Abstract
Aims/hypothesis
Ingested protein is a well-recognised stimulus for glucagon-like peptide-1 (GLP-1) release from intestinal L cells. This study aimed to characterise the molecular mechanisms employed by L cells to detect oligopeptides.
Methods
GLP-1 secretion from murine primary colonic cultures and Ca2+ dynamics in L cells were monitored in response to peptones and dipeptides. L cells were identified and purified based on their cell-specific expression of the fluorescent protein Venus, using GLU-Venus transgenic mice. Pharmacological tools and knockout mice were used to characterise candidate sensory pathways identified by expression analysis.
Results
GLP-1 secretion was triggered by peptones and di-/tripeptides, including the non-metabolisable glycine-sarcosine (Gly-Sar). Two sensory mechanisms involving peptide transporter-1 (PEPT1) and the calcium-sensing receptor (CaSR) were distinguishable. Responses to Gly-Sar (10 mmol/l) were abolished in the absence of extracellular Ca2+ or by the L-type calcium-channel blocker nifedipine (10 μmol/l) and were PEPT1-dependent, as demonstrated by their sensitivity to pH and 4-aminomethylbenzoic acid and the finding of impaired responses in tissue from Pept1 (also known as Slc15a1) knockout mice. Peptone (5 mg/ml)-stimulated Ca2+ responses were insensitive to nifedipine but were blocked by antagonists of CaSR. Peptone-stimulated GLP-1 secretion was not impaired in mice lacking the putative peptide-responsive receptor lysophosphatidic acid receptor 5 (LPAR5; also known as GPR92/93).
Conclusions/interpretation
Oligopeptides stimulate GLP-1 secretion through PEPT1-dependent electrogenic uptake and activation of CaSR. Both pathways are highly expressed in native L cells, and likely contribute to the ability of ingested protein to elevate plasma GLP-1 levels. Targeting nutrient-sensing pathways in L cells could be used to mobilise endogenous GLP-1 stores in humans, and could mimic some of the metabolic benefits of bariatric surgery.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-013-3037-3) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: CaSR, GLP-1, LPAR5, PEPT1
Introduction
Food ingestion triggers the secretion of a range of hormones from the gastrointestinal tract that coordinate nutrient absorption, insulin secretion and appetite. The ability of the enteroendocrine hormone glucagon-like peptide-1 (GLP-1) to enhance glucose-dependent insulin release and suppress appetite forms the basis for the clinical use of GLP-1 mimetics and dipeptidyl dipeptidase 4 inhibitors for the treatment of type 2 diabetes [1, 2]. GLP-1 levels rise dramatically after gastric bypass, and have been implicated in the improved insulin secretion that follows bariatric surgery [3, 4]. Such findings have triggered renewed interest in the idea of targeting the endogenous enteroendocrine system for the treatment of diabetes and obesity.
GLP-1 is only one of a family of gut peptides involved in the central and peripheral coordination of metabolism, acting alongside other enteroendocrine hormones such as cholecystokinin (CCK), glucose-dependent insulinotropic polypeptide (GIP) and peptide YY (PYY). These hormones are produced by an overlapping subset of enteroendocrine cells and are released into the bloodstream in varying proportions following the ingestion of carbohydrate, fat and protein. The stimulation of GIP and GLP-1 release following glucose ingestion forms the intestinal arm of the incretin axis, increasing the responsiveness of pancreatic beta cells to the rising plasma glucose levels. Co-ingestion of protein and fat, however, markedly enhances glucose-triggered GLP-1 and insulin secretion [5], and protein-rich meals have been associated with elevated PYY secretion and reduced postprandial hunger scores [6]. Understanding the mechanisms underlying protein-sensing in the enteroendocrine system might therefore identify strategies to increase GLP-1 release using orally available agents.
GLP-1-producing enteroendocrine cells are classically referred to as L cells, and have been identified in the human intestine as proximal as the duodenum. The number of L cells and the proportion that coexpress PYY increase along the longitudinal axis of the gastrointestinal tract [7]. Like most enteroendocrine cells, L cells are located in the epithelial layer, where they make direct contact with the luminal contents via their apical projections. The mechanisms underlying L cell carbohydrate sensing are relatively well characterised, and are critically dependent on electrogenic sugar uptake by the sodium-dependent glucose transporter 1 (SGLT1) [8–11]. How L cells detect ingested protein is more controversial.
We have previously reported on stimulation of GLP-1 secretion from primary colonic cultures and the enteroendocrine model cell line GLUTag by amino acids [12]. Glycine and alanine are strong stimulants of GLUTag cells through activation of the ionotropic glycine receptor [13], but this mechanism was not observed in colonic L cells in primary culture [8]. Glutamine, by contrast, was a strong stimulant in both the cell line and primary intestinal cultures [14] and was subsequently shown to stimulate GLP-1 secretion after ingestion in human volunteers and patients with type 2 diabetes [15]. Amino-acid-triggered secretion of GLPs has also been linked to activation of the calcium-sensing receptor (CaSR) [16], the umami taste receptor (Tas1R1/Tas1R3) [17] and G-protein-coupled receptor 6A (GPRC6A) [18].
In the current study, we investigated if and how larger protein fragments, such as peptones (relatively poorly defined breakdown products of proteins after enzymatic digestion) and oligopeptides, stimulate GLP-1 secretion in primary murine culture.
Methods
Materials and solutions
Reagents were supplied by Sigma Aldrich (Poole, UK), unless stated otherwise. The composition of standard bath solution used in all experiments was: 4.5 mmol/l KCl, 138 mmol/l NaCl, 4.2 mmol/l NaHCO3, 1.2 mmol/l NaH2PO4, 2.6 mmol/l CaCl2, 1.2 mmol/l MgCl2 and 10 mmol/l HEPES (adjusted to pH 7.4 with NaOH). For experiments in which pH was manipulated, the pH was adjusted to pH 8.0 with NaOH, or for the lower pH of 6.5, 10 mmol/l PIPES was used in place of HEPES. The Ca2+-free saline used in imaging experiments consisted of: 4.5 mmol/l KCl, 138 mmol/l NaCl, 4.2 mmol/l NaHCO3, 1.2 mmol/l NaH2PO4, 1.2 mmol/l MgCl2, 10 mmol/l HEPES and 0.5 mmol/l EGTA (pH 7.4). Calhex231-HCl and NPS2143-HCl were purchased from Tocris Bioscience (Bristol, UK). Peptone (P5905, Sigma) is an enzymatic meat hydrolysate.
Primary intestinal epithelial cell isolation and culture
Transgenic mice that express the yellow fluorescent protein Venus driven by the proglucagon promoter were generated using a bacterial artificial chromosome construct as previously described [8]. All animal procedures were approved by the local ethics committee and conformed to UK Home Office regulations. Pept1 (also known as Slc15a1) knockout mice were obtained from Deltagen (San Mateo, CA, USA) and backcrossed for ten generations to a C57BL/6 background. They were bred and kept in the same conditions as wild-type C57BL/6 mice in a specific pathogen-free animal facility at 22 ± 2°C and a 12:12 h light/dark cycle. Procedures involving Pept1 –/– mice were performed according to the German guidelines for animal care and approved by the state ethics committee under reference number 55.2-1-54-2531-39-10.
Lpar5 –/– mice were generated by Takeda Cambridge (Cambridge, UK). For generation of the targeting vectors, homology arms were PCR amplified from genomic DNA and cloned into a vector containing the internal ribosomal entry site β-galactosidase reporter gene, a neomycin selection marker and two thymidine kinase-negative selection markers. The linearised vector was electroporated into embryonic stem cells (129/SvEv) and homologous recombination was detected by screening PCR and southern blot. Chimeras were generated by blastocyst injection, bred with 129/SvEv animals and maintained inbred in this background. Homozygous animals were viable and fertile, without any obvious abnormalities.
For tissue preparation, mice aged 3–6 months were killed by cervical dislocation and the large intestine distal to the caecum was excised and stored in Leibovitz L-15 medium. The tissue was relieved of any attached tissue and luminal dietary content, chopped finely and digested in 0.4 mg/ml collagenase XI dissolved in DMEM (25 mmol/l glucose). The process of tissue digestion involved four cycles of incubations at 37°C followed by collection of the supernatant fraction and replacement with fresh warm collagenase. The supernatant fractions of the two last digests were centrifuged for 3 min at 300 g and the pellets were resuspended in DMEM containing 100 U/ml penicillin, 2 mmol/l l-glutamine, 10% FBS and 0.1 mg/ml streptomycin. Cells were plated on Matrigel (BD Bioscience, Oxford, UK) pre-coated 24-well plastic dishes or glass-bottom culture dishes (Mattek Ashland, MA, USA) and incubated in a humidified atmosphere containing 5% CO2 at 37°C for a period ranging between 24 h and 8 days. Cell culture media for the studies performed on Pept1 –/– mice and their littermate controls were purchased from Invitrogen (Paisley, UK).
GLUTag cell culture
The murine enteroendocrine cell lines GLUTag and STC-1 were cultured in DMEM containing 5.6 mmol/l (GLUTag) or 25 mmol/l (STC-1) glucose supplemented with 10% FCS (GLUTag) or 15% horse serum and 2.5% FCS (STC-1) and 2 mmol/l l-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin. The cells were grown in 75 cm2 flasks at 37°C and 5% CO2. Cultures were passaged when they reached 70% confluence and all the experiments were performed on cultures having undergone fewer than 30 passages.
GLP-1 secretion assay
At 24 h following seeding, cells were treated with test reagents dissolved in standard bath solution supplemented with 0.1% fatty-acid-free BSA, for 2 h at 37°C. Supernatant fraction and lysate samples from each well were collected and GLP-1 was assayed using fluorescence-based GLP-1 active (7-36) ELISA kits (Millipore, Watford, UK) or a ‘total-GLP-1’ assay (Meso Scale Discovery, Gaithersburg, MD, USA). The results were calculated as a percentage of the GLP-1 content (secreted plus intracellular) per well and were normalised to the basal secretion in saline buffer measured in parallel on the same day. All conditions were tested on two or more cultures established from one mouse each, unless stated otherwise, and the numbers of wells investigated are given in the figure legends.
Calcium imaging
Cells were loaded with 7 μmol/l fura-2-acetoxymethyl ester (fura-2-AM; Invitrogen) and 0.01% pluronic acid in a standard bath solution containing 10 mmol/l glucose and 375 μmol/l eserine for 15 min at 37°C and for a further 15 min at room temperature. An inverted fluorescence microscope (Olympus IX71, Olympus, Southend on Sea, UK) with a ×40 oil-immersion objective lens was used for signal visualisation. Venus was excited at 475 nm and fura-2 at 340 and 380 nm using a monochromator (Cairn Research, Faversham, UK) and a 75W xenon arc lamp, and emissions were recorded using an Orca ER camera (Hamamatsu, Welwyn Garden City, UK), a dichroic mirror and a 510 nm long pass filter. All images were collected on MetaFluor software (Molecular Devices, Wokingham, UK). The ratio of fura-2 emissions at 340 and 380 nm (340/380 ratio) was used to monitor changes in the intracellular calcium concentration, while Venus fluorescence was used to identify L cells. Solutions were perfused continuously at a rate of approximately 1 ml/min. Mean 340/380 ratios in the presence of the test agent were normalised to the respective mean ratio of the background of each cell, measured before the addition and after the washout of the test compound. Numbers (n) given in the figure legends refer to the number of individual cells investigated.
Quantitative RT-PCR
Populations of Venus-positive cells (L cells) or Venus-negative cells (non-L cells) of purity >95% were separated with a MoFlo cell sorter (Beckman Coulter, High Wycombe, UK) as previously described [8]. RNA was extracted from FACS-sorted cells by a microscale RNA isolation kit (Ambion, Austin, TX, USA) and was then reverse transcribed to cDNA according to standard protocols. The appropriate amount of first-strand cDNA template was mixed with specific TaqMan primers (Applied Biosystems, Foster City, CA, USA), water and PCR Master Mix (Applied Biosystems), and quantitative RT-PCR was conducted using a 7900HT Fast Real-Time PCR system (Applied Biosystems). β-Actin was used as the normalisation control. The primer/probe pairs used in this study were from Takeda for lysophosphatidic acid receptor 5 (Lpar5; also known as Gpr92/93): forward primer: CGTCGGGCGCCATCT; reverse primer: AGCGGTCCACGTTGATGAG; probe: FAM-CCAGATGAACATGTACGGC-MGB; or from Applied Biosystems: Casr: Mm00607939_s1; GprC6A: Mm00443375_m1; Pept1/Slc15a1: Mm04209483_ml; Pept2/Slc15a2: Mm00451610_ml; β-actin: Mm00607939_s1. All experiments were performed on at least three cDNAs isolated from one mouse each.
Data analysis
The results are expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism 4.01 (San Diego, CA, USA). One-way ANOVAs with post hoc Dunnett’s or Bonferroni’s tests were performed on log-transformed secretion data, as these data are heteroscedastic, and ΔCt expression data. Statistical significance for Ca2+ imaging data was assessed by Student’s t test. Values were regarded as significant when p < 0.05.
Results
GLP-1 secretion was triggered by products of protein digestion
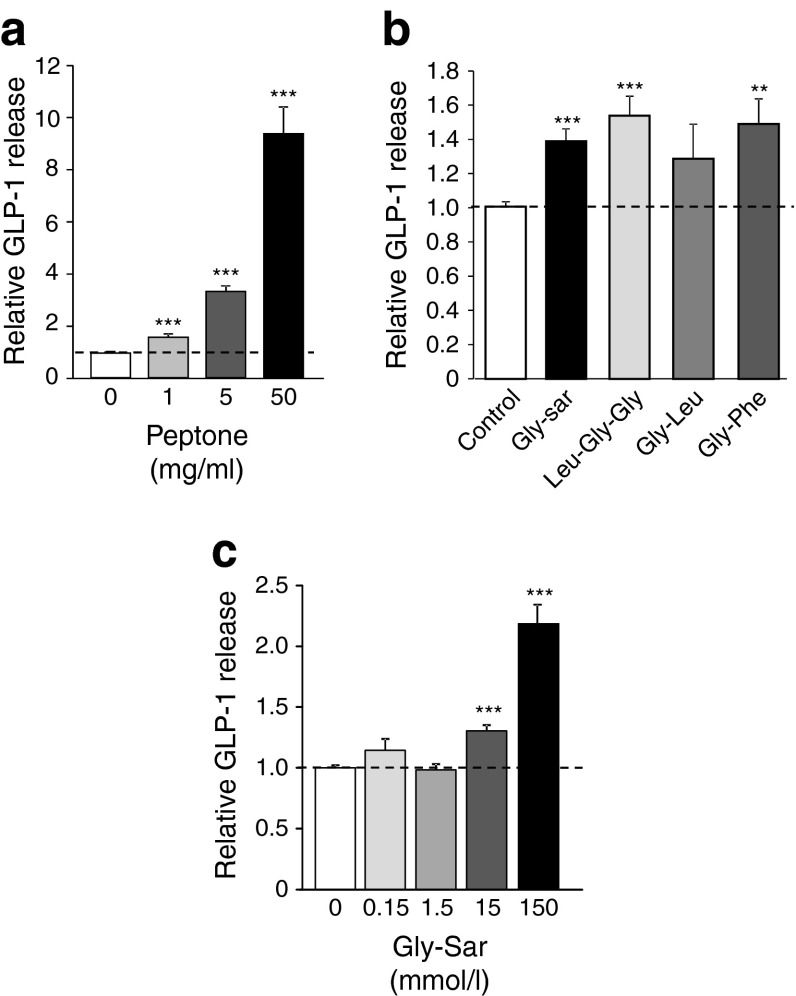
GLP-1 secretion from mouse colonic primary cultures was dose-dependently stimulated during 2 h incubations with an enzymatic meat hydrolysate (peptone). At the highest tested concentration of 50 mg/ml, secretion was enhanced approximately ninefold (Fig. 1a), while the lower peptone concentration of 5 mg/ml peptone triggered a more modest approximately threefold response. Synthetic di- and tripeptides also increased GLP-1 release, although to a lesser extent than peptone. Thus, glycine-leucine (Gly-Leu), glycine-phenylalanine (Gly-Phe), leucine-glycine-glycine (Leu-Gly-Gly) and the non-hydrolysable dipeptide glycine-sarcosine (Gly-Sar) enhanced GLP-1 release by approximately 1.4-fold at a concentration of 10 mmol/l each (Fig. 1b). At the higher concentration of 150 mmol/l (equivalent to 22 mg/ml) Gly-Sar was less effective than peptone, increasing secretion by approximately twofold (Fig. 1c).
Fig. 1.
GLP-1 secretion is stimulated by oligopeptides. GLP-1 secretion triggered by (a) 0, 1, 5 and 50 mg/ml peptone (n = 34, 7, 24 and 19, respectively); (b) Gly-Sar (n = 18), Leu-Gly-Gly (n = 6), Gly-Leu (n = 6) or Gly-Phe (n = 6) (each at 10 mmol/l); (c) 0, 0.15, 1.5, 15 and 150 mmol/l Gly-Sar (n = 27, 12, 11, 28 and 12, respectively). In all cases, mixed primary cultures from murine colon were incubated for 2 h under control conditions (standard bath solution) or in the additional presence of oligopeptides, as indicated. GLP-1 secretion in each well is expressed relative to the basal secretion (control) measured in parallel on the same day and indicated by the dashed line. Data represent the mean ± SEM. **p < 0.01, ***p < 0.001 compared with their respective controls by one-way ANOVA with post hoc Dunnett’s test
Protein digestion products trigger elevation of cytoplasmic calcium
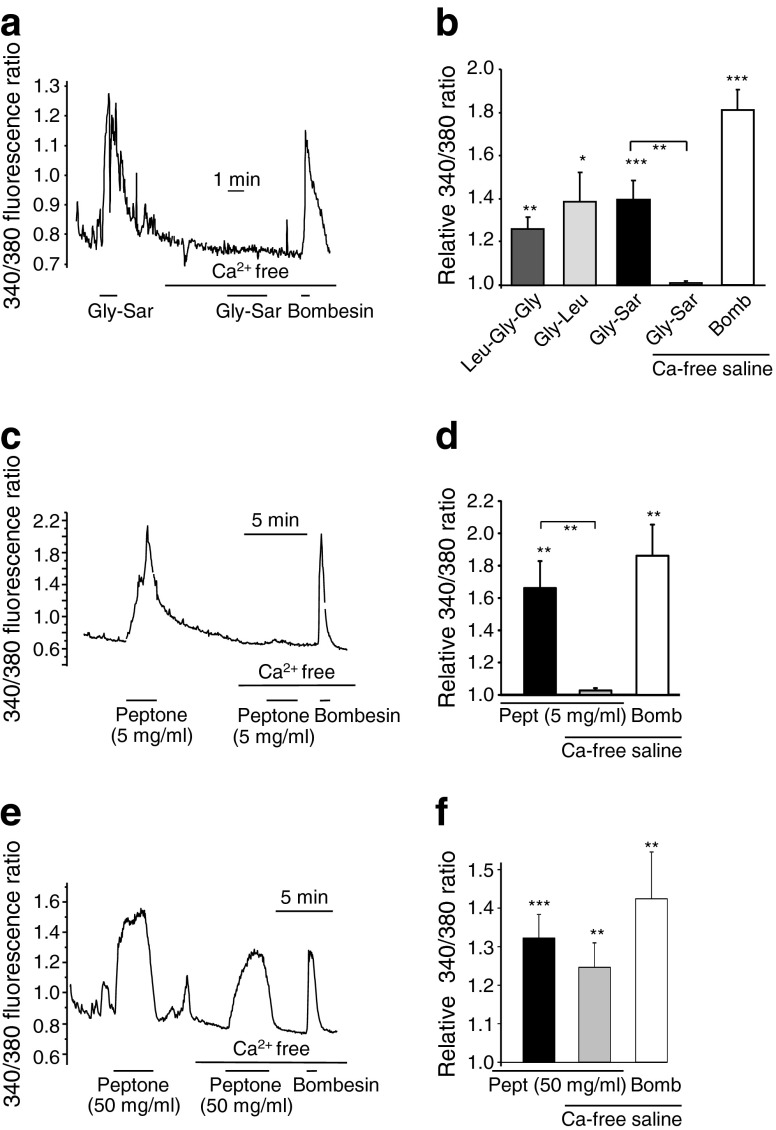
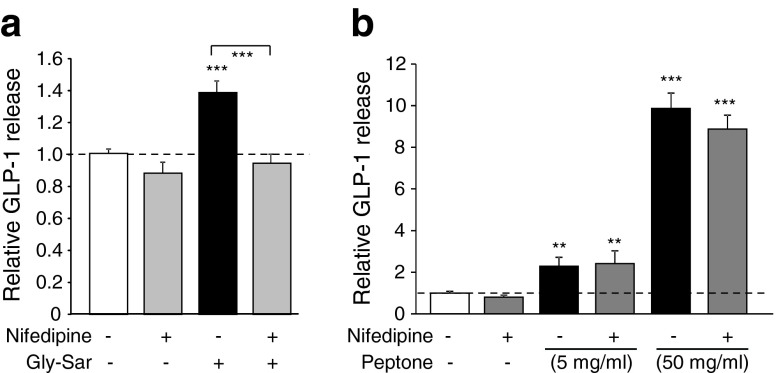
To investigate the molecular mechanisms underlying peptone- and oligopeptide-triggered responses, cytosolic Ca2+ fluctuations were monitored in identified primary L cells in mixed colonic cultures from GLU-Venus mice after loading with fura-2-AM. Di-/tripeptides (10 mmol/l) and peptone (5 or 50 mg/ml) triggered calcium elevation in L cells in standard saline buffer (Fig. 2). In Ca2+-free buffer, however, responses to Gly-Sar and 5 mg/ml peptone were abolished, whereas 50 mg/ml peptone or bombesin remained effective (Fig. 2). The dependence on extracellular Ca2+ for responses to dipeptides or 5 mg/ml peptone suggests that Ca2+ either enters L cells across the plasma membrane or is required externally for activation of a sensory pathway. To distinguish between these possibilities, we tested the effect of the voltage-gated calcium-channel blocker nifedipine. Whereas nifedipine (10 μmol/l) abolished secretion triggered by Gly-Sar, it did not prevent the response to 5 mg/ml peptone (Fig. 3), suggesting that these stimuli recruit distinct signalling pathways.
Fig. 2.
Oligopeptide-triggered Ca2+ responses in identified L cells in primary culture. (a, c, e) Fluorescent 340/380 ratio changes in primary identified L cells loaded with fura-2-AM and stimulated with (a) Gly-Sar (10 mmol/l), (c) 5 mg/ml peptone or (e) 50 mg/ml peptone in the presence and absence of extracellular Ca2+ and bombesin (100 nmol/l), as indicated. (b) Mean calcium changes in L cells following the addition of Leu-Gly-Gly (10 mmol/l, n = 5), Gly-Leu (10 mmol/l, n = 6), Gly-Sar (10 mmol/l) in the presence (n = 15) or absence (n = 5) of extracellular Ca2+, and bombesin in the absence of extracellular Ca2+ (Bomb, 100 nmol/l, n = 5), as indicated. (d) Mean calcium changes in L cells following the addition of peptone (Pept, 5 mg/ml) in the presence (n = 14) and absence (n = 9) of extracellular Ca2+, and bombesin (Bomb, 100 nmol/l, n = 9) in the absence of extracellular Ca2+, as indicated. (f) Mean calcium changes in L cells following the addition of peptone (Pept, 50 mg/ml) in the presence (n = 12) and absence (n = 11) of extracellular Ca2+, and bombesin (Bomb, 100 nmol/l, n = 11) in the absence of extracellular Ca2+, as indicated. (b, d, f) 340/380 ratios in the presence of the test agent were normalised to the mean of the background ratios of each cell measured before the addition and after washout of the test compound. Data represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared with baseline and between conditions by one- and two-sample Student’s t test
Fig. 3.
Gly-Sar- but not peptone-stimulated GLP-1 secretion relies on activation of L-type Ca2+ channels. (a) Mixed primary cultures from murine colon were incubated for 2 h under control conditions (standard bath solution) with (n = 9) or without (n = 18) nifedipine (10 μmol/l) or in the presence of Gly-Sar (10 mmol/l) with (n = 8) or without (n = 18) the addition of nifedipine, as indicated. (b) Mixed primary cultures from murine colon were incubated for 2 h under control conditions (standard bath solution) with (n = 6) or without (n = 12) nifedipine (10 μmol/l) or in the presence of 5 mg/ml peptone (with [n = 12] and without [n = 12] nifedipine) or 50 mg/ml peptone (with [n = 6] and without [n = 6] nifedipine), as indicated. GLP-1 secretion in each well is expressed relative to the basal secretion (control) measured in parallel on the same day and indicated by the dashed line. Data represent the mean ± SEM. **p < 0.01, ***p < 0.001 compared with their respective controls by one-way ANOVA with post hoc Dunnett’s test
Expression and function of PEPTs
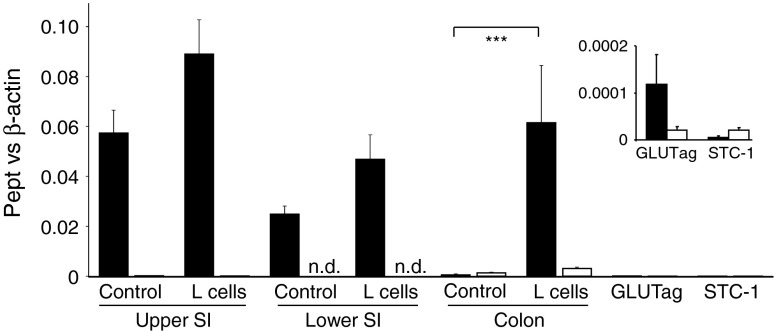
The nifedipine sensitivity of Gly-Sar-triggered GLP-1 secretion suggests that dipeptides trigger a depolarisation-dependent pathway in L cells. Based on our previous findings that the electrogenicity of brush-border SGLTs underlies glucose sensing in enteroendocrine cells, we investigated the expression and function of electrogenic proton-coupled PEPT1 and PEPT2 in L cells (Fig. 4). Pept1 expression was detected in L cells from the small intestine and colon, as well as in non-fluorescent small intestinal controls. It was low in control cells from the colon, and barely detectable in STC-1 and GLUTag cells. Pept2 was only poorly expressed in all the preparations examined.
Fig. 4.
Pept1, but not Pept2, is expressed in L cells. Expression of Pept1 (black bars) and Pept2 (white bars) mRNA relative to β-actin, as assessed by RT-PCR in FACS-sorted L and non-L cells (control) from the upper and lower small intestine (SI) and colon, and GLUTag and STC-1 model cell lines. Data are presented as the geometric mean and upper SEM of the data (n ≥ 3 each). Significance comparisons between L and non-L cells were calculated by ANOVA with post hoc Bonferroni analysis on the ΔCt data, ***p < 0.001. The inset shows the data for the cell lines on an amplified y-axis scale
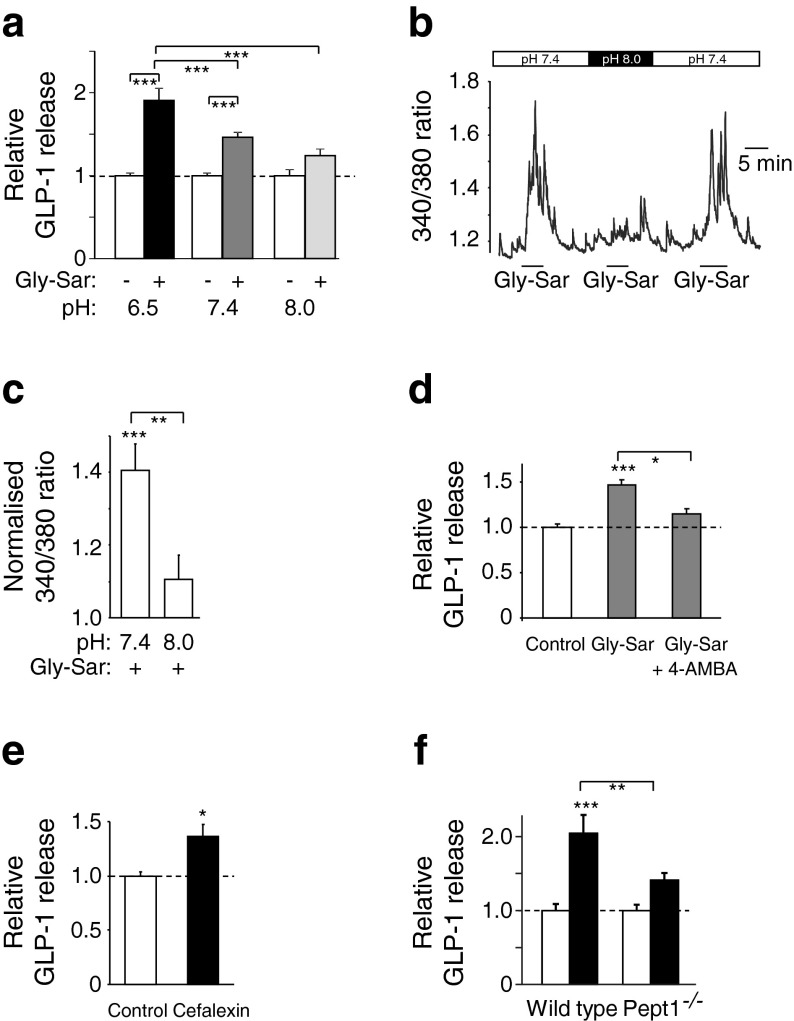
PEPT1 uses inwardly directed proton gradients to drive di- and tripeptide influx. We therefore predicted that any PEPT1-dependent triggering mechanism would exhibit pH dependence, with smaller responses occurring at high pH. Consistent with this idea, GLP-1 secretion triggered by Gly-Sar was greatest at pH 6.5 (1.91 ± 0.14-fold of baseline, p < 0.001, n = 14) and not significant at pH 8 (1.24 ± 0.08-fold of baseline, n = 8; Fig. 5a). Similarly, in Ca2+ imaging experiments, Gly-Sar-triggered Ca2+ responses were largely abolished at pH 8 (Fig. 5b, c). Secretory responses to Gly-Sar were inhibited by the non-translocated inhibitor of PEPT1, 4-aminomethylbenzoic acid (4-AMBA, 5 mmol/l; Fig. 5d) [19]. By contrast, 4-AMBA (5 mmol/l) did not inhibit peptone (5 or 50 mg/ml)-stimulated secretion (electronic supplementary material [ESM] Fig. 1). Supporting a functional coupling between PEPT1 and L cell activation, secretion was enhanced 1.4 ± 0.1-fold above baseline (p < 0.05) by the PEPT1 substrate cefalexin (5 mmol/l; Fig. 5e). We further evaluated the role of PEPT1 in L cell di-/tripeptide sensing using colonic cultures from Pept1 –/– mice. Basal GLP-1 release was unchanged at approximately 4% of total GLP-1 content per 2 h in both the wild-type and knockout groups, but secretion triggered by Gly-Sar (20 mmol/l) was significantly impaired in cultures from the Pept1 –/– mice (Fig. 5f).
Fig. 5.
PEPT1 underlies Gly-Sar-stimulated GLP-1 secretion. (a) GLP-1 secretion in response to Gly-Sar (15 mmol/l) at pH 6.5 (n = 14), pH 7.4 (n = 15) and pH 8.0 (n = 8), as indicated. Mixed primary cultures from murine colon were incubated for 2 h at different pHs in the presence and absence of Gly-Sar. GLP-1 secretion in Gly-Sar-containing wells is expressed relative to basal secretion (control) measured at the same pH in parallel on the same day and indicated by the dashed line. Data represent the mean ± SEM. ***p < 0.001 compared with their respective controls by one-way ANOVA with post hoc Bonferroni analysis. (b) Fluorescent 340/380 ratio changes of a primary identified L cell loaded with fura-2-AM and stimulated with Gly-Sar (10 mmol/l) at pH 7.4 and 8.0, as indicated. (c) Mean calcium changes in L cells following the addition of Gly-Sar (10 mmol/l) at pH 7.4 (n = 8) and 8.0 (n = 8), as indicated; 340/380 ratios in the presence of the test agent were normalised to the mean of the background ratios of each cell measured before the addition and after washout of the test compound. Data represent the mean ± SEM. **p < 0.01, ***p < 0.001 compared with baseline and between conditions by Student’s t test. (d) GLP-1 secretion in response to Gly-Sar (10 mmol/l) in the presence (n = 9) and absence (n = 9) of 4-AMBA (5 mmol/l). (e) GLP-1 secretion in response to cefalexin (5 mmol/l, n = 7). (f) GLP-1 secretion in the presence (black bars, n = 12) and absence (white bars, n = 12) of Gly-Sar (20 mmol/l) in cultures from wild-type and Pept1 –/– mice. (d–f) Mixed primary colonic cultures were incubated for 2 h under control conditions (standard bath solution) or in the additional presence of the indicated agents. GLP-1 secretion in each well is expressed relative to the basal secretion (control) measured in parallel on the same day and indicated by the dashed line. Data represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared with their respective controls by Student’s t test (e) or one-way ANOVA with post hoc Bonferroni analysis (d, f)
G-protein-coupled receptor candidates for peptone sensing
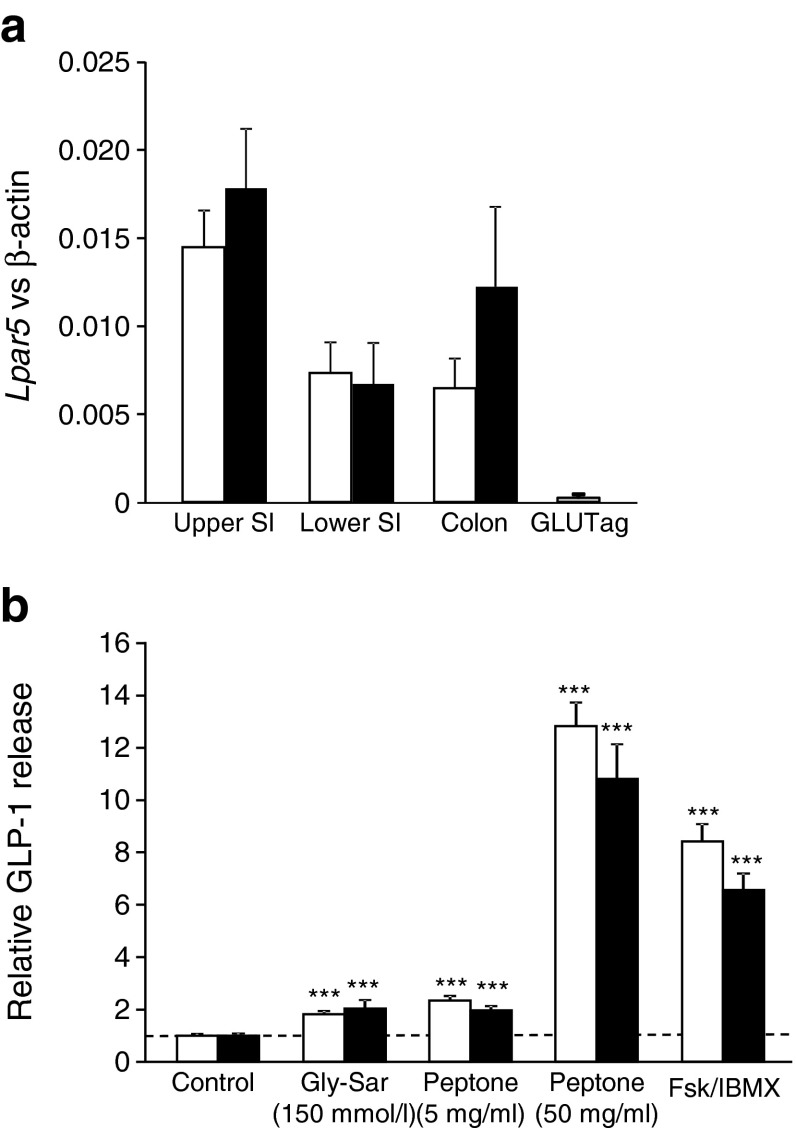
As LPAR5 (also known as GPR92/93) has been widely reported as a candidate peptone sensor and overexpression of LPAR5 in STC-1 cells has been linked to peptone-stimulated CCK release [20], we initially assessed the expression profile of this receptor and its potential involvement in GLP-1 secretion. Lpar5 was found to be expressed at similar levels in L cells and control cells along the gastrointestinal tract, but peptone- and dipeptide-triggered secretion was not impaired in colonic cultures prepared from Lpar5 –/– mice compared with their littermate controls (Fig. 6).
Fig. 6.
Peptone-stimulated GLP-1 secretion is not dependent on LPAR5. (a) Expression of Lpar5 mRNA relative to β-actin assessed by RT-PCR in FACS-sorted L cells (black bars) and non-L cells (white bars, control) from the upper and lower small intestine (SI) and colon, and the GLUTag model cell line. Data are presented as the geometric mean and upper SEM of the data. No significant differences between L and non-L cells were observed by ANOVA of the ΔCt data (n ≥ 3 each). (b) Mixed primary cultures from murine colon isolated from wild-type controls (white bars) or Lpar5 –/– animals (black bars) were incubated for 2 h under control conditions (standard bath solution, n = 14) or in the additional presence of Gly-Sar (150 mmol/l, n = 8), 5 mg/ml peptone (n = 14), 50 mg/ml peptone (n = 14) or glucose plus forskolin (Fsk) and isobutylmethylxanthine (IBMX; 10 mmol/l, 10 μmol/l and 10 μmol/l, respectively; Fsk/IBMX, n = 14), as indicated. GLP-1 secretion in each well is expressed relative to the basal secretion (control) measured in parallel on the same day and indicated by the dashed line. Data represent the mean ± SEM. ***p < 0.001 compared with their respective controls by one-way ANOVA with post hoc Bonferroni analysis. No significant difference between genotypes was observed
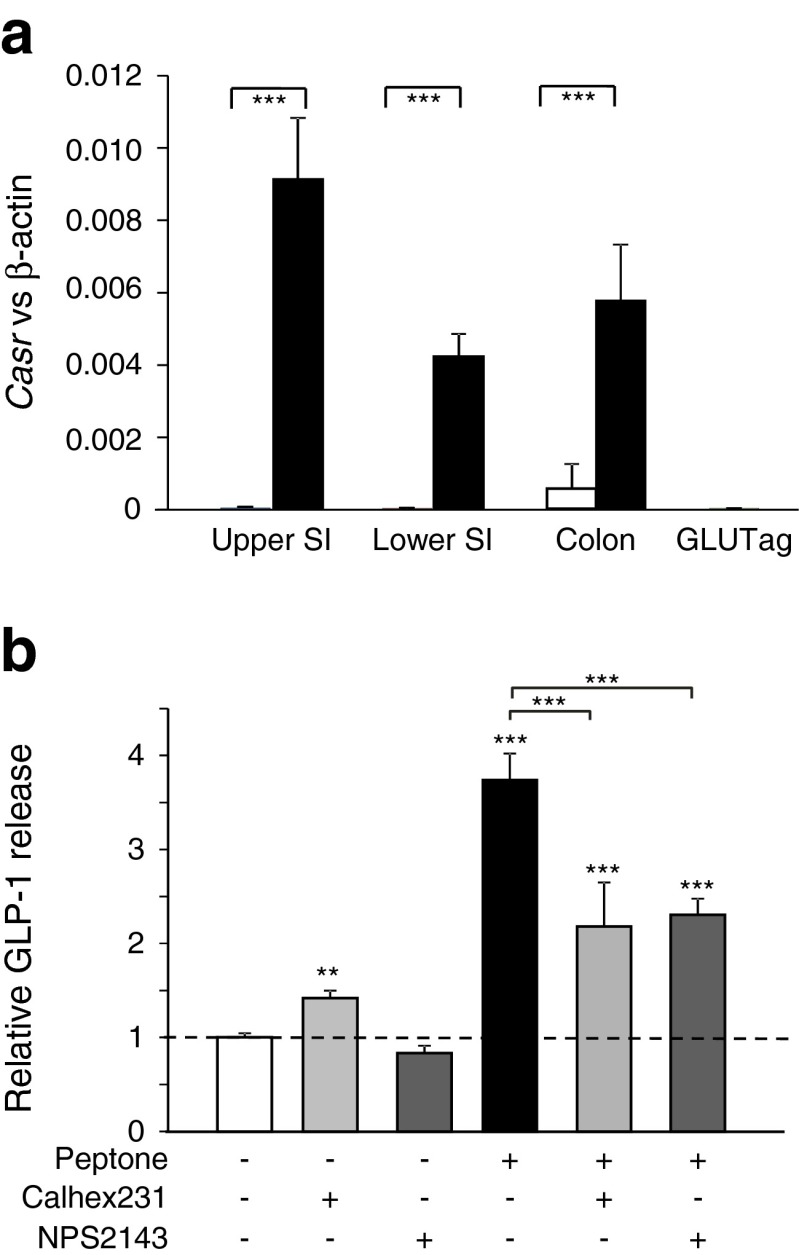
An alternative candidate for peptone sensing is CaSR, which has been implicated in amino-acid-triggered secretion of gastrin [21], CCK [22], GIP, PYY and GLP-1 [16], and was found by structural modelling to have a binding site large enough to accommodate oligopeptides [23]. mRNA for CaSR was detected in L cells isolated by flow cytometry from the small or large intestine, while expression was lower in non-fluorescent (non-L cell) populations and GLUTag cells (Fig. 7a). In support of the idea that CaSR is functionally expressed in L cells, the CaSR agonist calindol (10 μmol/l) [24] increased GLP-1 secretion from small intestinal cultures by 1.8 ± 0.3-fold (n = 6; p = 0.02 by single-sample Student’s t test). To evaluate the potential involvement of CaSR in peptone signalling, we tested the effects of NPS2143 and Calhex231, selective Ca2+-sensing receptor antagonists [25, 26]. NPS2143 (5 μmol/l) and Calhex231 (10 μmol/l) both significantly lowered peptone-triggered GLP-1 secretion from primary colonic cultures (Fig. 7b).
Fig. 7.
Role of CaSR in peptone-stimulated GLP-1 secretion. (a) Casr expression in L cells: expression of Casr mRNA relative to β-actin assessed by RT-PCR in FACS-sorted L cells (black bars) and non-L cells (white bars, control) from the upper and lower small intestine (SI) and colon, and GLUTag cells. Data are presented as the geometric mean and upper SEM of the data, calculated from ΔCt values (n ≥ 3 each). Significance comparisons between L and non-L cells were calculated by ANOVA with post hoc Bonferroni’s test on the ΔCt data, ***p < 0.001. (b) Casr inhibition ameliorates peptone-stimulated GLP-1 secretion: mixed primary cultures from murine colon were incubated for 2 h under control conditions (standard bath solution, n = 19) or in the additional presence of Calhex231 (10 μmol/l, n = 6), NPS2143 (5 μmol/l, n = 5) and/or peptone (5 mg/ml) (n = 13 without other addition, n = 6 with Calhex231, n = 10 with NPS2143), as indicated. GLP-1 secretion in each well is expressed relative to the basal secretion (control) measured in parallel on the same day and indicated by the dashed line. Data represent the mean ± SEM. **p < 0.01, ***p < 0.001 compared with their respective controls by one-way ANOVA with post hoc Bonferroni analysis
Discussion
There is considerable debate about the mechanisms by which enteroendocrine cells detect protein ingestion. A number of receptors and transporters have been identified that may underlie sensitivity to amino acids, but data suggest that larger di-, tri- and oligopeptides also stimulate release of hormones such as CCK and GLP-1. Our findings implicate CaSR and PEPT1 as sensors for these larger protein digestion products in primary L cells. Both pathways are sensitive to external calcium: CaSR because it requires Ca2+ binding to the extracellular Venus flytrap domain, and PEPT1 because it triggers membrane depolarisation and subsequent opening of voltage-gated Ca2+ currents. Interestingly, GLUTag cells, which have barely detectable expression of Casr and Pept1, were not activated by Gly-Sar and did not exhibit a greater secretory response to peptone than to glutamine alone (ESM Fig. 2).
CaSR has previously been implicated in amino acid (especially phenylalanine)-triggered secretion of gastrin [21], CCK [22], GIP, PYY and GLP-1 [16]. Structural studies, however, suggest that CaSR could also bind di-, tri- and oligopeptides [23]. Our data show that Casr was highly and specifically expressed in primary L cells, that Ca2+ responses to 5 mg/ml peptone in L cells were abolished by removal of extracellular Ca2+ and that peptone-triggered GLP-1 secretion was sensitive to the CaSR antagonists NPS2143 and Calhex231. The related receptor, GPRC6A, was recently demonstrated to underlie ornithine-stimulated GLP-1 secretion from GLUTag cells [18], but our data indicate that the cell line expresses relatively higher levels of Gprc6a and lower levels of Casr than primary L cells. Consistent with this observation, calindol, which inhibits GPRC6A but stimulates CaSR signalling [24], increased rather than decreased GLP-1 secretion from primary intestinal cultures.
Contrary to the Ca2+ responsiveness of Gly-Sar and 5 mg/ml peptone, the higher peptone concentration of 50 mg/ml peptone elicited a Ca2+ response in Ca2+-free medium. This is close to the solubility limit of peptone, and may trigger non-specific pathways. Our data show, however, that the secretory response is unlikely to represent an osmolar artefact, as 150 mmol/l Gly-Sar did not mimic the response, and also does not involve LPAR5, as knockout of this receptor had no effect on GLP-1 secretion triggered by low- or high-concentration peptone.
Meat hydrolysate has previously been shown to stimulate GLP-1 secretion in vascularly perfused rat jejunum/ileum, STC-1 cells [27] and the human GLP-1-secreting NCI-H716 cell line [28]. In the last of these, it was linked to activation of extracellular signal-regulated kinase (ERK)1/2, as demonstrated by the inhibitory effect of U0126 – a blocker of mitogen-activated protein kinase kinase (MEK)1/2. CaSR, which is known also to couple to mitogen-activated protein kinase (MAPK) and ERK1/2 in some cell types [29], may provide an underlying basis for these observations. Although meat hydrolysate triggered ERK phosphorylation in NCI-H716 cells within 2–15 min, this was not sufficient to trigger GLP-1 release as other activators of ERK, such as epidermal or fibroblast growth factor, did not enhance GLP-1 secretion [30]. These data suggest the existence of additional pathways that synergise with the ERK1/2–MAPK pathway.
The nifedipine sensitivity of Gly-Sar-triggered GLP-1 release from primary cultures suggests the recruitment of pathways dependent upon membrane depolarisation, which our data indicate may involve the proton-coupled transporter PEPT1. PEPT1 is a brush-border transporter of di- and tripeptides and is also responsible for uptake of peptide-based drugs such as the cephalosporin family of antibiotics [31]. The idea that electrogenic H+-coupled influx of dipeptides by PEPT1 could act as a trigger for GLP-1 release arose from our previous reports that luminal sugar sensing by L cells is directly linked to Na+-coupled glucose uptake by SGLT1, and that glutamine similarly triggers Ca2+ elevation in L cells via Na+-coupled amino acid transport [12, 32]. A PEPT1-dependent dipeptide-sensing pathway has been difficult to demonstrate using the cell line models GLUTag and STC-1, because they express endogenous Pept1 only very poorly compared with native L cells, although transfection of STC-1 cells with Pept1 has been reported to confer responsiveness to peptides [33]. In primary L cells, by contrast, we observed responses to the non-metabolisable PEPT1 substrate Gly-Sar that were pH-dependent, blocked by the competitive antagonist 4-AMBA and significantly reduced in Pept1 knockout mice. Taken together with the sensitivity of Gly-Sar-triggered GLP-1 release to nifedipine, our data are consistent with the idea that the depolarising effect of H+-dependent peptide uptake in L cells leads to GLP-1 secretion through increased electrical activity. The apical localisation of PEPT1, like several other electrogenic transporters, provides an explanation for how enteroendocrine cells can distinguish between luminal and basolateral nutrient supplies [34].
Conclusion
Nutritional effects on the gut endocrine system are critical for the coordinated digestion, absorption and peripheral disposal of ingested food, and the regulation of appetite. Increased nutrient delivery to the lower gut following gastric bypass surgery triggers dramatically elevated postprandial GLP-1 levels, contributing to improved beta cell function and resolution of many cases of type 2 diabetes [3]. Harnessing the endogenous secretory capability of the enteroendocrine system is a strategy currently under development for the treatment of diabetes and obesity, aiming to mimic the beneficial effects of bariatric surgery. As colonic expression of both Pept1 and Casr is relatively specific for L cells, and activation of both clearly stimulates hormone secretion, these pathways might offer exploitable targets for the mobilisation of intestinal GLP-1 and PYY.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 70 kb)
(PDF 69 kb)
Acknowledgements
The authors wish to thank Laura Parton, Helen Heffron and Johannes Grosse (Takeda, Cambridge, UK) for the gift of knockout tissue and reagents regarding LPAR5. Asan Ramzan (previously of the University of Cambridge, Cambridge, UK) contributed to data showing the pH-dependence of Gly-Sar acquired during his Masters degree in the FMG/FR laboratories. GLUTag cells were a kind gift from Daniel Drucker (Lunenfeld-Tanenbaum Research Institute, Toronto, ON, Canada). Meso Scale Discovery ‘total-GLP-1’ assays were performed by Keith Burling at the MRC Centre for Obesity and Related Disorders (University of Cambridge, Cambridge, UK).
Funding
This research was funded by Wellcome Trust grants to FMG and FR (WT088357/Z/09/Z and WT084210/Z/07/Z) and Full4Health (FP7/2011-2015, grant agreement no. 266408). Research in HD’s laboratory was funded by a Deutsche Forschungsgemeinschaft grant (DA 190/10-1).
Duality of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
ED, RP, GT, HEP, JH, BR, TZ and HD acquired, analysed and interpreted the data. FMG and FR designed the study, interpreted data and drafted the manuscript. All authors contributed to critical revision of the manuscript and approved the final version.
Abbreviations
- 4-AMBA
4-Aminomethylbenzoic acid
- CaSR
Calcium-sensing receptor
- CCK
Cholecystokinin
- ERK
Extracellular signal-regulated kinase
- Fura-2-AM
Fura-2-acetoxymethyl ester
- GIP
Glucose-dependent insulinotropic polypeptide
- GLP
Glucagon-like peptide
- GPRC6A
G-protein-coupled receptor 6A
- LPAR5
Lysophosphatidic acid receptor 5
- MAPK
Mitogen-activated protein kinase
- PEPT
Peptide transporter
- PYY
Peptide YY
- SGLT
Sodium-dependent glucose transporter
Footnotes
Eleftheria Diakogiannaki and Ramona Pais contributed equally to this manuscript.
Contributor Information
Fiona M. Gribble, Email: fmg23@cam.ac.uk
Frank Reimann, Email: fr222@cam.ac.uk.
References
- 1.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 2.Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med. 2011;124:S3–S18. doi: 10.1016/j.amjmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen NB, Dirksen C, Bojsen-Møller KN et al (2013) Exaggerated glucagon-like peptide-1 response is important for the improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 62:3044–3052 [DOI] [PMC free article] [PubMed]
- 4.Morínigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–1601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]
- 5.Ahlkvist L, Vikman J, Pacini G, Ahrén B. Synergism by individual macronutrients explains the marked early GLP-1 and islet hormone responses to mixed meal challenge in mice. Regul Pept. 2012;178:29–35. doi: 10.1016/j.regpep.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Habib AM, Richards P, Cairns LS, et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52:289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorboulev V, Schürmann A, Vallon V, et al. Na+-d-glucose cotransporter SGLT1 is Pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–196. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker HE, Adriaenssens A, Rogers G, et al. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55:2445–2455. doi: 10.1007/s00125-012-2585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reimann F, Williams L, da Silva XG, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47:1592–1601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- 13.Gameiro A, Reimann F, Habib AM, et al. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J Physiol. 2005;569:761–772. doi: 10.1113/jphysiol.2005.098962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology. 2011;152:405–413. doi: 10.1210/en.2010-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenfield JR, Farooqi IS, Keogh JM, et al. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89:106–113. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590:2917–2936. doi: 10.1113/jphysiol.2011.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JH, Inoue T, Higashiyama M, et al. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–473. doi: 10.1124/jpet.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oya M, Kitaguchi T, Pais R, Reimann F, Gribble F, Tsuboi T. The G protein-coupled receptor family c group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J Biol Chem. 2013;288:4513–4521. doi: 10.1074/jbc.M112.402677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meredith D, Boyd CA, Bronk JR, et al. 4-Aminomethylbenzoic acid is a non-translocated competitive inhibitor of the epithelial peptide transporter PepT1. J Physiol. 1998;512:629–634. doi: 10.1111/j.1469-7793.1998.629bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi S, Lee M, Shiu AL, Yo SJ, Halldén G, Aponte GW. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1366–G1375. doi: 10.1152/ajpgi.00516.2006. [DOI] [PubMed] [Google Scholar]
- 21.Feng J, Petersen CD, Coy DH, et al. Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci U S A. 2010;107:17791–17796. doi: 10.1073/pnas.1009078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liou AP, Sei Y, Zhao X, et al. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G538–G546. doi: 10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Yao Y, Kuang D, Hampson DR. Activation of family C G-protein-coupled receptors by the tripeptide glutathione. J Biol Chem. 2006;281:8864–8870. doi: 10.1074/jbc.M512865200. [DOI] [PubMed] [Google Scholar]
- 24.Faure H, Gorojankina T, Rice N, et al. Molecular determinants of non-competitive antagonist binding to the mouse GPRC6A receptor. Cell Calcium. 2009;46:323–332. doi: 10.1016/j.ceca.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Nemeth EF, Delmar EG, Heaton WL, et al. Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J Pharmacol Exp Ther. 2001;299:323–331. [PubMed] [Google Scholar]
- 26.Kessler A, Faure H, Petrel C, et al. N1-Benzoyl-N2-[1-(1-naphthyl)ethyl]-trans-1,2-diaminocyclohexanes: development of 4-chlorophenylcarboxamide (calhex 231) as a new calcium sensing receptor ligand demonstrating potent calcilytic activity. J Med Chem. 2006;49:5119–5128. doi: 10.1021/jm051233+. [DOI] [PubMed] [Google Scholar]
- 27.Cordier-Bussat M, Bernard C, Levenez F, et al. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes. 1998;47:1038–1045. doi: 10.2337/diabetes.47.7.1038. [DOI] [PubMed] [Google Scholar]
- 28.Reimer RA, Darimont C, Gremlich S, Nicolas-Métral V, Rüegg UT, Macé K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology. 2001;142:4522–4528. doi: 10.1210/en.142.10.4522. [DOI] [PubMed] [Google Scholar]
- 29.Huang C, Miller RT. The calcium-sensing receptor and its interacting proteins. J Cell Mol Med. 2007;11:923–934. doi: 10.1111/j.1582-4934.2007.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reimer RA. Meat hydrolysate and essential amino acid-induced glucagon-like peptide-1 secretion, in the human NCI-H716 enteroendocrine cell line, is regulated by extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinases. J Endocrinol. 2006;191:159–170. doi: 10.1677/joe.1.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- 32.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- 33.Matsumura K, Miki T, Jhomori T, Gonoi T, Seino S. Possible role of PEPT1 in gastrointestinal hormone secretion. Biochem Biophys Res Commun. 2005;336:1028–1032. doi: 10.1016/j.bbrc.2005.08.259. [DOI] [PubMed] [Google Scholar]
- 34.Liou AP, Chavez DI, Espero E, Hao S, Wank SA, Raybould HE. Protein hydrolysate-induced cholecystokinin secretion from enteroendocrine cells is indirectly mediated by the intestinal oligopeptide transporter PepT1. Am J Physiol Gastrointest Liver Physiol. 2011;300:G895–G902. doi: 10.1152/ajpgi.00521.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 70 kb)
(PDF 69 kb)