Abstract
This randomized, multicenter study compared the efficacy of docetaxel with or without capecitabine following fluorouracil/epirubicin/cyclophosphamide (FEC) therapy in operable breast cancer and investigated the role of Ki67 as a predictive biomarker. Patients were randomized to 4 cycles of docetaxel/capecitabine (docetaxel: 75 mg/m2 on day 1; capecitabine: 1,650 mg/m2 on days 1–14 every 3 weeks) or docetaxel alone (75 mg/m2 on day 1 every 3 weeks) after completion of 4 cycles of FEC (5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2 and cyclophosphamide 500 mg/m2 on day 1 every 3 weeks). The primary endpoint was the pathological complete response (pCR) rate. Predictive factor analysis was conducted using clinicopathological markers, including hormone receptors and Ki67 labeling index (Ki67LI). A total of 477 patients were randomized; the overall response in the docetaxel/capecitabine and docetaxel groups was 88.3 and 87.4 %, respectively. There were no significant differences in the pCR rate (docetaxel/capecitabine: 23 %; docetaxel: 24 %; p = 0.748), disease-free survival, or overall survival. However, patients with mid-range Ki67LI (10–20 %) showed a trend towards improved pCR rate with docetaxel/capecitabine compared to docetaxel alone. Furthermore, multivariate logistic regression analysis showed pre-treatment Ki67LI (odds ratio 1.031; 95 % CI 1.014–1.048; p = 0.0004) to be a significant predictor of pCR in this neoadjuvant treatment setting. Docetaxel/capecitabine (after 4 cycles of FEC) did not generate significant improvement in pCR compared to docetaxel alone. However, exploratory analyses suggested that assessment of pre-treatment Ki67LI may be a useful tool in the identification of responders to preoperative docetaxel/capecitabine in early-stage breast cancer.
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-013-2691-y) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Neoadjuvant chemotherapy, Ki67, Capecitabine, Pathological complete response, Docetaxel
Introduction
Neoadjuvant chemotherapy has become increasingly significant in the treatment of operable early-stage breast cancer, with the advantage of the potential to downgrade tumors and increase the rate of breast conserving surgery (BCS) in patients that may have otherwise required a mastectomy [1]. Results from the National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol B-18 trial demonstrated an increased likelihood in BCS in breast cancer patients treated with a neoadjuvant anthracycline-based regimen [1]. Although the B-18 trial did not demonstrate a survival advantage in patients treated with preoperative chemotherapy, it established pathological complete response (pCR) as a prognostic marker for disease-free survival (DFS). Indeed, pCR after neoadjuvant chemotherapy is considered a marker for favorable prognosis in breast cancer patients [2].
As such, clinical and molecular biomarkers capable of predicting pCR have been assessed following neoadjuvant treatment in breast cancer patients [3, 4]. In particular, the proliferation marker Ki67 has been reported to have predictive and prognostic value in patients with invasive breast cancer who received a range of neoadjuvant chemotherapy regimens, including anthracycline-based regimen without taxanes and anthracycline and taxane-based protocols [5].
While neoadjuvant treatment with anthracycline-based regimens is highly effective in the treatment of breast cancer, the sequential addition of a taxane to an anthracycline-based neoadjuvant regimen has been demonstrated to induce additive efficacy. In the NSABP B-27 trial, the sequential addition of docetaxel after doxorubicin and cyclophosphamide (AC) therapy doubled the rate of pCR, increased clinical response and increased the proportion of negative axillary nodes in early breast cancer patients [6]. In addition, 5-fluorouracil–epirubicin and cyclophosphamide (FEC) followed by docetaxel as neoadjuvant chemotherapy in the Japan Breast Cancer Research Group (JBCRG) 01 trial resulted in a pCR rate of 16 % with BCS possible for 85 % of the patients assessed [7].
In addition to inducing increased efficacy with anthracyclines, docetaxel has demonstrated significant synergy with the oral prodrug capecitabine [8]. Capecitabine is converted to 5-fluorouracil in a three-step process catalyzed by thymidine phosphorylase (TP) [9] and exhibits tumor specificity by exploiting the significantly higher activity of TP in tumor tissue in comparison to healthy tissue [8, 9]. Docetaxel has been demonstrated to upregulate TP expression in tumor tissues, possibly accounting for the synergistic effect observed with capecitabine [8]. Clinical studies have shown that single-agent capecitabine was an active and tolerable treatment for metastatic breast cancer (MBC) with disease progression during and after anthracycline and taxane therapy, achieving response rates of 20–29 % and a median survival in excess of 1 year [10, 11].
On the basis of these findings, the docetaxel/capecitabine regimen has been demonstrated to be well tolerated and effective for neoadjuvant treatment of stage II/III or locally advanced breast cancer [12–14]. Another study by O’Shaugnessy and colleagues also demonstrated a superior clinical response and survival outcome when the docetaxel/capecitabine regimen was compared with docetaxel alone in women with anthracycline-pretreated MBC [15]. However, these studies [12–15] did not undertake analyses to identify the tumor characteristics that define patients likely to respond to neoadjuvant docetaxel/capecitabine treatment.
Our randomized trial compared the efficacy of preoperative FEC followed by docetaxel with or without capecitabine in patients with early-stage breast cancer and assessed biomarkers that may be used to identify responders, in order to establish individualized treatment regimens.
Patients and methods
Study design
This multicenter, randomized, open study compared the efficacy of 4 cycles of FEC followed by 4 cycles of docetaxel and capecitabine or 4 cycles of docetaxel alone as neoadjuvant chemotherapy in patients with operable breast cancer. The study was approved by the Institutional Review Board of the Organisation of Oncology and Translational Research and conducted according to the Declaration of Helsinki. The primary endpoint was the pCR rate; secondary endpoints included toxicity, clinical response, frequency of breast and axillary lymph node conservation surgery, DFS, and overall survival (OS).
Patient eligibility
Women (20–70 years) with histologically confirmed operable invasive breast adenocarcinoma (T1C-3, N0, M0 (>1 cm)/T1-3, N1, M0) were eligible. In women without clinically suspicious axillary adenopathy, the primary breast tumor had to be >1 cm in diameter; patients with clinically suspicious axillary adenopathy could present with a primary tumor of any size (in accordance with cancer staging as per the American Joint Committee on Cancer).
Inclusion criteria were as follows: no prior treatment for breast cancer, Eastern Cooperative Oncology Group performance status of 0–1, white blood cell count >4,000–12,000 mm3 or neutrophil count >2,000 mm3, platelets >100,000 mm3, hemoglobin >9.5 g/dL, bilirubin <1.25× institutional upper limit of normal (ULN), creatinine <1.5× institutional ULN, creatinine clearance >30 mL/min, aspartate aminotransferase and alanine aminotransferase <1.5× institutional ULN, a normal electrocardiogram for cardiac function, and left ventricular ejection fraction of >60 %.
Exclusion criteria included uncontrolled medical conditions, significant interstitial pneumonia or pulmonary fibrosis, suspected of infection with fever, symptomatic varicella, required treatment for pleural or pericardial effusions, severe edema, severe peripheral neuropathy, required steroid pre-treatment, severe psychiatric disorders, inflammatory breast cancer, bilateral cancer (if both tumors were within the inclusion criteria, bilateral cancer was not excluded), and a history of other malignancies within the last 5 years (except for adequately treated non-melanoma skin cancer or carcinoma in situ of the cervix).
Study treatment
Patients were scheduled to receive 4 cycles of intravenous FEC (5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, cyclophosphamide 500 mg/m2) on day 1 every 3 weeks. Patients who completed 4 FEC cycles were randomly assigned to receive either 4 cycles of docetaxel (75 mg/m2, on day 1) plus capecitabine (825 mg/m2 twice daily on days 1–14) or 4 cycles of docetaxel alone (75 mg/m2, on day 1) every 3 weeks. For patients with a creatinine clearance of 30–50 mL/min, the initial dose of capecitabine was reduced to 75 % of the planned dose. Patients with disease progression while on FEC were excluded from randomization. A maximum 25 % dose reduction and 3-week dose delay were permitted for adverse events. Whereas a 75 % dose level was used as the initial dose for patients with low creatinine clearance, a further 25 % dose reduction was permitted for adverse events. Treatment prior to docetaxel comprised dexamethasone (8 mg oral; administered the morning and night before docetaxel). In addition, dexamethasone (10 mg intravenous) was administered 30 min before docetaxel. If a patient missed the 8 mg oral dexamethasone, the 10 mg intravenous dose was still administered and docetaxel administration occurred as planned. Primary surgery was undertaken within 3–6 weeks of neoadjuvant chemotherapy completion. Supportive care and postoperative endocrine or radiation therapy were administered at the investigator’s discretion. No patients received trastuzumab before surgery, as it was not approved in Japan at the time of the study.
Study assessments
Pre-enrolment assessments included medical history, physical examination, blood chemistry, bilateral mammogram, bone and computed tomography scans. Initial diagnosis of invasive adenocarcinoma was made by core needle biopsy. Estrogen receptor (ER) and progesterone receptor (PgR) status were confirmed by immunohistochemistry (IHC) before randomization. Human epidermal growth factor receptor 2 (HER2) status was confirmed by IHC or fluorescent in situ hybridization. For biomarker analysis, IHC was undertaken using a mouse anti-human TP monoclonal antibody (Chugai Pharmaceutical Co., Japan). TP immunoreactivity was detected in the cytoplasm of carcinoma cells and semi-quantitative evaluation was undertaken using >1,000 carcinoma cells in each case. Ki67 immunostaining was performed using MIB1 monoclonal antibody (Dako Co.Ltd.) as previously described [16]. Briefly, Ki67 was stained after overnight preparation using a 1:100 dilution of the antibody. Evaluation of Ki67 was performed by counting ≥1,000 carcinoma cells from each patient in the hot spots and the percentage of immunoreactivity was subsequently determined by a labelling index [17].
Clinicopathological assessments were undertaken at the central laboratory (Department of Anatomic Pathology, Tohoku University, Graduate School of Medicine, Japan). The clinical response was evaluated in accordance with the Response Evaluation Criteria In Solid Tumors guidelines. Tumor response evaluation was performed after cycles 4 and 8, and after each cycle where possible pCR was defined as no histological evidence of invasive carcinoma, or the appearance of only non-invasive or in situ carcinoma on pathologic examination of the surgical specimen. When histological diagnosis of pCR was difficult based on hematoxylin-eosin-stained tissue sections, irrespective of whether carcinoma cells were present as ductal carcinoma in situ components, immunohistochemistry of myoepithelial markers such as cytokeratin 5/6 and p63 was used to determine the presence of invasive carcinoma [18–20]. Toxicity was graded and reported according to the NCI Common Terminology Criteria for Adverse Events version 3.
Statistical analysis
Following a reported 16 % pCR rate when FEC was followed by docetaxel alone in the JBCRG 01 trial [7], it was determined that 434 assessable patients were required for randomization to achieve 80 % power for the detection of an increase in the proportion of pCR rate of the docetaxel/capecitabine versus docetaxel group. Differences in pCR rates were calculated using a one-sided Chi square test with Schouten collection at the alpha level of 5 %; 95 % confidence interval (CI) was also calculated. In predictive factor analysis, the interaction of pCR with Ki67 as a continuous variable was explored using the subpopulation treatment effect pattern plots (STEPP) method. For each risk factor, the odds ratio (OR) for pCR and 95 % CI was calculated using simple and multivariate logistic regression models. DFS and OS were calculated using the Kaplan–Meier method. For each prognostic factor, hazard ratio (HR) for DFS and 95 % CI was calculated using the simple Cox model. Factors associated with DFS in univariate analysis were included in the multivariate Cox model.
Results
Patient population
A total of 504 patients were enrolled into the study (15 centers in Japan, 1 in China, and 1 in Hong Kong), 27 of whom withdrew during FEC therapy. Following FEC therapy, 239 patients were randomly assigned to the docetaxel/capecitabine group and 238 patients to the docetaxel alone group; all 477 patients were included in the intent-to-treat (ITT) population. Patients randomized to both groups were well balanced with respect to age, menopausal status, and baseline tumor characteristics (Table 1).
Table 1.
Baseline patient demographics and clinical characteristics
| Number | Total | FEC only | FEC + T | FEC + TX | p value |
|---|---|---|---|---|---|
| 504 | 27 | 238 | 239 | ||
| Age | |||||
| Median | 49.0 | 47.0 | 49.0 | 49.0 | W:0.8769 |
| Range | 25.0–70.0 | 28.0–65.0 | 25.0–68.0 | 25.0–70.0 | |
| Menopausal status | |||||
| Premenopausal | 282 (56.0 %) | 16 (59.3 %) | 133 (55.9 %) | 133 (55.6 %) | C:0.9590 |
| Postmenopausal | 222 (44.0 %) | 11 (40.7 %) | 105 (44.1 %) | 106 (44.4 %) | |
| Initial tumor size | |||||
| Median | 3.5 | 3.5 | 3.5 | 3.5 | W:0.7508 |
| Range | 0.8–10.5 | 2.0–10.5 | 0.8– 8.0 | 1.0– 9.0 | |
| Axillary lymph nodes* | |||||
| Positive | 280 (55.6 %) | 12 (44.4 %) | 134 (56.3 %) | 134 (56.1 %) | C:0.9586 |
| Negative | 224 (44.4 %) | 15 (55.6 %) | 104 (43.7 %) | 105 (43.9 %) | |
| Clinical stage | |||||
| I | 5 (1.0 %) | 0 (0.0 %) | 2 (0.8 %) | 3 (1.3 %) | C:0.9170 |
| IIA | 218 (43.3 %) | 12 (44.4 %) | 100 (42.0 %) | 106 (44.4 %) | |
| IIB | 226 (44.8 %) | 11 (40.7 %) | 110 (46.2 %) | 105 (43.9 %) | |
| IIIA | 55 (10.9 %) | 4 (14.8 %) | 26 (10.9 %) | 25 (10.5 %) | |
| Histologic type | |||||
| Infiltrating ductal carcinoma | 491 (97.4 %) | 25 (92.6 %) | 233 (97.9 %) | 233 (97.5 %) | C:0.1087 |
| Infiltrating lobular carcinoma | 8 (1.6 %) | 1 (3.7 %) | 1 (0.4 %) | 6 (2.5 %) | |
| Mucinous carcinoma | 1 (0.2 %) | 0 (0.0 %) | 1 (0.4 %) | 0 (0.0 %) | |
| Invasive micropapillary carcinoma | 1 (0.2 %) | 0 (0.0 %) | 1 (0.4 %) | 0 (0.0 %) | |
| Infiltrated apocrine carcinoma | 2 (0.4 %) | 0 (0.0 %) | 2 (0.8 %) | 0 (0.0 %) | |
| Invasive small cell carcinoma | 1 (0.2 %) | 1 (3.7 %) | 0 (0.0 %) | 0 (0.0 %) | |
| Histologic type | |||||
| Infiltrating ductal carcinoma | 491 (97.4 %) | 25 (92.6 %) | 233 (97.9 %) | 233 (97.5 %) | C:0.7657 |
| Otherwise | 13 (2.6 %) | 2 (7.4 %) | 5 (2.1 %) | 6 (2.5 %) | |
| Nuclear grade | |||||
| G1 | 86 (17.1 %) | 8 (29.6 %) | 42 (17.6 %) | 36 (15.1 %) | C:0.6716 |
| G2 | 243 (48.2 %) | 14 (51.9 %) | 110 (46.2 %) | 119 (49.8 %) | |
| G3 | 167 (33.1 %) | 5 (18.5 %) | 81 (34.0 %) | 81 (33.9 %) | |
| NA/ND | 8 (1.6 %) | 0 (0.0 %) | 5 (2.1 %) | 3 (1.3 %) | |
| ER | |||||
| Positive | 327 (64.9 %) | 15 (55.6 %) | 157 (66.0 %) | 155 (64.9 %) | C:0.7423 |
| Negative | 163 (32.3 %) | 9 (33.3 %) | 75 (31.5 %) | 79 (33.1 %) | |
| NA/ND | 14 (2.8 %) | 3 (11.1 %) | 6 (2.5 %) | 5 (2.1 %) | |
| PgR | |||||
| Positive | 242 (48.0 %) | 10 (37.0 %) | 113 (47.5 %) | 119 (49.8 %) | C:0.5775 |
| Negative | 246 (48.8 %) | 14 (51.9 %) | 119 (50.0 %) | 113 (47.3 %) | |
| NA/ND | 10 (2.0 %) | 3 (11.1 %) | 6 (2.5 %) | 1 (0.4 %) | |
| ER/PgR* | |||||
| Positive | 331 (65.7 %) | 15 (55.6 %) | 158 (66.4 %) | 158 (66.1 %) | C:0.8930 |
| Negative | 159 (31.5 %) | 9 (33.3 %) | 74 (31.1 %) | 76 (31.8 %) | |
| NA/ND | 14 (2.8 %) | 3 (11.1 %) | 6 (2.5 %) | 5 (2.1 %) | |
| HER2* | |||||
| Positive | 99 (19.6 %) | 7 (25.9 %) | 44 (18.5 %) | 48 (20.1 %) | C:0.6576 |
| Negative | 380 (75.4 %) | 17 (63.0 %) | 183 (76.9 %) | 180 (75.3 %) | |
| NA/ND | 25 (5.0 %) | 3 (11.1 %) | 11 (4.6 %) | 11 (4.6 %) |
ER estrogen receptor, FEC fluorouracil/epirubicin/cyclophosphamide, HER2 Human epidermal growth factor receptor 2, NA not available, ND no data, PgR progesterone receptor, T docetaxel alone, TX docetaxle plus capecitabine
Treatment administration and study completion
No significant differences were observed in the delivery of FEC therapy between the treatment groups. However, the relative dose intensities for docetaxel were significantly lower in the docetaxel/capecitabine group than in the docetaxel alone group (p = 0.0006). A 25 % dose reduction was required for 33 % (79/239) of patients in the docetaxel/capecitabine group and 5.9 % (14/238) of patients in the docetaxel alone group. The rate of completion after the initial dose was significantly lower in the docetaxel/capecitabine group compared with the docetaxel alone group (44.8 and 88.7 %, respectively; p < 0.0001). Study discontinuation was significantly higher in the docetaxel/capecitabine (53/239; 22 %) group compared to docetaxel alone (13/238, 5.5 %; p < 0.0001). The majority of study withdrawals were attributed to drug toxicity (docetaxel/capecitabine: 31/53 patient; docetaxel alone: 9/13 patients; Fig. 1).
Fig. 1.
Study completion. FEC: fluorouracil/epirubicin/cyclophosphamide; T: docetaxel alone; TX: docetaxel plus capecitabine
Clinical and pathological response
The overall response rate (cCR and cPR) was 88.3 % (211/239) in the docetaxel/capecitabine group and 87.4 % (208/238) in the docetaxel group; no significant differences in clinical response were noted. The proportion of BCS was 70.7 % (169/239) in the docetaxel/capecitabine group and 71.4 % (170/238) in the docetaxel group; the proportion of axillary lymph node conservation surgery was 28.9 % (69/239) and 27.7 % (66/238), respectively (data not shown).
The pCR rate was 23 % in the docetaxel/capecitabine group and 24 % in the docetaxel group (p = 0.748; Table 2a). However, we observed an interesting trend in the subset of patients who had discontinued treatment or received a 25 % dose reduction. Despite treatment withdrawal, 12/53 in the docetaxel/capecitabine group and 1/13 in the docetaxel group achieved a pCR with rates of 22.6 and 7.7 %, respectively. A similar trend was observed in the 33.1 % (79/239) and 5.9 % (14/238) who received a 25 % dose reduction and achieved pCR rates of 24.1 % (19/79) and 14.3 % (2/14), respectively (Table 2b). Although not statistically significant, pCR rates were higher in the docetaxel/capecitabine group in comparison to the docetaxel group in this subpopulation.
Table 2.
Pathological response by (a) central assessment, (b) central assessment in patients who discontinued or received a reduced dose
| FEC (n = 27) | TX (n = 239) | T (n = 238) | Difference | p value | |
|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | (TX-T) (95 %CI) | ||
| (a) | |||||
| pCR | 7.4 | 23 (17.8–28.9) | 24.4 (19.1–30.3) | −1.4 (−9.0 to 6.3) | 0.7476 |
| pINV | 48.1 (28.7–68.1) | 72.4 (66.3–78.0) | 71.4 (65.2–77.1) | 1 | |
| Missing* | 44.4 (25.5–64.7) | 4.6 (2.3–8.1) | 4.2 (2.0–6.7) | 0.4 | |
| (b) | |||||
| pCR | 7.4 | 23 (17.8–28.9) | 24.4 (19.1–30.3) | −1.4 (−9.0 to 6.3) | 0.7476 |
| With discontinuation | (n = 12/53) | (n = 1/13) | |||
| pCR | – | 22.6 (12.3–36.2) | 7.7 (0.2–36.0) | 14.9 (−3.4 to 33.3) | |
| With dose reduction | (n = 19/79) | (n = 2/14) | |||
| pCR | – | 24.1 (15.1–35.0) | 14.3 (1.8–42.8) | 9.8 (−10.8 to 30.4) |
pCR pathological complete response, pINV pathological presence of invasive tumor, * patients missing post-baseline mainly due to discontinuation as a result of toxicity, CI confidence interval, FEC 5-fluorouracil–epirubicin–cyclophosphamide, TX docetaxel plus capecitabine, T docetaxel alone
Disease-free and overall survival
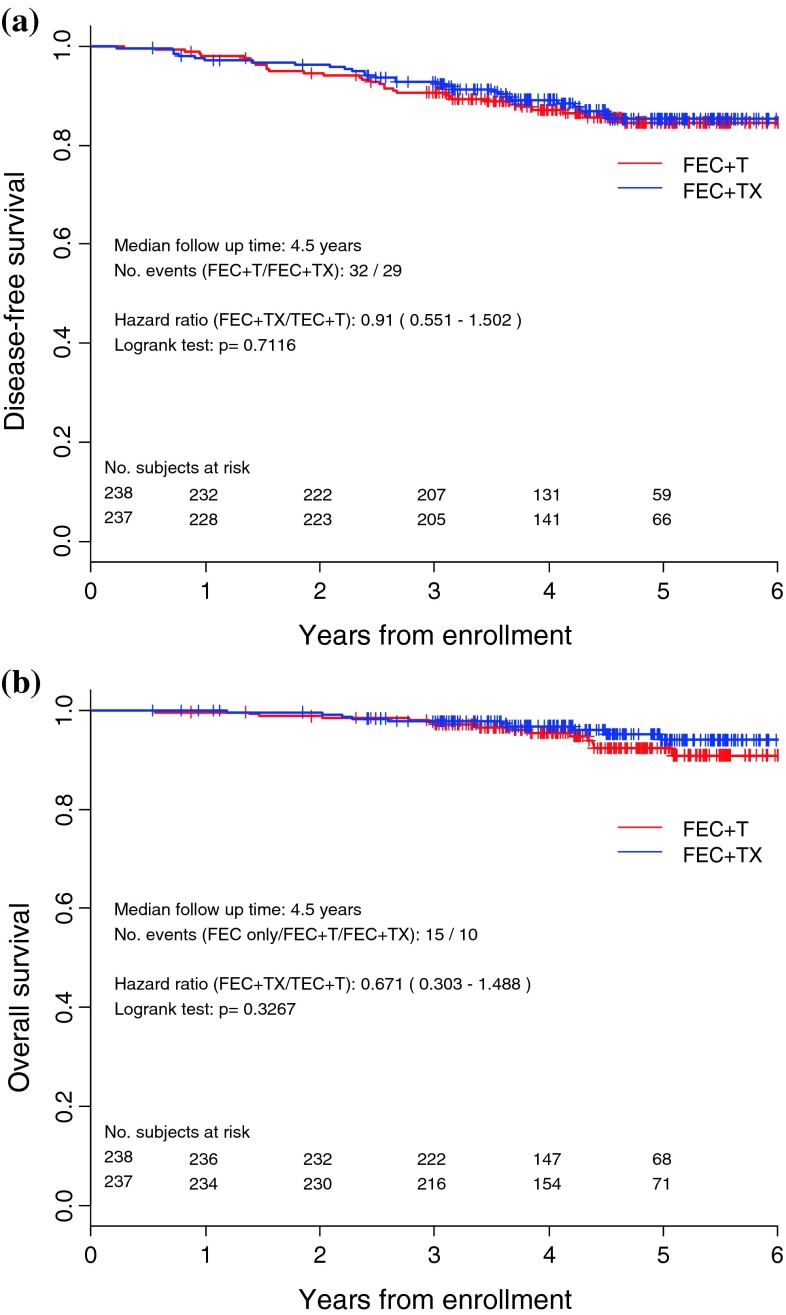
After a median 4.5-year follow-up, the 3-year DFS was estimated at 92.7 % in the docetaxel/capecitabine group and 90.7 % in the docetaxel group. Four patients were excluded from the ITT population due to missing data. A total of 29 events occurred in the docetaxel/capecitabine group and 32 in the docetaxel group, with a HR of 0.910 (95 % CI 0.551–1.502; Fig. 2a). During follow-up, 10 deaths occurred in the docetaxel/capecitabine group and 15 in the docetaxel group, with a point of estimate HR of 0.671 (95 % CI 0.303–1.488; Fig. 2b).
Fig. 2.
a Disease-free survival. b Overall survival. FEC: fluorouracil/epirubicin/cyclophosphamide; T: docetaxel alone; TX: docetaxel plus capecitabine
Predictive factor analyses for pathological response and survival status
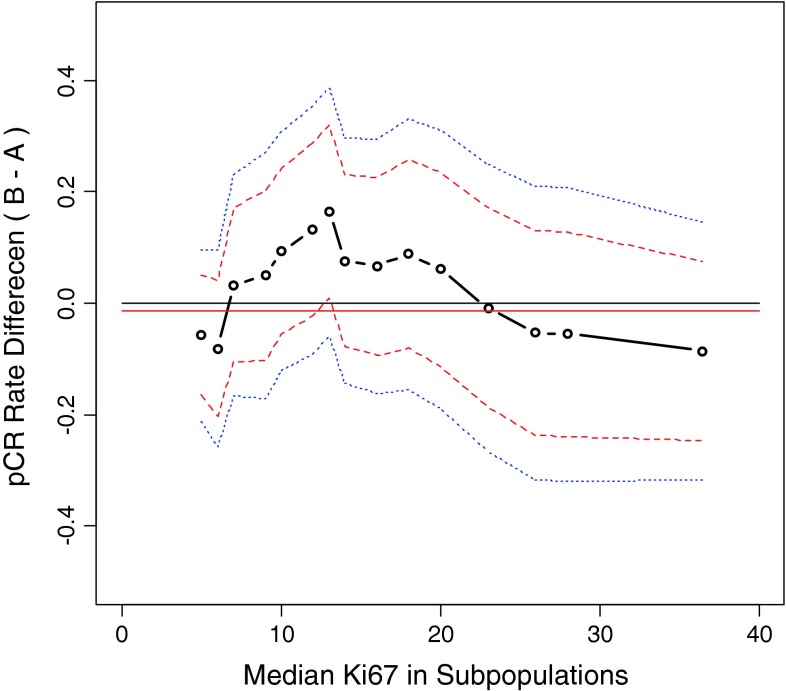
Subpopulation analysis for pathological response showed no significant difference between treatment groups (data not shown). To identify predictive factors for pathological response using age and Ki67 as continuous variables, an overlapping subpopulation of 84 patients was constructed and analyzed using the STEPP method. Although no statistical significance was achieved, STEPP analysis indicated a trend in favor of improved pCR rate in patients with mid-range of Ki67LI (10–20 %) following docetaxel/capecitabine compared with docetaxel alone (Fig. 3). To further investigate the predictive value of Ki67 relative to pCR, univariate and multiple logistic regression models were fitted to calculate the odds ratio (OR) and 95 % CI for each risk factor.
Fig. 3.
STEPP analysis of the treatment effect of docetaxel/capecitabine compared with single-agent docetaxel as measured by pCR. Values >0 suggested that the combination regimen was better; <0 indicated that single-agent docetaxel was better. Difference in pCR is shown (dashed black lines) with corresponding 95 % CI (dashed red lines) and corresponding 95 % confidence band (dashed blue lines). Overall difference in pCR (solid horizontal red line) is shown
Univariate analysis showed that nuclear grading, ER and/or PgR status, HER2 status, baseline Ki67 and TP-SI were all strongly associated with pCR (Table 3a). Multivariate analysis was performed using the predictive variables identified in the univariate analysis. To evaluate the effect of Ki67, a multivariate logistic regression analysis was undertaken in 410 patients with available baseline data for nuclear grading, ER and/or PgR, HER2, and Ki67. In the first model, all of these factors continued to be 15 % significant predictors for pCR. In the final model, pre-treatment levels of Ki67 proved to be a predictive factor for pCR, with an OR of 1.031 (95 % CI 1.014–1.048; p = 0.0004). Using this model, the random cross-validated sensitivity and specificity were 83.3 and 63.4 %, respectively (Table 3b).
Table 3.
Prediction of pCR using (a) simple logistic regression model, (b) multiple logistic regression model with Ki67
| Factor | # pat | # res | OR | 95 %CI | p value |
|---|---|---|---|---|---|
| (a) | |||||
| Age | |||||
| ≤49 | 248 | 56 | 0.880 | 0.577–1.343 | 0.5534 |
| ≥50 | 229 | 57 | 1 | ||
| Initial tumor size | |||||
| ≤2.0 | 29 | 7 | 1.047 | 0.409–2.682 | 0.9919 |
| 2.1–4.0 | 315 | 75 | 1.028 | 0.637–1.659 | |
| ≥4.1 | 133 | 31 | 1 | ||
| Axillary lymph node | |||||
| Positive | 268 | 62 | 0.932 | 0.610–1.426 | 0.7467 |
| Negative | 209 | 51 | 1 | ||
| Menopausal status | |||||
| Pre | 266 | 60 | 0.868 | 0.568–1.326 | 0.5135 |
| Post | 211 | 53 | |||
| Stage | |||||
| I/IIa | 54 | 211 | 1.849 | 0.818–4.179 | 0.3355 |
| IIb | 51 | 215 | 1.671 | 0.738–3.786 | |
| III | 8 | 51 | 1 | ||
| Nuclear grading | |||||
| G1 | 78 | 9 | 0.240 | 0.112–0.517 | <.0001 |
| G2 | 229 | 46 | 0.463 | 0.293–0.731 | |
| G3 | 162 | 57 | 1 | ||
| ER and/or PgR | |||||
| Positive | 327 | 58 | 0.265 | 0.167–0.422 | <.0001 |
| Negative | 116 | 52 | 1 | ||
| HER2 | |||||
| Positive | 62 | 33 | 4.552 | 2.604–7.958 | <.0001 |
| Negative | 380 | 76 | 1 | ||
| Baseline of Ki67 (%) | |||||
| ≥10 | 299 | 95 | 4.572 | 2.348–8.903 | <.0001 |
| <10 | 119 | 11 | 1 | ||
| Continuous | 418 | 1.043 | 1.027–1.059 | <.0001 | |
| TP-CI | |||||
| 1 + , 2 + , 3+ | 282 | 73 | 1.715 | 0.851–3.456 | 0.1316 |
| 0 | 65 | 11 | 1 | ||
| 2 + , 3+ | 119 | 33 | 1.332 | 0.801–2.213 | 0.2690 |
| 0, 1+ | 228 | 51 | 1 | ||
| TP-SI | |||||
| 1 + , 2 + , 3+ | 324 | 84 | 4.025 | 0.929–17.438 | 0.0627 |
| 0 | 25 | 2 | 1 | ||
| 2 + , 3+ | 197 | 59 | 1.979 | 1.182–3.315 | 0.0095 |
| 0, 1+ | 152 | 27 | 1 |
| OR | 95 %CI | p value | Sensitivity specificity | ROC (95 % CI) | Contrast with final model | |
|---|---|---|---|---|---|---|
| (b) | ||||||
| Grading | ||||||
| 1 | 0.312 | 0.129–0.756 | 0.0027 | Random cv | Apparent | |
| 2 | 0.461 | 0.274–0.773 | Sen: 0.8113 | 0.7510 | ||
| 3 | 1 | (0.6034, 0.8958) | (0.6999, 0.8021) | |||
| ER and/or PgR | ||||||
| Positive | 0.384 | 0.230–0.642 | 0.0003 | |||
| Negative | 1 | Spe: 0.6097 | Random cv | |||
| HER2 | ||||||
| Positive | 3.816 | 2.056–7.081 | <.0001 | (0.5517, 0.7391) | 0.7353 | |
| Negative | 1 | (0.6664, 0.7901) | ||||
| Grading | ||||||
| 1 | 0.402 | 0.163–0.991 | 0.00281 | Random cv | Apparent | Apparent |
| 2 | 0.536 | 0.316–0.909 | Sen: 0.8000 | 0.7657 | 0.0147 | |
| 3 | 1 | (0.6599, 0.8889) | (0.7172, 0.8143) | (−0.0055, 0.0350) | ||
| ER and/or PgR | ||||||
| Positive | 00.413 | 0.247–0.692 | 0.0008 | |||
| Negative | 1 | Spe: 0.6458 | ||||
| HER2 | ||||||
| Positive | 3.522 | 1.890–6.563 | <.0001 | (0.5792, 0.7452) | Random cv | Random cv |
| Negative | 0.7489 | 0.0168 | ||||
| Ki67 (%) | ||||||
| ≥10 | 2.718 | 1.331–5.549 | 0.0061 | (0.6827, 0.7986) | (−0.0303, 0.041) | |
| <10 | 1 | |||||
| Grading | ||||||
| 1 | 0.418 | 0.169–1.035 | 0.0298 | Random cv | Apparent | Apparent |
| 2 | 0.530 | 0.312–0.900 | Sen: 0.8333 | 0.7774 | 0.0264 | |
| 3 | 1 | (0.6735, 0.9400) | (0.7289, 0.8259) | (0.0015, 0.0513) | ||
| ER and/or PgR | ||||||
| Positive | 0.447 | 0.265–0.754 | 0.0026 | |||
| Negative | 1 | Spe: 0.6344 | Random cv | Random cv | ||
| HER2 | ||||||
| Positive | 3.794 | 2.038–7.065 | <0.0001 | (0.5063, 0.7713) | 0.7607 | 0.0274 |
| Negative | 1 | (0.6993, 0.8099) | (−0.0175, 0.0596) | |||
| Ki67 (continuous) | 1.031 | 1.014–1.048 | 0.0004 |
#pat number of patients, #res number of responders, CI confidence interval, ER estrogen receptor, HER2 human epidermal growth factor receptor 2, OR odds ratio, PgR progesterone receptor, TP-CI thymidine phosphorylase, interstitial, TP-SI thymidine phosphorylase, stromal
ER estrogen receptor, HER2 human epidermal growth factor receptor 2, PgR progesterone receptor, OR odds ratio
Predictive factors for DFS were analyzed using a multiple Cox model in a landmark analysis (Online Resource). When pCR and postKi67 were included in the final model, tumor stage (I, IIa/III: HR 0.144, 95 % CI 0.051–0.404; IIb/III: HR 0,264, 95 % CI 0.107–0.651; p = 0.0006), cancer cell TP status (continuous variables: HR 0.966, 95 % CI 0.941–0.993; p = 0.0125), pCR, and post-treatment Ki67LI (pCR/Ki67 <10/≥10: HR 0.269, 95 % CI 0.110–0.655; p = 0.0038) were all significantly associated with DFS (Table 4).
Table 4.
Hazard ratio for disease-free survival using a multiple cox model in landmark analysis
| Factors | HR | (95 % CI) | p value |
|---|---|---|---|
| The final model | |||
| Stage | |||
| I/IIa | 0.160 | 0.059–0.436 | 0.0016 |
| IIb | 0.390 | 0.170–0.893 | |
| III | 1 | ||
| ER and/or PgR | |||
| Positive | 0.468 | 0.235–0.932 | 0.0308 |
| Negative | 0.974 | 0.953–0.996 | 0.0193 |
| TP-CP | |||
| Continuous | 1 | ||
| Extended model 1 | |||
| Stage | |||
| I/IIa | 0.170 | 0.065–0.444 | 0.0011 |
| IIb | 0.360 | 0.165–0.787 | |
| III | 1 | ||
| ER and/or PgR | |||
| Positive | 0.327 | 0.160–0.670 | 0.0023 |
| Negative | 1 | ||
| TP-CP | |||
| Continuous | 0.975 | 0.954–0.997 | 0.0253 |
| pCR | |||
| Responder | 0.191 | 0.052–0.696 | 0.0121 |
| Nonresponder | |||
| Extended model 2 | |||
| Stage | |||
| I/IIa | 0.133 | 0.051–0.349 | 0.0002 |
| IIb | 0.308 | 0.134–0.706 | |
| III | 1 | ||
| ER and/or PgR | |||
| Positive | 0.441 | 0.221–0.878 | 0.0198 |
| Negative | 1 | ||
| TP-CP | |||
| Continuous | 0.974 | 0.953–0.996 | 0.0199 |
| Treatment | |||
| Completion | 0.633 | 0.209–1.917 | 0.3560 |
| Reduction | 1.125 | 0.339–3.729 | |
| Discontinuation | 1 | ||
| Extended model 3 | |||
| Stage | |||
| I/IIa | 0.134 | 0.051–0.350 | 0.0002 |
| IIb | 0.309 | 0.135–0.706 | |
| III | 1 | ||
| ER and/or PgR | |||
| Positive | 0.439 | 0.220–0.878 | 0.0200 |
| Negative | 1 | ||
| TP-CP | |||
| Continuous | 0.974 | 0.953–0.996 | 0.0183 |
| Treatment | |||
| Completion | 0.584 | 0.278–1.226 | 0.1554 |
| Otherwise | 1 | ||
| Extended model 4 | |||
| Stage | |||
| I/IIa | 0.153 | 0.056–0.419 | 0.0006 |
| IIb | 0.279 | 0.116–0.673 | |
| III | 1 | ||
| ER and/or PgR | |||
| Positive | 0.577 | 0.229–1.454 | 0.2433 |
| Negative | 1 | ||
| TP-CP | |||
| Continuous | 0.967 | 0.941–0.993 | 0.0144 |
| p CR & PostKi67 | |||
| Responder | 0.137 | 0.034–0.549 | 0.0140 |
| PostKi67 < 10 | 0.388 | 0.143–1.052 | |
| PostKi67≧10 | 1 | ||
| Extended model 5 | |||
| Stage | |||
| I/IIa | 0.144 | 0.051–0.404 | 0.0006 |
| IIb | 0.264 | 0.107–0.651 | |
| III | 1 | ||
| ER and/or PgR | |||
| Positive | 0.756 | 0.334–1.712 | 0.5030 |
| Negative | 1 | ||
| TP-CP | |||
| Continuous | 0.966 | 0.941–0.993 | 0.0125 |
| pCR & PostKi67 | |||
| Responder | 0.269 | 0.110–0.655 | 0.0038 |
| PostKi67 < 10 | |||
| PostKi67≧10 | 1 | ||
| Extended model 5 | |||
| Stage | |||
| I/IIa | 0.200 | 0.072–0.561 | 0.0031 |
| IIb | 0.264 | 0.103–0.676 | |
| III | 1 | ||
| ER and/or PgR | |||
| Positive | 0.385 | 0.152–0.977 | 0.0445 |
| Negative | 1 | ||
| TP-CP | |||
| Continuous | 0.970 | 0.943–0.997 | 0.0301 |
ER estrogen receptor, HER2 human epidermal growth factor receptor 2, PgR progesterone receptor, OR odds ratio, TP-CP thymidine phosphorylase, plasma
Safety profile
The frequency of major adverse events (≥grade 3) of docetaxel/capecitabine and docetaxel group were as follows: leukopenia (36 and 34 %, respectively), neutropenia (38 and 34 %, respectively), febrile neutropenia (8 and 5 %, respectively), and hand-foot syndrome (15 and 2 %, respectively). Docetaxel/capecitabine was associated with more capecitabine-related toxicity, including hand-foot syndrome, nausea, mucositis, and increased alanine aminotransferase. Six serious adverse events were reported for 3 patients in the docetaxel/capecitabine group (pneumonitic cough, muscle pain, neutropenia fever) and 3 patients in the docetaxel group (suicide, loss of eyesight of left eye, hematological toxicity). The event of suicide in the docetaxel alone group occurred after completion of treatment and was considered as unrelated to study treatment.
Discussion
We have presented results from a randomized study comparing preoperative capecitabine/docetaxel with docetaxel alone after FEC in early-stage breast cancer, and have identified Ki67 as a predictive biomarker that may be used to identify patients likely to respond to this neoadjuvant regimen.
In contrast to previous reports, we observed no difference in the pCR rate between the docetaxel/capecitabine and the docetaxel group. Our observation was similar to that from the GeparQuattro study, in which docetaxel/capecitabine did not improve pCR rate in comparison to docetaxel after epirubicin/cyclophosphamide treatment in the neoadjuvant setting [21]. Although a 16 % pCR rate was expected in the docetaxel group based on previous observations [5], the pCR rate in our study was higher (24 %). The variation in clinical outcome may be attributed to the currently limited means with which to select patient subpopulations most likely to respond to a given treatment regimen.
The docetaxel/capecitabine regimen was less well tolerated than docetaxel alone, with withdrawal rates of 22.2 and 5.5 % and dose reduction rates of 33.1 and 5.9 %, respectively. Despite treatment withdrawals and dose reductions, achievement of higher pCR rates in the docetaxel/capecitabine group in comparison to the docetaxel group in this subpopulation suggests that dose reduction does not negatively impact capecitabine efficacy. Our data confirms a similar observation in a MBC study, which reported no significant effect on efficacy when dose reduction occurred in 65 and 36 % of patients receiving the docetaxel/capecitabine regimen and docetaxel alone, respectively. However, an increased risk of disease progression was seen in patients with a dose reduction to 50 % of the starting dose in the docetaxel group (HR 1.91) [15]. As reported by other groups [22], our data demonstrate that the capecitabine dose can be reduced to minimize adverse effects without compromising efficacy. It was, however, interesting to observe that patients who discontinued or received a dose reduction in the docetaxel/capecitabine group achieved a higher pCR compared with the docetaxel alone group, while there was no difference in pCR between both groups in patients that completed the study at the original dose. Although the reason for this observation is unclear, the observation that the relative dose intensity for docetaxel was significantly lower in the combination arm compared with the single agent docetaxel arm may at least in part, account for the lack of difference in pCR. In addition, levels of toxicity may have had an impact on drug delivery and thus, pCR.
In addition to comparing the efficacy of neoadjuvant docetaxel/capecitabine with docetaxel alone, our study also sought to identify biomarkers that can identify patients likely to respond to treatment with docetaxel/capecitabine in early-stage breast cancer. Previously identified biomarkers, such as nuclear grading, ER and/or PgR status, HER2 status and Ki67, correlated with pCR in our study, as in other published studies [23]. Of particular interest was pre-treatment Ki67LI, which had a strong correlation with pCR and added to the predictive value of the multivariate logistic regression model. Indeed, data from several other studies suggest that high Ki67 levels in breast cancer are a predictive factor for pCR rate [5, 24–27]. This effect was present in our study, as patients with ≥10 % pre-treatment Ki67LI achieved a higher pCR rate in both the docetaxel/capecitabine (32.6 %) and docetaxel alone (31 %) groups, in comparison to patients with <10 % pre-treatment Ki67LI (pCR rates 6.5, 12.3 %, respectively). These findings support the suggestion that detection of pre-treatment Ki67LI could identify patients most likely to benefit from neoadjuvant chemotherapy. The prognostic value of Ki67 was also confirmed in our study, as post-treatment Ki67LI and pCR were significantly associated with DFS using a multiple Cox model in a landmark analysis. Thus, prognostic and predictive value was detected for Ki67, showing it to be a feasible marker for development of individualized treatment options for early-stage breast cancer patients.
To our knowledge, this is the first multicenter randomized study showing that assessment of pre- and post-treatment Ki67 may be a useful tool in predicting pCR and DFS with neoadjuvant docetaxel treatment with or without capecitabine in patients with early-stage breast cancer. Although further studies are required, our data suggests that the routine detection of the Ki67 proliferation marker in early-stage breast cancer could be a useful prognostic tool for the identification of patients most likely respond to preoperative docetaxel with or without capecitabine. As such, in addition to the current leading parameters (ER, PgR, and HER2 status), we propose that Ki67 should be included in the list of required routine biological markers that are used to define treatment recommendations in patients with early-stage breast cancer. Indeed, detection of predictive biomarkers prior to chemotherapy is likely to prove to be of the greatest advantage for neoadjuvant chemotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was made possible by the generous support and supply of knowledge by Product Research Department, Kamakura Research Laboratories, Chugai Pharmaceutical Co., Ltd. and Sanofi K.K., Japan. This work was supported by an unconditional grant from Sanofi K.K., Japan.
Conflict of interest
The authors have declared no conflicts of interest.
References
- 1.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: 9-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 3.Rouzier R, Perou C, Symmans W, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 4.Nolen B, Marks KJ, Ta’san S, et al. Serum biomarker profiles and response to neoadjuvant chemotherapy for locally advanced breast cancer. Breast Cancer Res. 2008;10:R45. doi: 10.1186/bcr2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fasching PA, Heusinger K, Haeberle L, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 7.Toi M, Nakamura S, Kuroi K, et al. Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res Treat. 2008;110:531–539. doi: 10.1007/s10549-007-9744-z. [DOI] [PubMed] [Google Scholar]
- 8.Sawada N, Ishikawa T, Fukase Y, et al. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res. 1998;4:1013–1019. [PubMed] [Google Scholar]
- 9.Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274–1281. doi: 10.1016/S0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 10.Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 11.Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92:1759–1768. doi: 10.1002/1097-0142(20011001)92:7<1759::AID-CNCR1691>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Jinno H, Sakata M, Hayashida T, et al. A phase II trial of capecitabine and docetaxel followed by 5-fluorouracil/epirubicin/cyclophosphamide (FEC) as preoperative treatment in women with stage II/III breast cancer. Ann Oncol. 2010;21:1262–1266. doi: 10.1093/annonc/mdp428. [DOI] [PubMed] [Google Scholar]
- 13.Natoli C, Cianchetti E, Tinari N, et al. A phase II study of dose-dense epirubicin plus cyclophosphamide followed by docetaxel plus capecitabine and pegfilgrastim support as preoperative therapy for patients with stage II, IIIA breast cancer. Ann Oncol. 2007;18:1015–1020. doi: 10.1093/annonc/mdm076. [DOI] [PubMed] [Google Scholar]
- 14.Lebowitz PF, Eng-Wong J, Swain SM, et al. A phase II trial of neoadjuvant docetaxel and capecitabine for locally advanced breast cancer. Clin Cancer Res. 2004;10:6764–6769. doi: 10.1158/1078-0432.CCR-04-0976. [DOI] [PubMed] [Google Scholar]
- 15.O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002;20:2812–2823. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Bouzubar N, Walker KJ, Griffiths K, et al. Ki67 immunostaining in primary breast cancer: pathological and clinical associations. Br J Cancer. 1989;59:943–947. doi: 10.1038/bjc.1989.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikami Y, Ueno T, Yoshimura K, Tsuda H, Kurosimi M, Masuda S et al. (2013) Inter-observer concordance of Ki67 labeling index in breast cancer. Japan Breast Cancer Research Group (JBCRG) Ki67 Ring Study. Cancer Sci. doi:10.1111/cas.12245 [DOI] [PMC free article] [PubMed]
- 18.Stefanou D, Batistatou A, Nonni A, Arkoumani E, Agnantis NJ. p63 expression in benign and malignant breast lesions. Histol Histopathol. 2004;19(2):465–471. doi: 10.14670/HH-19.465. [DOI] [PubMed] [Google Scholar]
- 19.Dewar R, Fadare O, Gilmore H, Gown AM. Best practices in diagnostic immunohistochemistry: myoepithelial markers in breast pathology. Arch Pathol Lab Med. 2011;135(4):422–429. doi: 10.5858/2010-0336-CP.1. [DOI] [PubMed] [Google Scholar]
- 20.Werling RW, Hwang H, Yaziji H, Gown AM. Immunohistochemical distinction of invasive from noninvasive breast lesions: a comparative study of p63 versus calponin and smooth muscle myosin heavy chain. Am J Surg Pathol. 2003;27(1):82–90. doi: 10.1097/00000478-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 21.von Minckwitz G, Rezai M, Loibl S, et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro Study. J Clin Oncol. 2010;28(12):2015–2023. doi: 10.1200/JCO.2009.23.8303. [DOI] [PubMed] [Google Scholar]
- 22.Leonard R, O’Shaughnessy J, Vukelja S, et al. Detailed analysis of a randomized phase III trial: can the tolerability of capecitabine plus docetaxel be improved without compromising its survival advantage? Ann Oncol. 2006;17:1379–1385. doi: 10.1093/annonc/mdl134. [DOI] [PubMed] [Google Scholar]
- 23.Caudle AS, Ganzalez-Angulo AM, Hunt KL, et al. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(11):1821–1828. doi: 10.1200/JCO.2009.25.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E, et al. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48(18):3342–3354. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang GC, Qian XK, Guo ZB et al (2012) Pre-treatment hormal receptor status and Ki67 index predict pathological complete response to neoadjuvant trastuzumab/taxanes but not disease-free survival in HER2-positive breast cancer patients. Med Oncol 29(5):3222–3231 [DOI] [PubMed]
- 27.Luporsi E, Andre F, Spyratos F et al (2012) Ki-67: level of evidence and methodoligical considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat 132(3):895–915 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.