Abstract
Over the last seven years, seven targeted agents have been approved in the treatment of advanced or metastatic renal cell cancer, changing the therapeutic approach and prognosis of the disease dramatically. The latest agent with demonstrated efficacy is axitinib (Inlyta®). This new generation of tyrosine kinase agent differs from previously existing agents by its greater activity potency of inhibition of vascular endothelial growth factor-receptor (VEGFR1-3). This efficacy has been tested in phase II and III clinical trials. Axitinib is the only targeted agent that benefits from recommended titration, with intra-patient dose escalation. The toxicity profile of the drug is tolerable. This paper reviews the mechanism of action of axitinib, its metabolism, and its pharmacokinetic profile. Clinical data of efficacy and safety is also detailed. The agent has been integrated in the international therapeutic guidelines, as a standard in treatment of renal cell cancer patients, previously treated through antiangiogenic therapy.
Keywords: axitinib, safety, efficacy, renal cell cancer
Introduction
Renal cell carcinoma (RCC) accounts for 2% to 3% of all cancers, with an increasing incidence of diagnosis. This represents 88,400 patients diagnosed per year in Europe, and 200,000 worldwide.1,2 Unfortunately, one third of the patients are diagnosed with metastatic or advanced disease. The mortality rate is approximately 100,000 deaths annually.1 Several histologic subtypes are described including clear cell carcinoma, tubulopapillary carcinoma, chromophobe RCC, and collecting duct RCC.3 The most frequently reported upon is clear cell carcinoma. Activation of different molecular alterations has been implicated in the development of renal cancer. Thus, in the hereditary form of tumors, such as Von Hippel Lindau (VHL) disease, the gene implicated is the VHL tumor suppressor gene. In most of the sporadic presentations of the disease, mutations or silencing of the gene are also reported. These mutations resulted in the accumulation of the hypoxia-inducible factor (HIF)-1α. After binding to HIF-β, the complex is able to act as a transcriptional factor complex, which translocates into the nucleus and induces the production of growth factors such as vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF). Binding to their respective tyrosine kinase receptors, these factors will induce endothelial cell growth, proliferation, and migration. According to the key role of this angiogenesis in the development of the disease, several agents have been developed in order to target the VEGF signaling pathway. These agents are represented by the tyrosine kinase inhibitors (TKI) and bevacizumab, a monoclonal antibody targeting the VEGF ligand.4 The other class of agents with demonstrated therapeutic effect is the mammalian target of rapamycin inhibitors (mTOR I). In the last decade, these targeted therapies have demonstrated their activity in the treatment of advanced kidney cancer and have led to a revolution in the therapeutic approach to the disease.
Until recently, six agents were approved for the treatment of advanced or metastatic RCC, including bevacizumab (+ interferon),5 TKI (sunitinib, sorafenib, pazopanib),6–8 and mTOR I (everolimus, temsirolimus).9,10 The latest agent with demonstrated activity, recently approved by the FDA (in January 2012) and the European Medicines Agency (in September 2012), belongs to a new generation of TKI: axitinib, which is more than a me-too drug.11,12 This agent has demonstrated promising activity in phase II trials for the treatment of various solid tumors in addition to RCC, including metastatic melanoma,13 thyroid cancer,14 and advanced non-small cell lung cancer.15 This review will focus on axitinib, describing the mechanism of activity, metabolism, and pharmacokinetics. Data concerning efficacy and toxicity in the treatment of RCC will be reported, based on the results of phase I,16 II,17–19 and III trials.20,21 Finally, the place of axitinib in international therapeutic guidelines will be emphasized.22,23
Mechanism of Action, Metabolism, and Pharmacokinetic Profile
Mechanism of action
Axitinib, a second generation targeted drug, is a potent and highly selective inhibitor of vascular endothelial growth factor receptor (VEGFR) tyrosine kinase 1, 2, and 3. This small molecule is an indazole derivative, C22H18N4OS, with a molecular weight of 386.47 Da. Its synthesis is described in U.S. Patent 6,534,524 (Fig. 1).
Figure 1.
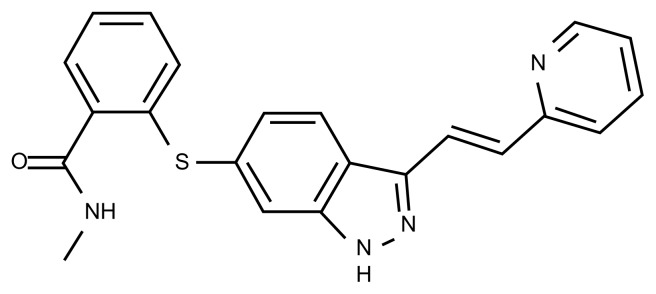
Structure of axitinib.
In vitro at picomolar concentrations, axitinib competitively binds to the intracellular ATP site domain of the VEGFR. Its small structure enables a close fit into a tunnel inside the kinase domain. It results in a stabilized and inactive conformation of the kinase and in the inhibition of signal transduction by VEGF. In endothelial cells, blockade of VEGF/VEGFR pathways 2 and 3 leads to a reduction in phosphorylation of endothelial nitric oxide synthase (eNOS), protein kinase B (AKT), and mitogen activated protein kinases (ERK ½). At nanomolar concentrations, axitinib also inhibits platelet derived growth factor receptors α and β (PDGFRα/β) and c-Kit.22 The in vitro half-maximal inhibitory concentration (IC50) of axitinib against VEGFR 1 to 3 were 0.1–0.3 nmol/L.25 This IC50 is 10-fold lower for the VEGF family of receptors than for other TKIs such as pazopanib, sunitinib, or sorafenib.26–28 Concerning other non-VEGF tyrosine kinase receptors (PDGF, fibroblast growth factor, colony-stimulating factor), the IC50 of axitinib was 1.6 to >1000 nmol/L (versus 6–880 nmol/L with other agents).29 These results testify to the high potency and selectivity of axitinib against VEGFRs (Table 1).26–28 Blockade of the VEGFR induces major changes of tumor vasculature in mouse models. In vivo, dynamic contrast enhanced magnetic resonance imaging (DCE-MRI), which is an imaging technique that can measure the density, integrity, and leakiness of tissue vasculature, showed that axitinib decreases the tumor blood flow/permeability. Maximum reduction in tumor endothelial transfer constant (Ktrans)— an indicator of vascular leakage to the extracellular space—was observed on day 7 after dosing and was correlated with decreased micro-vessel density, cellular viability, and tumor growth.
Table 1.
Inhibitory concentrations (IC50 in nmol) for targets with multitargeted TKIs.
| Drug | VEGFR1 | VEGFR2 | VEGFR3 | PDGFRα | PDGFRβ | c-Kit | RET | RAF | FLT3 |
|---|---|---|---|---|---|---|---|---|---|
| Axitinib9 | 0.1 | 0.2 | 0.1–0.3 | 5 | 1.6 | 1.7 | >1000 | NA | >1000 |
| Pazopanib24 | 10 | 30 | 47 | 71 | 84 | 74 | >1000 | NA | >1000 |
| Sunitinib25 | 10 | 10 | 10 | 5–10 | 10 | 13 | 100–200 | NA | 1–10 |
| Sorafenib26 | NA | 90 | 20 | 50–60 | 50–60 | 68 | 100–150 | 5–10 | 46 |
Abbreviation: NA, Not available.
Metabolism and pharmacokinetic profile
Pharmacokinetic analyses have been obtained from polled data of 17 trials, in both healthy volunteers and cancer patients.11 A two-compartment disposition model with first-order absorption and lag-time adequately describes the axitinib concentration-time profile. After a single dose of 5 mg in the fed state, axitinib is rapidly absorbed with peak plasma concentrations occurring at 2–6 h post dosing. The median time (tmax) ranged from 2.5–4.1 h. The rate of the drug’s absorption was higher in the fasting state with a peak concentration occurring 1–2 h after dosing.30 Moreover, Pithavala et al30 demonstrated in healthy volunteers that axitinib XLI Form film-coated immediate-release (FCIR) could be administered regardless of the fasting or fed state without significant impact on its pharmacokinetics.30 Twice daily administration of 5 mg of axitinib was associated in ≈1.4 fold accumulation compared to single administration. The oral bioavaibility of axitinib is 58%. Although a low pH results in the highest solubility of axitinib, studies have demonstrated that the effect of pH on absorption of axitinib was not clinically significant. As a measure of precaution, antacids or proton pump inhibitors should be administered at times other than 2 h before and 2 h after drug dosing.11 Axitinib is highly bound (>99%) to human plasma proteins with preferential binding to albumin and moderate binding to α1-acid glycoprotein. The plasma half-life ranges between 2.5 and 6.1 h and a steady-state is attained within 15 days. Linear correlations have been reported between dose and maximum plasma concentration, in addition to area under plasma concentration-time curve.16 The mean apparent volume of distribution in patients receiving 5 mg twice daily was 160L.11 Axitinib is primarily metabolized in the liver by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP1A2, CYP2C19, uridine diphosphate glucuronosyl-transferase (UGT) 1A1, and the drug transporters P-glycoprotein. Two metabolites are produced: an N-glucuronide metabolite and a sulfoxide. Both are ≥400-fold less potent against VEGFR-2 in vitro than axitinib.11 Less than one percent of the absorbed drug remained unchanged in the urine. Otherwise, a recent meta-analysis found no statistically significant associations between the polymorphism of genes encoding these enzymes and the transporters and axitinib pharmacokinetics.31 As observed in a phase 1 trial, axitinib pharmacokinetics are affected by CYP inducers (rifampicin, phenytoin, etc.) and inhibitors (ketoconazole, etc.). They may also be affected by drugs that are substrates or inhibitors of P-glycoprotein. Specific data on patients with mild to moderate hepatic impairment have been obtained through a phase I study, and showed an association between drug exposure and hepatic impairment, thus justifying the need for dose reduction in patients with moderate impairment.32 Mild to severe renal impairment, frequent in this population, is not expected to affect axitinib clearance. Thus no dosage adjustment is required. However, in end-stage renal disease, caution must be taken in case of administration, because of the lack of data.
Clinical Studies
Safety
Phase I
The first-in-human (FIH) phase I study of axitinib was conducted in 36 patients with a refractory solid tumor, including 6 RCC patients. The starting dose of axitinib was 5 mg twice a day with the plan to increase to a dose level of 30 mg twice a day. The dose limiting toxicities reported were hypertension and stomatitis. In terms of efficacy, 2 objective responses were reported from the 6 patients with RCC; 3 minor responses were also declared, one concerning a patient with RCC. This phase I study enabled the maximum tolerated dose to be determined, and 5 mg twice a day was determined to be the recommended dose for the phase II trials.16
Efficacy
Phase II trials
According to the interesting results observed in the phase I trial, axitinib was tested in kidney cancer patients in 2 phase II trials, which involved different populations of patients according to previous treatment received.17,18
The first trial enrolled patients with cytokine-refractory disease.17 Axitinib was administered at a fixed dose of 5 mg twice daily continuously with a 28-day cycle, until progressive disease or unacceptable toxicity. In patients who did not develop grade 2 toxicity or more, nor tumor response in the first 8 weeks, an increase of 20% of the dose of axitinib was permitted. All except 3 out of the 52 patients were evaluable both for efficacy and tolerability. Axitinib was administered at a median dose of 8.83 mg/day, and with a median duration of 9.4 months [0.1–32.0]. The overall response rate (ORR) was 44.2% (95% CI, 30.5–58.7), with 2 complete responses (4%) and 21 partial responses (40%). Furthermore, stable disease was reported in 22 patients (42%) for 8 weeks or longer, while 13 patients had stable disease for 24 weeks or longer. The median time to progression (TTP) was 15.7 months (95% CI, 8.4–23.4) and the median overall survival (OS) was 29.9 months (95% CI, 20.3—not estimable). Fifteen patients needed a dose reduction for grade 3 adverse effects including diarrhea (2), fatigue (2), dehydration (1), myalgia (1), and gout (1), or multiple grade 2 toxicities including hypertension (7). No hematological toxicity was reported. Nevertheless, 2 patients developed secondary erythrocytosis, with concomitant increased erythropoietin. The frequent adverse effects reported were: diarrhea, hypertension, fatigue, nausea, and hoarseness. Thirty patients developed hypertension, with grade 3–4 for 8 patients. The drug related hypertension was resolved under antihypertensive therapy, except for 8 patients who presented severe baseline hypertension.
The second phase II trial reported by Rini et al enrolled 62 patients, with sorafenib-refractory metastatic RCC (100% of the patients) in a single step, multicenter open-label study.18 Nearly 75% of the patients were heavily pre-treated with two or more prior systemic therapies (22.6% had received sunitinib). The main clinical characteristics were: median age 60 years old (35–77), 67.7% male, performance status 0 (33.9%) or 1 (66.1%), and clear cell or mixed histology (95.2%). One specificity of this trial was the titration allowed of axitinib, according to the inter-patient variability of drug exposure previously reported.18 Thus, an increase of axitinib from 5 mg twice a day to 7 mg twice daily, and finally 10 mg twice daily was managed, after a 2 week period, in patients who did not develop adverse events greater than grade 2, nor hypertension (definition: 2 measurements of >150/90 mmHg). This titration was possible in 33 patients (53.2%); 20 (32.3%) received 7 mg twice a day, and 13 (21.0%) received 10 mg twice a day.
All patients were evaluable for efficacy, with an ORR of 22.6%. Median progression free survival (PFS) was 7.4 months (95% CI, 6.7–11.0) and the reported median OS reached 13.6 months (95% CI, 8.4–18.8). In terms of tolerability, the toxicity profile of axitinib was consistent with the one reported with the VEGFR-TKI family with grade 3–4 adverse effects such as hand-foot syndrome (16.1%), fatigue (16.1%), dyspnea (14.5%), diarrhea (14.5%), and hypertension (16.1%). As previously reported17 hematological toxicity appeared to be minor or mild without grade 4 adverse effects. For 12 patients out of the 22 who did stop treatment after adverse effects, the final interruption was related to the toxicity of axitinib. Temporary interruptions were unavoidable/inevitable in 45 patients (72.6%) and dose reductions occurred in 28 patients (45.2%).
Phase III trial32
Based on the promising results of the 2 phase II studies, axitinib was tested in a phase III trial in comparison to sorafenib, in the second line setting, in patients with metastatic or advanced RCC progressive under a first line treatment with sunitinib or bevacizumab plus interferon, temsirolimus, or cytokines.20,33 The AXIS trial was a multicenter randomized (1:1) controlled trial, with 723 patients enrolled between September 2008 and July 2010. Patients enrolled were randomized between sorafenib at a fixed dose of 400 mg twice a day continuously and axitinib with a starting dose of 5 mg twice a day continuously, and the possibility of titration with a first level of 7 mg twice a day and successively 10 mg twice a day. This increase was allowed after 15 days of treatment in patients who did not develop hypertension, without antihypertensive medication, and no adverse effect above grade 2. The primary objective was PFS. Secondary endpoints were OS, RR, duration of response, and time to symptom deterioration. Concerning the previous line of treatment, 389 (54%) patients had received sunitinib, 251 (35%) cytokines, 59 (8%) bevacizumab, and 24 (3%) temsirolimus. The dose was increased above 5 mg twice a day in 37% of patients in the axitinib arm (132 patients). The median PFS was 6.7 months for the axitinib group and 4.7 months for the sorafenib group, with an HR:0.665 (95% CI 0.544–0.812, P < 0.001). Furthermore, the superiority of axitinib was demonstrated in subgroups of patients treated by cytokines, 12.1 months versus 6.5 (HR:0.464, 95% CI, 0.318–0.676, P < 0.0001) and also in patients previously treated by sunitinib, 4.8 months compared to 3.4 months (HR:0.741, 95% CI, 0.573–0.958, P < 0.0107). The ORR was 19% for the axitinib arm and 9% for sorafenib (P = 0.0001).20 The toxicity profile was as expected considering the phase II data. More recently, data on the updated efficacy and safety have been reported.34 The investigator-assessed PFS was 8.3 months and 5.7 respectively, (HR:0.656, 95% CI, 0.552–0.779, P < 0.0001). There was no difference in OS; the median OS was 20.1 months for the axitinib group and 19.2 months for the sorafenib arm. In post-hoc analysis of the results of the phase II trial conducted by Rini et al, Dutcher et al, reported an ORR of 27.6% in patients previously treated only by cytokines, of 25% in those who had received only sorafenib, and of 7.1% in those who had received sunitinib and sorafenib.35 Moreover, data of RR according to the previous treatment received by the patients included in the AXIS trial have been reported as 11.3% (95% CI, 7.2–16.7) in the sunitinib pretreated group, and 32.5% in the cytokine-pretreated patients (95% CI, 24.5–41.5). The statistically significant advantage of axitinib in median PFS was observed in the global population but also in two main subgroups of patients (sunitinib pretreated and cytokine pretreated). Moreover, patients with prolonged exposure to sunitinib, meaning TKI sensibility, had greater benefit from axitinib. The PFS reported was 6.3 months in axitinib group versus 4.6 months for sorafenib group, in patients treated with sunitinib for at least 9 months. In terms of tolerability, the most frequent adverse events, in more than 30% of the patients treated with axitinib, were: diarrhea (55%, all grade), hypertension (40% all grade), fatigue (39%), decreased appetite (34%), nausea (32%), and dysphonia (31%). Furthermore, hypertension, nausea, dysphonia, and hypothyroidism were more common with axitinib, whereas palmar-plantar erythrodysesthesia, alopecia, and rash were more frequent with sorafenib. Concerning the serious adverse effects, at least grade 3 or biological abnormalities, hypertension (17%), diarrhea (11%), and fatigue (10%) were reported in the axitinib group while palmar-plantar syndrome, hypophosphatemia, lipase elevation, and hypertension were reported in the sorafenib arm.34 Finally, an increase in hemoglobin was reported in 31 patients (9%) treated with axitinib. No toxic deaths were reported in the axitinib group. Axitinib compared favorably, considering the risk of deterioration of symptoms, with functional assessment of cancer therapy—Kidney Cancer Symposium Index (FKSI-15), with a 17% reduction (P = 0.020).35
The risk of hand-foot skin reaction (HFSR) under axitinib was evaluated according to data reported of 6 clinical trials (one phase III, AXIS trial,18 and 5 phase II studies13–15,17,18,31,33).36 Out of the 984 patients included, the overall incidence of all grade and high grade HFSR was 29.2% (95% CI, 14.0–51.1) and 9.6% (95% CI, 4.2–20.7), respectively. Despite the increased specificity for VEGFR and limited inhibitory effects on other multikinase target receptors, including PDGFRs c-Kit and Flt-3, axitinib is associated with HFSR with a greater incidence than pazopanib and significantly less incidence when compared to sorafenib.36
Recently, data reported by Rini et al in patients exposed for a long period (9 patients receiving axitinib for at least two years), suggested the safety of the drug even with a cumulative exposure of 5 years or more of targeted agents.37 Thus, the rates of common selected adverse events appeared to be highest during the first year of therapy (fatigue, diarrhea, hypertension, nausea, anorexia, arthralgia, hoarseness). Two unexpected severe effects (at least grade 3), were reported after 2 years of axitinib treatment, in long survivors, 5 years or more, one myocardial ischemia, and one small bowel obstruction. Nevertheless, no detail on the causality was warranted.
Logically, the efficacy of axitinib has been tested in the front line setting.21 The AGILE trial is a randomized phase III trial comparing axitinib to sorafenib in untreated patients (n = 288). Participating patients were allocated to axitinib (n = 192) or sorafenib (n = 96). The starting dose of axitinib was 5 mg twice daily, with a possibility of titration to 7 mg twice daily, and then 10 mg twice daily as per the previously described criteria in the AXIS trial. The fixed dose of sorafenib remained stable during the treatment, at 400 mg twice a day. Unfortunately, the study did not meet statistical significance with a median PFS of 10.1 months (7.2–12.1) in the experimental arm, and 6.5 months (4.7–8.3) in the sorafenib group, (HR:0.77, 95% CI, 0.56–1.05, P = 0.038). Thus, axitinib remains indicated in the second line setting. Nevertheless, in the pre-planned subgroup of patients with good performance status (PS = 0), the median PFS reported was significantly superior in the axitinib group of patients with 13.7 (10.1–19.4) and 6.6 (4.7–9.9) in the sorafenib group (HR:0.64, 95% CI, 0.42–0.99, P = 0.022).
Predictive Factors of Efficacy
The pharmacodynamic parameter
The use of DCE-MRI for evaluating changes in the volume transfer coefficient of the contrast agent (Ktrans) was studied in the phase I trial of axitinib.38 The measurement was performed at baseline and at day 2. A linear correlation was found between axitinib exposure and changes in Ktrans and initial AUC. A decrease of 50% or more in Ktrans indicated vascular response, and corresponded to an AUC > 200 ng · h/mL. Data suggests that a dose-dependent effect of axitinib on endothelial cells may exist.
Pharmacokinetic-pharmacodynamic parameters
The results of exploratory post-hoc pharmacokinetic analyses conducted on long-term survivors who participated in the phase II trial, suggest that numerically longer OS, longer PFS, and higher ORR are reported in patients who presented a 2-hour post-dose concentration on day 1 of the cycle 1 ranging from 45.2 to 56.4 ng/mL.39 These results are in accordance with previous results from Rixe et al, suggesting that higher exposure of axitinib at the end of the first cycle of treatment was correlated to a longer median of OS.40
The predictive value of developing hypertension during exposition to axitinib has been suggested in a polled retrospective analysis of 230 patients treated for four different solid tumor types.40 Patients with a diastolic blood pressure (dBP) of 90 mmHg or more presented an increase in ORR compared to patients who did not.
In specific treatment-naïve kidney cancer patients, an increase of the dBP of 15 mmHg or more appeared to be associated with better clinical outcomes than an increase in dBP of less than 15 mmHg.19
The results of the post-hoc 12-week landmark analysis from the AXIS trial have been reported. The median OS was longer in patients with a dBP of at least 90 mmHg than in those with less than 90 mmHg, with 20.7 months (95% CI, 18.4–24.6) and 12.9 months (10.1–20.4), respectively (P = 0.0116). Similar results have been observed with systolic blood pressure (sBP) of more or less than 140 mmHg. In multivariate analysis, the development of high dBP, or sBP, was an independent predictor of OS; HR = 0.627 (95% CI, 0.507–0.776) for dBP 90 mmHg or higher, and HR = 0.490 (95% CI, 0.391–0.613) for sBP 140 mmHg or higher. Nevertheless, as reported in the phase II titration trial, no difference was observed in PFS, in the 8-week and 12-week analysis between patients who did develop hypertension and those who did not.33
Recently, in February 2013, Rini et al presented the results of a dedicated phase II trial in the frontline setting, testing the potential benefit of titration, with 3 arms of treatment: (1) 5 mg twice daily or less depending on the tolerability; (2) randomization on patients with safe titration, meaning no grade 2 adverse effect in the past 2 weeks of treatment, no hypertension or need of antihypertensive therapy, with two arms of titration, one active with axitinib increase of 7 mg twice and finally 10 mg twice daily; and (3) a placebo titration.41 Ninety-one patients were not randomized, 56 were randomized between placebo and axitinib titration. The ORR was significantly higher in the axitinib titration 54% (40–67) compared to the placebo titration group 34% (22–48), respectively (1-sided P = 0.019). The ORR was 59% (49–70) in the non-randomized arm. Finally, the PFS from first dose did not differ in case of active titration, compared to placebo, with a median of 14.5 months (9.2–24.5) versus 15.7 months (8.3–19.4) respectively (HR:0.85, 95% CI, 0.54–1.35, P = 0.244). In the non-randomized arm, the median PFS was 16.6 months (11.2–22.5).
Place in Therapy
According to the results of the phase III AXIS trial, with demonstrated efficacy of axitinib in previously treated patients in the second line setting, the drug was integrated in the updates to international guidelines.22,23 Axitinib (Inlyta®) has been approved and is recommended in patients with advanced or metastatic clear cell carcinoma who have failed on one previous regimen in US (FDA), and on a previous treatment with sunitinib or cytokines in Europe (EMA). The starting dose is 5 mg twice daily, with or without food, every 12 hours. Furthermore, it is the only targeted agent to date with recommended titration at day 14, according to normal blood pressure without antihypertensive therapy and in the absence of adverse events of more than grade 2, with a first increase to 7 mg twice daily, and a possible new increase 14 days latter to 10 mg twice daily, according to the same criteria.
Conclusions
Axitinib is the first TKI agent with prospectively confirmed antitumor activity, even after prior exposure to VEGFR-TKI.18–21 Lack of cross-resistance has been suggested according to data of sequential use of targeted agents, for example sunitinib after sorafenib or the reverse, but only in retrospective studies.42,43 In 2012, this agent therefore became a new standard in the second line setting after prior exposure to antiangiogenic agents, joining everolimus. The mTOR I has demonstrated efficacy, compared in a double blind RECORD-1 trial9 to placebo in patients who were previously exposed to at least one systemic regimen. Because of these differences, no cross-trial comparison can be made. Nevertheless, standard guidelines have integrated both agents with different mechanisms of action in the second line setting.22,23 One major point now is to determine the best therapeutic sequence, which may differ from one patient to another.44 This emphasizes the importance of developing biomarkers. Nevertheless, only a prospective randomized trial comparing TKI to an mTOR I agent in patients previously exposed to an antiangiogenic agent will help to determine the best sequence of treatment. Three trials are being conducted. The first one, the INTORSECT, compared the efficacy of temsirolimus and sorafenib. The results were presented at the 37th Congress of the European Society of Medical Oncology in October 2012.45 No difference in median PFS has been reported between temsirolimus and sorafenib, at 4.28 and 3.91 months, respectively, with OS of 12.27 and 16.64 months, respectively.
Recently, Figlin et al46 presented a new therapeutic paradigm of second-line treatment, depending on the sensitivity or resistance to VEGF agents, observed in the front-line setting.46 The definition of predictive factors may be a first step in the optimization of management of these agents, especially the development of high blood pressure.41
Some patients appear to have a prolonged benefit. Rini et al reported data on long-term responders to axitinib.21 This retrospective analysis considered 11 patients out of the 52 included in the phase II trial, continuing axitinib in an access protocol. The estimated 5-year OS rate was 20.6% (95% CI, 10.9–32.4).
According to the high RR reported, particularly regarding the RR observed with other agents (sunitinib,6 bevacizumab,7 pazopanib8) even though not comparable, between 26% and 46%, axitinib may be of great interest in the neoadjuvant setting. This hypothesis is being tested in a phase II trial of Axipan to assess the efficacy of the drug to enable partial nephrectomy after treatment of a local tumor of at least 7 centimeters (NCT 01599754).47
To date, no combination of targeted agents has been reported feasible in terms of safety, despite non-overlapping toxicities. These associations have been particularly established with the first generation of TKI, including sunitinib and sorafenib. Whether the more specific inhibition of axitinib allows combination is being evaluated in 2 phase I trials: the EVAX trial, testing the association of everolimus and axitinib (NCT 01334073),48 and another trial combining temsirolimus and axitinib (NCT 01529138).49 Both trials include patients with solid tumors, not only kidney cancer.
Table 2.
Summary of phase II of axitinib in treatment of RCC.
Table 3.
Summary of phase III trials of axitinib in treatment of RCC.
Footnotes
Author Contributions
Analyzed the data: MGG. Wrote the first draft of the manuscript: MGG. Contributed to the writing of the manuscript: LF, AQ, MGG. Agree with manuscript results and conclusions: MGG, LF, AQ, AR. Jointly developed the structure and arguments for the paper: MGG, AR. Made critical revisions and approved final version: MGG, AR. All authors reviewed and approved of the final manuscript.
Competing Interests
MG-G reports honoraria from Novartis, Janssen and GlaxoSmithKline, and board memberships for Pfizer and Sanofi. Other authors report no competing interests.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
Funding
Author(s) disclose no funding sources.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;27(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46(4):765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 4.Algaba F, Akaza H, López-Beltrán A, et al. Current pathology keys of renal cell carcinoma. Eur Urol. 2011;60(4):634–43. doi: 10.1016/j.eururo.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Albiges L. Vascular endothelial growth factor-targeted therapy for the treatment of renal cell carcinoma. Drugs. 2011;71(9):1179–91. doi: 10.2165/11591410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal cell carcinoma. N Engl J Med. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 10.Hudes G, Carducci M, Tomczak P, et al. Temserolimus, interferon alfa, or both for advanced renal-cell carcinoma. New Engl J Med. 2007;356(22):2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 11.Pfizer Inc. Inlyta (axitinib®) oral tablets: US prescribing information (online) http://labeling.pfizer.com/ShowLabeling.aspx?id=759.
- 12.European Medicines Agency. Inlyta®film-coated tablets: summary of product characteristics (online) http://www.emea.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002406//WC500132188.pdf.
- 13.Fruehauf JP, Lutzky J, McDermott DF, et al. Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1,2,3, in patients with metastatic melanoma. Clin Cancer Res. 2011;17(23):7462–9. doi: 10.1158/1078-0432.CCR-11-0534. [DOI] [PubMed] [Google Scholar]
- 14.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26(29):4708–13. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiller JH, Larson T, Ou SH, et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol. 2009;27(23):3836–41. doi: 10.1200/JCO.2008.20.8355. [DOI] [PubMed] [Google Scholar]
- 16.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol. 2005;23(24):5474–83. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]
- 17.Rini B, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27(27):4462–8. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 18.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8(11):975–84. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 19.Rini B, Grünwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell cancer (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(Suppl):4503. [Google Scholar]
- 20.Rini B, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomized phase 3 trial. Lancet. 2011;378(9807):1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 21.Hutson T, Gallardo J, Lesovoy V, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2013;31(Suppl) doi: 10.1016/S1470-2045(13)70465-0. Abstr LBA348. [DOI] [PubMed] [Google Scholar]
- 22.Escudier B, Eisen T, Porta C, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii65–71. doi: 10.1093/annonc/mds227. [DOI] [PubMed] [Google Scholar]
- 23.NCCN guidelines NCCN Website. 2012. [ nccn.org]
- 24.Kelly RJ, Rixe O. Axitinib-a selective inhibitor of the vascular endothelial growth factor (VEGF) receptor. Target Oncol. 2009;4(4):297–305. doi: 10.1007/s11523-009-0126-9. [DOI] [PubMed] [Google Scholar]
- 25.Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14(22):7272–83. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6(7):2012–21. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 27.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamics relationship. Clin Cancer Res. 2003;9(1):327–37. [PubMed] [Google Scholar]
- 28.Wilhelm SM, Carter C, Tang L, et al. BAY-43-9006 exhibits broad oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 29.Escudier B, Gore M. Axitinib for the management of metastatic renal cell carcinoma. Drugs R D. 2011;11(2):113–26. doi: 10.2165/11591240-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pithavala YK, Chen Y, Toh M, et al. Evaluation of the effect of food on the pharmacokinetics of axitinib in healthy volunteers. Cancer Chemother Pharmacol. 2012;70(1):103–12. doi: 10.1007/s00280-012-1888-9. [DOI] [PubMed] [Google Scholar]
- 31.Brennan M, Williams JA, Chen Y, Tortorici M, Pithavala Y, Liu YC. Meta-analysis of contribution of genetic polymorphisms in drug-metabolizing enzymes or transporters to axitinib pharmacokinetics. Eur J Clin Pharmacol. 2012;68(5):645–55. doi: 10.1007/s00228-011-1171-8. [DOI] [PubMed] [Google Scholar]
- 32.Tortorici MA, Toh M, Rahavendran SV, et al. Influence of mild and moderate hepatic impairment on axitinib pharmacokinetics. Invest New Drugs. 2011;29(6):1370–80. doi: 10.1007/s10637-010-9477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14(6):552–62. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 34.Dutcher JP, Wilding GR, Hudes WM, et al. Sequential axitinib (AG-013736) therapy of patients (pts) with metastatic clear cell renal cell cancer (RCC) refractory to sunitinib and sorafenib, cytokines and sorafenib, or sorafenib alone. J Clin Oncol. 2008;26(Suppl 15):5127. [Google Scholar]
- 35.Cella D, Escudier B, Rini B, et al. Patient-reported outcomes (PROs) in a phase III AXIS trial of axitinib versus sorafenib as a second-line therapy for metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2011;29(Suppl):4504. [Google Scholar]
- 36.Fischer A, Wu S, Ho AL, Lacouture ME. The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor:a systemic review of literature and metaanalysis. Invest New Drugs. 2013;31(3):787–97. doi: 10.1007/s10637-013-9927-x. [DOI] [PubMed] [Google Scholar]
- 37.Rini B, de La Motte Rouge T, Harzstark AL, et al. Five-year survival in patients with cytokine-refractory metastatic renal cell carcinoma treated with axitinib. Clin Genitourin Cancer. 2013;11(2):107–14. doi: 10.1016/j.clgc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Liu G, Rugo HS, Wilding G, et al. Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: results from a phase I study. J Clin Oncol. 2005;23(24):5464–73. doi: 10.1200/JCO.2005.04.143. [DOI] [PubMed] [Google Scholar]
- 39.Rixe O, Dutcher J, Motzer R, et al. Diastolic Blood Pressure (dBP) and pharmacokinetics (PK) as predictors of axitinib efficacy in metastatic renal cell cancer (mRCC) J Clin Oncol. 2009;27(Suppl):5045. [Google Scholar]
- 40.Rini BI, Schiller JH, Fruehauf JP, et al. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin Cancer Res. 2011;17(11):3841–9. doi: 10.1158/1078-0432.CCR-10-2806. [DOI] [PubMed] [Google Scholar]
- 41.Rini B, Gruenwald V, Mayer N, et al. Axitinib with or without dose titration for first-line metastatic renal cell carcinoma (mRCC): Unblinded results from a randomized phase II study. J Clin Oncol. 2013;31(Suppl):LBA349. [Google Scholar]
- 42.Eichelberg C, Heuer R, Chun FK, et al. Sequential use of the tyrosine kinase inhibitors, sorafenib and sunitinib in metastatic renal cell carcinoma: a retrospective outcome analysis. Eur Urol. 2008;54(6):1373–8. doi: 10.1016/j.eururo.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 43.Sablin MP, Negrier S, Ravaud A, et al. Sequential sorafenib and sunitinib for renal cell carcinoma. J Urol. 2009;182(1):29–34. doi: 10.1016/j.juro.2009.02.119. [DOI] [PubMed] [Google Scholar]
- 44.Calvo E, Ravaud A, Bellmunt J. What is the optimal therapy for patients with metastatic renal cell carcinoma who progress on an initial VEGFr- TKI? Cancer Treat Rev. 2013;39(4):366–74. doi: 10.1016/j.ctrv.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Hutson T, Escudier B, Esteban E, et al. Temsirolimus versus Sorafenib as second line therapy in metastatic renal cell carcinoma: results from the INTORSECT trial. Ann Oncol. 2012;23(Suppl):LBA22. [Google Scholar]
- 46.Figlin R. Permissiveness/Redundancy of the VEGF Pathway: Why Better VEGF-TKIs Don’t Translate into Better Clinical Therapies. GUASCO. 2013 [Google Scholar]
- 47.http://clinicaltrials.gov/ct2/show/NCT01263769?term=Neoadjuvant+AND+axi.
- 48.http://clinicaltrials.gov/ct2/show/NCT01334073.
- 49.http://clinicaltrials.gov/show/NCT01529138.


