Abstract
Background
The capacity of pneumococcal vaccination to confer memory in HIV-infected children is critical for durable protection.
Methods
HIV-infected children 2–<19 years administered two doses of pneumococcal conjugate vaccine (PCV7) and one dose of polysaccharide vaccine (PPV) on HAART were randomized four-five years later to receive a PCV7 or PPV booster. Total and high avidity antibodies to serotypes 1 (PPV) and 6B and 14 (PCV7 and PPV) were determined by ELISA. Memory was defined as persistence of ≥0.5 mcg/mL of serotype-specific antibody on day 0 or change from <0.5 mcg/mL to ≥0.5 mcg/mL between day 0 and week 1, or, ≥4-fold antibody rise between day 0 and week 1.
Results
Prior to boosting, four to five years after the previous PCV7-PCV7-PPV series, geometric mean concentrations (GMCs) were 0.46 mcg/mL (serotype 1), 1.31 mcg/mL (serotype 6B), and 1.47 mcg/mL (serotype 14), with concentrations ≥0.5 mcg/mL in 41% (serotype 1) to 82% (serotypes 6B and 14). Memory based on antibody concentration ≥0.5 mcg/mL before or 1 week after boosting with PCV7 or PPV was demonstrated in 42–61% for serotype 1 and 87–94% for serotypes 6B and 14, with lower rates based on day 0 to week 1 ≥4-fold antibody rise (serotype 1, 3–13%; serotype 6B, 13–31%; serotype 14, 29–53%). Antibody concentrations post-boosting were greater following PCV7 than PPV for serotypes 6B and 14. Ratios of highly avid to total antibody pre- and post-boosting were 0.5–0.8. Predictors of memory included higher CD4% (nadir before HAART and at P1024 and P1061s entry), CD19% (at P1024 and P1061s entry), and antibody response after the PCV7-PCV7-PPV primary series and lower viral load (at P1024 and P1061s entry) and age.
Conclusions
Protective antibody concentrations, high avidity, and booster responses to PCV7 or PPV indicative of memory were present four-five years after PCV7-PCV7-PPV in HIV-infected children on HAART.
Keywords: pneumococcal, vaccine, memory, HIV, children
Introduction
Infections caused by Streptococcus pneumoniae remain an important problem in HIV-infected children and adults, even where highly active antiretroviral therapy (HAART) is widely used [1–4]. Pneumococcal conjugate vaccines (PCVs) prevent invasive pneumococcal disease in HIV-infected children and adults [5–6]. A 3-dose series of 9-valent PCV administered to HIV-infected infants in South Africa reduced invasive disease caused by vaccine serotypes by 65%, although efficacy was lower than the 83% efficacy in HIV-uninfected children [5, 7]. After a mean of six years, efficacy in these young HIV-infected children fell to 39%, compared with 78% efficacy in HIV-uninfected children. Serotype-specific antibody levels were lower in HIV-infected children compared with HIV-uninfected counterparts before and after a subsequent PCV booster dose. Similarly, among HIV-infected adults in Malawi with a prior pneumococcal infection, efficacy of 7-valent PCV decreased from 85% in the first year after a 2-dose series to 25% in subsequent years [6]. These observations suggest waning protection following PCV in HIV-infected children and adults. In these studies, most subjects were not receiving antiretroviral therapy at primary vaccination or during follow-up. Whether HAART-associated immune preservation and/or reconstitution affect development of memory and persistence of protection is critical to understanding optimal timing of pneumococcal immunization, its long-term impact on HIV-infected children, and need for booster doses.
International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) study P1024 evaluated the immunogenicity of 2 doses of 7-valent PCV followed by 1 dose of 23-valent pneumococcal polysaccharide vaccine (PPV) in HIV-infected children on HAART. Vaccination was immunogenic, with antibody responses comparable to those of healthy children and generally higher than in antiretroviral-naïve South African infants [8]. This report focuses on a substudy of P1024, IMPAACT P1061s, which evaluated persistence of antibody and memory 4–5 years following PCV7-PCV7-PPV vaccination.
Materials and Methods
Study population
HIV-infected children 2–<19 years old were eligible for P1024 if they fit into immunologic strata based on nadir CD4% prior to HAART and CD4% at screening: stratum 1, <15% and <15%; stratum 2, <15% and ≥15%; stratum 3, 15%–≤25% and ≥15%; and stratum 4, ≥25% and ≥25%. Additional inclusion criteria included perinatal infection (strata 2–4 only), stable HAART regimen (≥3 antiretrovirals from ≥2 classes) for ≥6 months (≥3 months for stratum 1), and an HIV RNA PCR (Roche Amplicor Monitor Assay) <30,000 copies/mL (<60,000 copies/mL for stratum 1), and no prior PCV. Subjects received PCV7 at entry and 8-weeks and PPV at 16-weeks. Subjects who enrolled in P1024 June 2001–March 2002 were eligible for P1061s, which enrolled February 2006–August 2006 at 26/39 sites that participated in P1024. Subjects were maintained in the same strata to which they were classified in P1024.
Study protocol
Informed consent was obtained and human experimentation guidelines of the US Department of Health and Human Services and participating institutions were followed. Subjects who received two doses of PCV7 and one dose of PPV in P1024 without ≥ grade 3 adverse events or allergic reactions related to PCV7 or PPV and had not received additional doses of either vaccine since the conclusion of P1024 qualified to receive one dose of PCV7 (Pneumococcal 7-Valent Conjugate Vaccine, Prevnar; Wyeth-Lederle Vaccines; 0.5 mL intramuscular) or PPV (Polyvalent Pneumococcal Vaccine, PNEUMOVAX 23; Merck & Co.; 0.5 mL intramuscular) at P1061s entry, based on 1:1 random assignment within strata. Hepatitis B virus and measles-mumps-rubella vaccines were also administered [9–10]. Pneumococcal antibody concentrations were measured at entry and 1 and 4 weeks post-booster, and plasma HIV RNA viral load (VL) and lymphocyte subsets were measured at entry.
Laboratory assays and immunologic definitions
IgG antibodies to pneumococcal serotypes 1 (PPV-containing only) and 6B and 14 (PCV7- and PPV-containing) were determined by ELISA [8]. These serotypes were selected because serotype 6B is among the least immunogenic serotypes and might be sensitive to differences between PCV7 and PPV and among immunologic strata; serotype 14 represents the most common serotype causing invasive disease in U.S. children prior to PCV7; and protection against serotype 1 waned quickly in South African HIV-infected children, suggesting the need to understand antibody persistence for this serotype [11]. Serotype-specific antibody avidity was determined using a modification employing 0.5M NaSCN as a chaotropic agent to elute low affinity antibody [12]. Control serum (SF89, Dr. Carl Frasch, Food and Drug Administration, Bethesda, MD) was used as the standard to determine serotype-specific antibody concentrations.
A uniform threshold for assessing antibody persistence and memory to each serotype was used as suggested by World Health Organization guidance [13–14]. Although a concentration of 0.35 mcg/mL is considered a correlate of protection by licensing agencies, we selected a threshold concentration of 0.5 mcg/ml to reflect higher pre-vaccination antibody concentrations and poorer antibody function in pediatric AIDS patients that suggest that greater antibody quantities may be necessary for protection in HIV-infected children against invasive infection and pneumonia [15]. Correlation of ELISA antibody at this level with opsonophagocytic activity for serotypes 6B and 14 has been demonstrated in HIV-infected children [14].
Immunologic memory was defined as: (1) antibody persistence with ≥0.5 mcg/mL of serotype-specific antibody on day 0 or change from <0.5 mcg/mL to ≥0.5 mcg/mL between day 0 and week 1, or, (2) ≥4-fold antibody rise between day 0 and week 1. Combined response (memory or primary response) was defined as (1) antibody concentration ≥0.5 mcg/mL at day 0, week 1, or week 4, or, (2) ≥4-fold rise in antibody concentration between day 0 and week 1 or day 0 and week 4 (Supplementary Text and Supplementary Table 1). Avidity results were expressed as the avidity ratio, the proportion of total ELISA antibody that remained bound after elution.
Statistical analysis
All subjects who received 2 doses of PCV7 and 1 dose of PPV in P1024, had neither vaccine between the last P1024 visit and P1061s entry, and had pneumococcal serologies at P1061s entry were included in analyses of antibody persistence. Timepoints included weeks -212, -188, and -140 relative to P1061s entry (P1024 timepoints corresponding to 8, 32, and 80 weeks after the conclusion of the PCV7-PCV7-PPV regimen) and P1061s entry (week 0). Subjects who received a PCV7 or PPV booster in P1061s and had serologic results at entry, week 1 (6–13 days), and week 4 (25–36 days) were included in analyses of memory. Because only one P1061s subject was in immune stratum 1, this subject was included in analyses in which strata were combined but was not included in comparisons among strata. Comparisons utilized Fisher’s Exact test for categorical variables; Kruskal-Wallis, Wilcoxon Rank Sum, and Wilcoxon Signed Rank tests for continuous variables; and McNemar’s test for comparison of response rates between timepoints. Univariate logistic regression analyses assessing predictors of memory focused on parameters predictive of PCV7-PCV7-PPV response in P1024.
Results
Population characteristics
One hundred one of 224 eligible P1024 subjects enrolled in P1061s. Forty-three received PCV7 and 41 received PPV. Eleven did not qualify for PCV7 or PPV because they had received PPV between P1024 and P1061s and six due to PCV7- or PPV-associated adverse events in P1024. Sixty-seven of 84 vaccine recipients had data available at each timepoint for inclusion in analyses of memory (Table 1). Only 5/67 had a CD4% that would no longer have placed them in their original P1024 strata. Higher immune stratum was associated with younger age (p = 0.02, PCV7 recipients), higher nadir CD4% (p <0.001, PCV7 and PPV), higher P1024 CD4% (p <0.01, PPV), lower P1024 VL (p = 0.05, PPV), greater P1061s CD4% (p <0.01, PPV), and lower P1061s VL. Median time from conclusion of the P1024 PCV7-PCV7-PPV regimen to P1061s entry was 4.2 years.
Table 1.
Characteristics of the analyzed population.
| Parametera (%, except as noted) |
Pneumococcal Conjugate Vaccine 7 | Pneumococcal Polysaccharide Vaccine | P- Valueb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All Subjects | Immune Stratum | All Subjects | Immune Stratum | ||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||||
| N | 36 | 0 | 14 | 14 | 8 | 31 | 1 | 10 | 12 | 8 | |
| Age | |||||||||||
| Median, years | 13.8 | NAc | 15.5 | 14.2 | 10.8 | 14.6 | 21.9 | 15.1 | 13.4 | 12.8 | 0.55 |
| Range, years | 8.3–21.8 | 8.5–21.9 | |||||||||
| Interquartile range, years | 12.0–15.8 | 11.1–16.6 | |||||||||
| Male sex | 42 | NA | 36 | 50 | 38 | 55 | 0 | 60 | 50 | 63 | 0.33 |
| Race/ethnicity | 0.78 | ||||||||||
| White Non-Hispanic | 17 | NA | 21 | 14 | 13 | 10 | 0 | 0 | 25 | 0 | |
| Black Non-Hispanic | 61 | NA | 50 | 79 | 50 | 65 | 100 | 80 | 42 | 75 | |
| Hispanic | 22 | NA | 29 | 7 | 38 | 26 | 0 | 20 | 33 | 25 | |
| Other | 0 | NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CDC clinical classification | 0.39 | ||||||||||
| N (not symptomatic) | 6 | NA | 0 | 7 | 12 | 13 | 0 | 0 | 33 | 0 | |
| A (mildly symptomatic) | 22 | NA | 21 | 14 | 38 | 35 | 0 | 40 | 33 | 38 | |
| B (moderately symptomatic) | 47 | NA | 36 | 57 | 50 | 32 | 0 | 30 | 17 | 62 | |
| C (severely symptomatic) | 25 | NA | 43 | 21 | 0 | 19 | 100 | 30 | 17 | 0 | |
| Pre-HAART Nadir CD4% | |||||||||||
| Median | 16 | NA | 11 | 18 | 30 | 18 | 4 | 9 | 18 | 32 | 0.67 |
| <15% | 39 | NA | 100 | 0 | 0 | 35 | 100 | 100 | 0 | 0 | 0.95 |
| 15%–<25% | 39 | NA | 0 | 100 | 0 | 39 | 0 | 0 | 100 | 0 | |
| ≥25% | 22 | NA | 0 | 0 | 100 | 26 | 0 | 0 | 0 | 100 | |
| P1024 Screening CD4% | |||||||||||
| Median | 33 | NA | 31 | 33 | 40 | 35 | 12 | 28 | 38 | 37 | 0.88 |
| <15% | 0 | NA | 0 | 0 | 0 | 3 | 100 | 0 | 0 | 0 | 0.33 |
| 15–<25% | 14 | NA | 29 | 7 | 0 | 6 | 0 | 20 | 0 | 0 | |
| ≥25% | 86 | NA | 71 | 93 | 100 | 90 | 0 | 80 | 100 | 100 | |
| P1024 HIV RNA level | |||||||||||
| ≤400 copies/mL | 78 | NA | 57 | 93 | 88 | 68 | 0 | 50 | 75 | 88 | 0.63 |
| 401–5000 copies/mL | 11 | NA | 21 | 0 | 13 | 13 | 0 | 10 | 17 | 13 | |
| >5000 copies/mL | 11 | NA | 21 | 7 | 0 | 19 | 100 | 40 | 8 | 0 | |
| P1061s CD4% | |||||||||||
| Median | 33 | NA | 33 | 29 | 35 | 34 | 0 | 29 | 36 | 38 | 0.31 |
| <15% | 8 | NA | 0 | 21 | 0 | 10 | 100 | 20 | 0 | 0 | 1.00 |
| 15–25% | 11 | NA | 14 | 14 | 0 | 13 | 0 | 20 | 17 | 0 | |
| ≥25% | 81 | NA | 86 | 64 | 100 | 77 | 0 | 60 | 83 | 100 | |
| P1061s HIV RNA level | |||||||||||
| ≤400 copies/mL | 58 | NA | 57 | 57 | 63 | 74 | 0 | 60 | 92 | 75 | 0.34 |
| 401–5000 copies/mL | 22 | NA | 21 | 14 | 38 | 10 | 0 | 20 | 8 | 0 | |
| >5000 copies/mL | 19 | NA | 21 | 29 | 0 | 16 | 100 | 20 | 0 | 25 | |
| Interval, P1024 week 16 (PPV dose) to P1061s entry | |||||||||||
| Median, years | 4.3 | NA | 4.2 | 4.3 | 4.2 | 4.2 | 4.1 | 4.3 | 4.2 | 4.1 | 0.20 |
| Range, years | 4.0–4.8 | 3.8–4.5 | |||||||||
| Percent not receiving | |||||||||||
| HAART at P1061s entry | 11 | NA | 7 | 14 | 12 | 6 | 100 | 0 | 0 | 12 | 0.68 |
Note: N = number of subjects in each subgroup.
Data are percentage of subjects, unless otherwise indicated.
Comparison of subjects who received pneumococcal conjugate vaccine (immune strata combined) v. subjects who received pneumococcal polysaccharide vaccine (immune strata combined).
Not applicable.
P1024 baseline characteristics and serologic results were similar between subjects who qualified for P1061s memory analyses and the remainder of the P1024 PCV7/PPV analysis group except for trends toward better virologic control at P1024 entry among subjects in P1061s analyses (VL <400 copies/mL, 73% v. 56%; 401–5000 copies/mL, 12% v. 22% ; >5000 copies/mL, 15% v. 22%; p=0.06) and a higher median P1024 CD4 count in the P1061s subset (940/mm3 v. 888/mm3, p = 0.06), with no difference in median P1024 CD4% (34% v. 33%, p = 0.28).
Antibody persistence
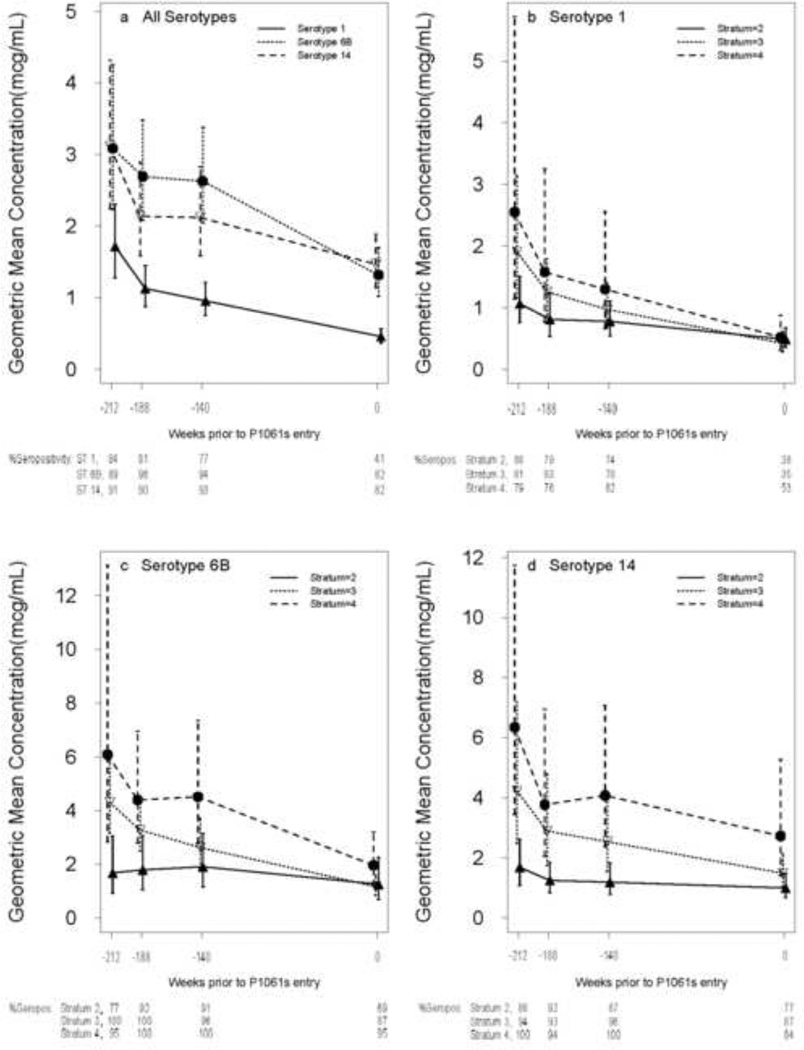
Antibody concentrations for serotypes 1, 6B, and 14 at each timepoint after the P1024 PCV7-PCV7-PPV regimen (week -212 relative to P1061s entry) until P1061s entry (week 0) were significantly lower than the previous timepoint (p ≤0.03), except for the week -188 to week -140 interval for serotypes 6B and 14 (Figure 1). Concentrations declined more rapidly between weeks -212 to -188 than between weeks -188 to -140 and weeks -140 to 0 (p <0.01, paired signed rank test). Among the 3 serotypes, there were no overall differences in rates of antibody decay between weeks -212 and 0. Curves for each immune stratum mirrored overall curves for each serotype. GMCs generally varied directly with stratum, with differences lessening as the interval from PCV7-PCV7-PPV increased. The slope of antibody fall between week -212 and week 0 was greater with increasing stratum for serotype 6B (p = 0.01, Kruskal-Wallis test).
Figure 1.
Persistence of antibody between P1024 and P1061s. Geometric mean concentrations and confidence intervals are shown for each of the three serotypes evaluated. Timepoints are at weeks -212, -188, and -140 relative to P1061s entry (8, 32, and 80 weeks after the conclusion of the PCV7-PCV7-PPV regimen in P1024) and at P1061s entry (week 0; a median of 220 weeks after the conclusion of the PCV7-PCV7-PPV series at P1024 week 16). Panel (a) depicts all immune strata combined for the three serotypes and panels (b) – (d) show the immune strata separately for each serotype. Immune stratum 1, which contained only one subject, is excluded from panels (b) – (d). Seventy-nine subjects were assessed at week -212, 73 at week -188, 70 at week -140, and 79 at week 0. The percent with antibody concentration ≥0.5 mcg/mL (% Seropos) at each timepoint are displayed below panel (a) for the three serotypes (STs; all immune strata combined) and below panels (b) – (d) for each immune stratum (excluding stratum 1). Note: the y-axis scale in panels (a) and (b) extends to 5 mcg/mL, while the y-axis scale in panels (c) and (d) extend to 12 mcg/mL.
GMCs at P1061s entry exceeded 0.5 mcg/mL for serotypes 6B and 14, but not serotype 1, and exceeded these subjects’ GMCs prior to P1024 entry, for all 3 serotypes: serotype 1, 0.46 mcg/mL v. 0.26 mcg/mL; serotype 6B, 1.31 mcg/mL v. 0.75 mcg/mL; serotype 14, 1.47 mcg/mL v. 0.32 mcg/mL (p ≤0.001, paired signed rank test). GMCs for each of immune strata 2–4 for serotypes 6B and 14, but only for stratum 4 for serotype 1, were ≥0.5 mcg/mL at P1061s entry. The GMC at P1061s entry was significantly higher with increasing immune stratum for serotype 14 only (p = 0.04, Kruskal-Wallis test). The proportions of subjects with antibody concentrations ≥0.5 mcg/mL at P1061s entry were 41% for serotype 1 and 82% for serotypes 6B and 14.
Antibody concentrations after P1061s vaccination
Among all strata combined, serotype 1 GMCs increased at weeks 1 and 4 only in PPV recipients, as expected, whereas GMCs increased at weeks 1 and 4 for serotypes 6B and 14 in PCV7 and PPV recipients (Table 2). The GMC for serotype 1 four weeks after boosting with PPV was similar to the GMC of 1.5 mcg/mL eight weeks after P1024 PCV7-PCV7-PPV vaccination, while for serotypes 6B and 14, GMCs four weeks after boosting with PCV7 or PPV exceeded GMCs of 3.23 and 3.55 mcg/mL, respectively, measured eight weeks after PCV7-PCV7-PPV. PCV7 recipients attained higher GMCs than PPV recipients for serotype 14 at weeks 1 and 4, with a similar pattern within immune strata. GMCs were generally greater in higher immune strata, with significant differences for serotype 14. Antibody concentrations of PCV7 and PPV recipients (combined) at week 1 correlated negatively with age for serotypes 6B (Rho = −.30, P = .01) and 14 (Rho = −.25, P = .04).
Table 2.
Geometric mean antibody concentrations and percentage with antibody levels ≥0.5 mcg/mL before and after booster vaccination in P1061s.
| ST | Week | Pneumococcal Conjugate Vaccine 7 (PCV7) | Pneumococcal Polysaccharide Vaccine (PPV) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject Group | Subject Group | ||||||||||||||||
| All (36) |
2 (14) |
3 (14) |
4 (8) |
All (36) |
2 (14) |
3 (14) |
4 (8) |
All (31) |
2 (10) |
3 (12) |
4 (8) |
All (31) |
2 (10) |
3 (12) |
4 (8) |
||
| GMC (95% CI) | %≥ 0.5 mcg/mL (95% CI) |
GMC (95% CI) | % ≥ 0.5 mcg/mL (95% CI) |
||||||||||||||
| 1 | 0 | 0.5 (0.3–0.7) | 0.6 (0.3–1.0) | 0.4 (0.2–0.8) | 0.6 (0.2–1.4) | 42 (26–59) | 43 (21–69) | 29 (10–58) | 63 (29–89) | 0.4 (0.3–0.6) | 0.4 (0.3–0.7) | 0.4 (0.2–0.7) | 0.6 (0.2–1.8) | 39 (23–57) | 30 (9–62) | 33 (12–65) | 63 (29–89) |
| 1a | 0.5 (0.3–0.7) | 0.5 (0.3–0.9) | 0.5 (0.2–0.9)b | 0.5 (0.2–1.2) | 39 (24–56) | 36 (15–63) | 29 (10–58) | 63 (29–89) | 0.8 (0.6–1.1)c | 0.6 (0.3–0.9) | 0.9 (0.5–1.6)b,c | 1.0 (0.3–2.9)c | 61d (43–77) | 50 (22–78) | 67 (35–88) | 75 (35–95) | |
| 4e | 0.6 (0.4–0.8)b | 0.7 (0.4–1.2) | 0.5 (0.3–1.0)b,c,f | 0.5 (0.2–1.1) | 53g (35–68) | 50 (23–77) | 50g (23–77) | 63 (29–89) | 1.5 (1.1–2.1)b,c,f | 0.9 (0.6–1.4)f | 1.9 (1.2–3.1)b,c,f | 2.2 (0.8–5.7)c,f | 94d,g,h (80–99) | 90h (56–99) | 100g (76–100) | 88 (50–99) | |
| 6B | 0 | 1.1 (0.7–1.7) | 0.9 (0.4–2.0) | 1.0 (0.5–2.0) | 1.7 (0.7–4.0) | 72 (56–86) | 57 (31–79) | 71 (42–90) | 100 (65–100) | 1.6 (1–2.5) | 2.0 (0.6–6.2) | 1.2 (0.8–2.0) | 2.0 (0.6–7.2) | 84 (67–93) | 80 (44–96) | 92 (65–100) | 75 (35–95) |
| 1 | 2.7 (1.8–4.3)c | 1.8 (0.9–3.4)c | 2.5 (1.1–5.6)c | 7.0 (2.8–17.4)c | 86 (72–94) | 79 (50–94) | 86 (58–97) | 100 (65–100) | 3.0 (1.7–5.1)c | 2.0 (0.6–6.6) | 2.9 (1.4–6.1)c | 6.0 (1.5–23.1)c | 87 (71–95) | 80 (44–96) | 92 (65–100) | 88 (50–99) | |
| 4 | 6.9 (4.6–10.4)c,f | 5.8 (3.1–10.8)c,f | 5.1 (2.5–10.4)c,f | 16.3 (6.5–40.6)c,f | 97h (86–100) | 100 (77–100) | 93 (69–100) | 100 (65–100) | 4.9 (2.9–8.4)c,f | 2.7 (0.7–10.0)c | 6.7 (3.7–12.2)c,f | 7.9 (2.3–27.4)c,f | 90 (75–97) | 80 (44–96) | 100 (76–100) | 88 (50–99) | |
| 14 | 0 | 1.6 (1.1–2.3) | 1.3 (0.7–2.7) | 1.4 (0.8–2.3) | 2.7 (1.0–7.4) | 83 (68–92) | 79 (50–94) | 86 (58–97) | 88 (50–99) | 1.3 (0.9–1.9) | 0.8 (0.5–1.4) | 1.3 (0.8–2.2) | 2.5 (0.8–8.4) | 84 (67–93) | 80 (44–96) | 92 (65–100) | 88 (50–99) |
| 1 | 6.5 (4.2–10.2)b,c | 4.5 (1.9–10.4)c,i | 6.2 (2.9–13.3)c,i | 13.4 (6.8–26.5)c,i | 94 (81–99) | 93 (69–100) | 93 (69–100) | 100 (65–100) | 3.0 (1.9–4.7)b,c | 1.6 (0.9–3)c,i | 3.3 (1.5–7.5)c,i | 6.6 (2.3–19.4)i | 90 (75–97) | 90 (56–99) | 92 (65–100) | 100 (65–100) | |
| 4 | 10.3 (7–15.2)b,c,f | 7.7 (4.2–14.3)b,c,f | 9.4 (4.3–20.3)c,f | 20.5 (11.8–35.5)f | 97 (86–100) | 100 (77–100) | 93 (69–100) | 100 (65–100) | 5.9 (4.0–8.9)b,c,f | 2.7 (1.5–4.9)b,f,i | 8.6 (5.1–14.4)c,f,i | 12.3 (5.7–26.7)c,f,i | 100 (90–100) | 100 (73–100) | 100 (76–100) | 100 (65–100) | |
Note: Subjects qualifying for memory analyses are divided according to whether they received a pneumococcal conjugate vaccine (PCV7) or pneumococcal polysaccharide vaccine (PPV) booster dose in P1061s. Subject group refers to all subjects (all immune strata combined), immune stratum 2, immune stratum 3, and immune stratum 4, and the number of subjects in each subject group is indicated in parentheses. The subject in immune stratum 1 is included within �all subjects�, but is not shown separately due to the small number of subjects in this immune stratum. ST = serotype, GMC = geometric mean concentration (mcg/mL), and CI = confidence interval.
Permitted window = 6–13 days
P ≤.05, antibody concentration, PCV7 recipients v. PPV recipients (Wilcoxon Rank Sum Test)
P ≤.05, antibody concentration v. previous timepoint (Paired Sign Rank Test)
P ≤.02, percent ≥0.5 mcg/mL v. previous timepoint (McNemar’s Exact Test)
Permitted window = 25–36 days, except for 1 subject whose 3rd serology was obtained at 84 days
P ≤.03, antibody concentration v. week 0 (Paired Sign Rank Test)
P <.01, percent ≥0.5 mcg/mL, PCV7 recipients v. PPV recipients (Fisher’s Exact Test)
P ≤.03, percent ≥0.5 mcg/mL v. week 0 (McNemar’s Exact Test)
P ≤ 0.05, antibody concentration among immune strata (Kruskal-Wallis Test)
The proportion with antibody concentrations ≥0.5 mcg/mL for serotype 1 increased only among PPV recipients, as expected, from 39% to 61% at week 1 and 94% at week 4. For serotypes 6B and 14, baseline rates of antibody concentrations ≥0.5 mcg/mL were already 72–84%, increasing in PCV7 and PPV recipients to 86–94% and 90–100% at weeks 1 and 4, respectively.
Antibody avidity
Avidity ratios ranged from 0.3–0.8 for serotype 1, 0.5–0.9 for serotype 6B, and 0.6–0.9 for serotype 14, with no consistent differences among study timepoints, PCV7 v. PPV booster recipients, or strata.
Memory responses
The proportions with memory based on antibody concentration ≥0.5 mcg/mL at entry or week 1 were lower for serotype 1 (42%, 61%) compared with serotypes 6B and 14 (87–94%), for which rates were similar following PCV7 or PPV booster (Table 3). Memory responses based on ≥4-fold rise at week 1 were substantially lower than those based on antibody concentration ≥0.5 mcg/mL, with the highest rates for serotype 14. Memory based on 4-fold rise was observed for serotypes 6B and 14 more frequently following PCV7 booster than PPV booster. Trends within immune strata mirrored overall trends. All subjects manifesting memory based on ≥4-fold rise at week 1 had antibody concentrations ≥0.5 mcg/mL at week 1.
Table 3.
Memory responses detected in P1061s.
| ST | Pneumococcal Conjugate Vaccine 7 (PCV7) | Pneumococcal Polysaccharide Vaccine (PPV) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject Group | Subject Group | |||||||||||||||
| All (36) |
2 (14) |
3 (14) |
4 (8) |
All (36) |
2 (14) |
3 (14) |
4 (8) |
All (31) |
2 (10) |
3 (12) |
4 (8) |
All (31) |
2 (10) |
3 (12) |
4 (8) |
|
| Antibody Concentration ≥0.5 mcg/mL At Entry or Week 1, % (95% CI) |
≥4-fold Rise Criterion, % (95% CI) |
Antibody Concentration ≥0.5 mcg/mL At Entry or Week 1, % (95% CI) |
≥4-fold Rise Criterion, % (95% CI) |
|||||||||||||
| 1 | 42 (26–59) | 43 (21–69) | 29 (10–58) | 63 (29–89) | 3 (0–14) | 0 (0–23) | 7 (0–31) | 0 (0–35) | 61 (43–77) | 50 (22–78) | 67 (35–88) | 75 (35–95) | 13 (5–29) | 0 (0–27) | 25 (7–55) | 13 (1–50) |
| 6B | 89 (74–96)a | 86 (58–97) | 86 (58–97) | 100 (65–100) | 31 (18–47)a | 21 (6–50) | 21 (6–50) | 63 (29–89) | 87 (71–95)b | 80 (44–96) | 92 (65–100) | 88 (50–99) | 13 (5–29) | 0 (0–27) | 17 (3–45) | 25 (5–65) |
| 14 | 94 (81–99)c | 93 (69–100) | 93 (69–100) | 100 (65–100) | 53 (35–68)c | 43 (21–69) | 57 (31–79) | 63 (29–89) | 94 (80–99)d | 90 (56–99) | 100 (76–100) | 100 (65–100) | 29 (16–47) | 20 (4–56) | 25 (7–55) | 50 (19–81) |
Note: Subjects qualifying for memory analyses are divided according to whether they received a pneumococcal conjugate vaccine (PCV7) or pneumococcal polysaccharide vaccine (PPV) booster dose in P1061s. Subject group refers to all subjects (all immune strata combined), immune stratum 2, immune stratum 3, and immune stratum 4, and the number of subjects in each subject group is indicated in parentheses. The subject in immune stratum 1 is included within �all subjects�, but is not shown separately due to the small number of subjects in this immune stratum. The percentages of subjects with memory defined according to an antibody concentration ≥0.5 mcg/mL at entry or week 1 and to a ≥4-fold rise criterion (4-fold or greater antibody rise between entry and week 1) are shown. ST = serotype, CI = confidence interval.
P <.01, antibody concentration ≥0.5 mcg/mL at entry or week 1 and ≥4-fold rise criterion, serotype 6B, PCV7 recipients v. serotype 1, PCV7 recipients (Fisher’s Exact Test)
P = .04, antibody concentration ≥0.5 mcg/mL at entry or week 1, serotype 6B, PPV recipients v. serotype 1, PPV recipients (Fisher’s Exact Test)
P <.0001, antibody concentration ≥0.5 mcg/mL at entry or week 1 and ≥4-fold rise criterion, serotype 14, PCV7 recipients v. serotype 1, PCV7 recipients (Fisher’s Exact Test)
P <.01, antibody concentration ≥0.5 mcg/mL at entry or week 1, serotype 14, PPV recipients v. serotype 1, PPV recipients (Fisher’s Exact Test)
Predictors of memory
Memory was associated with higher CD4% (nadir before HAART, P1024 screening, P1061s entry) and CD19% (P1024 and P1061s entry), younger age, lower P1024 and P1061s VL, and greater antibody concentration eight weeks after P1024 vaccination (Table 4).
Table 4.
Predictors of immunologic memory in P1061s.
| Variablea | Serotypeb | Memory Response Criterion | Odds Ratio (Confidence Interval) | P-value |
|---|---|---|---|---|
| Age | 1 | 4–fold response | .63 (.33–.96)c | .03 |
| 6B | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | 78 (.61–.99)c | .04 | |
| 14 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | .65 (.43–.90)c | .01 | |
| Nadir CD4% prior to HAART | 6B | 4–fold response | 1.08 (1.01–1.16)d | .02 |
| 14 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | 1.14 (1.01–1.35)d | .04 | |
| CD4% at P1024 screening | 1 | 4–fold response | 1.44 (1.13–2.20)d | .001 |
| 6B | 4–fold response | 1.1 (1.02–1.20)d | .02 | |
| CD4% at P1061s entry | 1 | 4–fold response | 1.39 (1.09–2.05)d | .003 |
| 1 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | 1.08 (1.00–1.19)d | .04 | |
| 14 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | 1.18 (1.07–1.38)d | .0007 | |
| CD19% at P1024 entry | 1 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | 1.39 (1.13–1.91)d | .0003 |
| CD19% at P1061s entry | 1 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | 1.15 (1.00–1.40)d | .05 |
| Logarithm P1024 viral load | 1 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | .22 (.04–.85)e | .03 |
| 14 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | .21 (.04–.84)e | .03 | |
| P1024 viral load ≤400 copies/mL (v. >400 copies/mL) | 14 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | 9.6 (1.14–202.19) | .04 |
| P1024 viral load ≤5000 copies/mL (v. >5000 copies/mL) | 1 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | 12.86 (1.69–270.89) | .01 |
| Logarithm P1061s viral load | 14 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | .30 (.09–.88)e | .03 |
| Logarithm P1024 antibody | 6B | 4–fold response | 3.07 (1.08–10.23)f | .03 |
| Concentration after PCV7-PCV7-PPV (P1024 Week 24) | 6B | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | 6.82 (1.54–42.63)f | .01 |
| 14 | Antibody concentration ≥0.5 mcg/mL at entry or week 1 | 18.3 (2.18–325.15)f | .005 |
Note: Odds ratios and Profile Likelihood confidence intervals for variables associated with memory with a Likelihood Ratio Test P-value <0.05 in univariate analyses are shown. Memory was analyzed using 2 definitions: antibody concentration ≥0.5 mcg/mL at entry or week 1; 4-fold or greater antibody rise between entry and week 1.
Other variables examined included immune stratum, time on P1061s entry HAART regimen, change in HAART due to virologic failure between P1024 and P1061s, logarithm of antibody concentration at P1024 entry, viral load ≤400 copies/mL v. >400 copies/mL at P1061s entry, viral load ≤5000 copies/mL v. >5000 copies/mL at P1061s entry.
Predictors for serotype 1 are shown only for subjects who received PPV in P1061s.
Odds ratio for every year increase
Odds ratio for every 1% increase
Odds ratio for every one logarithm (copies/mL) increase
Odds ratio for every one logarithm (mcg/mL) increase
Safety
No ≥ grade 3 adverse events related to vaccination were observed.
Discussion
Long-term protection against encapsulated bacteria requires sustained antibody levels, in addition to persistence of memory B cells [16–17]. A strong correlation between persistence of antibody and frequency of memory B cells has been observed, suggesting that memory is implied by persistence of antibody [17]. We used definitions of memory that included antibody persistence and anamnestic responses. Antibody concentrations at P1061s entry remained greater than at P1024 entry for all three serotypes, more than 4 years after PCV7-PCV7-PPV vaccination. For serotypes 6B and 14, present in PCV7 and PPV, GMCs exceeded 0.5 mcg/mL, while for serotype 1, present only in PPV, the GMC was slightly below this threshold. Antibody levels ≥0.5 mcg/mL persisted in 82% of subjects for PCV7-containing serotypes and in 42% for serotype 1. Booster vaccination with PCV7 or PPV increased GMCs and the proportion with antibody concentration ≥0.5 mcg/mL (except for serotype 1 in PCV7 recipients) at one week. GMCs 4 weeks after PCV7 or PPV for serotypes 6B and 14 were 1.5–3.0-fold higher than peak concentrations following previous PCV7-PCV7-PPV, consistent with booster responses. In contrast, the GMC after PPV boosting for serotype 1 was only comparable to that after the PCV7-PCV7-PPV regimen. Although U.S. guidelines recommending pneumococcal vaccination for HIV-infected children precluded having a control group of previously unvaccinated, HIV-infected children, our finding that antibody concentrations correlated inversely with age suggests that memory, rather than age-related immune maturation, was responsible for higher GMCs for serotypes 6B and 14 after boosting compared with GMCs after the previous vaccine series. Boosting with PCV7 yielded higher GMCs than with PPV for serotypes in both vaccines, in contrast to studies in healthy children [18], while comparably high proportions with antibody concentration ≥0.5 mcg/mL were achieved for these serotypes following either vaccine. Among subjects who received a PPV booster, despite the absence of an anamnestic response for serotype 1, a high percentage achieved antibody concentrations ≥0.5 mcg/mL for all 3 serotypes by week 4.
A lower proportion with persistence of antibody concentrations ≥0.5 mcg/mL, an increase in GMC after boosting to the same level achieved after previous vaccination, and a lower rate of memory for serotype 1 are consistent with its presentation as an unconjugated polysaccharide in PPV and the experience that PPV has modest immunogenicity, poor durability, and inconsistent efficacy in HAART-treated HIV-infected subjects [8]. In contrast, a large majority demonstrated antibody persistence and memory based on antibody concentration ≥0.5 mcg/mL at entry or week 1 for serotype 6B, a traditionally poor immunogen, and serotype 14. Memory for these serotypes was comparable to memory following measles vaccination (85%) in P1024/P1061s, but greater than for hepatitis B vaccine (45%) [9–10]. Antibody, in subjects boosted with PCV7 or PPV, was predominantly high avidity antibody, which is associated with memory and functional immunity [19–20]. Rates of memory were more modest with the 4-fold response criterion, similar to measles and hepatitis B vaccination, perhaps because of relatively high antibody concentrations pre-boosting [9–10, 21].
Antibody persistence and memory for PCV7 serotypes in children vaccinated while on HAART contrasts with waning efficacy and seropositivity and modest boosting after PCV vaccination in the absence of HAART in HIV-infected South African children [5]. Previous studies of HIV-infected children on HAART demonstrated low responses and short-lived antibody persistence following primary PCV7 vaccination and low responses and declining avidity after a PCV7 booster, mirroring findings in HIV-infected adults [14, 22–23]. Depletion of memory cells by previous PPV vaccination was proposed as a cause of impaired induction of memory [22]. In contrast, subjects in P1024/P1061s manifested memory (and lacked hyporesponsiveness) despite 75% having received ≥1 PPV doses prior to PCV7 [8]. Experience with Haemophilus influenzae type b conjugate vaccine in HIV-infected children is similarly conflicting [24–25]. Disparate results may reflect dissimilarities in HIV VL and CD4 profiles, vaccination schedules, age, and study endpoints [26]. Although several studies suggest that reductions in number or function of memory B cell subsets in HIV-infected individuals may limit memory [27–30], the present study suggests that a PCV7- and PPV-containing vaccine regimen does elicit memory in older HIV-infected children on HAART who have favorable CD4 counts and low HIV VL.
Higher CD4% (prior to HAART, at primary PCV7 vaccination, or at boosting) and lower VL (at primary PCV7 vaccination or boosting) were predictive of memory, similar to HBV vaccine-induced memory, but contrasting with live-virus measles vaccine, for which HIV VL, but not CD4 measures, was predictive. Greater antibody concentration after the PCV7-PCV7-PPV series was a strong predictor of memory, similar to findings for HBV and measles vaccines [9–10].
Antibody boosting due to carriage of serotypes 6B and/or 14 is a possible alternative explanation for antibody persistence we observed. This possibility is mitigated, however, by low pneumococcal carriage rates in adolescents and declines in carriage of PCV7 serotypes following introduction of universal infant/toddler PCV7 vaccination. Other possible study limitations include use of ELISA rather than functional antibody responses [24] and that protective antibody concentrations established in healthy populations may overestimate protection in HIV-infected children [31]. Nevertheless, we found antibody to have high avidity, suggesting adequate quality. The definition of memory based on ≥4-fold antibody increase one week after boosting likely underestimated memory if kinetics are delayed in HIV-infected children. Finally, power may have been limited by the number of subjects, chance associations may have occurred due to multiple comparisons, and exclusion of subjects with high HIV VL may limit generalizability.
Pneumococcal infections continue to be an important threat to HIV-infected children, even in the HAART era [1]. Whereas previous studies failed to demonstrate sustained immunogenicity and efficacy of PPV or PCV in HIV-infected children, our findings suggest that a PCV- and PPV-containing regimen in combination with HAART results in memory that persists at least 4–5 years and is likely to provide durable protection against invasive pneumococcal disease.
Supplementary Material
Highlights.
HIV-infected children were studied following pneumococcal vaccination on HAART
Subjects received 2 conjugate (PCV) and 1 polysaccharide (PPV) vaccine doses
4–5 years later, most had protective antibody concentrations for PCV serotypes
Antibody responses following a PCV or PPV booster were indicative of memory
Antibody measured was primarily high avidity antibody
Acknowledgments
Funding
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD [contract number N01-DK-9-001/HHSN267200800001C]. This work was also supported in part by the General Clinical Research Center Units funded by the National Center for Research Resources, National Institutes of Health [including MO1-RR00069, General Clinical Research Centers Program]. Wyeth-Lederle Vaccines and Merck and Co. provided study vaccines used in P1061s. Neither the funding agencies nor the pharmaceutical companies had involvement in the analysis and interpretation of data, writing of the manuscript, or submission for publication.
The authors appreciate the participation of patients and families in this study and the assistance of research personnel at the study sites. The authors thank Kenneth Stanley for his contributions to the data analysis plan.
Abbreviations
- PCV
pneumococcal conjugate vaccine
- PPV
pneumococcal polysaccharide vaccine
- VL
viral load
Additional members of the P1024 and P1061s Protocol Teams
Shirley Jankelevich, M.D. and Patrick Jean-Philippe, M.D., Pediatric Medicine Branch, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland; Jennifer Read, M.D., Pediatric, Adolescent, and Maternal AIDS Branch, National Institute of Child Health and Human Development, Bethesda, Maryland; Victoria Hadhazy, M.A., Alison Robbins, M.A., and Beth Sheeran, M.S., R.D., PACTG/IMPAACT Operations Office, Rockville, Maryland; Jane C. Lindsey, Sc.D. and Terence Fenton, Ed.D., Statistical and Data Analysis Center, Harvard School of Public Health, Boston, Massachusetts; Janice Hodge, R.N., B.S., Mary Caporale, MSc., M.P.H., Nancy Webb, M.S., and Courtney Ashton, Frontier Science and Technology Research Foundation, Amherst, New York; Dorothy Smith, M.S., C.P.N.P., University of Massachusetts Memorial Medical Center, Worcester, Massachusetts; Lourdes Angeli, RN, B.S.N., M.P.H., San Juan City Hospital, San Juan, Puerto Rico; John Pella, C.C.R.A., C.C.R.C., M.P.H., Westat, Inc., Rockville, Maryland; Paul Tran, R.Ph., Pharmaceutical Affairs Branch, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland; Howard M. Rosenblatt, MD, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas; Grace Aldrovandi, MD, Children's Hospital of Los Angeles, Los Angeles, California; Rachel Barrett, B.S., University of Colorado School of Medicine, Denver, Colorado; William Kabat, B.S., Chicago Children’s Memorial Hospital, Chicago, Illinois; Alan Shaw, Ph.D., Merck and Co., West Point, Pennsylvania; Daniel Isaacman, M.D. and Velma Keeley, B.S., Wyeth Pharmaceuticals, Collegeville, Pennsylvania; Paul Willems, M.D., GlaxoSmithKline Pharmaceuticals, Collegeville, Pennsylvania; Carol Gore, Panorama Village, Texas.
Participating sites and site personnel:
Chicago Childrens’ Memorial Hospital (Ram Yogev, M.D.), UMDNJ-New Jersey Medical School (Barry Dashefsky, M.D., Linda Bettica, R.N., Paul Palumbo, M.D.), Harlem Hospital (Elaine Abrams, M.D., Maxine Frere, R.N., Lisa Gaye Robinson, M.D.), Metropolitan Hospital Center (Mahrukh Bamji, M.D.), Long Beach Memorial Hospital (Audra Deveikis, Susan Marks, Karen Elkins, Lisa Melton), San Juan City Hospital (Eleanor Jimenez, M.D.), Los Angeles County Medical Center (James Homans, M.D., Ana Melendez, R.N., Andrea Kovacs, M.D.), University of Florida - Jacksonville (Mobeen H. Rathore, M.D., Ana Alvarez, M.D., Ayesha Mirza, M.D., Thomas Chiu, M.D.), University of California - San Diego (Stephen A. Spector, M.D., Rolando Viani, M.D., M.T.P., Mary Caffery, R.N., M.S.N., Lisa Stangl, C.P.N.P.), State University of New York at Stony Brook (Denise Ferraro, R.N., Silvia Muniz, Michell Davi, N.P.), University of Colorado School of Medicine and The Children’s Hospital (Jody Maes, M.D., Carol Salbenblatt, R.N., Suzanne Paul, B.S.N., M.S.N, F.N.P., Emily Barr, C.P.N.P., C.N.M., M.S.N.; Grant No. M01 RR00069, General Clinical Research Centers Program, National Center for Research Resources, NIH), University of Chicago Children’s Hospital, Schneider Children’s Hospital of North Shore – Long Island Jewish Health System (Vincent R. Bonagura, M.D., Susan J. Schuval, M.D., Connie Colter, M.S., C.P.N.P.), Children’s Hospital of Oakland (Ann Petru, M.D., Teresa Courville, R.N., M.N., Karen Gold, R.N., F.N.P., Lauren Poole, R.N., F. N.P.), St. Christopher’s Hospital for Children and Drexel University College of Medicine (Janet S. Chen, M.D., Jill A. Foster, M.D., Daniel H. Conway, M.D., Gary Koutsoubis), Boston Medical Center and Boston University School of Medicine (Ann Marie Regan, P.N.P.), State University of New York Downstate (H. Jack Moallem, M.D., Edward Handelsman, M.D., Denise Swindell, Jean Kaye, R.N.), Ramon Ruiz Arnau University (Wanda Figueroa, M.D.), Bronx Lebanon Hospital Center (Mavis Dummitt, R.N., Anantha Kallury, B.Pharm., Murli Purswani, M.D., Saroj Bakshi, M.D.), St. Lukes/Roosevelt Hospital Center (Emma Stuard, M.D., Steven Arpadi, M.D.), University of Massachusetts Medical Center (Katherine Luzuriaga, M.D., Dottie Smith), Children’s Hospital of Boston (Sandra Burchett, M.D., M.S., Lynne Lewis, R.N., M.S., C.P.N.P., Catherine Kneut, R.N., M.S., C.P.N.P.), North Broward Hospital District (Amy Inman, B.S., Linh Tran, Pharm.D., Guillermo Talero, M.D., Ann Puga, M.D.), University of Maryland (John Farley, M.D., Mary MacFadden), University of California – San Francisco Moffitt Hospital (Diane Wara, M.D.), New York University School of Medicine (Siham Akleh, R.N., Aditya Kaul, M.D., Sulachni Chandwani, M.D., Thomas Hastings, R.N.), University of Rochester Medical Center (Geoffrey A. Weinberg, M.D., Susan Laverty, R.N., Barbra Murante, M.S., R.N., P.N.P., Francis Gigliotti, M.D.), Yale University School of Medicine (Warren A. Andiman, M.D., Leslie Hurst, M.S., Sostena Romano, A.P.R.N.), University of Puerto Rico and University Children’s Hospital (Irma Rodriguez, M.D.), University of Connecticut Health Center/Connecticut Children’s Medical Center (Juan C. Salazar, M.D., M.P.H., Gail Karas, R.N., Lorraine Wells, R.N.), Texas Children’s Hospital and Baylor University (William Shearer, M.D.), Cook County Hospital (Jaime Martinez, M.D.), Cornell University – New York Presbyterian Hospital (Joseph Stavola, M.D.), Children’s Hospital National Medical Center (Hans Spiegel, M.D.), University of Florida – Gainesville (Robert Lawrence, M.D.), Tulane University and Charity Hospital of New Orleans (Russell Van Dyke, M.D., Cheryl Borne, R.N., Margaret Cowie, B.S.), University of Alabama at Birmingham (Robert Pass, M.D., Marilyn Crain, M.D., Newana Beatty).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Mark J. Abzug: no commercial or other association that poses a conflict of interest
Lin Ye Song: no commercial or other association that poses a conflict of interest
Myron J. Levin is a consultant to and shares intellectual property with Merck, Sharpe & Dohme.
Sharon A. Nachman: no commercial or other association that poses a conflict of interest
William Borkowsky: no commercial or other association that poses a conflict of interest
Stephen I. Pelton receives honoraria from Pfizer Inc, Merck Vaccines, and GSK bio for participation in advisory board meetings on pneumococcal vaccines and receives investigator-initiated grants from Merck Vaccines, Novartis Vaccines, and Pfizer, Inc.
References
- 1.Abzug MJ, Pelton SI. Prevention of invasive pneumococcal disease in HIV-infected children: expanding the toolbox. J Infect Dis. 2009;199:1109–1111. doi: 10.1086/597389. [DOI] [PubMed] [Google Scholar]
- 2.Flannery B, Heffernan RT, Harrison LH, Ray SM, Reingold AL, Hadler J, et al. Changes in invasive pneumococcal disease among HIV-infected adults living in the era of childhood pneumococcal immunization. Ann Intern Med. 2006;144:1–9. doi: 10.7326/0003-4819-144-1-200601030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Albrich WC, Baughman W, Schmotzer B, Farley MM. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1569–1576. doi: 10.1086/518149. [DOI] [PubMed] [Google Scholar]
- 4.Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Moore DP, Klugman KP, et al. The impact of antiretroviral treatment on the burden of invasive pneumococcal disease in South African children: a time series analysis. AIDS. 2011;25:453–462. doi: 10.1097/QAD.0b013e328341b7f1. [DOI] [PubMed] [Google Scholar]
- 5.Madhi SA, Klugman KP, Kuwanda L, Cutland C, Käyhty H, Adrian P. Quantitative and qualitative anamnestic immune responses to pneumococcal conjugate vaccine in HIV-infected and HIV-uninfected children 5 years after vaccination. J Infect Dis. 2009;199:1168–1176. doi: 10.1086/597388. [DOI] [PubMed] [Google Scholar]
- 6.French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, et al. A trial of 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362:812–822. doi: 10.1056/NEJMoa0903029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhi SA, Adrian P, Kuwanda L, Jassat W, Jones S, Little T, et al. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine. 2007;25:2451–2457. doi: 10.1016/j.vaccine.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Abzug MJ, Pelton SI, Song L, Fenton T, Levin MJ, Nachman SA, et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2006;25:920–929. doi: 10.1097/01.inf.0000237830.33228.c3. [DOI] [PubMed] [Google Scholar]
- 9.Abzug MJ, Warshaw M, Rosenblatt HM, Levin MJ, Nachman SA, Pelton SI, Borkowsky W, Fenton T International Maternal Pediatric Adolescent AIDS Clinical Trials Group P1024 and P1061s Protocol Teams. Immunogenicity and immunologic memory after hepatitis B virus booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis. 2009;200:935–946. doi: 10.1086/605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abzug MJ, Qin M, Levin MJ, Fenton T, Beeler JA, Bellini WJ, Audet S, Sowers SB, Borkowsky W, Nachman SA, Pelton SI, Rosenblatt HM International Maternal Pediatric Adolescent AIDS Clinical Trials Group P1024 and P1061s Protocol Teams. Immunogenicity, immunologic memory, and safety following measles revaccination in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis. 2012;206:512–522. doi: 10.1093/infdis/jis386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klugman KP, Madhi SA, Adegbola RA, Cutts F, Greenwood B, Hausdorff WP. Timing of serotype 1 pneumococcal disease suggests the need for evaluation of a booster dose. Vaccine. 2011;29:3372–3373. doi: 10.1016/j.vaccine.2011.02.089. [DOI] [PubMed] [Google Scholar]
- 12.Wuorimaa T, Dagan R, Väkeväinen M, Bailleux F, Haikala R, Yaich M, et al. Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J Infect Dis. 2001;184:1211–1215. doi: 10.1086/323648. [DOI] [PubMed] [Google Scholar]
- 13.Recommendations to assure the quality, safety, and efficacy of pneumococcal conjugate vaccines. Geneva: World Health Organization; 2009. [Accessed 3/7/2013]. 60th Meeting of the WHO Expert Committee on Biological Standardization. Available at: http://www.who.int/biologicals/areas/vaccines/pneumo/Pneumo_final_23APRIL_2010.pdf. [Google Scholar]
- 14.Tarrago D, Casal J, Ruiz-Contreras J, Ramos JT, Rojo P, Snippe H, et al. Assessment of antibody response elicited by a 7-valent pneumococcal conjugate vaccine in pediatric human immunodeficiency virus infection. Clin Diagn Lab Immunol. 2005;12:165–170. doi: 10.1128/CDLI.12.1.165-170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhi SA, Kuwanda L, Cutland C, Holm A, Käyhty H, Klugman KP. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis J. 2005;24:410–416. doi: 10.1097/01.inf.0000160942.84169.14. [DOI] [PubMed] [Google Scholar]
- 16.McVernon J, Johnson PD, Pollard AJ, Slack MP, Moxon ER. Immunologic memory in Haemophilus influenzae type b conjugate vaccine failure. Arch Dis Child. 2003;88:379–383. doi: 10.1136/adc.88.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanchard Rohner G, Snape MD, Kelly DF, John T, Morant A, Yu LM, et al. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J Immunol. 2008;180:2165–2173. doi: 10.4049/jimmunol.180.4.2165. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien KL, Hochmann M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007;7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 19.Usinger WR, Lucas AH. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. 1999;67:2366–2370. doi: 10.1128/iai.67.5.2366-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antttila M, Voutilainen M, Jantti V, Eskola J, Kayhty H. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin Exp Immunol. 1999;118:402–407. doi: 10.1046/j.1365-2249.1999.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thanee C, Pancharoen C, Likitnukul S, Luangwedchakarn V, Umrod P, Phasomsap C, et al. The immunogenicity and safety of pneumococcal conjugate vaccine in human immunodeficiency virus-infected Thai children. Vaccine. 2011;29:5886–5891. doi: 10.1016/j.vaccine.2011.06.072. [DOI] [PubMed] [Google Scholar]
- 22.Spoulou VI, Tsoumas DL, Papaevangelou VG, Mostrou GI, Theodoridou MC. Immunogenicity and immunological memory induced by a 7-valent pneumococcal CRM197 conjugate vaccine in symptomatic HIV-1 infected children. Vaccine. 2005;23:5289–5293. doi: 10.1016/j.vaccine.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Feikin DR, Elie CM, Goetz MB, Lennox JL, Carlone GM, Romero-Steiner S, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine. 2002;20:545–553. doi: 10.1016/s0264-410x(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 24.Spoulou VI, Tsoumas DL, Papaevangelou VG, Mostrou GI, Theodoridou MC. Haemophilus influenza type b conjugate vaccine-induced immunological memory in symptomatic HIV-1-infected children. AIDS. 2003;17:1396–1398. doi: 10.1097/00002030-200306130-00017. [DOI] [PubMed] [Google Scholar]
- 25.Rutstein RM, Rudy BJ, Cnaan A. Response of human immunodeficiency virus-exposed and –infected infants to Haemophilus influenza type b conjugate vaccine. Arch Pediatr Adol Med. 1996;150:838–842. doi: 10.1001/archpedi.1996.02170330064011. [DOI] [PubMed] [Google Scholar]
- 26.Bliss SJ, O’Brien KL, Janoff EN, Cotton MF, Musoke P, Coovadia H, et al. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect Dis. 2007;8:67–80. doi: 10.1016/S1473-3099(07)70242-6. [DOI] [PubMed] [Google Scholar]
- 27.Hart M, Steel A, Clark SA, Moyle G, Nelson M, Henderson DC, et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol. 2007;178:8212–8220. doi: 10.4049/jimmunol.178.12.8212. [DOI] [PubMed] [Google Scholar]
- 28.Titanji K, de Milito A, Cagigi A, Thorstensson R, Grützmeier S, Atlas A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 29.D’Orsogna LJ, Krueger RG, McKinnon EJ, French MA. Circulating memory B-cell subpopulations are affected differently by HIV infection and antiretroviral therapy. AIDS. 2007;21:1747–1752. doi: 10.1097/QAD.0b013e32828642c7. [DOI] [PubMed] [Google Scholar]
- 30.Iwajomo OH, Finn A, Moons P, Nkhata R, Sepako E, Ogunniyi AD, et al. Deteriorating pneumococcal-specific B-cell memory in minimally symptomatic African children with HIV infection. J Infect Dis. 2011;204:534–543. doi: 10.1093/infdis/jir316. [DOI] [PubMed] [Google Scholar]
- 31.Madhi SA, Adrian P, Cotton MF, McIntyre JA, Jean-Philippe P, Meadows S, et al. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis. 2010;202:355–361. doi: 10.1086/653704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.