Abstract
Purpose.
Nonarteritic anterior ischemic optic neuropathy (NAION) is the most common cause of sudden optic nerve–related vision loss in persons older than 50 in the United States. There currently is no treatment for this disorder. We previously showed that systemic administration of 15-deoxy, delta (12, 14) prostaglandin J2 (PGJ2) is neuroprotective in our rodent model of AION (rAION). In this study, we determined if a single intravitreal (IVT) injection of PGJ2 is neuroprotective after rAION, and if this method of administration is toxic to the retina, optic nerve, or both.
Methods.
Toxicity was assessed after a single IVT injection of PGJ2 in one eye and PBS in the contralateral eye of normal, adult Long-Evans rats. Efficacy was assessed by inducing rAION in one eye and injecting either PGJ2 or vehicle immediately following induction, with the fellow eye serving as naïve control. Visual evoked potentials (VEPs) and ERGs were performed before induction and at specific intervals thereafter. Animals were euthanized 30 days after induction, after which immunohistochemistry, transmission electron microscopy, and quantitative stereology of retinal ganglion cell (RGC) numbers were performed.
Results.
Toxicity: IVT PGJ2 did not alter the VEP or ERG compared with PBS-injected control eyes, and neither IVT PGJ2 nor PBS reduced overall RGC numbers. Efficacy: IVT PGJ2 preserved VEP amplitude, reduced optic nerve edema, and resulted in significant preservation of RGCs and axons in eyes with rAION.
Conclusions.
A single IVT injection of PGJ2 is nontoxic to the retina and optic nerve and neuroprotective when given immediately after rAION induction.
Keywords: nonarteritic anterior ischemic optic neuropathy, white matter, ischemia, 15d-Prostaglandin J2, intravitreal injection, neuroprotection
In a rat model of NAION, intravitreal injection of PGJ2 immediately after onset of NAION provides sustained retinal ganglion cell neuroprotection, as determined by visual evoked potential, optical coherence tomography, immunohistochemistry, ganglion cell counts, and transmission electron microscopy.
Introduction
Nonarteritic anterior ischemic optic neuropathy (NAION) is caused by ischemia in the optic nerve head (optic disc) and is the leading cause of sudden optic nerve (ON)-related vision loss in individuals older than 50 years. It has an incidence of 2.3 to 10.2 per 100,000 in the United States, resulting in 1500 to 6000 new cases per year.1,2 The pathophysiology of NAION is poorly understood, which may explain why there are no effective medical or surgical treatments for the condition.3 One of the key pathognomonic features of NAION is optic disc swelling that has been postulated to cause a compartment syndrome, resulting in progressive local ischemia and further ON damage.4 There is also evidence that inflammation at the site of the ischemic lesion may be responsible for some of the ON damage that occurs in NAION.5
Rodent and nonhuman primate models of NAION (rAION, pAION) have been developed by our laboratory6–8 and corroborated by other laboratories.9 In these models, the photosensitive dye rose bengal (RB) is injected intravenously and then activated by intraocular laser to create superoxide radicals that damage the vascular endothelium of optic disc capillaries. Although the mechanism by which ON damage is produced may not be identical to that which causes NAION, these models nevertheless produce ON damage that clinically, electrophysiologically, and histopathologically resembles the human condition,6–8 thus enabling a more thorough investigation of potential mechanisms of ON damage in patients with NAION and providing a basis with which to evaluate potential treatments.
Prostaglandin J2 (PGJ2) is a naturally occurring metabolite of prostaglandin D2, which is itself generated from prostaglandin H following stress-associated activation of either cyclooxygenase-1 or -2. PGJ2 acts by at least two partially independent cellular pathways. In addition to direct inhibitory effects on nuclear factor κB activity,10 PGJ2 is believed to be the primary natural ligand for the transcriptional factor perioxisomal proliferator activator receptor gamma11 and is neuroprotective in a variety of systems.12 A modified, active, and physiologically stable form of PGJ2 (15-deoxy 12,14 delta prostaglandin J2) is also neuroprotective,11 having been shown to be effective in reducing rAION-induced ON edema and preserving retinal ganglion cells (RGCs) following systemic administration.13 Work on understanding the mechanisms of these effects is ongoing.
Although PGJ2 can be given systemically, a number of potentially unwanted side effects can occur by this route of administration. These include apoptosis of newly formed oligodendrocytes,14,15 kidney epithelial toxicity,16 and system-specific interleukin and cytokine upregulation17 that can alter pulmonary reactivity. These toxic effects may potentially result from differential PGJ2 accumulation, relative concentration-dependent sensitivity of different tissues to PGJ2, or tissue-specific responses. Regardless of the cause, “off-target” drug effects require sustained vigilance following drug administration.
The intravitreal (IVT) administration of various agents has revolutionized the treatment of ocular disorders and has become the preferred route of administration for systemically active compounds. The success of IVT treatment has been largely responsible for the renewed corporate interest in treatment of ocular disease. This approach has shown great versatility for treatment of diseases as diverse as age-related macular degeneration,18 viral retinitis,19 uveitis,20 and iris neovascularization.21 IVT administration enables drug delivery to the targeted tissue in much higher concentrations than might be possible by systemic administration, potentially increasing the therapeutic response and reducing toxicity to nontargeted organs. In this study, we attempted to determine if a single IVT injection of PGJ2 was toxic to the retina or optic nerve in normal adult rats and if it was neuroprotective when administered immediately after induction of rAION.
Methods
Animals
All animal protocols were approved by the University of Maryland Baltimore Institutional Animal Care and Use Committee, and all animals were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Male Long-Evans rats (175–300 g), obtained from Harlan Labs (Indianapolis, IN) were kept in the University of Maryland Baltimore animal facility and were given food and water ad libitum. Animals were anesthetized for all procedures with an intraperitoneal injection of a mixture of ketamine/xylazine (80 mg/kg; 4 mg/kg).
Induction and Visualization of rAION
RAION was generated as described previously.6 Briefly, a specially designed contact lens was placed on the cornea of an anesthetized rat to visualize the retina and ON. RAION was then induced using an intravenous injection of RB (2.5 mM), followed by ON laser illumination (500 μm spot size, 50 mW power, 10–12 seconds' duration) via frequency-doubled YAG laser (535 nm; Iridex Corporation, Mountain View, CA), coupled to a slit-lamp biomicroscope (Haag-Streit 900; Haag-Streit, Mason, OH). The retinal fundus, including the ON, was photographed before rAION induction and 1 to 2 days postinduction using a Nikon D1X camera (Nikon Corporation, Tokyo, Japan), also coupled to the slit-lamp. The ocular fundi (n = 9) also were imaged in vivo using spectral-domain optical coherence tomography (SD-OCT) (Heidelberg Engineering, Heidelberg, Germany) using a plano-concave contact lens that enabled visualization of the retinal layers. These animals were imaged both preinduction and at 2 days postinduction. For quantification of ON edema, five optical sections (scans) were taken through the retina and optic nerve, and the diameter of the optic nerve was defined as the minimal distance between the two inner nuclear layers. Values of ON edema for baseline, PGJ2-treated, and PBS (vehicle)-treated eyes were compared using the Wilcoxon-Mann-Whitney Rank Sum test.
Intravitreal Injection
All IVT injections were performed without masking the identity of the drug or control under aseptic conditions using a 33-gauge needle attached to a syringe (Hamilton Bonaduz AG, Bonaduz, Switzerland). Both pupils were dilated using 1% tropicamide. The eye to be injected was irrigated and the surrounding orbital region of each animal swabbed with 5% povidone-iodine. Eyes were anesthetized with viscous tetracaine (Tetravisc; Ocusoft, Inc., Richmond, TX). Injections were made through the pars plana under direct visualization using a dissecting microscope and included methylene blue dye to confirm delivery. For toxicity studies, one eye was injected with 2 μL PGJ2 (Cayman Chemicals, Tel Aviv, Israel) in 20% ethanol-PBS at a concentration of 0.25 μg/μL (total, 0.5 μg), and the contralateral eye was injected with 2 μL PBS (i.e., vehicle). For efficacy studies, one eye was injected with either 2 μL PGJ2 or 2 μL PBS immediately following rAION induction, with the fellow eye serving as a naïve control. Tobramycin-decadron ophthalmic ointment was applied immediately after the injections.
Electrophysiology
All animals used for toxicity studies underwent Ganzfeld ERGs and flash-evoked VEPs both before (baseline studies) and after injections. For efficacy studies, only flash-evoked VEPs were recorded from the animals to preserve dark adaptation and, thus, to reduce amplitude variability. Animals were dark-adapted for at least 12 hours before recording, and their pupils were dilated with one drop of 1.0% tropicamide. ERGs (when recorded) and VEPs (all animals) were obtained at baseline and at 28 days posttreatment. In addition, ERGs and VEPs were recorded at 3 days and 10 days after treatment in the toxicity group, and at 1 and 2 weeks in the efficacy group.
Visual Evoked Potentials.
VEPs were recorded prior to ERGs. The active electrode (Oz) was placed in the midline of the animal's shaved skull directly anterior to the occipital protuberance, the reference electrode was placed in the midline frontal position (Fz), and the ground was placed on the tail using surface electrodes and EC2 electrode paste (Grass Instruments, New Bedford, MA). A black rubber eye occluder shielded the eye not being tested. VEPs were recorded using the LKC UTAS SunBurst electrophysiology recording system (LKC Technologies, Inc., Gaithersburg, MD). Responses were averaged 80 times per record, filtered between 0.1 and 100 Hz at a luminance of 0.3 cd.s/m2, and repeated multiple times for each eye until consistent waveforms were obtained. Results were analyzed using the Wilcoxon-Mann-Whitney Rank Sum test. Data analyses of the VEPs in the efficacy group were performed by two graders, one of whom was masked as to which animals were given IVT PGJ2.
Electroretinography.
ERGs were recorded bilaterally using the same UTAS SunBurst electrophysiology recording system used for recording VEPs. Active electrodes were constructed using electrostatic carpet fibers (Shieldex, Bremen, Germany) attached to an insulated lead; contact with the cornea was enhanced with a drop of viscous methylcellulose. Surface electrodes were used for the indifferent electrode (placed at the midline frontal position) and ground electrode (tail position) using Grass EC2 electrode paste. All test luminances conformed to the ISCEV standard.22
Euthanasia and Tissue Preparation
For all studies, animals were euthanized at 30 days postinjection. Deeply anesthetized animals were perfused with 4% paraformaldehyde-PBS (PF-PBS), following which tissues were isolated and postfixed in the same solution. Eyes with attached ONs were enucleated, punctured at the cornea, and postfixed in PF-PBS. Optic nerve tissues used for transmission electron microscopy (TEM) studies were fixed further in a mixture of paraformaldehyde-glutaraldehyde.
ON Preparation for TEM
Fixed ON tissue was impregnated with 5% uranyl acetate and lead citrate and embedded in Araldite-Epon. Sections 0.5-μm thick were cut and stained with toluidine blue and imaged with a ×100 oil immersion lens on an Olympus microscope (Olympus BX41; Olympus America, Inc., Center Valley, PA). Thin sections (200 nm) were floated onto copper grids and examined by TEM (Philips, Eindhoven, The Netherlands). Photographs were taken at ×2500 and ×4000 magnification with appropriate-size standards.
RGC Layer Stereology
Following fixation in 4% PF-PBS, retinas were dissected free of underlying tissues, the vitreous was partially digested using 1 mg/mL hyaluronidase (Sigma Chemicals, St. Louis, MO), and RGCs in the whole retinas were immunohistochemically identified using an anti-rabbit antibody to Brn3a (1:500; Santa Cruz Chemicals, Santa Cruz, CA). Primary antibody was detected following extensive washing using a Cy3-labeled donkey anti-rabbit secondary antibody (Jackson ImmunoResearch, West Point, PA). Postfixed retinas were flat-mounted using fluorescent mounting medium. The total retinal area was identified and outlined. RGCs were identified using a Nikon Eclipse E800 compound microscope (Nikon Corporation, Melville, NY) with a 410-nm excitation filter/450-nm pass filter cube. A ×60 air objective with a low numerical aperture was used to give a depth of field sufficient to penetrate the nerve fiber and RGC layers. Cells in the RGC layer were counted using a computer-driven microscope stage controlled by an optical fractionator linked to a stereological imaging package (Stereoinvestigator, Ver.6.0; Microbrightfield Bioscience, Williston, VT). Stereological analysis was performed using the stereoinvestigator package, using a sufficient number of random sites within each defined region. At least 800 cells, at a minimum of 50 sites, were counted per retina, a number greater than that required by the Schmitz-Hof equation for statistical validity.23 Seven animals per group were used for the initial toxicological studies and 10 animals per group were used for rAION neuroprotection assessment. Significance of the differences between groups was determined using a two-tailed Student's t-test.
Results
Toxicity Studies
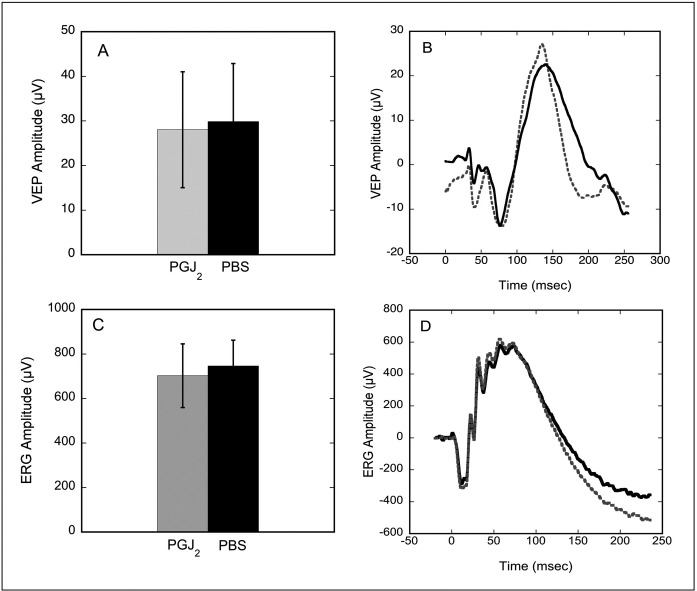
No ophthalmoscopically visible changes were observed in naïve (i.e., no rAION) eyes of animals at 3, 10, or 28 days post-IVT injection of either PGJ2 or PBS. In addition, there were no significant differences in VEP amplitudes between eyes injected with PGJ2 and eyes injected with PBS (Figs. 1A, 1B, n = 4, P = 1), and there were no significant differences in ERG amplitudes or implicit times between PBS-injected and PGJ2-injected eyes (Figs. 1C, 1D). Finally, no statistically significant differences in numbers of RGCs (i.e., Brn3a+ cells) were detected in retinas from eyes injected with PGJ2 versus PBS-injected eyes (P = 0.67) (Fig. 2).
Figure 1.
Electrophysiologic effects of an IVT injection of PGJ2 versus PBS into a normal eye. Data are shown for VEP (A, B) and ERG (C, D) amplitudes, 28 days post-IVT injection (n = 4). Gray bars (A, C) and dotted lines (B, D) indicate eyes injected with PGJ2, whereas black bars and solid lines indicate eyes injected with PBS. VEPs and ERGs recorded from eyes injected with PGJ2 are not significantly different from those recorded from eyes injected with PBS (P = 1 for VEP and 0.5 for ERG, Wilcoxon test). Error bars indicate ±1 SD.
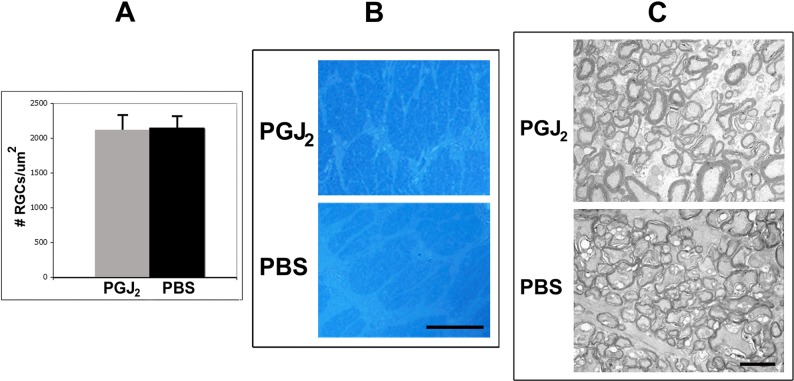
Figure 2.
Histologic effects of a single IVT injection of PGJ2 versus PBS into a normal eye. (A) RGC counts in eyes injected with PGJ2 (n = 5) do not differ significantly from those in eyes injected with PBS (n = 5) (P = 0.67). Optic nerve cross-sections show no differences in architecture after a single IVT injection of PGJ2 ([B, C], upper figures), compared with PBS ([B, C], lower figures), when examined using light microscopy (B) or TEM (C). Scale bars: 20 μm (B); 2 μm (C).
Similar to the results of electrophysiological testing, quantitative RGC stereology did not show significant differences in the number of Brn3a+ cells in the RGC layer of the retinas of PGJ2-injected eyes, compared with PBS-injected eyes, at 30 days postinjection (Fig. 2A), and no histological changes were detectable in the ONs of animals whose eyes were injected with PGJ2, compared with PBS, at 30 days postinduction (Figs. 2B, 2C). Thus, IVT administration of PGJ2 did not produce functional or histological evidence of ocular toxicity in these animals when given at the dose used in this study.
Efficacy Studies
One major action of systemically administered PGJ2 is the reduction of ON edema following induction of rAION.13 A single IVT injection of PGJ2 also substantially reduced ON edema in rAION-induced eyes when the injection was given immediately after induction (Fig. 3).
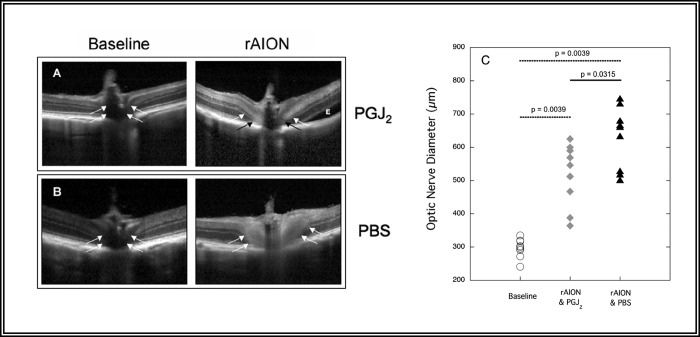
Figure 3.
OCT in rAION. (A, B) Raw data from two animals before (left column) and 2 days after (right column) induction of rAION followed immediately by a single IVT injection of either PGJ2 or PBS. (A) The upper panel shows the baseline (left) and 2-day post-rAION (right) OCTs in the eye of one animal given a single IVT injection of PGJ2 immediately after induction of rAION. (B) The lower panel shows the appearance at baseline (left) and 2 days following (right) an IVT injection of PBS in the eye of the second animal. The ON and perineural regions of the eye injected with PBS show substantially more swelling than the same regions in the eye injected with PGJ2 (arrows). “E” indicates an area of intraretinal edema. (C) Scatter plot of OCT data showing degree of optic nerve edema in eyes before induction of rAION (open circles, n = 18), 2 days after induction of rAION followed by PGJ2 injection (gray diamonds, n = 9), and 2 days after induction of rAION followed by PBS injection (black triangles, n = 9). Overall, optic nerves in eyes injected with PGJ2 are less edematous than eyes injected with PBS (P = 0.03).
Electrophysiologic Evidence for PGJ2 Neuroprotection
To evaluate further the neuroprotective effect of PGJ2 on ON function, we recorded VEPs at 1 week, 2 weeks, and 4 weeks after rAION induction (Fig. 4). We compared the VEP amplitudes from the injected and naïve eyes of the same animal to eliminate artifacts due to interanimal variations in VEP amplitude. VEP amplitudes in eyes injected with PGJ2 (n = 6) measured 110.6% of the fellow control eyes, whereas VEP amplitudes from eyes injected with PBS (n = 6) measured 42.4% (P = 0.004) of the control eyes. We did not compare VEP latencies in the two data sets because VEP latencies in rats vary considerably with minor changes in the state of dark adaptation.
Figure 4.
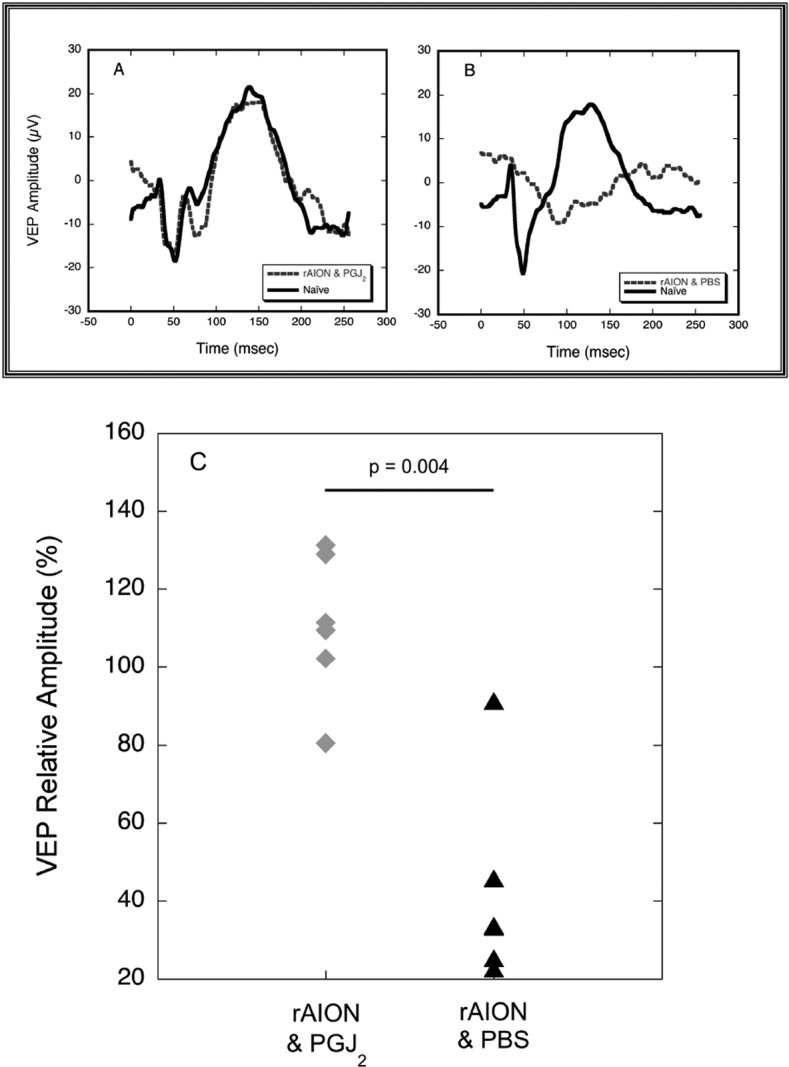
Effects of PGJ2 on VEPs in rAION. (A, B) VEPs 2 days post-rAION from the same two animals whose OCT results are shown in Figure 3. (A) The VEP waveform from the eye of the animal in which rAION was induced and then injected immediately with PGJ2 (dashed line) is nearly identical in amplitude and latency with the waveform from the animal's contralateral naïve (no rAION, uninjected) eye (solid line). (B) The VEP waveform from the eye of the animal in which rAION was induced and then injected immediately with PBS (dashed line) shows a substantial reduction in amplitude and increased latency compared with the fellow naïve eye (solid line). (C) Ratios of amplitudes in the treated versus untreated eye of animals receiving an IVT injection of PBS post-rAION induction (black triangles, n = 6) versus eyes of animals receiving an IVT injection of PGJ2 post-rAION induction (gray diamonds, n = 6). For clarity, data are shown for 1-week post-rAION; 2- and 4-week data were not significantly different. No significant change from normal is observed in the PGJ2-treated eyes (P = 0.92), whereas the animals treated with PBS show a 58% decline in the eye with rAION.
PGJ2-Associated Preservation of Retinal and ON Structure
We previously demonstrated by both histological and ultrastructural analyses that rAION results in both axon loss and postischemic demyelination.6,7 In the current study, ON histology using toluidine blue staining revealed increased preservation of ON axons and improvement in overall ON structure in eyes 30 days post-rAION induction followed immediately by a single IVT injection of PGJ2 compared with PBS-injected eyes. Striking differences were seen in the TEM analysis of ONs from PBS-injected eyes compared with ONs from PGJ2-injected eyes following rAION induction. Specifically, there were significant differences in the relative axon numbers, axon packing density, and degree of postischemic demyelination (Fig. 5). TEM of normal, control ONs revealed axon bundles separated by thin septae (Figs. 5A1, 5A4). The axons were of varying calibers, ranging from 0.2 to 2.0 μm in diameter. In PBS-injected eyes 30-day post-rAION induction, severe axon loss was apparent, with depletion of both large- and small-diameter axons (Figs. 5A2, 5A5, 5B). The interaxonal septae were greatly thickened, with regions in which there were only a few surviving axons. There were also areas with demyelinated axons and nearby debris (possibly myelin breakdown products). In contrast, ONs from eyes injected with PGJ2 immediately after rAION induction revealed many regions of relative axon preservation (Figs. 5A3, 5A6, 5B) containing well-myelinated axons.
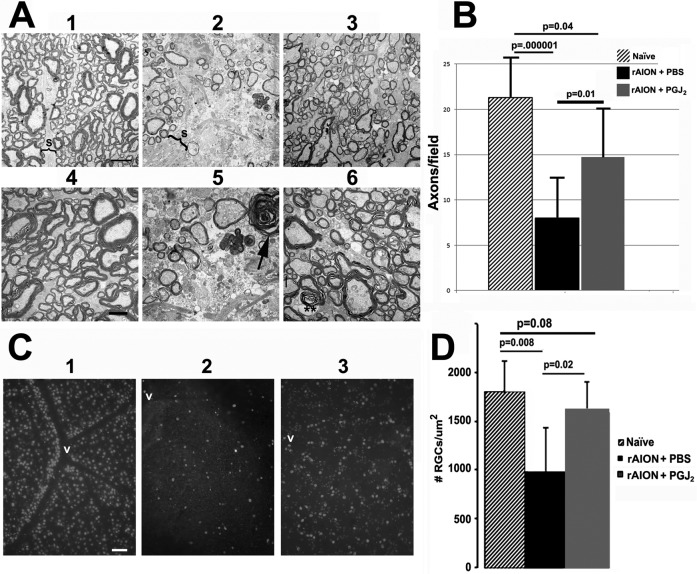
Figure 5.
Optic nerve and RGC stereological analysis of naive (no rAION, uninjected) eyes and in eyes after induction of rAION immediately followed by a single IVT injection of either PGJ2 or PBS. (A) TEM analysis: (1–3) are lower magnification (×2500); (4–6) are from the same general regions as seen in low power but at higher magnification (×4000). (1) Control ON: Axons (Ax) are relatively closely packed, with thin separating septae (S). (2) ON from an rAION-induced, PBS-injected eye. The septae (S) are greatly widened, with many fewer axons (Ax). (3) ON from an rAION-induced, PGJ2-injected eye shows axonal preservation. (4) Control ON: Normal myelinated axons in a näive (no rAION, uninjected) eye. (5) ON from an rAION-induced, PBS-injected eye. Compared with (4), the numbers of axons are greatly reduced, and there are degenerating axons showing demyelination (arrow). (6) ON from an rAION-induced, PGJ2-treated eye. Both large and small axons appear normally myelinated (double asterisks), and there are many more intact axons than in nerves from the eye injected with PBS (compare with [5]). Scale bars: 2 μm (A1–A3), 500 nm (A4–A6). (B) Axon quantification. Axons from 10 random fields were counted from micrographs obtained from lower-magnification TEMs. Mean ± SD of each group. The ONs from PGJ2-injected ONs at 30 days postinduction (gray bar) had more intact axons than ONs from PBS-injected eyes (black bar). Note the overlap of numbers of axons per field in PGJ2-injected eyes compared with naïve eyes. The number of axons in PGJ2-injected eyes compared with PBS-injected eyes was significant (2-tailed t-test, P ≤ 0.01). (C) Photomicrographs of representative retinal flat mounts of (1) naïve, (2) rAION-induced, PBS-injected, and (3) rAION-induced, PGJ2-injected eyes. RGCs are identified by Brn3a immunostaining. RGCs are more numerous near vessels (v) in the naïve eye. Scale bar: 50 μm. (D) Stereological analysis of Brn3a+ cells (RGCs) in naïve (no rAION, uninjected) eyes (hatched bar), rAION-induced, PBS-injected eyes (black bar), and rAION-induced, PGJ2-injected eyes (gray bar). Compared with naïve eyes, there was a significant loss of RGCs in rAION-induced eyes injected with PBS (P = 0.008), whereas eyes injected with PGJ2 immediately after induction of rAION showed a significant increase in the number of RGCs compared with PBS-injected eyes (P < 0.02).
Stereological analysis of Brn3a+ cells in the RGC layer (presumably RGCs) in the PGJ2-injected eyes compared with PBS-injected eyes also revealed striking differences in preservation after rAION induction (Fig. 5C). rAION normally results in significant RGC loss at 30 days postinduction. This loss was present in all eyes injected with PBS but not in eyes injected with PGJ2 (Fig. 5D).
Discussion
NAION is hypothesized to be caused initially by focal ON ischemia. The initial ischemia produces an inflammatory response5 that might result not only in direct damage to surrounding tissue but also in further edema, resulting in a compartment syndrome. This compartmentation results in further axon damage.4,5,24,25 Thus, treatments designed to reduce ON edema, the inflammatory response, or both are of primary clinical interest in the areas of both ON and brain ischemia treatment.
PGJ2 has been reported to have both beneficial and deleterious effects to cells of the central nervous system.14 In the present study, we found neither electrophysiologic nor histologic evidence of retinal or ON toxicity from PGJ2 when PGJ2 was administered intravitreally in the doses described above. Although toxicity was assessed in a relatively small number of animals, the IVT dose of PGJ2 that we used was much less than that used for systemic administration. Thus, this method of delivery may have minimized the potential systemic toxicity of PGJ2 while providing high transient levels of active drug to the area of greatest involvement: the anterior ON.
With respect to efficacy, the present study shows that IVT administration of PGJ2 immediately after induction of rAION reduces ON edema compared with IVT injection of PBS, when evaluated 2 days postinduction. This reduction is discernible both by direct visualization and by OCT. Other studies also have found that PGJ2 administration increases vascular perfusion in the ON head,13 and this may be, at least in part, the mechanism by which PGJ2 reduces edema and inflammation that develops in the region of the insult.13,26
The early reduction in postischemia edema in this model correlates with preservation of both ON axons and RGCs. Indeed, our histological and ultrastructural analyses of the ONs in PGJ2-injected eyes show a marked reduction in the extent of axon destruction and increased numbers of intact RGCs, compared with PBS-injected eyes. These data are consistent with the hypothesis that PGJ2 administration reduces edema, vascular compromise, and, possibly, inflammation within the ON at or near the site of ischemia.
Our electrophysiological results confirm that the structural effects of PGJ2 are mirrored by preservation of visual function in PGJ2-treated eyes; VEP amplitudes were substantially better from eyes injected with PGJ2 than from eyes injected with vehicle. In fact, the VEP amplitudes in PGJ2-injected eyes were virtually identical to amplitudes from unlasered, uninjected eyes.
We chose to wait 30 days post-rAION induction and IVT injection before euthanizing our animals to minimize the possibility that the neuroprotective effects of PGJ2 were transient and that dying cells would contribute to the counts of RGCs. The 30-day analysis point was chosen because a previous study from our laboratory revealed that the majority of RGC death following induction of rAION occurs within the first 21 days.27 The data from the current study indicate that intraocular administration of PGJ2 provides RGC protection lasting at least 30 days in our rAION model. Although this does not prove that the effects of PGJ2 last for the lifetime of the animal, the 30-day period is a substantial time frame in the life of an adult rat, which is approximately 24 months.
In summary, the results of the current study suggest that in rAION, IVT injection of PGJ2 provides neuroprotection that persists for at least 30 days and, thus, may be an effective neuroprotective treatment for NAION, although whether its mechanism is related solely to the reduction in postischemia edema, reduction in vascular congestion, reduction of intraneural inflammation, or a combination of these processes is unclear.
Although intraocular PGJ2 appears promising as a clinical treatment, there are a number of questions that remain to be answered. First, the drug was administered immediately after rAION induction and before visible development of ON edema. PGJ2 administration may be effective only in the very early stages of the condition, before the development of edema-associated vascular compromise or significant inflammation. Second, because NAION damage may result from a number of different mechanisms, it is possible that PGJ2 administration may be effective in only a subset of patients, even if given early in the course of the disease. Third, because rAION is a murine model of NAION, both nonhuman primate testing and, ultimately, a randomized clinical trial, must be performed to determine if PGJ2 administration is neuroprotective in the human condition. Finally, we injected our animals with a single dose of PGJ2. We do not know how long the drug remains active within the eye. In may be that multiple IVT injections of the drug are required to provide optimum visual results in patients with NAION. Regardless, PGJ2 is the first compound that has been shown in vivo to be a robust, long-term neuroprotective agent for acute ON ischemia in an animal model of NAION.
Acknowledgments
The authors thank Fernandino Vilson and David Bernstein for their assistance in stereological analysis.
The use of PGJ2 for NAION has been granted US patent #8,106,096 to the University of Maryland, Baltimore.
Supported by National Institutes of Health Grants EY015304 and EY019529 (SLB), by a Disney award from Research to Prevent Blindness (MAJ), and by a grant from the Hirschhorn Foundation (VT, NRM).
Disclosure: V. Touitou, None; M.A. Johnson, None; Y. Guo, None; N.R. Miller, None; S.L. Bernstein, P
References
- 1. Luneau K, Newman NJ, Biousse V. Ischemic optic neuropathies. Neurologist. 2008; 14: 341–354 [DOI] [PubMed] [Google Scholar]
- 2. Hattenhauer MG, Leavitt JA, Hodge DO, Grill R, Gray DT. Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997; 123: 103–107 [DOI] [PubMed] [Google Scholar]
- 3. Atkins EJ, Bruce BB, Newman NJ, Biousse V. Treatment of nonarteritic anterior ischemic optic neuropathy. Surv Ophthalmol. 2010; 55: 47–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kerr NM, Chew SS, Danesh-Meyer HV. Non-arteritic anterior ischaemic optic neuropathy: a review and update. J Clin Neurosci. 2009; 16: 994–1000 [DOI] [PubMed] [Google Scholar]
- 5. Salgado C, Vilson F, Miller NR, Bernstein SL. Cellular inflammation in nonarteritic anterior ischemic optic neuropathy and its primate model. Arch Ophthalmol. 2011; 129: 1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernstein SL, Guo Y, Kelman SE, Flower RW, Johnson MA. Functional and cellular responses in a novel rodent model of anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2003; 44: 4153–4162 [DOI] [PubMed] [Google Scholar]
- 7. Goldenberg-Cohen N, Guo Y, Margolis FL, Miller NR, Cohen Y, Bernstein SL. Oligodendrocyte dysfunction following induction of experimental anterior optic nerve ischemia. Invest Ophthalmol Vis Sci. 2005; 46: 2716–2725 [DOI] [PubMed] [Google Scholar]
- 8. Chen CS, Johnson MA, Flower RA, Slater BJ, Miller NR, Bernstein SL. A primate model of nonarteritic anterior ischemic optic neuropathy (pNAION). Invest Ophthalmol Vis Sci. 2008; 49: 2985–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chuman H, Maekubo T, Osako T, Kodama Y, Ishiai M, Nao-I N. Rodent model of nonarteritic ischemic optic neuropathy and its electrophysiological evaluation. Jpn J Ophthalmol. 2012; 56: 518–527 [DOI] [PubMed] [Google Scholar]
- 10. Zhao ML, Brosnan CF, Lee SC. 15-deoxy (12, 14)-PGJ2 inhibits astrocyte IL-1 signaling: inhibition of NF-kappaB and MAP kinase pathways and suppression of cytokine and chemokine expression. J Neuroimmunol. 2004; 153: 132–142 [DOI] [PubMed] [Google Scholar]
- 11. Lin TN, Cheung WM, Wu JS, et al. 15d-prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006; 26: 481–487 [DOI] [PubMed] [Google Scholar]
- 12. Zhuang H, Kim YS, Namiranian K, Doré S. Prostaglandins of J series control heme oxygenase expression: potential significance in modulating neuroinflammation. Ann N Y Acad Sci. 2003; 993: 208–216 [DOI] [PubMed] [Google Scholar]
- 13. Nicholson JD, Puche AC, Guo Y, Weinreich D, Slater BJ, Bernstein SL. PGJ(2) provides prolonged CNS stroke protection by reducing white matter edema. PLoS One. 2012; 7: e50021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiang Z, Lin T, Reeves SA. 15d-PGJ2 induces apoptosis of mouse oligodendrocyte precursor cells. J Neuroinflammation. 2007; 4: 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pierre SR, Lemmens MA, Figueiredo-Pereira ME. Subchronic infusion of the product of inflammation prostaglandin J2 models sporadic Parkinson's disease in mice. J Neuroinflammation. 2009; 6: 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang DS, Kwon CH, Park JY, et al. 15-deoxy-Delta12, 14-prostaglandin J2 induces renal epithelial cell death through NF-kappaB-dependent and MAPK-independent mechanism. Toxicol Appl Pharmacol. 2006; 216: 426–435 [DOI] [PubMed] [Google Scholar]
- 17. Chiba T, Ueki S, Ito W, et al. 15-Deoxy-Delta(12, 14)-prostaglandin J2 induces IL-8 and GM-CSF in a human airway epithelial cell line (NCI-H292). Int Arch Allergy Immunol. 2009; 149: 77–82 [DOI] [PubMed] [Google Scholar]
- 18. Nau JY. Avastin and lucentis for macular degeneration [in French]. Rev Med Suisse. 2012; 8: 1636–1637 [PubMed] [Google Scholar]
- 19. Cochereau-Massin I, Lehoang P, Lautier-Frau M, et al. Efficacy and tolerance of intravitreal ganciclovir in cytomegalovirus retinitis in acquired immune deficiency syndrome. Ophthalmology. 1991; 98: 1348–1353 [DOI] [PubMed] [Google Scholar]
- 20. Sallam A, Taylor SR, Habot-Wilner Z, et al. Repeat intravitreal triamcinolone acetonide injections in uveitis macular oedema. Acta Ophthalmol. 2012; 90: e323–e325 [DOI] [PubMed] [Google Scholar]
- 21. Jiang Y, Liang X, Li X, Tao Y, Wang K. Analysis of the clinical efficacy of intravitreal bevacizumab in the treatment of iris neovascularization caused by proliferative diabetic retinopathy. Acta Ophthalmol. 2009; 87: 736–740 [DOI] [PubMed] [Google Scholar]
- 22. Marmor M, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M; International Society for Clinical Electrophysiology of Vision. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009; 118: 69–77 [DOI] [PubMed] [Google Scholar]
- 23. Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005; 130: 813–831 [DOI] [PubMed] [Google Scholar]
- 24. Tesser RA, Niendorf ER, Levin LA. The morphology of an infarct in nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2003; 110: 2031–2035 [DOI] [PubMed] [Google Scholar]
- 25. Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2003; 23: 157–163 [DOI] [PubMed] [Google Scholar]
- 26. Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006; 12: 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmad AS, Ahmad M, Maruyama T, Narumiya S, Doré S. Prostaglandin D2 DP1 receptor is beneficial in ischemic stroke and in acute exicitotoxicity in young and old mice. Age. 2010; 32: 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]