been experimentally identified as integrin-regulatory miRNAs are predicted by in silico miRNA targeting software. For example, integrin β1 has been shown to be regulated by miR29, miR-124/506, miR-183, all of which were predicted by miRNA sequence analysis. In contrast, several other miRNAs, including miR-192, miR-338, and miR451, that have also been shown to target integrin β1 were not predicted by gene targeting software (15). These studies highlight the complication of integrin miRNA prediction. Moreover, it is now apparent that miRNA regulation of target genes is highly complex and can be effected by, among other things, variable miRNA expression threshold levels, below which miRNA will have little or no effect, or the presence of miRNA competitors that can alter binding to specific target genes (38). Thus, in general, in-silico miRNA predictions merely provide guidance to help direct future investigations aimed at understanding integrin expression and their functional regulation by miRNA.
Table 1.
Computationally predicted integirn α subunits-targeting miRNAs were identified using MicroRNA (www.microrna.org) and confirmed by Targetscan online software (www.targetscan.org). All sequences of target genes were defined by microRNA score < −0.1 and by TargetScan context+ score > 80.
| Gene symbol | ID | Chromsome site | miRNA | Target sequence | micrrna score | TargetScan Convert+ score* | Conserved condition |
|---|---|---|---|---|---|---|---|
| ITGA1 | Integrin alpha 1 | chr5 (q11.2) | hsa-miR-200b | UCUUU-GCUGAGCAGCU | −1.2825 | 99 | P |
| hsa-miR-200c | CAAUUUUCCAUUUCAGUAUU | −1.2825 | 99 | P | |||
| hsa-miR-429 | UUUUCCAUUUCAGUAUU | −1.2816 | 99 | P | |||
| hsa-miR-135a | UUGUCAUGAAAAGCCAU | −0.9526 | 95 | P | |||
| hsa-miR-203 | CAUUUCA | −1.0219 | 86 | P | |||
| hsa-miR-329 | GCUUUUUAGGUGUGU | −0.9223 | 95 | P | |||
| hsa-miR-362-3p | ACUGCUU-UUUAGGUGUGU | −0.919 | 96 | P | |||
| hsa-miR-135b | UUGUCAUGAAAAGCCAU | −0.9526 | 96 | P | |||
| hsa-miR-10a | CAACUCCUUGG---UACAGGGU | −0.6762 | 85 | P | |||
| hsa-miR-10b | AACUCCUUGGUACAGGGU | −0.3367 | 86 | P | |||
| ITGA2 | integrin alpha2 | chr5 (q11.2) | hsa-miR-195 | UUUAUGCUGCU | −0.2991 | 86 | C |
| hsa-miR-16 | AUUUA--UGCUGCU | −0.2799 | 88 | C | |||
| hsa-miR-15a | UCCAAGCAUG-ACAACUU | −0.2039 | 83 | C | |||
| hsa-miR-15b | UCAAUUUAUGCUGCU | −0.3017 | 81 | C | |||
| hsa-miR-497 | UCAAUUUAUGCUGCU | −0.3068 | 82 | C | |||
| hsa-miR-424 | UGCUGCU | −0.3042 | 81 | C | |||
| hsa-miR-27a | ACUGUGA | −0.712 | 88 | C | |||
| hsa-miR-27b | ACUGUGA | −0.712 | 88 | C | |||
| has-miR-19a | AAUAUUUCA---AUUUGCAC | −0.844 | 86 | C | |||
| has-miR-19b | AAUAUUUCA---AUUUGCAC | −0.844 | 86 | C | |||
| ITGA3 | Integrin alpha3 | chr17(q21.33) | has-miR-199a-5p | ACACUGG | −0.4432 | 81 | C |
| has-miR-199b-5p | ACACUGG | −0.4432 | 81 | C | |||
| has-miR-181a | AGCGACA-C-UUGAAUGU | −0.6484 | 87 | C | |||
| has-miR-181b | AGCGACACU--UGAAUGU | −0.6197 | 87 | C | |||
| has-miR-181c | AGCGACA-CUUGAAUGU | −0.6483 | 87 | C | |||
| has-miR-181d | AGCGACACU--UGAAUGU | −0.6197 | 87 | C | |||
| hsa-miR-101 | CAUGGUACUGU | −0.2294 | 84 | C | |||
| hsa-miR-506 | GUGCCUU | −0.5015 | 83 | C | |||
| hsa-miR-124 | CUCCUUGUGCCUU | −0.505 | 83 | C | |||
| ITGA4 | integrin alpha4 | chr2(q31.3) | has-miR-30a | UUUAAAAGACACUGUUUAC | −0.8 | 95 | C |
| has-miR-30b | UGUUUAC | −0.8036 | 92 | C | |||
| has-miR-30c | UGUUUAC | −0.8036 | 92 | C | |||
| has-miR-30d | UGUUUAC | −0.8036 | 93 | C | |||
| has-miR-30e | UAAAAGACACUGUUUAC | −0.8036 | 95 | C | |||
| ITGA5 | integrin alpha5 | chr12(q13.13) | has-miR-26a | CCAGCCCAGAGACAUACUUGA | −0.6745 | 84 | C |
| has-miR-26b | GACAUACUUGA | −0.6745 | 84 | C | |||
| hsa-miR-128 | CCCAUGCACUGUG | −0.4134 | 92 | C | |||
| hsa-miR-152 | CAUGCACUG | −0.2845 | 91 | C | |||
| has-miR-148a | UGCACUG | −0.2845 | 91 | C | |||
| has-miR-148b | AUGCACUG | −0.2845 | 91 | C | |||
| has-miR-367 | CUGUUGC--AAGUGCAAU | −1.0428 | 98 | C | |||
| has-miR-92a | CUGUUGCAAGUGCAAU | −1.0535 | 97 | C | |||
| has-miR-92b | CUGUUGCAAGUGCAAU | −2.107 | 97 | C | |||
| has-miR-363 | GUUGCAAGUGCAAU | −1.0535 | 97 | C | |||
| has-miR-25 | CUGUUGCAAGUGCAAU | −1.0561 | 97 | C | |||
| has-miR-32 | AACUCUGUUGCAAGUGCAAU | −1.0482 | 97 | C | |||
| ITGA6 | integrin alpha6 | chr2(q31.1) | hsa-miR-19a | GUUUUGCAC | −0.4404 | 84 | C |
| hsa-miR-19b | GUUUUGCAC | −0.4404 | 84 | C | |||
| has-miR-367 | CUAAAUGUGCAAU | −0.9819 | 88 | C | |||
| hsa-miR-32 | AAC-UA--AAUGUGCAAU | −0.9789 | 88 | C | |||
| hsa-miR-92a | AAAUGUGCAAU | −0.9819 | 85 | C | |||
| hsa-miR-25 | GUCUUAAACUAAAUGUGCAAU | −0.9758 | 84 | C | |||
| hsa-miR-363 | AAUGUGCAAU | −0.9789 | 84 | C | |||
| hsa-miR-92b | GUGCAAU | −0.9819 | 84 | C | |||
| has-miR-30a | UGUUUAC | −1.2059 | 87 | C | |||
| has-miR-30b | AAUAUAUUUUGUUUAC | −1.2028 | 87 | C | |||
| has-miR-30c | AAAAUAUAUUUUGUUUAC | −1.2028 | 87 | C | |||
| has-miR-30d | UGUUUAC | −1.2075 | 87 | C | |||
| has-miR-30e | UGUUUAC | −1.2059 | 86 | C | |||
| has-miR-143 | GUUAAAAAUG--UCAUCUC | −0.9666 | 94 | C | |||
| ITGA7 | integrin alpha7 | chr12(q13.2) | hsa-miR-124 | UCCCCGGAAGUGCCUU | −0.5703 | 95 | C |
| hsa-miR-506 | UCCCCGGAA--GUGCCUU | −0.5389 | 96 | C | |||
| ITGA8 | integrin alpha8 | chr10 (p13) | hsa-miR-8 | AGAAGACCAAAG | −0.3072 | 97 | P |
| hsa-miR-199a-5p | AACACUGG | −0.571 | 89 | P | |||
| hsa-miR-199b-5p | CAAAGACCUCAAACACUGG | −0.5746 | 88 | P | |||
| ITGA9 | integrin alpha9 | chr3 (p22.2) | hsa-miR-125a-5p | GCAUGGUCAA--CCCUCAGGG | −0.6957 | 80 | C |
| hsa-miR-125b | CUCAGGG | −0.6957 | 80 | C | |||
| ITGA10 | integrin alpha10 | chr1(q21.1) | hsa-miR-22 | AGUCCUCC--CUGGCAGCU | −0.4393 | 88 | C |
| ITGA11 | integrin alpha11 | chr15(q23) | hsa-miR-148a | UCUGGAAUGCACUG | −0.5994 | 96 | C |
| hsa-miR-148b | UCUGGAAUGCACUG | −0.5994 | 96 | C | |||
| hsa-miR-152 | UCUGGAAUGCACUG | −0.5994 | 96 | C | |||
| ITGAD | integrin alpha D | chr16(p11.2) | hsa-miR-382 | AAUCAACUUACAUGGAAACAACU | −0.9143 | 80 | P |
| hsa-miR-18a | GUGCUA-A-GCACCUU | −0.1672 | 85 | P | |||
| hsa-miR-190 | UAAUGU-UUUUACAUAUC | −0.6222 | 88 | P | |||
| hsa-miR-190b | UAAUGUUUUUACAUAUC | −0.2668 | 89 | P | |||
| hsa-miR-365 | UAUUUGUGGGCAUU | −0.8989 | 94 | P | |||
| ITGAE | integrin alpha E | chr17(p13.2) | hsa-miR-382 | AAUCAACUUACAUGGAAACAACU | −0.9143 | 80 | P |
| hsa-miR-18a | GUGCUA-A-GCACCUU | −0.1672 | 85 | P | |||
| hsa-miR-190 | UAAUGU-UUUUACAUAUC | −0.6222 | 88 | P | |||
| hsa-miR-190b | UAAUGUUUUUACAUAUC | −0.2668 | 89 | P | |||
| hsa-miR-365 | UAUUUGUGGGCAUU | −0.8989 | 94 | P | |||
| ITGAL | integrin alpha L | chr16(p11.2) | hsa-miR-23a | AAUGUGA | −0.9167 | 99 | C |
| hsa-miR-23b | UUAU-CCAAUAAAUGUGA | −0.9167 | 99 | C | |||
| ITGAM | integrin alpha M | chr16(p11.2) | hsa-miR-224 | UCAAU-GUGACUU | −0.1207 | 90 | P |
| hsa-miR-539 | CACCA---AU-AUUUCUC | −1.136 | 98 | P | |||
| hsa-miR-186 | AUUCUUU | −0.8343 | 85 | P | |||
| hsa-miR-342-3p | AUUCCAUUGUGUGAG | −0.7084 | 80 | P | |||
| hsa-miR-340 | UGGUAGCA-UACUUUAUA | −0.77 | 90 | P | |||
| hsa-miR-185 | AGCAGC--UUCUCUCC | −0.2405 | 87 | P | |||
| ITGAV | integrin alpha V | chr2 (q32.1) | hsa-miR-142-3p | ACACUAC | −0.9501 | 95 | C |
| hsa-miR-135a | AUAUCAUAAUGCUUAAAGCCAU | −0.6978 | 88 | C | |||
| hsa-miR-135b | AUAUCAUAAUGCUUAAAGCCAU | −0.6978 | 90 | C | |||
| hsa-miR-25 | CAAGUGCAAU | −0.8261 | 94 | C | |||
| hsa-miR-367 | GUCAUUGUUCUCAAGUGCAAU | −0.8046 | 96 | C | |||
| hsa-miR-363 | AUUGUUCUCAAGUGCAAU | −0.8118 | 94 | C | |||
| hsa-miR-92a | CAAGUGCAAU | −0.8261 | 94 | C | |||
| hsa-miR-92b | CAAGUGCAAU | −0.8261 | 93 | C | |||
| hsa-miR-32 | CAUUGUUCUCAAGUGCAAU | −0.8154 | 94 | C | |||
| ITGAW | integrin alpha W | not available | not available | ||||
| ITGAX | integrin alpha X | chr16(p11.2) | hsa-miR-491-5p | GCGAGUUUUCCCCAC | −0.4861 | 90 | P |
| hsa-miR-150 | CUGCUUCCUGUCUUUGGGAG | −0.5063 | 97 | P | |||
| hsa-miR-103b | ACAGUUCUGAAU-AUGCUGC | −0.6088 | 99 | P | |||
| hsa-miR-425 | AGUGAAUUAGUGUCAU | −0.4593 | 84 | P | |||
| hsa-miR-145 | AACUGGA | −0.1668 | 85 | P | |||
| hsa-miR-335 | UACCGCUCUUG | −0.2027 | 84 | P | |||
| ITGA2B | integrin alpha-IIB | chr17(q21.31) | hsa-miR-24 | GACUGAGCC | −0.4374 | 88 | P |
Table 2.
Computationally predicted integrin β subunits-targeting miRNAs were identified using MicroRNA (www.microrna.org) and confirmed by Targetscan online software (www.targetscan.org). All sequences of target genes were defined by microRNA score < −0.1 and by TargetScan context+ score > 80.
| Gene symbol | ID | Chromsome site | miRNA | Target sequence | microrna score | TargetScan Convert+ score* | Conserved condition |
|---|---|---|---|---|---|---|---|
| ITGB1 | integrin beta1 | chr10(q11.22) | hsa-miR-124 | GUGCCUU | −1.1982 | 94 | C |
| hsa-miR-506 | GUGCCUU | −1.203 | 93 | C | |||
| hsa-miR-183 | UUACUUUGAGUUAGUGCCAU | −0.9096 | 82 | C | |||
| hsa-miR-29a | GUUUAAUGUCUGGUGCUCAU | −0.6906 | 81 | C | |||
| hsa-miR-29b | UUUGUUUAAUGUCUGGUGCU | −0.6906 | 80 | C | |||
| hsa-miR-29c | GUUUAAUGUCUGGUGCU | −0.6906 | 81 | C | |||
| ITGB2 | integrin beta2 | chr21(q22.3) | hsa-miR-876-5p | AUUAAC-CAGAAAUCC | −1.028 | 97 | P |
| hsa-miR-335 | AUGGCUUGCCACAGCUCUUG | −0.843 | 97 | P | |||
| hsa-miR-1271 | GGUGCCAA | −0.827 | 89 | P | |||
| hsa-miR-96 | AAAGGUG---GUGCCAA | −0.7773 | 88 | P | |||
| hsa-miR-10b | CAGGGU | −0.2881 | 86 | P | |||
| hsa-miR-10a | CAGGGU | −0.2856 | 86 | P | |||
| ITGB3 | integrin beta3 | chr17(q21.32) | hsa-miR-30a-d | UCCCUGCCAUCAUGUUUAC | −0.3691 | 86–87 | P |
| hsa-miR-30e | UCCCUGCCAUCAUGUUUAC | −0.3632 | 87 | P | |||
| hsa-miR-150 | UGGGGUA--GGUUGGGAG | −0.2092 | 89 | P | |||
| hsa-miR-222 | CCUGAUGUAGC | −0.3796 | 81 | P | |||
| hsa-miR-302a-e | AU-GUAGCACUU | −0.7835 | 93 | P | |||
| hsa-miR-520a-e | UCCUGA-UGUAGCACUU | −0.7762 | 94 | P | |||
| hsa-miR-373 | UCUCCUGAU-GUAGCACUU | −0.7798 | 93 | P | |||
| ITGB4 | integrin beta4 | chr17(q25.1) | hsa-miR-9 | AAACCUAUUUGUAACCAAAG | −0.8263 | 97 | C |
| ITGB5 | integrin beta5 | chr3(q21.2) | hsa-miR-495 | UGU-CCGUGUUUGUU | −0.8726 | 85 | P |
| hsa-miR-421 | GGCUGUUGAGAUGCCUGUUGA | −0.5167 | 90 | C | |||
| hsa-miR-486-5p | CAGUGCCUGUACAGG | −0.234 | 94 | P | |||
| ITGB6 | integrin beta6 | chr2(q24.2) | none | none | none | none | |
| ITGB7 | integrin beta7 | chr12(q13.13) | hsa-miR-499-5p | GUAUACAAU-AAAGUCUUA | −0.8023 | 85 | P |
| ITGB8 | integrin beta8 | chr7(p21.1) | hsa-miR-145 | AACUGGA | −0.3691 | 99 | C |
| hsa-miR-372 | CUAGUGUCGUUGUAGCACUU | −0.3666 | 91 | C | |||
| hsa-miR-302a-e | ACUAGUGUCGUUGUAGCACUU | −0.3702 | 88–89 | C | |||
| hsa-miR-17 | UGUCGUUGU-AGCACUUU | −0.5923 | 97 | C | |||
| hsa-miR-93 | ACUAGUGUCGUUGUAGCACUUU | −0.596 | 97 | C | |||
| hsa-miR-106 | UGUCGUUGU-AGCACUUU | −0.5923 | 97 | C | |||
| hsa-miR-20a-b | UGUCGUUGU-AGCACUUU | −0.596 | 96 | C | |||
| hsa-miR-19a-b | UAUACAUUUGCAC | −0.8825 | 94 | C | |||
| hsa-miR-145 | AACUGGA | −0.7663 | 99 | C | |||
| hsa-miR-23a-c | AAUGUGA | −-0.344 | 85 | C | |||
| hsa-miR-139-5p | AAAGAGCUGACACUGUAG | −0.1317 | 82 | C | |||
| hsa-miR-373 | GUUGUAGCACUU | −0.3745 | 87 | C |
Future basic and clinical research on the role of miRNA in regulating integrin expression are likely to focus on several key areas, including: finding new integrin-regulating miRNAs through in silico prediction and experimental confirmation; identifying integrin polymorphisms, including intronic and 3’-UTR polymorphisms which regulate miRNA binding(24); defining miRNA structural modifications which influence integrin expression; and finally, development of new miRNA-modifying drugs. Advances in these areas offer great promise and may lead to new pharmaceutical approaches for the treatment of cancers and other diseases.
Summary
A large family of outside-in signaling proteins, integrins are involved in many critical cellular processes, including angiogenesis, proliferation, and tumorogenesis. Elucidating the mechanisms of integrin regulation is crucial to understanding integrin function. Moreover, while the role of integrins in the biology of a variety of cancers is now widely described, there is growing evidence that integrins may also be important determinants of a diverse and vast range of diseases including fibrotic lung and renal diseases (39,40), acute lung injury (41), graft-versus-host disease (31) and even multiple sclerosis as treatments specifically targeting integrins in patients with multplie sclerosis have recently shown promise (42). Accordingly, identification of pharmacologic therapies directed at integrins in a variety of clinical contexts is a robust area of ongoing investigation. Among the various mechanisms regulating integrin expression, miRNAs have emerged as particularly promising targets in this regard. As the complete spectrum of miRNAs capable of regulating most integrins remains undefined this will undoubtedly remain an important and active area of investigation for the near future and beyond.
Figure 3. MicroRNAs regulate integrin αV and β subunits that associate with αV.
Integrin αV associates with β3, β5, β6, β8, and exclusively interact with RGD extracellular matrices, such as fibronectin, vitronectin, and fibrinogen. In this group, integrin αV and associated subunits β3, β5, β8, but not β6, have been confirmed to be regulated by miRNAs. One specific integrin α subunit, αIIb, forms a complex with integrin β3 and is regulated by distinct miRNA as shown.
Acknowledgements
All authors have read the journal's policy on conflicts of interest and have none to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amiel J, de Pontual L, Henrion-Caude A. Advances in genetics. 2012;80:1–36. doi: 10.1016/B978-0-12-404742-6.00001-6. [DOI] [PubMed] [Google Scholar]
- 2.Henrion-Caude A, Girard M, Amiel J. Current gene therapy. 2012;12(4):292–300. doi: 10.2174/156652312802083602. [DOI] [PubMed] [Google Scholar]
- 3.Guo W, Giancotti FG. Nature reviews. 2004;5(10):816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 4.Hogervorst F, Kuikman I, von dem Borne AE, Sonnenberg A. The EMBO journal. 1990;9(3):765–770. doi: 10.1002/j.1460-2075.1990.tb08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barczyk M, Carracedo S, Gullberg D. Cell and tissue research. 2010;339(1):269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augoff K, Das M, Bialkowska K, McCue B, Plow EF, Sossey-Alaoui K. Mol Cancer Res. 2011;9(11):1500–1508. doi: 10.1158/1541-7786.MCR-11-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Biochemical and biophysical research communications. 2011;412(1):74–79. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 8.Kwiecinski M, Elfimova N, Noetel A, Tox U, Steffen HM, Hacker U, Nischt R, Dienes HP, Odenthal M. Laboratory investigation; a journal of technical methods and pathology. 2012;92(7):978–987. doi: 10.1038/labinvest.2012.70. [DOI] [PubMed] [Google Scholar]
- 9.Kwiecinski M, Noetel A, Elfimova N, Trebicka J, Schievenbusch S, Strack I, Molnar L, von Brandenstein M, Tox U, Nischt R, Coutelle O, Dienes HP, Odenthal M. PloS one. 2011;6(9):e24568. doi: 10.1371/journal.pone.0024568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Taylor NE, Lu L, Usa K, Cowley AW, Jr, Ferreri NR, Yeo NC, Liang M. Hypertension. 2010;55(4):974–982. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler A, Thomson D, Giles K, Maleki S, Mreich E, Wheeler H, Leedman P, Biggs M, Cook R, Little N, Robinson B, McDonald K. Eur J Cancer. 2011;47(6):953–963. doi: 10.1016/j.ejca.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Hunt S, Jones AV, Hinsley EE, Whawell SA, Lambert DW. FEBS letters. 2011;585(1):187–192. doi: 10.1016/j.febslet.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 13.Cao X, Pfaff SL, Gage FH. Genes & development. 2007;21(5):531–536. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. The Journal of biological chemistry. 2011;285(8):5461–5471. doi: 10.1074/jbc.M109.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuchiya S, Oku M, Imanaka Y, Kunimoto R, Okuno Y, Terasawa K, Sato F, Tsujimoto G, Shimizu K. Nucleic acids research. 2009;37(11):3821–3827. doi: 10.1093/nar/gkp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelmohsen K, Hutchison ER, Lee EK, Kuwano Y, Kim MM, Masuda K, Srikantan S, Subaran SS, Marasa BS, Mattson MP, Gorospe M. Molecular and cellular biology. 2010;30(17):4197–4210. doi: 10.1128/MCB.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang L, Deng Z, Shatseva T, Yang J, Peng C, Du WW, Yee AJ, Ang LC, He C, Shan SW, Yang BB. Oncogene. 2011;30(7):806–821. doi: 10.1038/onc.2010.465. [DOI] [PubMed] [Google Scholar]
- 18.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. Science (New York, N.Y. 2009;324(5935):1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 19.Valastyan S, Chang A, Benaich N, Reinhardt F, Weinberg RA. Genes & development. 2011;25(6):646–659. doi: 10.1101/gad.2004211. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Valastyan S, Chang A, Benaich N, Reinhardt F, Weinberg RA. Cancer research. 2010;70(12):5147–5154. doi: 10.1158/0008-5472.CAN-10-0410. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Giancotti FG. Trends in pharmacological sciences. 2007;28(10):506–511. doi: 10.1016/j.tips.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Gerson KD, Shearstone JR, Maddula VS, Seligmann BE, Mercurio AM. The Journal of biological chemistry. 2012;287(13):9835–9844. doi: 10.1074/jbc.M111.317727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes AE, Bradley DT, Campbell M, Lechner J, Dash DP, Simpson DA, Willoughby CE. American journal of human genetics. 2011;89(5):628–633. doi: 10.1016/j.ajhg.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brendle A, Lei H, Brandt A, Johansson R, Enquist K, Henriksson R, Hemminki K, Lenner P, Forsti A. Carcinogenesis. 2008;29(7):1394–1399. doi: 10.1093/carcin/bgn126. [DOI] [PubMed] [Google Scholar]
- 25.Slezak S, Jin P, Caruccio L, Ren J, Bennett M, Zia N, Adams S, Wang E, Ascensao J, Schechter G, Stroncek D. Journal of translational medicine. 2009;7:39. doi: 10.1186/1479-5876-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayadas TN, Cullere X. Trends in immunology. 2005;26(7):388–395. doi: 10.1016/j.it.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Wang Y, Liang Y, Zhao M, Long H, Ding S, Yin H, Lu Q. Arthritis and rheumatism. 2010 doi: 10.1002/art.30196. [DOI] [PubMed] [Google Scholar]
- 28.Conrad AT, Dittel BN. Cell research. 2011;21(2):213–216. doi: 10.1038/cr.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q, Zhang M, Jiang X, Zhang Z, Dai L, Min S, Wu X, He Q, Liu J, Zhang Y, Zhang Z, Yang R. International journal of cancer. 2011;129(11):2662–2673. doi: 10.1002/ijc.25921. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Varambally S, Maher CA, Cao Q, Chockley P, Toubai T, Malter C, Nieves E, Tawara I, Wang Y, Ward PA, Chinnaiyan A, Reddy P. Blood. 2011;117(23):6172–6183. doi: 10.1182/blood-2010-12-325647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leonhardt F, Grundmann S, Behe M, Bluhm F, Dumont RA, Braun F, Fani M, Riesner K, Prinz G, Hechinger AK, Gerlach UV, Dierbach H, Penack O, Schmitt-Graff A, Finke J, Weber WA, Zeiser R. Blood. 2013;121(17):3307–3318. doi: 10.1182/blood-2012-07-442665. [DOI] [PubMed] [Google Scholar]
- 32.Muller DW, Bosserhoff AK. Oncogene. 2008;27(52):6698–6706. doi: 10.1038/onc.2008.282. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Chen B, Blystone SD, McHugh KP, Ross FP, Ramos DM. Invasion & metastasis. 1998;18(1):1–14. doi: 10.1159/000024494. [DOI] [PubMed] [Google Scholar]
- 34.Lu J, Guo S, Ebert BL, Zhang H, Peng X, Bosco J, Pretz J, Schlanger R, Wang JY, Mak RH, Dombkowski DM, Preffer FI, Scadden DT, Golub TR. Developmental cell. 2008;14(6):843–853. doi: 10.1016/j.devcel.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro F, Gutman D, Meire E, Caceres M, Rigoutsos I, Bentwich Z, Lieberman J. Blood. 2009;114(10):2181–2192. doi: 10.1182/blood-2009-02-205062. [DOI] [PubMed] [Google Scholar]
- 36.Yu X, Cohen DM, Chen CS. Stem cells (Dayton, Ohio) 2012 doi: 10.1002/stem.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SK, Teng Y, Wong HK, Ng TK, Huang L, Lei P, Choy KW, Liu Y, Zhang M, Lam DS, Yam GH, Pang CP. PloS one. 2011;6(6):e21249. doi: 10.1371/journal.pone.0021249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebert MS, Sharp PA. Cell. 2012;149(3):515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson NC, Sheppard D. Biochimica et biophysica acta. 2013;1832(7):891–896. doi: 10.1016/j.bbadis.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pozzi A, Zent R. J Am Soc Nephrol. 2013 [Google Scholar]
- 41.Chen W, Sammani S, Mitra S, Ma SF, Garcia JG, Jacobson JR. American journal of physiology. 2012;303(4):L279–285. doi: 10.1152/ajplung.00361.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodman SL, Picard M. Trends in pharmacological sciences. 2012;33(7):405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]