Abstract
Objective
Pregnant adolescents have high rates of poor birth outcomes, but the causes are unclear. We present a prospective, longitudinal study of pregnant adolescents assessing associations between maternal psychobiological stress indices and offspring gestational age at birth and birthweight.
Method
Healthy nulliparous pregnant adolescents were recruited (n = 205) and followed during pregnancy. Ambulatory assessments over 24 h of perceived psychological stress (collected every 30 min) and salivary cortisol (6 samples) and a summary questionnaire, the Perceived Stress Scale, were collected at three time points (13–16, 24–27, and 34–37 gestational weeks). Corticotropin-releasing hormone, C-reactive protein, and interleukin 6 were assayed from blood taken at the latter 2 sessions. A final sample of 119 participants was selected for analyses.
Results
The ambulatory assessment of perceived psychological stress was positively correlated with the Perceived Stress Scale (r = .20, p = .03) but neither was associated with any of the biological assays (all ps > .20). Based on backward selection regression models that included all stress variables and relevant covariates, the ambulatory assessments of perceived psychological stress and cortisol — though not the Perceived Stress Scale — were negatively associated with gestational age at birth (F(4, 107) = 3.38, p = .01) while cortisol was negatively related to birthweight (F(5, 107) = 14.83, p < .0001).
Conclusions
Targeted interventions to reduce psychological and biological indicators of heightened stress during pregnancy may have positive public health benefits for the offspring given the associations of shortened gestation and lower birthweight with risk for poor mental and physical health outcomes.
Keywords: Pregnancy, Adolescence, Cortisol, Stress, Gestational age at birth, Birthweight
Introduction
Despite recent declines, the U.S. adolescent pregnancy rate is among the highest in the developed world — about 367,678 births per year comprising about 9% of all U.S. births [1]. Adolescent pregnancies have high rates of poor birth outcomes that are known to set the stage for poor physical and mental health trajectories [2–12]. Specifically, compared to adult women (aged 20–34), adolescents (aged 15–19) have 20% higher rates of preterm birth (defined as ≤37 gestational weeks at birth) and low birthweight (below 2500 g): 13.6% versus 11.4% and 9.6% versus 7.8%, respectively [1]. Earlier born and lower-birthweight babies are at risk for adverse health conditions such as epilepsy (see [10]), compromised cognitive development [2,5,6,8,11], lower school achievement [2], psychiatric syndromes including attention deficit hyperactivity disorder [5,7], anxiety and depression [9] and other social–emotional problems [5]. A recent neuroimaging study found that even normal variation in birthweight predicted differences across the lifespan in brain structure such that lower birthweight related to less cortical thickness, providing neural evidence that these basic characteristics of birth outcomes — timing and weight — are meaningful variables with functional significance for the future life course [12]. Independent of the tight coupling of adolescent pregnancy with poverty [3,4], it is unclear why adolescent pregnancies pose a risk to the offspring’s future health.
Research indicates that maternal antenatal psychological stress —the appraisal that situational demands exceed one’s personal resources (and the associated mood states, e.g., irritated, frustrated, angry) [13,14] — is negatively associated with birth outcomes [15–21], though some studies fail to show an association [22] and others have inconsistent findings (e.g., a positive association with birthweight yet a negative association with gestational age at birth [23]). Few studies have examined the role of antenatal psychological stress in adolescent pregnancy despite this population’s increased risk for it as well as poor birth outcomes. Several factors point to the likelihood of high psychological stress in pregnant adolescents: they often live in poverty [3,4], are confronting significant social upheaval [24,25], and have histories of adverse childhoods [26], including as the victims of prior physical or sexual abuse [23,27–31], with the associated risk for current poor psychosocial adjustment. To date, there are only four published reports on psychological stress in adolescent pregnancy in relation to birth outcomes. Three found associations between higher stress or anxiety and adverse birth outcomes [32–34], and one did not [35]. However, two that showed significant results focused on the stressful sequelae of trauma [32,33] as opposed to current perceived psychological stress that is the focus of adult studies, and the one that showed null results [35] had a very small sample size (n = 38). Moreover, recent research on perceived psychological stress and birth outcomes of adult pregnancies has begun to utilize a more ecologically-valid and reliable approach to its assessment [22,36] — diary reporting of mood states or emotional experiences on an electronic device multiple times during participants’ daily lives [37–41] — but this approach has not yet been applied to pregnant adolescents.
Stress is a biopsychosocial process that involves multiple systems [42]; an emerging literature with adult pregnant women is examining the impact of psychological, endocrine and immune stress systems on birth outcomes. Of the few studies considering maternal antenatal stress as a factor in adolescents’ poor birth outcomes, only one [35] included biological assays. Yet findings from the adult research that includes biological factors have been significant and informative. For example, recent studies have found a negative association between maternal antenatal cortisol awakening response (CAR)/diurnal salivary cortisol and gestational age at birth, birthweight and birth length [22,43].
There also is strong evidence for the role of immune system inflammatory pathways in pregnancy and parturition [44], and studies have linked maternal stress to elevated inflammatory markers during pregnancy [45–47]. One recent study demonstrated associations between elevated IL-6 and TNF-α and earlier gestational age at birth [45]. It is known that the endocrine and inflammatory systems interact and co-regulate [48]; however, as noted in a recent review [49], there are no studies examining the influence of psychological stress, HPA axis activation, and inflammation in the same model in relation to birth outcomes.
We conducted a prospective longitudinal observational study of pregnant adolescents to assess the effect of maternal antenatal stress on two key birth outcomes, gestational age at birth and birthweight. Participants were assessed at three time points (early, middle, and late pregnancy). We measured perceived psychological stress with the Perceived Stress Scale (PSS) [50] and also used the “ambulatory” approach, ecological momentary assessment (EMA), to collect momentary assessments of current mood states associated with perceived psychological stress such as “nervous”, “angry”, “strained”, “irritated”, “worried”, “stressed”, “frustrated” and “lonely” [14,50,51]. EMA offers an advantage in that momentary ratings of experiences are not dependent on recall as are traditional assessments such as the Perceived Stress Scale (PSS) [52]. Diurnal salivary cortisol was collected at the same time points. Corticotropin releasing hormone (CRH), interleukin-6 (IL-6) and C-reactive protein (CRP) were assayed from blood at the 2nd and 3rd study sessions. We hypothesized that (1) greater perceived psychological stress would predict poor birth outcomes; (2) higher cortisol would be associated with greater perceived psychological stress and poor birth outcomes; and (3) higher levels of CRH and inflammation would be associated with poor birth outcomes.
Methods
Participants
Nulliparous pregnant adolescents, ages 14–19,1 were recruited through the Departments of Obstetrics and Gynecology at Columbia University Medical Center (CUMC) and Weill Cornell Medical College and flyers posted in the CUMC vicinity. To reduce unwanted between-subject variance, only nulliparous pregnant adolescents were enrolled. All had a healthy pregnancy at the time of recruitment. Participants were excluded if they acknowledged smoking or use of recreational drugs, lacked fluency in English, or were multiparous. Participants also were excluded on the basis of frequent use of the following: nitrates, steroids, beta blockers, triptans, and psychiatric medications.
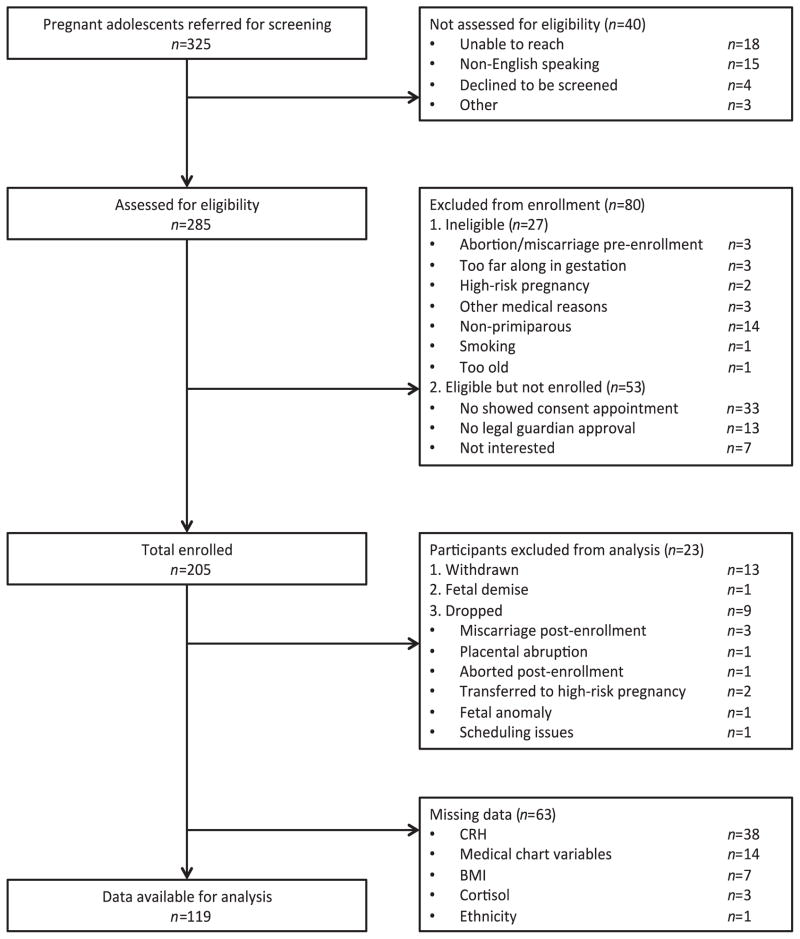
We enrolled 205 participants (see Fig. 1 for the complete enrollment flow chart). Data analysis was performed on a final sample of n = 119 adolescents and their infants.2 All enrolled participants provided written-informed consent, and all procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute/ CUMC.
Fig. 1.
Flow chart showing complete information on participant enrollment and final number for data analyses.
Study procedure
Mood and salivary cortisol assessment took place between 13–16, 24–27 and 34–37 gestational weeks (+/−1 week). Blood measures were collected at the 2nd and 3rd study sessions. (Fig. 2 illustrates the study protocol.) Birth outcome information was culled from participants’ medical charts. Participants also had one, randomly scheduled, urine toxicology screen to test for use of cannabinoids, amphetamines, benzodiazepines, opioids, and cotinine.3
Fig. 2.
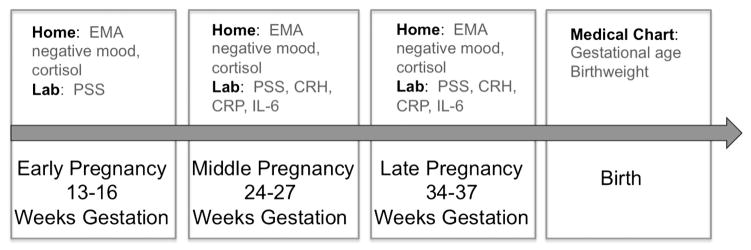
Study procedure depicting measures collected at each time point. Measures were gathered in the laboratory, in the participant’s home, or using the medical chart. The arrow represents time. Measures are listed above the arrow and time points of the study are listed below. CRH = corticotropin-releasing hormone; CRP = C-reactive protein; EMA = ecological momentary assessment; IL-6 = interleukin 6; PSS = Perceived Stress Scale.
Perceived psychological stress
The PSS [52] is a 14-item instrument designed to measure the degree to which participants appraise their lives over the past month as “unpredictable, uncontrollable, and overloading” — i.e., high on perceived psychological stress [13]. On the PSS, respondents rate the frequency of specific experiences on a 5-point scale from “never” to “very often” (e.g., “In the last month how often have you found that you could not cope with all the things that you had to do?”). The PSS has been shown to have adequate reliability, reporting a coefficient alpha of .84 to .86 [50].
Following other research utilizing EMA assessments with adolescents [14,53], for 24-hour periods participants provided ratings on 18 mood states using a personal digital assistant (PDA). Mood states were scored on a 5-point Likert scale and included the following: cheerful, cooperative, responsible, caring, proud, friendly, relaxed, productive, hard-working, lonely, nervous, angry, frustrated, competitive, strained, worried, irritated, stressed (see below for the construction of the negative mood calculation). Participants were instructed to provide ratings every 30 min while awake at the time an automated ambulatory blood pressure unit inflated (data not included in this study). Participants were able to enter mood ratings at additional times. They were incentivized to provide ratings, earning $0.50 per entry.
Salivary cortisol
Diurnal salivary cortisol was collected over 48 h beginning at the time of the study session visit. Subsequent samples were collected on the following schedule: at waking; 45 min, 2.5 h, 3.5 h, and 8 h after waking; and at 10 PM or before going to bed. (Participants continued the schedule of collection at the next collection point relative to their study visit.) Salivette tubes (Sarstedt, Newton, NC) were used for cortisol collection. The cotton used for each sample was kept in a bottle with a Medication Event Monitoring System (MEMS) cap (Aardex, Union City, CA) to record the time of opening. Once used, the cotton was placed in a Salivette tube. After being returned to the lab, samples were kept frozen until assayed using a commercial ELISA kit (Salimetrics, State College, PA). Only samples from the second collection day, which included all time points and is equal to a total of 6 potential samples, were used in the analyses.
Cytokines and CRH
10 ml blood samples were collected in EDTA tubes. Samples were placed on ice, spun down and frozen at −80 °C within 60 min of collection. IL-6 and CRP were assayed using high sensitivity commercial ELISA kits (HS-IL-6: R+D Systems, Minneapolis, MN; Zymutest HS-CRP: Diapharma, West Chester, OH). Plasma CRH was measured by radioimmunoassay as previously described [54] with the following modifications: For the extraction procedure, 1 ml of each plasma sample was mixed with 4 ml ice-cold methanol in glass tubes, vortexed for 1 min and incubated on ice for 20 min. Tubes were spun at 3500 rpm for 15 min at 4 °C. The top layer was transferred to a clean glass tube on ice. 0.5 ml of ice-cold methanol was added to the remainder fraction, vortexed and spun again at 3500 rpm for 15 min at 4 °C. The top layer was added to the first removed aliquot and dried in a vacuum centrifuge. Samples were concentrated twofold during reconstitution in an assay buffer and incubated at 4 °C for 48 h. CRH antibody (Abcam, Cambridge, MA) was diluted 1:106. CRH (Sigma, St. Louis, MO) was diluted for the standard curve of 0–2500 pg/ml and CRH I125 tracer (PerkinElmer, Waltham, MA) to 4000 counts/tube. Extraction efficiency was 60%. The detection limit was 18 pg/ml and the intra-assay coefficient of variation was <10%.
Birth outcomes
Gestational age at birth and birthweight were determined from the medical record as was information on the presence of pregnancy complications (defined as infection, preeclampsia/hypertension, vascular complications, diabetes mellitus, and other), C-section, and sex of the infant. Gestational age at birth was determined based on the medical record reporting of dating based on ultrasound examinations and last reported menstrual cycle.
Data preparation
Body mass index (BMI) was calculated using pre-pregnancy weight from self-report and measured height, both ascertained at the first study session. To create an index of EMA perceived psychological stress, hereafter referenced as EMA negative mood, all items from the mood assessments were entered into an exploratory factor analysis using maximum likelihood estimation as the fitting method. Items with loadings below 0.5 were excluded from further analysis. The negative mood index at each time point was calculated based on the average of the following items: angry, frustrated, irritated, and stressed. All items included in the EMA negative mood index are associated with perceived stress [14,50,51]. Because of variability in the number of diary entries (see below), a weighted score was calculated as follows: For each person, for each diary entry, the mean of the four mood states (angry, frustrated, irritated and stressed) was calculated. Next, for each person, for each time point, the average of the four mood states was calculated. Then, the weight for each study session was applied to that average by multiplying the two terms, yielding the weighted score for that session. A weight was determined by dividing the number of diary entries at the session of interest by the total number of diary entries across all study sessions. The final weighted score was calculated by taking an average of the weighted scores across all sessions. (Average negative mood in session 1 * # of entries in session 1 / total number of entries + average negative mood in session 2 * # of entries in session 2 / total number of entries + average negative mood in session 3 * # of entries in session 3 / total number of entries in session 3.) Area under the curve (AUC) cortisol [55] was calculated using units of μg/dl and requiring the presence of at least 4/6 cortisol samples within the 24-hour period. CRH was adjusted for gestational age at time of collection as follows: log transformed CRH values were regressed on estimated due date then residuals were used as the CRH predictor variable in the models [56]. To adjust birthweight in relation to gestational age, we followed recent approaches that characterize the non-linear relationship and identify birthweight as rising with greater gestational age, with steeper slopes during the 3rd trimester, and leveling off beyond 40 weeks [57]. We added gestational age at birth, squared gestational age at birth, and cubed gestational age at birth to our model selection approaches (described below). The cubed gestational age at birth was removed during the model selection process to arrive at optimal model fitness (see details below). Log transformations were performed on CRH, CRP, IL-6, gestational age at birth and corrected birthweight variables as the raw values of these variables were not found to be normally distributed.
Data analysis and criteria for missing data
To test the influence of psychological and biological stress variables on gestational age at birth and birthweight, backward selection regression models were designed including both a priori predictor variables (i.e., EMA negative mood, PSS, cortisol, CRH, CRP, IL-6) and covariates (i.e., birth complications, C-section, ethnicity, pre-pregnancy BMI, infant sex). Specifically, backward selection regression uses a backward elimination technique for model selection, starting from the full model including all possible independent effects, and then removes variables at each step to give the lowest value of the Akaike Information Corrected Criterion (AICC) and then deems the model most parsimonious at the point when deleting a variable would increase the AICC statistic. (Table 1 lists each of the variables entered into the excluding the outcome and ‘other’ variables.) These models included average values of predictor stress variables across pregnancy. Participants were excluded from analysis if average data were missing from any variable in the model. SAS 9.3 was used for all statistical analyses. All tests were two-tailed with the alpha set at 0.05. The White test was used to evaluate the variance of residuals as homogeneous (p > .05) for both the birthweight and gestational age models. Kolmogorov–Smirnov tests were used to test for normal distribution. Due to significant missing data, the fully conditional specification (FCS) method was used to impute missing values for additional analyses. The missing values of each predictor/control variable were imputed by regression on all the others. Outcome variables were not imputed. The final models of log transformed birthweight and log transformed gestational age at birth were rerun with multiple imputations.
Table 1.
Descriptives
| Variables | N | Mean | Std dev | Min | Max |
|---|---|---|---|---|---|
| Outcome variables | |||||
| Birthweight (grams) | 118 | 3206.99 | 567.14 | 947.00 | 4525.00 |
| Gestational age at birth (weeks) | 119 | 39.03 | 2.28 | 26.57 | 41.57 |
| Predictor variables | |||||
| Maternal pre-pregnancy BMI | 119 | 25.97 | 6.32 | 16.57 | 47.6 |
| EMA negative mood | 119 | 0.61 | 0.30 | 0.33 | 1.93 |
| Perceived Stress Scale | 119 | 26.40 | 5.46 | 12.00 | 40.33 |
| Cortisol (μg/dl)a | 119 | 3.02 | 0.92 | 1.00 | 6.21 |
| Corticotropin releasing hormone (CRH) (pg/ml) | 119 | 659.09 | 590.16 | 18.00 | 4664.00 |
| C-reactive protein (CRP) (mg/ml) | 119 | 8.33 | 7.86 | 0.01 | 43.23 |
| Interleukin-6 (IL-6) (pg/ml) | 119 | 1.97 | 1.19 | 0.50 | 7.54 |
| Pregnancy complications | |||||
| No | 59 | – | – | – | – |
| Yes | 60 | – | – | – | – |
| C-section | |||||
| No | 89 | – | – | – | – |
| Yes | 29 | – | – | – | – |
| Ethnicity | |||||
| Hispanic | 107 | – | – | – | – |
| Non-Hispanic | 12 | – | – | – | – |
| Infant sex | |||||
| Male | 73 | – | – | – | – |
| Female | 46 | – | – | – | – |
| Other | |||||
| Maternal age (years) | 119 | 17.88 | 1.15 | 14.00 | 20.00 |
| EMA — total # of entries | 119 | 50.25 | 23.44 | 4.00 | 118.00 |
| EMAb — # of entries each day | 119 | 19.61 | 8.07 | 3.00 | 59.00 |
| Cortisol — total # samples submitted | 119 | 13.59 | 3.72 | 4.00 | 19.00 |
| Cortisol — # of samples each day | 119 | 5.78 | 0.56 | 4.00 | 7.00 |
Std dev = standard deviation; BMI = body mass index; EMA = ecological momentary assessment; Min = minimum; Max = maximum.
Area under the curve with respect to ground.
As previously described, EMA negative mood data were weighted to account for variability in the number of responses.
Results
Missing data
As noted, 63 participants had missing data for at least one variable, which excluded them from the analyses (see Fig. 1).
Descriptives
Descriptive statistics are provided in Table 1. In all, 89.9% of the pregnant adolescents were Hispanic, 50.4% experienced birth complications, 61.3% of the babies were male, 8.4% were born preterm, and 10.1% were born with low birthweight. National rates for pregnant Hispanic adolescents are as follows: 12.7% were born preterm and 7.9% were born with low birthweight [1]. Compared to the enrolled sample (n = 205), Wilcoxon–Mann–Whitney tests showed that the participants who were included in the analyses (n = 119) did not differ on any of the demographic variables, e.g., BMI, ethnicity (all ps > .05).
EMA negative mood, cortisol collection, and PSS
Participants submitted on average 50.25 (sd = 23.44) total EMA negative mood entries and on average, 19.61 (sd = 8.07) entries a day; they provided 13.59 (sd = 3.72) total salivary cortisol samples and on average 5.78 (sd = 0.56) a day, indicating reasonable compliance with the study protocol (Table 1). Specifically, with respect to the EMA diary reporting, the average of nearly 20 entries over a 24-hour period suggests almost one entry every 30 min (as requested) for a 12-hour period. However, as previously indicated, given the range of number of responses (see Table 1), the EMA negative mood data were weighted to control for variability in response compliance. For cortisol, the average total of nearly 14 (out of a possible 18 samples) and of almost 6 daily samples shows overall good conformity with the study protocol. More specifically, across each study period, the following proportion of participants supplied >5 cortisol samples (and the others included in models supplied 4): 86.5%, 91.3%, and 91.1%, at the 1st, 2nd and 3rd sessions, respectively. The average PSS score was 26.4 (sd = 5.46). This score is comparable to those obtained by DiPietro et al. [58] and Evans et al. [59], though notably closer to that of the depression, anxiety and comorbid groups of Evans et al. [59].
Correlations
Correlations between the predictor and outcome variables were assessed (Table 2). A modest positive correlation between EMA negative mood and PSS score (r = .20, p = .03) was detected, but there were no correlations between psychological and biological markers of stress (all ps > .20).
Table 2.
Correlations between predictor and outcome variables in backward selection regression models
| Pre-pregnancy Body Mass Index | Perceived Stress Scale | EMA Negative Mood | C-reactive Protein (CRP)a | Interleukin-6 (IL-6)a | Corticotropin- releasing Hormone (CRH)a | Cortisolb | Maternal Age | Gestational Age at Birtha | Birthweighta,c | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-pregnancy Body Mass Index | ||||||||||
| Perceived Stress Scale | −0.04 0.64 |
|||||||||
| EMA Negative Mood | −0.05 0.61 |
0.20 0.03 |
||||||||
| C-reactive Protein (CRP)a | 0.43 0.00 |
−0.06 0.50 |
−0.02 0.87 |
|||||||
| Interleukin-6 (IL-6)a | 0.31 0.00 |
−0.12 0.20 |
−0.08 0.41 |
0.31 0.00 |
||||||
| Corticotropin- releasing Hormone (CRH)a | −0.17 0.06 |
−0.04 0.70 |
−0.07 0.47 |
−0.01 0.91 |
0.06 0.52 |
|||||
| Cortisolb | −0.17 0.07 |
0.06 0.50 |
−0.02 0.79 |
−0.07 0.42 |
0.12 0.20 |
0.20 0.03 |
||||
| Maternal Age | 0.12 0.18 |
−0.12 0.20 |
0.07 0.46 |
0.19 0.04 |
0.15 0.1 |
0.09 0.34 |
0.03 0.71 |
|||
| Gestational Age at Birtha | 0.10 0.26 |
0.04 0.64 |
−0.40 0.00 |
−0.07 0.47 |
−0.11 0.25 |
−0.16 0.08 |
−0.28 0.00 |
−0.03 0.71 |
||
| Birthweighta,c | 0.12 0.20 |
−0.12 0.20 |
0.11 0.25 |
0.03 0.78 |
0.09 0.32 |
−0.11 0.25 |
−0.10 0.28 |
0.04 0.66 |
0 1.00 |
Pearson correlation coefficients (r-values) appear at the top of each cell. p-Values (two-tailed test) appear at the bottom of each cell. All n’s and units of measurement are listed in Table 1.
Log-transformed variable.
Area under the curve with respect to ground.
Quadratic correction for gestational age at birth.
Maternal stress and birth outcomes
Tables 3 and 4 show results from backward selection regression models that provide some support for hypotheses 1 and 2; they indicate that across all participants included in analyses, EMA negative mood and cortisol were each negatively associated with gestational age at birth; there are only trend associations predicting birthweight. However, despite log transforming the gestational age at birth and birthweight variables, these models did not reach criteria testing for normality: Normality test of residuals: Kolmogorov–Smirnov test, D = 0.16, p < .01, and D = 0.14, p < .01, respectively.
Table 3.
Backward selection regression model: gestational age at birtha (n = 119) F(4,114) = 11.29, p < .0001
| Variables | Estimate (β) | Standard error | p-Value |
|---|---|---|---|
| Perceived Stress Scale | 0 | 0 | .080 |
| EMA negative mood | −0.10 | 0.02 | <.001 |
| Corticotropin-releasing hormone (pg/ml)a | −0.01 | 0.01 | .113 |
| Cortisol (μg/dl)b | −0.02 | 0.01 | .001 |
Bold indicates significant effects.
Log-transformed variable.
Area under the curve with respect to ground.
Table 4.
Backward selection regression model: birthweighta (n = 118) F(5,112) = 51.01, p < .0001
| Variables | Estimate (β) | Standard error | p-Value |
|---|---|---|---|
| Gestational age at birth (weeks) | 19.94 | 9.68 | .042 |
| Gestational age at birth * gestational age at birth (weeks) | −2.39 | 1.36 | .083 |
| Infant sex (male = 0, female = 1) | −0.04 | 0.02 | .099 |
| Perceived Stress Scale | −0 | 0 | .087 |
| EMA negative mood | 0.08 | 0.04 | .087 |
Bold indicates significant effects.
Log-transformed variable.
Based on data inspection of boxplots, we identified 7 infants with gestational ages at birth that qualified as outliers (each was below 36 weeks) and 5 with birthweights that qualified as outliers (4 below 2200 g and one above 4000 g). Tables 5 and 6 show results from backward selection regression models excluding outliers; they indicate that EMA negative mood and cortisol were each negatively associated with gestational age at birth and that cortisol was negatively associated with birthweight. The results of normality test of residuals were as follows: D = 0.08, p < .06, and D = 0.08, p < .09, respectively.
Table 5.
Backward selection regression model: gestational age at birtha (n = 112) F(4,107) = 3.38, p < .0120
| Variables | Estimate (β) | Standard error | p-Value |
|---|---|---|---|
| Perceived Stress Scale | 0 | 0 | .098 |
| EMA negative mood | −0.02 | 0.01 | .043 |
| Corticotropin-releasing hormone (pg/ml)a | −0 | 0 | .280 |
| Cortisol (μg/dl)b | −0.01 | 0 | .013 |
Bold indicates significant effects.
Log-transformed variable.
Area under the curve with respect to ground.
Table 6.
Backward selection regression model: birthweighta (n = 113) F(5,107) = 14.83, p < .0001
| Variables | Estimate (β) | Standard error | p-Value |
|---|---|---|---|
| Gestational age at birth (weeks) | −34.79 | 16.24 | .034 |
| Gestational age at birth * gestational age at birth (weeks) | 5.01 | 2.24 | .028 |
| Infant sex (male = 0, female = 1) | −0.06 | 0.02 | .011 |
| Ethnicity (non-Hispanic = 0, Hispanic = 1) | −0.08 | 0.03 | .030 |
| Cortisol (μg/dl)b | −0.02 | 0.01 | .034 |
Bold indicates significant effects.
Log-transformed variable.
Area under the curve with respect to ground.
Finally, because our sample size was significantly reduced due to missing data (see Fig. 1), we applied imputation methods to our data (see above). Tables 7 and 8 indicate that for these analyses, the results for gestational age at birth are similar to those from the complete data set; for birthweight, while cortisol remains in the model, it no longer is a significant variable.
Table 7.
Backward selection regression model: gestational age at birtha (n = 173)
| Variables | Estimate (β) | Standard error | 95% confidence limits | p-Value | |
|---|---|---|---|---|---|
| Perceived Stress Scale | 0 | 0 | −0 | 0 | .320 |
| EMA negative mood | −0.04 | 0.01 | −0.06 | −0.02 | <.001 |
| Corticotropin-releasing hormone (pg/ml)a | −0.01 | 0.01 | −0.03 | 0.01 | .312 |
| Cortisol (μg/dl)b | −0.02 | 0.01 | −0.03 | −0.01 | .004 |
Bold indicates significant effects.
Log-transformed variable.
Area under the curve with respect to ground.
Table 8.
Backward selection regression model: birthweighta (n = 176)
| Variables | Estimate (β) | Standard error | 95% confidence limits | p-Value | |
|---|---|---|---|---|---|
| Gestational age at birth (weeks) | 33.80 | 7.90 | 18.29 | 49.31 | <.001 |
| Gestational age at birth * gestational age at birth (weeks) | −4.39 | 1.12 | −6.58 | −2.20 | <.001 |
| Infant sex (male = 0, female = 1) | −0.04 | 0.02 | −0.08 | −0 | .037 |
| Ethnicity (non-Hispanic = 0, Hispanic = 1) | −0.05 | 0.03 | −0.11 | 0.01 | .134 |
| Cortisol (μg/dl)b | −0.02 | 0.01 | −0.04 | 0.01 | .185 |
Bold indicates significant effects.
Log-transformed variable.
Area under the curve with respect to ground.
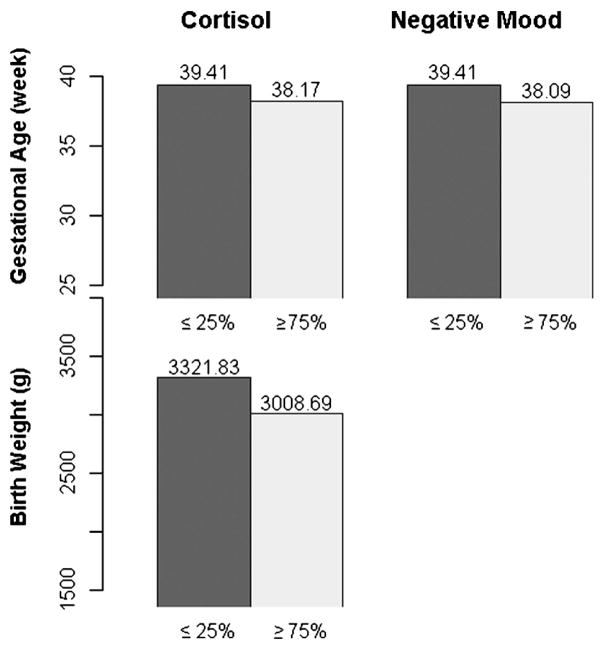
To visually represent the associations between the significant maternal stress variables and infant birth outcomes, Fig. 3 shows average gestational age at birth and birthweight in relation to adolescents with the lowest quartile values for cortisol and EMA negative mood versus those with the highest quartile across all participants.
Fig. 3.
The left bars (dark) represent participants with average cortisol or average EMA negative mood ≤25%; the right bars (light) are for those with values ≥75%. Bar height: mean of outcomes (gestational age or birthweight).
Removal of other outliers and other confounds
Because the number of EMA mood assessments and cortisol samples varied, boxplots for these variables were assessed to identify possible outliers. Two outliers were identified for EMA mood and one outlier was identified for cortisol. To determine whether these participants unduly influenced the results, the primary models were rerun with these participants excluded. The results were unchanged. Because 34 participants (28.57%) had urinary tract infections (UTIs) at some point during pregnancy, we re-ran the two birth outcome models excluding these participants. The results were unchanged.
Discussion
Using a psychobiological approach to adolescent risk for poor birth outcomes, we assessed the influence of maternal antenatal perceived psychological (EMA PSS), endocrine (cortisol, CRH) and inflammatory (CRP, IL-6) stress markers averaged over the course of pregnancy on gestational age at birth and birthweight. Several notable results were found. First, averaged over the course of pregnancy, EMA negative mood and cortisol were found to relate negatively to gestational age at birth while cortisol was associated with earlier birthweight; no effects of the inflammatory markers were found. Second, we found that it was EMA negative mood, but not the PSS, that was associated with birth outcomes. Importantly, these results were found with a sample of pregnant adolescents recruited through routine prenatal care appointments, drug-free according to random urine screens, and with rates of preterm birth and low birthweight below the national averages; thus maternal antenatal stress effects were found in a sample possibly shifted towards health compared to the norms of this population.
Birth outcomes — average stress effects
Gestational age at birth is a robust predictor of cognitive and health outcomes, and it is becoming increasingly recognized that the effects extend from early preterm through full term [2,7,60]. For example, a recent study showed that gestational age at birth — treated continuously among term babies alone — related positively to academic ability at 8 years of age [2]. Negative associations between the maternal psychological [15,16,18,19,21,23] and endocrine [22,43] stress systems and infant gestational age at birth have been well documented. Here, treating gestational age at birth as a continuous measure, we found that both maternal EMA negative mood and AUC cortisol were negatively associated with earlier birth age. These results were stable with and without outliers included (7 children born prior to 36 weeks), and in our imputation model. Specifically, even when we removed the 7 outlier infants — possibly those most affected by maternal stress as suggested by the change in the model estimates — we found that for every unit increase in AUC (μg/dl) cortisol, gestational age at birth dropped by 0.79%; when the weighted EMA negative mood score increased from the median (0.51) to the 90th quantile (0.92), gestational age at birth dropped by 0.88%.4 We did not observe effects of the immune markers.
Though the pathways to gestational age at birth and birthweight may differ, as with gestational age at birth, birthweight has been shown to be a robust predictor of cognitive and health outcomes [5–7,9–11], and the effects hold up within the healthy range of birthweight [6,8,61]. Similar to gestational age at birth, negative associations between birthweight and the maternal psychological [15,18,20] and endocrine [62] stress systems have been demonstrated, though there is at least one report of psychological stress indicating effects in the opposite direction [23]. In the present study, treating birthweight as a continuous measure and correcting for gestational age at birth, in support of our hypotheses, we found a negative association between maternal AUC cortisol and birthweight, though this association only emerged once birthweight outliers were removed from analyses, and was not maintained in the imputation model (cortisol was kept in the model but did not reach significance). Specifically, the results showed that for every unit increase in AUC (μg/dl) cortisol, birthweight dropped by 2.43%.
The impact of elevations in maternal cortisol and perceived psychological stress (as reported via EMA assessment of negative mood) was comparable to that found in relation to smoking during pregnancy. Specifically, across all babies, those exposed to the highest compared to the lowest quartile of maternal cortisol had birthweights of 3008.69 versus 3321.83 g and gestational ages at birth of 38.17 versus 39.41 weeks; for EMA negative mood the results were 38.09 versus 39.41 gestational weeks at birth (see Fig. 3). In two reports of pregnant women smokers versus non-smokers, the results were 38.3 versus 38.6 weeks [63] or 3139 versus 3409 g (unadjusted) and 38.6 versus 39.5 weeks [64].
EMA vs. PSS
As noted, EMA negative mood was associated negatively with gestational age at birth. Though it has been found to relate to a variety of interesting and important outcomes [50,65], and though it related positively to EMA negative mood in the current study, the PSS was not found to have predictive value on birth outcomes relative to the other variables included in the models. It is of interest to note that the PSS is a one-time measurement depending on participant recall of the past month whereas the EMA method gathers ratings in the present. Thus, EMA may be a more sensitive method of assessing perceived psychological stress (and associated emotional states) [22] in relation to biological or health variables, with the current study adding to a growing body of work demonstrating such results [53,66,67]. One prior study of adult pregnancy, which did not find an effect of EMA on birth outcomes, had a much smaller sample size and range of gestational ages at birth [22].
A psychobiological approach
The present study offers the advantage that the influence of psychological stress was assessed along with the influence of endocrine and immune stress markers on birth outcomes. The results based on average indices of psychological and biological stress revealed an influence of both EMA and endocrine stress measures, but none were found for the inflammatory markers. This is consistent with a recent study that also failed to find a relationship between circulating levels of immune system markers, IL-6 and TNF-α, and birth outcomes [68]. Associations between inflammatory markers and preterm birth are more commonly found in local tissues such as amniotic fluid and vaginocervical fluid [69,70], though a recent study has demonstrated associations between elevated systemic IL-6 and TNF-α (but not CRP) and reduced gestational age at birth [45]. We also did not find any associations between maternal psychological reports of stress using PSS or EMA and assays of the biological effectors of stress, e.g., cortisol, CRH or inflammatory systems, suggesting that psychological stress and cortisol have independent effects on birth outcomes. Our failure to demonstrate an association between maternal perceived stress and cortisol is consistent with other reports in the literature [71,72]. Our findings contrast with a report by Entringer et al. [22] showing a positive correlation between EMA negative mood and cortisol in adults. On the other hand, the few existing studies on pregnant adolescents have identified aspects of cortisol regulation that differ from adults, and thus may indicate a unique psychobiological regulation that contributes to our null results predicted based on some findings with adults. For example, pregnant adolescents who scored lower on self-reports of anxiety and depression had greater cortisol reactivity to a novel situation [73]. In other research, pregnant adolescents who scored lower on a measure of state anxiety had higher resting cortisol — the opposite of what would be predicted [14,74].
While use of an EMA approach to stress measurement for the characterization of maternal antenatal stress appears to be more sensitive than retrospective self reports (even those spanning just a few weeks, as is typical) for predicting birth outcomes, we were unable to demonstrate an association between the EMA measure and any of our hypothesized endocrine or immune mediators. Other studies have also failed to demonstrate a physiological mediator of the effects of stress on birth outcomes (see [75]). It is possible that the effects of maternal psychological stress on birth outcomes are mediated through other physiological mechanisms that were not included here, such as glucocorticoid feedback sensitivity, the autonomic nervous system, or endocrine and immune changes in specific tissues.
Limitations and future directions
Our study has several limitations that should be noted. Our results may not generalize to other pregnant adolescent populations. There may have been self-selection bias among our participants as ours was a longitudinal study that involved several time points of data collection and a large amount of interaction between the researchers and participants. It is possible that our participants were ones who found the study to be a source of social support during pregnancy and were able to remain engaged. In addition, exclusion criteria such as drinking or smoking may limit the generalizability of our results. The sample also was largely Hispanic so the results may not generalize to other ethnic/racial groups. In addition, though our sample was relatively compliant with the extensive protocol demands, there were significant missing data; the failure of the imputation model of birthweight to be consistent with the findings based on the smaller, albeit complete, data set underscores the need to replicate our findings. An important consideration for research of stress during pregnancy is the population that is being studied. Several studies have focused on low-income women due to their increased risk for life stress [42,76]; we chose the adolescent period, one that is also characterized by increased risk for stress, as well as poor birth outcomes. Whether these results generalize to adult pregnant women is an empirical question.
Summary and conclusions
The present study is the first in pregnant adolescents to examine perceived psychological stress and stress biomarkers in relation to birth outcomes. Strengths of the study include: (1) dimensional approach to birth outcomes, (2) EMA mood assessment and (3) endocrine stress biomarkers, including ambulatory cortisol collection. Overall, it was found that even in a sample of pregnant adolescents with better birth outcomes than the national averages, higher average EMA negative mood and cortisol during pregnancy related to lower gestational age at birth and that higher cortisol predicted lower birthweight. Of note, only these measures that had repeated ambulatory assessments were associated with birth outcomes. Targeted interventions to reduce psychological and biological indicators of heightened stress during pregnancy may have positive public health benefits for the offspring given the associations of shortened gestation and lower birthweight with risk for poor mental and physical health outcomes.
Acknowledgments
The authors wish to thank the young women who participated in this study, our top-notch research assistants, Sophie Foss, Laura Kurzius, and Willa Marquis, for dedicated help with participant engagement and all of the data collection, Ruth Yang for performance of CRH, IL-6, and cortisol assays, and Dr. Laraine McDonough for her helpful comments on this manuscript. This research was supported by the National Institute of Mental Health (MH077144-01A2 to CM and 5T32MH018264-28 to JS) and the National Center for Advancing Translational Sciences, National Institutes of Health UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156.
Footnotes
To qualify for the study, participants needed to fall within this age range prior to 19 weeks of gestation.
A benign tumor was found on the neck of a fetus in the 3rd trimester. The infant was born otherwise healthy, of normal weight and at term.
One participant tested positive for cannabinoid use twice during pregnancy.
Due to the nature of log-transformed variables, these outcomes can only be reported in terms of percent, not raw units.
Competing interest statement
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf. The authors have no competing interests to report.
References
- 1.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Wilson EC, Mathews TJ. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1–71. [PubMed] [Google Scholar]
- 2.Noble KG, Fifer WP, Rauh VA, Nomura Y, Andrews HF. Academic achievement varies with gestational age among children born at term. Pediatrics. 2012;130:e257–64. doi: 10.1542/peds.2011-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felice ME, Feinstein RA, Fisher MM, Kaplan DW, Olmedo LF, Rome ES, et al. Adolescent pregnancy—current trends and issues: 1998 American Academy of Pediatrics Committee on Adolescence, 1998–1999. Pediatrics. 1999;103:516–20. doi: 10.1542/peds.103.2.516. [DOI] [PubMed] [Google Scholar]
- 4.Darroch JE. Adolescent pregnancy trends and demographics. Curr Womens Health Rep. 2001;1:102–10. [PubMed] [Google Scholar]
- 5.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288:728–37. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 6.Heinonen K, Raikkonen K, Pesonen AK, Kajantie E, Andersson S, Eriksson JG, et al. Prenatal and postnatal growth and cognitive abilities at 56 months of age: a longitudinal study of infants born at term. Pediatrics. 2008;121:e1325–33. doi: 10.1542/peds.2007-1172. [DOI] [PubMed] [Google Scholar]
- 7.Linnet KM, Wisborg K, Agerbo E, Secher NJ, Thomsen PH, Henriksen TB. Gestational age, birth weight, and the risk of hyperkinetic disorder. Arch Dis Child. 2006;91:1–6. doi: 10.1136/adc.2005.088872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matte TD, Bresnahan M, Begg MD, Susser E. Influence of variation in birth weight within normal range and within sibships on IQ at age 7 years: cohort study. BMJ. 2001;323:310–4. doi: 10.1136/bmj.323.7308.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomura Y, Wickramaratne PJ, Pilowsky DJ, Newcorn JH, Bruder-Costello B, Davey C, et al. Low birth weight and risk of affective disorders and selected medical illness in offspring at high and low risk for depression. Compr Psychiatry. 2007;48:470–8. doi: 10.1016/j.comppsych.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paneth NS. The problem of low birth weight. Future Child. 1995;5:19–34. [PubMed] [Google Scholar]
- 11.Richards M, Hardy R, Kuh D, Wadsworth ME. Birthweight, postnatal growth and cognitive function in a national UK birth cohort. Int J Epidemiol. 2002;31:342–8. [PubMed] [Google Scholar]
- 12.Walhovd KB, Fjell AM, Brown TT, Kuperman JM, Chung Y, Hagler DJ, et al. Long-term influence of normal variation in neonatal characteristics on human brain development. Proc Natl Acad Sci U S A. 2012;109:20089–94. doi: 10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen S. Contrasting the Hassles Scale and the Perceived Stress Scale: who’s really measuring appraised stress? Am Psychol. 1986;41:716–8. [Google Scholar]
- 14.DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Copper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, et al. The preterm prediction study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. Am J Obstet Gynecol. 1996;175:1286–92. doi: 10.1016/s0002-9378(96)70042-x. [DOI] [PubMed] [Google Scholar]
- 16.Hedegaard M, Henriksen TB, Secher NJ, Hatch MC, Sabroe S. Do stressful life events affect duration of gestation and risk of preterm delivery? Epidemiology. 1996;7:339–45. doi: 10.1097/00001648-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Lobel M. Conceptualizations, measurement, and effects of prenatal maternal stress on birth outcomes. J Behav Med. 1994;17:225–72. doi: 10.1007/BF01857952. [DOI] [PubMed] [Google Scholar]
- 18.Lobel M, Dunkel-Schetter C, Scrimshaw SC. Prenatal maternal stress and prematurity: a prospective study of socioeconomically disadvantaged women. Health Psychol. 1992;11:32–40. doi: 10.1037//0278-6133.11.1.32. [DOI] [PubMed] [Google Scholar]
- 19.Misra DP, O’Campo P, Strobino D. Testing a sociomedical model for preterm delivery. Paediatr Perinat Epidemiol. 2001;15:110–22. doi: 10.1046/j.1365-3016.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 20.Sable MR, Wilkinson DS. Impact of perceived stress, major life events and pregnancy attitudes on low birth weight. Fam Plann Perspect. 2000;32:288–94. [PubMed] [Google Scholar]
- 21.Zhu P, Tao F, Hao J, Sun Y, Jiang X. Prenatal life events stress: implications for preterm birth and infant birthweight. Am J Obstet Gynecol. 2010;203:34, e1–8. doi: 10.1016/j.ajog.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Entringer S, Buss C, Andersen J, Chicz-DeMet A, Wadhwa PD. Ecological momentary assessment of maternal cortisol profiles over a multiple-day period predicts the length of human gestation. Psychosom Med. 2011;73:469–74. doi: 10.1097/PSY.0b013e31821fbf9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tegethoff M, Greene N, Olsen J, Meyer AH, Meinlschmidt G. Maternal psychosocial adversity during pregnancy is associated with length of gestation and offspring size at birth: evidence from a population-based cohort study. Psychosom Med. 2010;72:419–26. doi: 10.1097/PSY.0b013e3181d2f0b0. [DOI] [PubMed] [Google Scholar]
- 24.Szigethy EM, Ruiz P. Depression among pregnant adolescents: an integrated treatment approach. Am J Psychiatry. 2001;158:22–7. doi: 10.1176/appi.ajp.158.1.22. [DOI] [PubMed] [Google Scholar]
- 25.Stevens-Simon C, Beach RK, McGregor JA. Does incomplete growth and development predispose teenagers to preterm delivery? A template for research. J Perinatol. 2002;22:315–23. doi: 10.1038/sj.jp.7210694. [DOI] [PubMed] [Google Scholar]
- 26.Hillis SD, Anda RF, Dube SR, Felitti VJ, Marchbanks PA, Marks JS. The association between adverse childhood experiences and adolescent pregnancy, long-term psychosocial consequences, and fetal death. Pediatrics. 2004;113:320–7. doi: 10.1542/peds.113.2.320. [DOI] [PubMed] [Google Scholar]
- 27.Saewyc EM, Magee LL, Pettingell SE. Teenage pregnancy and associated risk behaviors among sexually abused adolescents. Perspect Sex Reprod Health. 2004;36:98–105. doi: 10.1363/psrh.36.98.04. [DOI] [PubMed] [Google Scholar]
- 28.Dietz PM, Spitz AM, Anda RF, Williamson DF, McMahon PM, Santelli JS, et al. Unintended pregnancy among adult women exposed to abuse or household dysfunction during their childhood. JAMA. 1999;282:1359–64. doi: 10.1001/jama.282.14.1359. [DOI] [PubMed] [Google Scholar]
- 29.Fiscella K, Kitzman HJ, Cole RE, Sidora KJ, Olds D. Does child abuse predict adolescent pregnancy? Pediatrics. 1998;101:620–4. doi: 10.1542/peds.101.4.620. [DOI] [PubMed] [Google Scholar]
- 30.Stock JL, Bell MA, Boyer DK, Connell FA. Adolescent pregnancy and sexual risk-taking among sexually abused girls. Fam Plann Perspect. 1997;29:200–3. 27. [PubMed] [Google Scholar]
- 31.Boyer D, Fine D. Sexual abuse as a factor in adolescent pregnancy and child maltreatment. Fam Plann Perspect. 1992;24:4–11. 9. [PubMed] [Google Scholar]
- 32.Stevens-Simon C, Kaplan DW, McAnarney ER. Factors associated with preterm delivery among pregnant adolescents. J Adolesc Health. 1993;14:340–2. doi: 10.1016/1054-139x(93)90185-r. [DOI] [PubMed] [Google Scholar]
- 33.Stevens-Simon C, McAnarney ER. Childhood victimization: relationship to adolescent pregnancy outcome. Child Abuse Negl. 1994;18:569–75. doi: 10.1016/0145-2134(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 34.Miller HL. The effect of maternal stress on fetal birth weight. In: Humphrey JH, editor. Human stress: current selected research. New York: AMS Press; 1987. pp. 103–19. [Google Scholar]
- 35.McCool WF, Dorn LD, Susman EJ. The relation of cortisol reactivity and anxiety to perinatal outcome in primiparous adolescents. Res Nurs Health. 1994;17:411–20. doi: 10.1002/nur.4770170604. [DOI] [PubMed] [Google Scholar]
- 36.Giesbrecht GF, Campbell T, Letourneau N, Kaplan BJ. Advancing gestation does not attenuate biobehavioural coherence between psychological distress and cortisol. Biol Psychol. 2013;93:45–51. doi: 10.1016/j.biopsycho.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 37.McLean SA, Williams DA, Harris RE, Kop WJ, Groner KH, Ambrose K, et al. Momentary relationship between cortisol secretion and symptoms in patients with fibromyalgia. Arthritis Rheum. 2005;52:3660–9. doi: 10.1002/art.21372. [DOI] [PubMed] [Google Scholar]
- 38.Henker B, Whalen CK, Jamner LD, Delfino RJ. Anxiety, affect, and activity in teenagers: monitoring daily life with electronic diaries. J Am Acad Child Adolesc Psychiatry. 2002;41:660–70. doi: 10.1097/00004583-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Steptoe A, Willemsen G. The influence of low job control on ambulatory blood pressure and perceived stress over the working day in men and women from the Whitehall II cohort. J Hypertens. 2004;22:915–20. doi: 10.1097/00004872-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Steptoe A, Gibson EL, Hamer M, Wardle J. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology. 2007;32:56–64. doi: 10.1016/j.psyneuen.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Brondolo E, Rieppi R, Erickson SA, Bagiella E, Shapiro PA, McKinley P, et al. Hostility, interpersonal interactions, and ambulatory blood pressure. Psychosom Med. 2003;65:1003–11. doi: 10.1097/01.psy.0000097329.53585.a1. [DOI] [PubMed] [Google Scholar]
- 42.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 43.Buss C, Entringer S, Reyes JF, Chicz-DeMet A, Sandman CA, Waffarn F, et al. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2009;201:398, e1–8. doi: 10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 44.Piccinni MP, Maggi E, Romagnani S. Role of hormone-controlled T-cell cytokines in the maintenance of pregnancy. Biochem Soc Trans. 2000;28:212–5. doi: 10.1042/bst0280212. [DOI] [PubMed] [Google Scholar]
- 45.Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun. 2012;26:650–9. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom Med. 2005;67:625–31. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- 47.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–50. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–25. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 49.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531–58. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 50.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 51.Cacioppo JT, Hawkley LC, Berntson GG. The anatomy of loneliness. Curr Dir Psychol Sci. 2003;12:71–4. [Google Scholar]
- 52.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 53.Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–79. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Schurmeyer TH, Avgerinos PC, Gold PW, Gallucci WT, Tomai TP, Cutler GB, et al. Human corticotropin-releasing factor in man: pharmacokinetic properties and dose–response of plasma adrenocorticotropin and cortisol secretion. J Clin Endocrinol Metab. 1984;59:1103–8. doi: 10.1210/jcem-59-6-1103. [DOI] [PubMed] [Google Scholar]
- 55.Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69:651–9. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- 56.Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-Demet A, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. 2006;77:573–87. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 59.Evans LM, Myers MM, Monk C. Pregnant women’s cortisol is elevated with anxiety and depression — but only when comorbid. Arch Womens Ment Health. 2008;11:239–48. doi: 10.1007/s00737-008-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baron IS, Litman FR, Ahronovich MD, Baker R. Late preterm birth: a review of medical and neuropsychological childhood outcomes. Neuropsychol Rev. 2012;22:438–50. doi: 10.1007/s11065-012-9210-5. [DOI] [PubMed] [Google Scholar]
- 61.Broekman BF, Chan YH, Chong YS, Quek SC, Fung D, Low YL, et al. The influence of birth size on intelligence in healthy children. Pediatrics. 2009;123:e1011–6. doi: 10.1542/peds.2008-3344. [DOI] [PubMed] [Google Scholar]
- 62.Bolten MI, Wurmser H, Buske-Kirschbaum A, Papousek M, Pirke KM, Hellhammer D. Cortisol levels in pregnancy as a psychobiological predictor for birth weight. Arch Womens Ment Health. 2010;14:33–41. doi: 10.1007/s00737-010-0183-1. [DOI] [PubMed] [Google Scholar]
- 63.Li CQ, Windsor RA, Perkins L, Goldenberg RL, Lowe JB. The impact on infant birth weight and gestational age of cotinine-validated smoking reduction during pregnancy. JAMA. 1993;269:1519–24. [PubMed] [Google Scholar]
- 64.McCowan LM, Dekker GA, Chan E, Stewart A, Chappell LC, Hunter M, et al. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ. 2009;338:b1081. doi: 10.1136/bmj.b1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen S, Doyle WJ, Skoner DP. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosom Med. 1999;61:175–80. doi: 10.1097/00006842-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Hawkley LC, Burleson MH, Berntson GG, Cacioppo JT. Loneliness in everyday life: cardiovascular activity, psychosocial context, and health behaviors. J Pers Soc Psychol. 2003;85:105–20. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- 67.Gallo LC, Matthews KA. Adolescents’ attachment orientation influences ambulatory blood pressure responses to everyday social interactions. Psychosom Med. 2006;68:253–61. doi: 10.1097/01.psy.0000204633.33599.81. [DOI] [PubMed] [Google Scholar]
- 68.Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosom Med. 2011;73:656–63. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116:393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 70.Sykes L, MacIntyre DA, Yap XJ, Teoh TG, Bennett PR. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediators Inflamm. 2012;2012:967629. doi: 10.1155/2012/967629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Werner E, Zhao Y, Evans L, Kinsella M, Kurzius L, Altincatal A, et al. Higher maternal pre-natal cortisol and younger age predict greater infant reactivity to novelty at 4 months: an observation-based study. Dev Psychobiol. 2012 doi: 10.1002/dev.21066. http://dx.doi.org/10.1002/dev.21066 (in press) [DOI] [PMC free article] [PubMed]
- 72.Voegtline KM, Costigan KA, Kivlighan KT, Laudenslager ML, Henderson JL, Dipietro JA. Concurrent levels of maternal salivary cortisol are unrelated to self-reported psychological measures in low-risk pregnant women. Arch Womens Ment Health. 2013;16:101–8. doi: 10.1007/s00737-012-0321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dorn LD, Susman EJ, Petersen AC. Cortisol reactivity and anxiety and depression in pregnant adolescents: a longitudinal perspective. Psychoneuroendocrinology. 1993;18:219–39. doi: 10.1016/0306-4530(93)90006-7. [DOI] [PubMed] [Google Scholar]
- 74.Rondo PH, Vaz AJ, Moraes F, Tomkins A. The relationship between salivary cortisol concentrations and anxiety in adolescent and non-adolescent pregnant women. Braz J Med Biol Res. 2004;37:1403–9. doi: 10.1590/s0100-879x2004000900016. [DOI] [PubMed] [Google Scholar]
- 75.Mancuso RA, Schetter CD, Rini CM, Roesch SC, Hobel CJ. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosom Med. 2004;66:762–9. doi: 10.1097/01.psy.0000138284.70670.d5. [DOI] [PubMed] [Google Scholar]
- 76.Braveman P, Marchi K, Egerter S, Kim S, Metzler M, Stancil T, et al. Poverty, near-poverty, and hardship around the time of pregnancy. Matern Child Health J. 2010;14:20–35. doi: 10.1007/s10995-008-0427-0. [DOI] [PubMed] [Google Scholar]