Abstract
Fetal gallstones and cholelithiasis, detected by routine third trimester ultrasound, have been described in the literature with controversial clinical significance. We report a case of fetal cholelithiasis detected at 35 weeks gestation during a routine scan. The diagnosis was performed using an integrated 2-dimensional (2-D) and 3-dimensional (3-D) ultrasound approach in order to obtain a better definition of the fetal gallbladder and its content. A neonatal follow-up was achieved. The present study has a twofold purpose: firstly, to update the diagnostic approach using the innovative 3-D modalities and secondly, to review the management of this condition during fetal and postnatal life.
Keywords: fetal cholelithiasis, prenatal diagnosis, third trimester, ultrasound, management
Introduction
Fetal cholelithiasis is uncommon during pregnancy although its frequency of diagnosis has increased over the last years, probably due to both the improved accuracy and the increasing use of ultrasound examination in clinical practice.1–5 Despite the remarkable number of fetal scans performed annually worldwide, little is known about the causes, natural history, and clinical significance of echogenic foci in the gallbladder during fetal life, unlike in the pediatric or adult patient.6–8 We report a case of cholelithiasis in the fetal gallbladder identified sonographically in the late third trimester of pregnancy during a routine obstetric scan.
Case Report
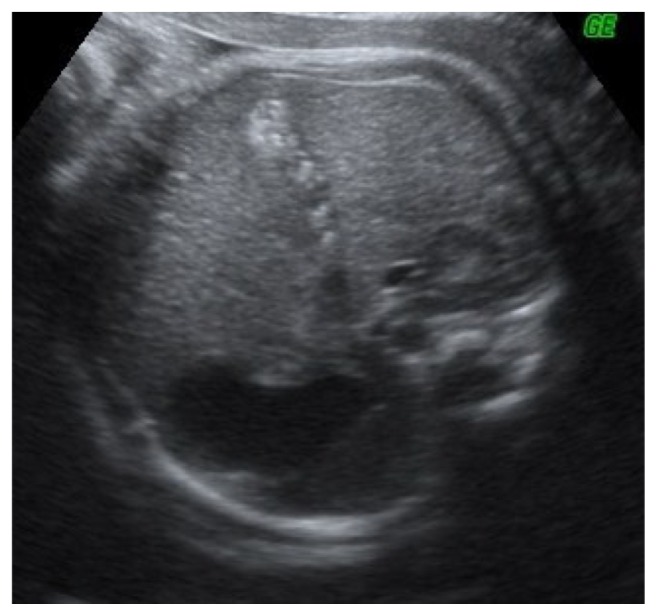
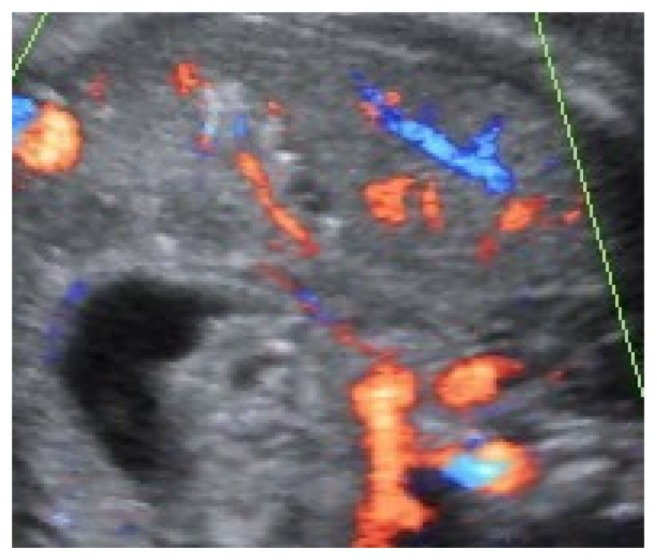
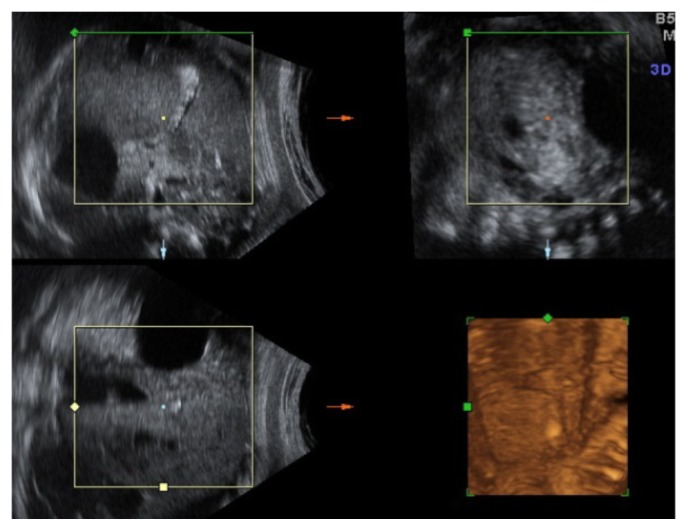
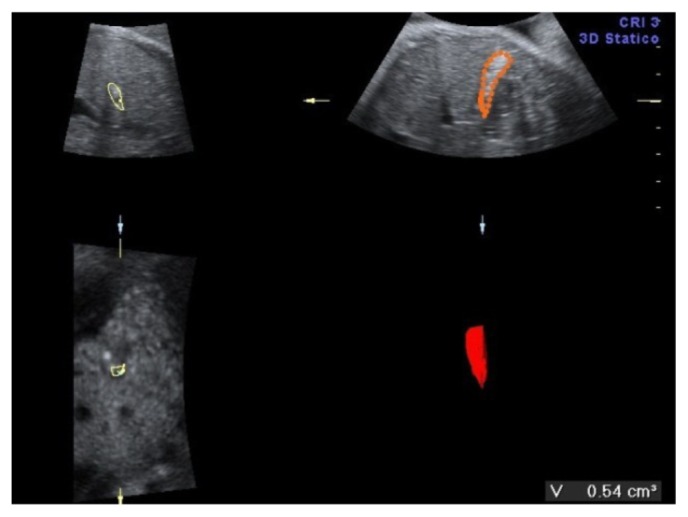
A 34-year-old-woman, gravid 2, para 1, was referred at 35 weeks of gestation for a routine obstetric ultrasound examination in our prenatal center. High risk pregnancy as the result of a preexisting maternal medical condition or one that had appeared later was excluded. A healthy prepregnancy body mass index (BMI) and a correct weight gain during pregnancy were recorded. A previous ultrasound examination performed in another center at 23 weeks demonstrated a live singleton fetus with no apparent structural abnormalities and normal morphological development. An ultrasound evaluation was achieved before 35 weeks. In the third trimester, a traditional scan and traditional organ system evaluation scan of the fetus was unremarkable except for a large nonshadowing multiple small echogenic foci within the gallbladder in a transverse image through the fetal abdomen (Fig. 1). An integrated 2-dimensional (2-D) and 3-dimensional (3-D) scan was realized, using different 3-D imaging techniques (rendering, tomographic ultrasound imaging [TUI], virtual organ computer-aided analysis [VOCAL], angio-power Doppler) (Figs. 2–4) in order to detail the relationship between the gallbladder and the cholelithiasis. A female infant weighing 3650 grams was born at 38 weeks of gestation by cesarean section for previous abdominal delivery, with Apgar scores of 9 and 10 at 1 and 5 minutes, respectively. At 1 day of age, there was no evidence of jaundice, hematological incompatibility, or sepsis. A satisfactory health status was confirmed by bilirubin and neonatal screening test for metabolic pathologies. At 2 days of life, an abdominal ultrasonography examination was achieved. A normal-sized gallbladder with minimal neonatal cholelithiasis was detected. In consideration of the liver chemistry including hematological indices within normal ranges and reassuring ultrasound result, the infant was discharged on the third day of life. An ultrasound follow-up was performed at 2 months of life, revealing a normal size gallbladder deprived of echogenic stones, biliary tract anomalies, or obstructions. In consideration of the resolution of abnormal findings, further evaluation was not planned.
Figure 1.
Fetal cholelithiasis in third trimester detected by ultrasound scan. Stefania Triunfo is the author of all ultrasound images of this paper.
Figure 2.
Abdominal vascular flow in fetus with cholelithiasis in third trimester.
Figure 4.
Rendering of fetal gallbladder.
Discussion
In a review in 1928, Potter identified several descriptions of neonatal gallstones and cited 2 poorly documented cases of fetal cholelithiasis diagnosed at the time of autopsy9, though the first prenatal diagnosis by Beretsky and Lankin occurred in 1983.2 Anomalies of the gallbladder, including sludge and gallstones, are extremely uncommon in fetal life, with an incidence of 0.42%, as reported recently in a large cohort of 9235 pregnancies, with a slightly greater male predilection.5,10
In the last decades, maternal conditions and fetal or obstetrical predisposing risk factors have been proposed to have a causative role, but none of them appears conclusive (Table 1). In the condition placental abruption, Fanaroff suggested that the placental cross of hemoglobin transformed into bilirubin increases the indirect bilirubin levels, responsible for sludge or gallstones in the fetal gallbladder.11 Conversely, Brown proposed that an increase in estrogen levels might predispose to the formation of pigment stones stimulating the secretion of cholesterol and reducing the synthesis of biliary acids.5 In some cases, maternal narcotic use, methadone particularly, should modify gastrointestinal activity with an increased transition time and the formation of solid echogenic material.12,13 Augmented cellular degradation is known to predispose to calculus formation in postnatal life. Thus, a corresponding situation could be involved during hemolytic anemia and blood group incompatibilities; both conditions are recognized as predisposing factors in the development of echogenic material in the bile.1–4 In addition to the risk factors previously described in the literature, Kiserud suggested that chromosomal aberrations, cardiac malformations, gastroschisis, influence of prostaglandin, and possibly prenatal leukemoid reaction may cause the presence of material in the fetal gallbladder.14 Taking into consideration the largest single series reported by Brown and the equally significant results reported by Kiserud, characterized by scans performed for indications, it can be concluded that in the centers receiving a variety of referrals, more than half of the cases with echodense contents of the fetal gallbladder have maternal or fetal complications.5,14
Table 1.
Maternal and fetal condition associated with gallstones in the fetal population.
| Maternal |
| Placental abruption |
| Increased estrogen levels |
| Narcotics use |
| Prolonged fast |
| Diabetes of any type |
| Enteral nutrition |
| Pharmacological treatment (ceftriaxone, furosemide, prostaglandin E2) |
| Intoxication with denatured oil treated with steroid |
| Sepsis |
| Fetal |
| Rhesus or ABO blood group incompatibility |
| Congenital anomalies (cardiovascular, gastrointestinal, urinary) |
| Twin pregnancy with fetal demise of one twin |
| Genetic anomalies (trisomy 21) |
| Chromosomal aberrations (translocation 10;11) |
| Growth restriction |
| Oligohydramnios |
| Hepatitis |
| Prenatal leukemoid reaction |
| Idiopathic |
Sonographically, after 14 weeks gestation, the fetal gallbladder can be recognized as an anechoic, oblong, teardrop-like structure with a thin echogenic wall, located at the inferior surface of the right lobe of the liver. Accurate assessment and identification of the fetal gallbladder require prior visualization of the umbilical vein, because they have a similar appearance. After 20 weeks of gestation, the fetal gallbladder can be visualized in 37.5% to 64.7% of the cases.4 Stones and sludge identified in the fetal gallbladder are almost absent in the second trimester, resulting in a specific phenomenon of the third trimester due to abnormal production, composition, and mode of transportation of bile in the biliary tract.2,15 A wide spectrum of findings regarding homogeneity, echogenicity, and degree of acoustic shadowing can be detected.5,16 Echogenic material in the gallbladder may be visualized by transverse sections through the fetal abdomen in the form of single, multiple, or diffuse foci.8 Frequently, the echogenic material completely fills the gallbladder lumen.6 Unlike in the adult patient, this material produces different degrees of acoustic shadowing. Small echogenic foci from 2 to 3 mm in size and sludge may not cause shadowing if they do not lie in the center of, or in the focal range of, the beam, while foci greater than 3 mm may produce acoustic. Schirmer underlined the importance of the nature of the calculi, concluding that none of calcified (radiopaque) stones disappeared spontaneously after birth, contrary to the cholesterol, urate, or pigment stones (nonradiopaque).17 In the literature, cases of fetal gallstone diagnoses complicated by contracted gallbladder with a stone localization have been reported as no clear finding.1,15 In this condition, the calculi could be confused with liver calcification, calcified liver mass, or meconium peritonitis. In an attempt to precisely specify the relationship between the gallbladder, its content, and nearby structures, we speculated whether a 3-D approach might provide more information in diagnostic processing than a traditional 2-D ultrasonography. By an offline processing, 3-D rendering surface and TUI showed the relationship between the gallbladder and the cholelithiasis. The volume of the gallbladder was measured by VOCAL, and the visualization of the vessels surrounding the gallbladder was demonstrated by angio-power Doppler. Overall, in contrast to conventional 2-D ultrasound that only allows imaging of single planes, the 3-D approach offers several image displays that do not exist in 2-D imaging. In obstetrics, in particular, it offers a precise demonstration of the normal and abnormal anatomy of the fetus to the obstetrician. Digital storage of volumes permits virtual examinations by reloading of volumes and navigating through them in the absence of the patient. The value of a 3-D scan is indisputable in specific clinical situations when the traditional scan results are poorly defined, not precise, or not clerly interpreted. In literature, there is evidence suggesting that 3-D ultrasound improves the diagnosis increasing the visualization quality of the anatomical landmarks, with minimal impact on the examination time required. Furthermore, cardiac or brain volumes can be successfully analyzed off-line locally or sent by Internet to experts for review.18 To date, after an initial enthusiasm for the innovative techniques in obstetric ultrasound and their application in several conditions, an in-depth revaluation about the possible risks related to use, or better the abuse, has been required.18 3-D modalities should be used only in selected cases requiring an effective investigation and analyzed postprocessing only by skilled operators. Generally, fetal cholelithiasis appears as a quite clear finding, detectable easily for anatomical reasons and for advanced gestational age at diagnosis time.18
Complications are very rare and, in most series, gallstones disappear spontaneously within the first year of life. Only 1 case of a laparoscopic cholecystectomy in a 16-day-old infant has been reported.19 In the review of Suchet et al, 30 cases of fetal cholelithiasis with a long-term follow-up in 21 out of 30 babies were described.12 In only 3 patients, a persistent echogenic material in the gallbladder after 2 weeks of life was observed without any abdominal symptoms.12 Stringer et al reported a series of 3 male fetuses examined sonographically in late gestation with evidence of gallbladder cholelithiasis.13 Postnatally, all 3 were asymptomatic, but they had variable ultrasound findings. In 2 of these, the abnormalities noted sonographically resolved spontaneously in 6 weeks, while in third, a persistent gallbladder “calculus” until 6 months has been detected.15 In the 2 cases described by Suma et al, the fetal gallstones showed complete resolution at the follow-up sonography at 2 and 3 months of life without any treatment.3 Munjuluri et al described 2 cases treated with ursodeoxycholic acid (dosage 5–7 mg/kg/die), reporting the complete resolution of gallstones at 2 and 4 months of life, respectively.16 Recently, Iroh Tam and Angelides described the first case of fetal gallstones in 3 siblings (a male term neonate with gallstones and 2 male twins by dizygotic dichorionic pregnancy, twin a with gallstones and twin b with sludge in the gallbladder).1 Both siblings with gallstones were treated with ursodeoxycholic acid after birth with complete resolution. In both cases, the therapeutic decision was based on undocumented side effects of the infant health and for obtaining a more rapid resolution of the stones.16 In consideration of previous studies and in our experience, fetal cholelithiasis is confirmed to be a self-limiting disease without complications and with a spontaneous resolution in the first year of life. Although no morbidity that would be amenable to treatment has been documented, there are still theoretical neonatal risks related to the cholelithiasis therapy, including the possibility of recurrance by the end of treatment with a rare long-term freedom from stones.20 Therefore, if further investigations are necessary in order to clarify the potential risks of the pharmacological approach, we discourage some forms of therapy such as ursodeoxycholic acid administration due to unknown and potential side effects in childhood or adulthood.
In conclusion, several questions remain unanswered about the etiology, the risks of further gallstone formation, and long-term effects of pharmacological treatment in cholelithiasis detected during pregnancy. Attention has to be reserved for potential maternal or fetal risk factors. A close follow-up is indicated in these patients until spontaneous resolution is demonstrated by traditional ultrasound. The 3-D imaging techniques might show some advantages in difficult clinical conditions, through an off-line processing of stored images. Considering the high rate of spontaneous resolution, the best practice is to reassure parents and to observe the clinical evolution with periodic ultrasound examinations without any treatment. Only future follow-up studies will be able to respond fully to current open questions.
Figure 3.
VOCAL of fetal gallbladder.
Table 2.
Fetal characteristics, ultrasound findings and neonatal outcome in case reports in the literature.
| Study | No of cases | Sex | Gestational ageat detection | Sonographic findings | Outcome |
|---|---|---|---|---|---|
| Beretsky et al2 | 1 | F | 36 | Multiple foci | Resolution within 1 month |
| Heijne et al7 | 1 | M | 34 | Multiple foci | Resolution within 3 months |
| Klingensmith et al (1988)24 | 1 | M | 37 | Multiple foci | Resolution within 6 weeks |
| Abbitt et al6 | 1 | M | 33 | Multiple foci | Resolution within 10 months |
| Broussin et al (1990) | 3 | M-M-M | 33-38-36 | Multiple foci | Unknown-resolution within 3 months and 6 weeks |
| Suchet et al12 | 1 | Not reported | 35 | Multiple foci | Resolution within 10 days |
| Petrikowsky et al21 | 5 | M-M-F-M-F | 33–36 | Multiple foci | Resolution at birth |
| Stringer et al15 | 3 | M-M-M | 32-35-32 | Multiple foci | Resolution within 6 weeks, 1 month, 6 weeks |
| Kiserud et al14 | 6 | M-M-F-M-F-M | From 33 to 38 | 4 with multiple foci 2 Sludge | Resolution within 6 weeks |
| Nishi et al22 | 1 | F | 34 | Multiple foci | Resolution at birth |
| Hertzberg et al23 | 2 | F-M | 31–36 | Multiple foci | Resolution within 2 months, at birth |
| Munjuluri et al (2005)16 | 2 | F-M | 34–34 | Multiple foci | Resolution within 8 weeks |
| Cancho Candela et al10 | 11 | Not reported | From 29 to 38 | 4 with single focus 7 with multiple foci | Resolution within 7 weeks |
| Sheiner et al (2006)8 | 4 | M-M-M-M | From 29 to 32 | Multiple foci | Resolution within 1 month |
| Triunfo et al (2013) | 1 | F | 35 | Multiple small foci | Resolution at birth |
Footnotes
Author Contributions
Conceived and designed the experiments: ST, PR, PF. Analyzed the data: ST, PR. Wrote the first draft of the manuscript: ST. Contributed to the writing of the manuscript: AG, PF. Agree with manuscript results and conclusions: ST, PR, PF, AG, GS. Jointly developed the structure and arguments for the paper: ST, AG, PF. Made critical revisions and approved final version: ST, PR, PF, AG, GS. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
References
- 1.Iroh Tam PY, Angelides A. Perinatal detection of gallstones in siblings. Am J Perinatol. 2010;27:771–4. doi: 10.1055/s-0030-1254239. [DOI] [PubMed] [Google Scholar]
- 2.Beretsky I, Lankin DH. Diagnosis of fetal cholelithiasis using real-time high-resolution imaging employing digital detection. J Ultrasound Med. 1983;2:381–3. doi: 10.7863/jum.1983.2.8.381. [DOI] [PubMed] [Google Scholar]
- 3.Suma V, Marini A, Bucci N, Toffoluti T, Talenti E. Fetal gallstones: sonographic and clinical observations. Ultrasound Obstet Gynecol. 1998;12:439–41. doi: 10.1046/j.1469-0705.1998.12060439.x. [DOI] [PubMed] [Google Scholar]
- 4.Agnifili A, Verzaro R, Carducci G, et al. Fetal cholelithiasis: a prospective study of incidence, predisposing factors, and ultrasonographic and clinical features. Clin Pediatr. 1999;38:371–3. doi: 10.1177/000992289903800610. [DOI] [PubMed] [Google Scholar]
- 5.Brown DL, Teele RL, Doubilet PM, DiSalvo DN, Benson CB, Van Alstyne GA. Echogenic material in the fetal gallbladder: sonographic and clinical observations. Radiology. 1992;182:73–6. doi: 10.1148/radiology.182.1.1727312. [DOI] [PubMed] [Google Scholar]
- 6.Abbitt PL, McIlhenny J. Prenatal detection of gallstones. J Clin Ultrasound. 1990;18:202–4. doi: 10.1002/jcu.1870180313. [DOI] [PubMed] [Google Scholar]
- 7.Heijne L, Ednay D. The development of fetal gallstones demonstrated by ultrasound. Radiography. 1985;51:155–6. [PubMed] [Google Scholar]
- 8.Sheiner E, Abramowicz JS, Hershkovitz R. Fetal gallstones detected by routine third trimester ultrasound. Int J Gynaecol Obstet. 2006;92:255–6. doi: 10.1016/j.ijgo.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Potter AH. Gall-bladder disease in young subjects. Surg Gynecol Obstet. 1928;46:795–808. [Google Scholar]
- 10.Cancho Candela R, Díaz González J, Perandones Fernández C, Viñuela Rueda B, Relea Sarabia A, Andrés de Llano JM. Echogenic material in fetal gallbladder: prenatal diagnosis and postnatal follow-up [in Spanish] An Pediatr (Barc) 2004;61:326–9. doi: 10.1016/s1695-4033(04)78396-4. [DOI] [PubMed] [Google Scholar]
- 11.Fanaroff AA, Martin RJ, Miler MJ. Identification and management of high-risk problems in the neonate. In: Creasy RK, Resnik R, editors. Maternal-Fetal Medicine, Principles and Practice. 2nd ed. Philadelphia, PA: Saunders; 1989. pp. 1176–9. [Google Scholar]
- 12.Suchet IB, Labatte MF, Dyck CS, Salgado LA. Fetal cholelithiasis: a case report and review of the literature. J Clin Ultrasound. 1993;21:198–202. doi: 10.1002/jcu.1870210309. [DOI] [PubMed] [Google Scholar]
- 13.Devonald KJ, Ellwood DA, Colditz PB. The variable appearances of fetal gallstones. J Ultrasound Med. 1992;11:579–85. doi: 10.7863/jum.1992.11.11.579. [DOI] [PubMed] [Google Scholar]
- 14.Kiserud T, Gjellnd K, Bogno H, Waardal M, Reigstad H, Rosendahl K. Echogenic material in the fetal gallbladder and fetal disease. Ultrasound Obstet Gynecol. 1997;10:103–6. doi: 10.1046/j.1469-0705.1997.10020103.x. [DOI] [PubMed] [Google Scholar]
- 15.Stringer MD, Lim P, Cave M, Martinez D, Liford RJ. Fetal gallstones. J Pediatr Surg. 1996;31:1589–91. doi: 10.1016/s0022-3468(96)90189-x. [DOI] [PubMed] [Google Scholar]
- 16.Munjuluri N, Elgharaby N, Acolet D, Kadir RA. Fetal gallstones. Fetal Diagn Ther. 2005;20:241–3. doi: 10.1159/000085077. [DOI] [PubMed] [Google Scholar]
- 17.Schiermer WJ, Grisoni ER, Gauder MWL. The spectrum of cholelithiasis in the first year of life. J Pediatr Surg. 1989;24:1064–7. doi: 10.1016/s0022-3468(89)80216-7. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo G, Pietrolucci M, Aiello E, Mammarella S, Bosi C, Arduini D. The role of three-dimensional ultrasound in the diagnosis of fetal congenital anomalies: a review. Minerva Ginecol. 2011;63:401–10. [PubMed] [Google Scholar]
- 19.Gertner M, Farmer D. Laparoscopic cholecystecomy in a 16-day-old infant with chronic cholelithiasis. Journal of Pediatric Surgery. 2004;39:17–9. doi: 10.1016/j.jpedsurg.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 20.Svenssono J, Makin E. Gallstone disease in children. Semin Pediatr Surg. 2012;21:256–65. doi: 10.1053/j.sempedsurg.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Petrikowsky B, Klein V, Holsten N. Sludge in fetal gallbladder: natural history and neonatal outcome. Br J Radiol. 1996;69:1017–8. doi: 10.1259/0007-1285-69-827-1017. [DOI] [PubMed] [Google Scholar]
- 22.Nishi T. Ultrasonographic diagnosis of fetal cholelithiasis. J Obstet Res. 1997;23:251–25. doi: 10.1111/j.1447-0756.1997.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 23.Hertzberg BC, Kiwer MA. Fetal gallstones in a contracted gallbladder: potential to imulate hepatic or peritoneal calcification. J Ultrasound Med. 1998;17:667–80. doi: 10.7863/jum.1998.17.10.667. [DOI] [PubMed] [Google Scholar]
- 24.Klingensmith WC, 3rd, Cioffi-Ragan DT. Fetal gallstones. Radiology. 1988 Apr;167:143–4. doi: 10.1148/radiology.167.1.3279452. [DOI] [PubMed] [Google Scholar]