Abstract
Anatabine is a Solanaceae plant family alkaloid marketed in the United States as a dietary supplement. It has demonstrated anti-inflammatory effects in vivo and in vitro, and may be useful for musculoskeletal aches and pains. The purpose of this internet-based survey study was to provide more information about anatabine users who report benefits for joint pain or stiffness. Of the 282 survey respondents, 232 (82%) reported a benefit from anatabine supplementation for one or more joint pain conditions, most commonly the knee, wrists/hands/fingers, shoulder, and back, most often due to osteoarthritis or injury to the joint. Mean scores of joint pain and stiffness were significantly (P < 0.0001) reduced after starting anatabine supplementation, and for most respondents joint pain was virtually eliminated. Around 90% of all individuals rated the effect of anatabine supplementation as good or excellent for joint pain, stiffness, functionality, and overall effects. These results provide evidence that anatabine supplementation can lead to substantial improvement of musculoskeletal aches, pains, and stiffness, and can provide benefits in some individuals for various medical conditions in multiple joint locations.
Keywords: anatabine, dietary supplement, joint pain, inflammation, musculoskeletal, online survey
Introduction
Chronic joint pain disorders are among the most common health afflictions in the United States (US) population, and are often exacerbated by excessive or persistent inflammation. Arthritis is the primary cause of recurring joint pain in the US, and afflicts an estimated 50 million American adults over the age of 18.1 Musculoskeletal and joint disorders become more common with increasing age; US Centers for Disease Control (CDC) data show that an estimated 46 million adults over age 45 have chronic joint symptoms, and 50% of the US population over age 65 will receive a diagnosis of arthritis at some time from their doctor.1 As the US population continues to age, the prevalence of musculoskeletal and joint pain disorders will continue to grow.
Joint disorders are characterized by a variety of signs and symptoms associated with excessive inflammation such as stiffness, swelling, pain, and tenderness. Treatments for joint pain are often targeted to reduce chronic inflammation at the site and may include non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin, ibuprofen, and naproxen.2 Oral, topical, or injectable corticosteroids may be needed for more severe cases of inflammation. Unfortunately, medications such as these do not work well for all patients,3 and have a number of potentially serious side effects that become more common with higher dosage, prolonged usage, and increased age.4,5 For these reasons, many people turn to alternative treatments to reduce inflammation and joint pain, including using dietary supplements like fish oil, chondroitin sulfate, and glucosamine.6
A unique compound that may assist the body in maintaining a healthy level of inflammation and reduce pain caused by musculoskeletal disorders is anatabine, a naturally occurring alkaloid found in Solanaceae family plants and vegetables such as peppers, potatoes, tomatoes, eggplant, and tobacco. Multiple preclinical studies have demonstrated that anatabine has anti-inflammatory effects in vitro and in vivo.7–10 For example, anatabine has been shown to inhibit NF-κB and STAT3 phosphorylation in animal models of Alzheimer’s disease and multiple sclerosis, acting to reduce the production of pro-inflammatory cytokines and other markers of inflammation, and to reduce cell and tissue damage caused by overproduction of these molecules.7–9 Anatabine administration was found to attenuate multiple manifestations of thyroid autoimmunity in an animal model of Hashimoto’s thyroiditis, and in vitro it dose-dependently suppressed macrophage production of iNOS and COX-2, two enzymes that increase dramatically during proinflammatory states.10
Anatabine is currently marketed in the US as a dietary supplement intended to provide anti-inflammatory support; however its effectiveness for individuals suffering from joint pain disorders that may be associated with chronic or excessive inflammation is unknown. The purpose of this internet-based survey study was to evaluate the characteristics of anatabine users, including information about: why they use the supplement; how they use the supplement (amount used per day, length of time used, etc.); and whether they report any discernible effects from using the supplement. Survey respondents who reported beneficial effects of anatabine supplementation for conditions that cause joint pain or stiffness completed additional survey questions about their particular joint pain conditions, and how the supplement affected or benefitted the conditions. These data will be useful in understanding the types of individuals who may derive benefit from dietary supplementation with anatabine, and in helping to plan clinical studies of the effects of anatabine supplementation in subjects with conditions that cause joint pain or stiffness.
Methods
Overall study design and plan
The protocol for this study received Institutional Review Board (IRB) approval prior to subject recruitment, and all subjects were required to provide online informed consent before receiving access to the internet survey. Potentially eligible subjects received an email invitation to participate in the survey in order to obtain approximately 200 completed surveys. Individuals were eligible to participate in the study if they had purchased the anatabine supplement (brand name Anatabloc®) more than once through Rock Creek Pharmaceuticals, Inc.’s (Gloucester, MA) product website (as determined from their ordering history), or if they had reported benefits from taking the anatabine supplement through testimonials received by email, in posts on social media websites (eg, Facebook, Twitter, etc.), or by posting to the customer comments/review sections of online marketplaces (eg, Amazon, GNC, etc.). This population was selected as likely to represent regular anatabine dietary supplement users who were not intimidated by internet communication and were likely to participate.
Characteristics of the survey
The survey included questions about the respondent’s demographics, general health, and anatabine supplement use. Survey respondents who reported beneficial effects from anatabine supplementation for conditions that cause joint pain or stiffness completed additional nested survey questions to learn more about their particular joint pain condition, and how the supplement affected or benefitted the condition. This information was to be used to characterize and provide insight into how and why individuals were using the supplement, and about any benefits they experienced from using it.
All individuals eligible for the survey received an email containing a brief explanation of the purpose of the survey, an invitation to participate, and a link to the survey website. Once an individual clicked on the link, they were taken to a webpage containing an informed consent statement. Individuals who agreed to participate by clicking “agree” on the informed consent page were taken to the survey pages and presented with questions about the nature of their anatabine supplement usage and their perceived effects from use of the supplement. The survey was intended to take approximately 15 minutes to complete. Those individuals who did not complete the survey within three business days received a follow-up email reminding them about the survey, and asking them to complete the survey.
Statistical methods
The primary outcome was to assess the characteristics of individuals who have repeatedly purchased the anatabine supplement. Summaries included the sample size, mean, standard deviation (SD), minimum, and maximum. Frequency distributions were calculated for all qualitative responses. To evaluate self-reported effectiveness of the supplement, the mean difference between pre and post ratings of joint pain and stiffness within the joint pain cohort was compared using a Student’s t-test for significance (P < 0.05). No safety assessments were performed in this survey.
Results
Study subjects (survey respondents)
Email invitations were sent to a total of 706 individuals. Of these, 700 (99%) were receiving an ongoing, pre-billed monthly shipment of the supplement, and 6 (1%) were individuals who had provided unsolicited testimonials about their experience using the supplement, and supplied an email address or other contact information. Of the 706 invited individuals, 282 (40%) responded to the invitation by clicking “agree” on the informed consent webpage, and were thus considered survey respondents. Of the 282 survey respondents, 232 (82%) reported a benefit from their use of the anatabine supplement for one or more joint pain conditions, and therefore completed additional questions related to their particular condition(s) and the effects they had experienced. These 232 individuals comprise the joint pain relief cohort.
Demographics and characteristics of anatabine supplement use
Demographics for all survey respondents and for the subset of respondents who comprise the joint pain relief cohort are shown in Table 1. A summary of the characteristics of anatabine supplement use by all survey respondents and the joint pain relief cohort is shown in Table 2A and B.
Table 1.
Demographics of all survey respondents and individuals in the joint pain relief cohort.
| Parameter | All survey respondents (N = 282) | Joint pain relief cohort (N = 232) |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 60.4 (9.76) | 60.3 (9.32) |
| Median | 61 | 61 |
| Min, max | 18, 85 | 35, 85 |
| Ethnic origin, n (%) | ||
| Caucasian | 265 (94.0) | 218 (94.0) |
| African American | 2 (0.7) | 2 (0.9) |
| Asian | 7 (2.5) | 5 (2.1) |
| Other | 3 (1.1) | 3 (1.3) |
| Prefer not to answer | 5 (1.8) | 4 (1.7) |
| Gender, n (%) | ||
| Male | 235 (83.3) | 191 (82.3) |
| Female | 47 (16.7) | 41 (17.7) |
| Height (inches) | ||
| Mean (SD) | 69.9 (3.27) | 70.0 (3.29) |
| Median | 70 | 70 |
| Min, max | 61, 81 | 61, 81 |
| Weight (pounds) | ||
| Mean (SD) | 189.3 (37.44) | 191.4 (37.59) |
| Median | 185 | 189 |
| Min, max | 100, 285 | 110, 285 |
Table 2.
Characteristics of anatabine supplement use by all survey respondents and individuals in the joint pain relief cohort.
| Parameter | All survey respondents (N = 282) n (%) | Joint pain relief cohort (N = 232) n (%) |
|---|---|---|
| (A) | ||
| How long have you used the anatabine supplement? | ||
| < 2 months | 0 (0) | 0 (0) |
| 2 to 3 months | 1 (0.4) | 1 (0.4) |
| 3 to 4 months | 2 (0.7) | 2 (8.6) |
| 4 to 6 months | 17 (6.0) | 12 (5.2) |
| > 6 months | 262 (92.9) | 217 (93.5) |
| Why did you initially buy the anatabine supplement? | ||
| Minimize inflammation due to athletics/exercise | 29 (10.3) | 25 (10.8) |
| Maintain/improve overall health | 75 (26.6) | 60 (25.9) |
| For an inflammatory medical condition | 59 (20.9) | 51 (22.0) |
| Concerned about chronic inflammation in general | 102 (36.2) | 84 (36.2) |
| Other | 17 (6.0) | 12 (5.2) |
| Do you use the anatabine supplement primarily for help with joint pain or stiffness? | ||
| Yes, and it has helped | 141 (50.0) | |
| No, but it has helped | 91 (32.3) | |
| Yes, but it has not helped | 11 (3.9) | |
| No, and it has not helped | 39 (13.8) | |
| (B) | ||
| Amount (mg) of the anatabine supplement used each day? | ||
| Mean (SD) | 6.4 (2.18) | 6.5 (2.29) |
| Median | 6 | 6 |
| Min, max | 2, 20 | 2, 20 |
| Anatabine (mg) per kg of body weight used each day | ||
| Mean (SD) | 0.076 (0.028) | 0.076 (0.028) |
| Median | 0.071 | 0.071 |
| Min, max | 0.024, 0.22 | 0.024, 0.22 |
Of the 282 survey respondents, 141 (50%) reported that they were using the anatabine supplement primarily for joint pain or stiffness, and that it was providing beneficial effects. An additional 91 (32%) subjects indicated that the supplement helped with their joint pain or stiffness although that was not the primary reason they were using the product. In all, 232 of the 282 (82%) survey respondents reported that anatabine was helping ease their joint pain or stiffness. In contrast, only 11 (4%) respondents indicated that they were not feeling relief from joint pain or stiffness although that was the primary reason they were using the supplement.
Results for additional questions answered by the joint pain relief cohort
Locations and medical diagnoses of affected joints
The individuals who comprised the joint pain relief cohort were asked to select the joint(s) that had benefitted from anatabine supplementation. These subjects were also asked to specify the illness or medical condition that was the cause of the pain or stiffness for each affected joint they selected. Individuals who reported multiple affected joints could select the same medical condition for all affected joints, or a different condition for each joint.
The 232 individuals in this cohort reported a total of 472 affected joints that responded favorably to anatabine supplementation, suggesting that many individuals experienced benefits at multiple joint locations. The joints selected most often were: knee (107 individuals; or 46% of individuals in the cohort); wrists, hands, and fingers (93; 40%); shoulder (79; 34%); and back (76; 33%). When taken as a percentage of the total number of cases of positively affected joints, the knee consisted of 23% of the total responses (107 of 472), the wrists/hands/fingers consisted of 20% of the responses (93 of 472), the shoulder 17% of total responses (79 of 472), and the back 16% of total responses (76 of 472).
The most frequently reported causes for joint pain were osteoarthritis (99 times) and injury to the joint (105 times) (Table 3). In many cases, individuals did not know the medical reason for their joint pain, as evidenced by 143 responses of ‘do not know.’ Osteoarthritis was chosen as the medical diagnosis by over 20% of the individuals indicating benefits for each of the following joints: wrists, hands, fingers (35.5%); hip (27.1%); and knee (20.6%).
Table 3.
Medical reason for joint pain by joint location for individuals in the joint pain relief cohort.
| Medical reason for joint pain | Total number of times selected (%)b | Joints helped by anatabinea | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Shoulder (N = 79) n (%) | Elbow (N = 18) n (%) | Wrist, hands, fingers (N = 93) n (%) | Hip (N = 48) n (%) | Knee (N = 107) n (%) | Ankle (N = 20) n (%) | Foot, toes (N = 31) n (%) | Back (N = 76) n (%) | ||
| Injury to the joint | 105 (22.2) | 31 (39.2) | 2 (11.1) | 11 (11.8) | 5 (10.4) | 33 (30.8) | 6 (30.0) | 5 (16.1) | 12 (15.8) |
| Osteoarthritis | 99 (21.0) | 10 (12.7) | 1 (5.6) | 33 (35.5) | 13 (27.1) | 22 (20.6) | 3 (15.0) | 6 (19.4) | 11 (14.5) |
| Tendinitis | 30 (6.4) | 5 (6.3) | 7 (38.9) | 7 (7.5) | 0 | 4 (3.7) | 3 (15.0) | 3 (9.7) | 1 (1.3) |
| Rheumatoid arthritis | 18 (3.8) | 1 (1.3) | 0 | 7 (7.5) | 1 (2.1) | 5 (4.7) | 0 | 2 (6.5) | 2 (2.6) |
| Bursitis | 8 (1.7) | 4 (5.1) | 1 (5.6) | 0 | 2 (4.2) | 1 (0.9) | 0 | 0 | 0 |
| Fibromyalgia | 8 (1.7) | 1 (1.3) | 1 (5.6) | 1 (1.1) | 2 (4.2) | 1 (0.9) | 0 | 0 | 2 (2.6) |
| Neuropathy | 6 (1.3) | 0 | 1 (5.6) | 0 | 1 (2.1) | 1 (0.9) | 1 (5.0) | 1 (3.2) | 1 (1.3) |
| Gout | 3 (0.6) | 0 | 0 | 0 | 0 | 0 | 1 (5.0) | 2 (6.5) | 0 |
| Lupus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Do not know | 143 (30.3) | 22 (27.9) | 4 (22.2) | 24 (25.8) | 22 (45.8) | 32 (29.9) | 4 (20.0) | 6 (19.4) | 29 (38.2) |
| Other | 52 (11.0) | 5 (6.3) | 1 (5.6) | 10 (10.8) | 2 (4.2) | 8 (7.5) | 2 (10.0) | 6 (19.4) | 18 (23.7) |
Notes:
Each individual could pick more than one joint location, thus these numbers do not sum to N = 232, but instead sum to 472;
472 instances of joint pain relief were reported by the 232 individuals who comprised the joint pain relief cohort, thus the percentages of total responses were calculated using a denominator of 472.
Length of time suffering from joint pain conditions
Individuals in the joint pain relief cohort were asked how long they had suffered from each of the joint condition(s) that they reported (total of 472 joint pain reports). There was quite a bit of variability in the length of time these individuals reported suffering from their specific joint pain conditions; however, the average amount of time reported across all joint locations was about 10 years, and the median amount of time was 7 years, indicating that many of these individuals had been experiencing musculoskeletal ailments for a very long period of time (Table 4). Of note, one individual in this cohort reported relief from a back pain condition (spondylolisthesis) that had been an issue for 68 years.
Table 4.
Length of time (years) suffering from joint pain by joint location for individuals in the joint pain relief cohort.
| Overallb | Joints helped by anatabinea | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Shoulder (N = 79) | Elbow (N = 18) | Wrist, hands, fingers (N = 93) | Hip (N = 48) | Knee (N = 107) | Ankle (N = 20) | Foot, toes (N = 31) | Back (N = 76) | ||
| Mean (SD) | 10 (10.06) | 8 (8.30) | 8 (8.28) | 8 (7.15) | 9 (7.68) | 11 (10.81) | 10 (9.71) | 10 (10.83) | 16 (13.47) |
| Median | 7 | 5 | 6 | 6 | 7 | 6 | 9 | 6 | 11 |
| Min, max | 0, 68 | 0.5, 40 | 0.3, 30 | 0.2, 40 | 0.2, 30 | 0.3, 50 | 0.5, 28 | 1, 50 | 0, 68 |
Notes:
Each individual could pick more than one joint location, thus these numbers do not sum to N = 232. The total number of joint location responses was 472;
472 instances of joint pain relief were reported by the 232 individuals who comprised the joint pain relief cohort, thus the descriptive statistics for “Overall” were calculated from all 472 reports.
Almost three-quarters (74%; 347 of 472) of joint conditions were reported to have started within the past 10 years, approximately 16% (79 of 472) started between 11 and 20 years ago, 9% (44 of 472) started between 21 and 40 years ago, and 1% (6 of 472) started over 40 years ago.
Length of time to experience joint pain benefits
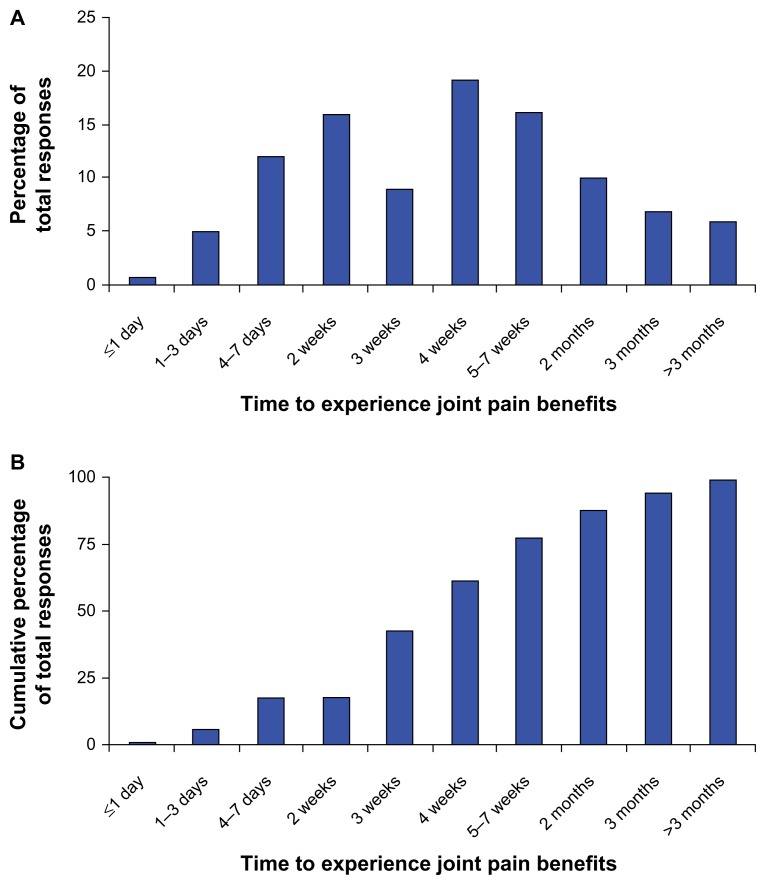
Individuals in the joint pain relief cohort were asked how long it took to experience results from the anatabine supplement. The length of time to experience joint benefits ranged from within one day to more than three months, indicating that there was quite a bit of variability in responsiveness to anatabine supplementation for joint pain and stiffness (Fig. 1A). Overall, the most frequently cited length of time to experience results was about four weeks, which was chosen 90 times out of the total 472 responses (a total of 19% of the responses) for affected joints. When the length of time to respond was viewed cumulatively, the supplement provided positive benefits in four weeks or less for 289 of the 472 (61%) joint conditions reported, and in two months or less for 412 of the 472 (87%) joint conditions (Fig. 1B).
Figure 1.
Length of time to experience joint pain benefits for all 472 reported cases (A) and cumulative response rate for all joint pain cases over time (B).
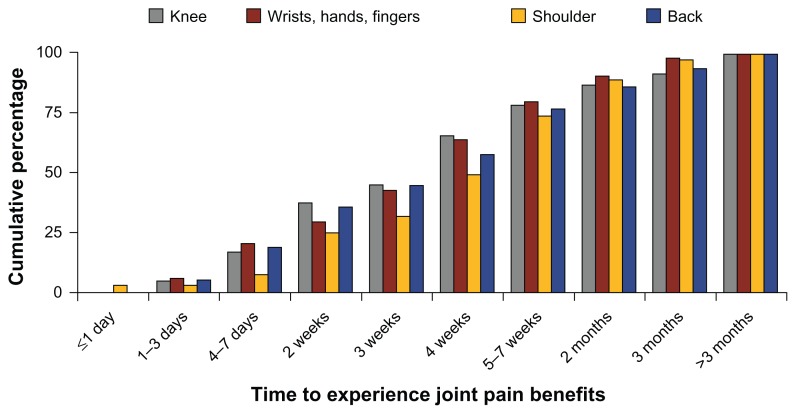
When viewed by specific joint locations, there was substantial interindividual variability in the length of time it took to notice benefits. For example, of the 107 individuals reporting benefits for the knee, 21% said it took about two weeks to notice effects, and another 21% said it took four weeks. This variation in time for individuals to see an effect was apparent for all of the joint locations. In other words, no particular joint location appeared to respond to anatabine supplementation more quickly than any other. Cumulatively, 65% (70 of 107) of individuals reporting benefits for the knee responded favorably in four weeks or less, and 87% (93 of 107) reported benefits by two months (Fig. 2). For the wrists/hands/fingers, 63% (59 of 93) of individuals reported a beneficial effect in four weeks or less, and that figure reached 90% (84 of 93) within two months of starting the anatabine supplement. There was no statistical relationship between the amount of anatabine (in mg) per kilogram of body weight used each day, and the length of time that individuals reported using the supplement before observing beneficial effects on joint pain (data not shown).
Figure 2.
Cumulative response rate for specific joint pain locations over time.
Return of joint pain symptoms upon supplement discontinuation
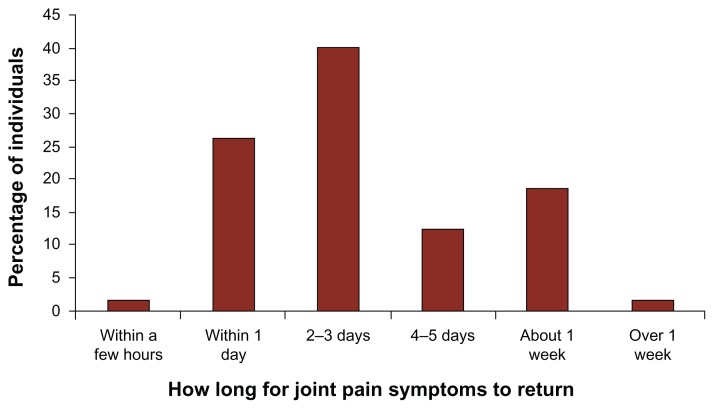
Individuals in the joint pain relief cohort were asked if their joint pain symptoms returned if they forgot to take, or intentionally stopped taking the anatabine supplement. Of the 78 respondents who stopped taking the supplement for some period of time for any reason, 83% experienced a noticeable return of their joint pain symptoms. Forty-four of 65 (68%) respondents indicated that their symptoms returned within 2–3 days or less, and 64 of 65 (98%) indicated that their symptoms returned within one week or less (Fig. 3). Almost all of the respondents (64 of 65, or 98%) who had stopped using anatabine and felt their joint pain symptoms return subsequently felt those symptoms decrease once they resumed using the supplement.
Figure 3.
Length of time for joint pain symptoms to return for individuals in the joint pain relief cohort who stopped anatabine supplementation for any reason.
Ratings of supplement efficacy
Individuals in the joint pain relief cohort were asked to rate their joint pain and/or stiffness before and after they began using anatabine for each affected joint (472 total responses) on a scale between 1 and 10, with 1 being no pain/stiffness at all, and 10 being the worst pain/stiffness imaginable. The results showed that 69% (325 of 472) of the joint pain cases were rated as a 5 or above before using anatabine, and 98% (464 of 472) of the cases were rated a 4 or below since using anatabine. Importantly, 147 (31%) and 205 (43%) cases were rated as a 1 or 2, respectively, after anatabine supplementation began, suggesting that for almost three-quarters (74%) of the total cases, anatabine supplementation greatly reduced pain and stiffness.
Change scores for the mean ratings of pain and stiffness before and after initiation of supplementation were calculated to determine if they were statistically different. The mean (SD) pre and post scores were 5.4 (1.74) and 2.0 (0.98), respectively, indicating a statistically significant (P < 0.0001) reduction in ratings of pain and stiffness following the initiation of anatabine supplementation.
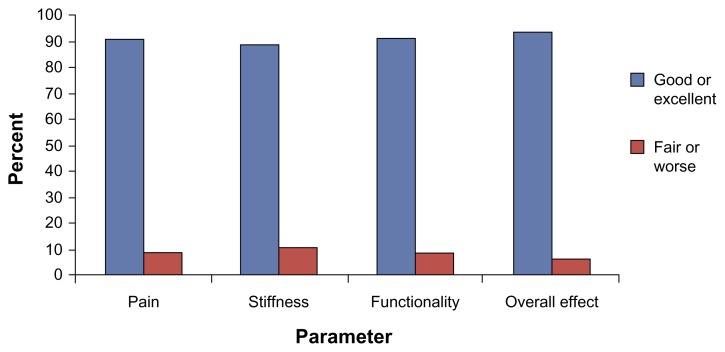
Individuals in the joint pain relief cohort were also asked to rate the effectiveness of the anatabine supplement for joint pain, stiffness, functionality, and overall impression on a scale anchored on one end by ‘Made Worse’ and the other end by ‘Excellent’. Over half of all individuals in the cohort rated the effects of anatabine as ‘Good’ for each component, and over one-third rated the effects as ‘Excellent’. The total numbers of individuals who rated the effects of anatabine as ‘Good’ or ‘Excellent’ for each of the components were: Pain—211 (91%); Stiffness—207 (89%); Functionality—212 (91%); and, Overall—218 (94%) (Fig. 4).
Figure 4.
Ratings of the effects of anatabine on joint pain, stiffness, functionality, and overall.
Use of other supplements and medications for joint pain
Of the 232 individuals in the joint pain relief cohort, 210 (90%) reported that they currently were not regularly taking any medications (over the counter or prescription) for joint pain or stiffness, and 157 (68%) said they did not take any other dietary supplements in addition to anatabine each day to help with joint pain or stiffness. Interestingly, 60% (138 of 232) of individuals reported that they had cut back on the use of pain relievers or other medications for joint pain or stiffness while using anatabine, and of those 138 individuals, 134 (97%) had cut back their use of these types of medications by at least half. Further, 91 (66%) of those individuals reported completely stopping their use of any medications for joint pain while using anatabine.
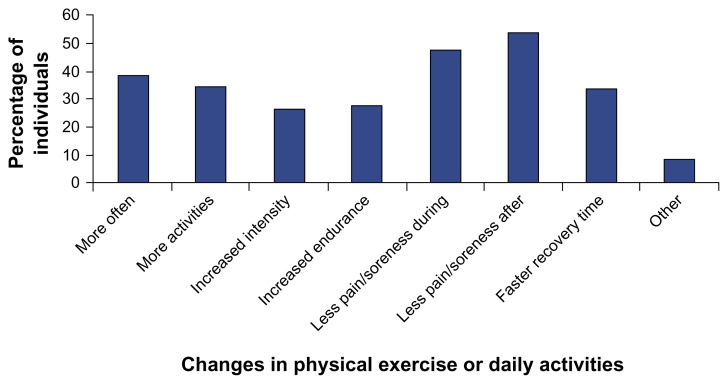
Changes in physical exercise or daily activities
Individuals in the joint pain relief cohort were asked if they had noticed any changes in their physical exercise or daily activities (they were allowed to choose more than one answer). Many of these individuals indicated that they felt less pain or soreness during (47% of the respondents) and after (53% of the respondents) exercise or daily activities (Fig. 5). Other frequently chosen answers were: able to engage in exercise or daily activities more often (38%); able to engage in a wider range of exercise or daily activities (35%); and, faster recovery time after exercise or daily activities (34%). Many respondents chose multiple answers in response to this question, suggesting anatabine supplementation significantly aided their ability to engage in physical activities.
Figure 5.
Changes in physical exercise or daily activities reported by individuals in the joint pain relief cohort.
Discussion
Collectively, the results from this online survey study of users of a dietary supplement containing the Solanaceae alkaloid anatabine offer evidence that this supplement provides beneficial effects for several common and often debilitating musculoskeletal ailments. Survey respondents consisted primarily of individuals who were long-term users of the supplement, but were not recruited based upon any prior knowledge of musculoskeletal effects or satisfaction with the supplement’s effects. Over 80% of the survey respondents reported joint pain benefits, suggesting that a large percentage of long-term users of this dietary supplement are using the supplement due to—and are deriving benefits for— musculoskeletal complaints.
The survey respondents were mostly male and around 60 years of age, which places them in a demographic that would be expected to experience numerous musculoskeletal aches and pains.1 Many of these individuals reported that they engage in a number of recreational and athletic activities that have either been hindered significantly by, or have exacerbated, ongoing musculoskeletal condition(s). The most commonly affected joints were the knee, the wrists/hands/fingers, the shoulder, and the back, and osteoarthritis and injury to the joint were the most commonly cited medical causes of joint pain. In many cases the actual reason for the painful condition was not known. Interestingly, rheumatoid arthritis and tendinitis made up a relatively minor fraction of the known reasons for joint pain, although these are fairly common causes of joint pain in an older and physically active population.11,12
Many of the musculoskeletal conditions affecting individuals in the joint pain relief cohort were long-term issues, and often required treatment with NSAIDs or prescription medications in addition to the use of other dietary supplements. Half (50%) of the reported joint pain conditions were ongoing for at least seven years, and almost 10% of the conditions were reported as ongoing for 20–40 years. Most of these individuals reported cutting back on the use of medications and pain relievers for joint pain and stiffness, and about two-thirds reported completely stopping their use of joint pain medications since they began using the anatabine supplement. These results indicate that anatabine can affect chronic musculoskeletal complaints that have persisted for many years, and that may be unresponsive to the most commonly used interventions.
Anatabine is a unique dietary supplement that has been marketed in the US for anti-inflammatory support since 2011. Although humans are exposed to anatabine on a daily basis through consumption of common foods from the Solanaceae plant family such as peppers, potatoes, and tomatoes, the greatest exposure to anatabine and related alkaloids comes from the use of tobacco products.13–15 Several epidemiological studies have found that cigarette smoking is inversely related to the incidence of several autoimmune diseases characterized by excessive inflammation, including Hashimoto’s thyroiditis, type 1 diabetes mellitus, Parkinson’s disease, and ulcerative colitis.16–20 Interestingly, two large, longitudinal epidemiological studies found that smokers also have lower rates of osteoarthritis than non-smokers, 21,22 and the protective effects of smoking on osteoarthritis are recognized on the CDC’s website. 23 However, two recent meta-analyses have concluded that the data supporting a protective effect of smoking on the development and progression of osteoarthritis are not compelling, and may actually be false, although controlled studies are needed.24,25
It has been hypothesized that nicotine may be the active component in tobacco responsible for these protective, anti-inflammatory effects, and data from a recent case-control study of 490 people showed that eating greater amounts of Solanaceae vegetables such as peppers, tomatoes, tomato juice, and potatoes was associated with a reduced risk of developing Parkinson’s disease.26 This relationship was not evident for consumption of all other vegetables combined, suggesting a specific protective effect from constituents found only in Solanaceae vegetables such as nicotine or related alkaloids. Although several preclinical and clinical studies investigating nicotine as a treatment for autoimmune and neurodegenerative diseases have shown promising results,27–30 the toxicity and abuse potential of nicotine limit its therapeutic utility.31,32
Anatabine has a chemical structure somewhat similar to nicotine, but lacks the 3-pyridylmethylamine moiety common to most nicotinic alkaloids that is believed to be responsible for their toxicity.33 Preclinical studies and initial studies in humans (unpublished data) suggest that anatabine has a better safety profile relative to nicotine, and has a unique mechanism of action relative to popular dietary supplements used to treat joint pain such as omega-3 fatty acids, glucosamine, and chondroitin sulfate. Fish oils and omega-3 fatty acids are believed to act by changing the physical and chemical nature of the cell membrane and membrane protein-mediated responses to inflammation.34 Glucosamine and chondroitin sulfate are raw materials for healthy cartilage and are aimed at improving joint mechanics through cartilage repair rather than by directly affecting inflammation, although their benefits for joint conditions such as osteoarthritis are largely unproven.35 Anatabine has been shown in preclinical studies to interfere with the intracellular activation of pro-inflammatory transcription factors NF-κB and STAT3, and to reduce the downstream production of multiple pro-inflammatory cytokines and other pro-inflammatory mediators.7–10 These effects may be mediated by activation of specific cholinergic receptor subtypes, such as alpha-7, that are known to potentiate anti-inflammatory effects, and are the target of new therapeutics in development for a wide range of diseases.36,37 However, the precise mechanism of action for anatabine has yet to be elucidated.
There are several limitations to this study. First, this is a questionnaire-based survey study and not a controlled trial, and thus does not control for concomitant pain medication and other dietary supplements that individuals may have taken for reasons other than joint pain. Second, the study relies on self-report information from a sample of individuals chosen based upon their repeated supplement purchases, and thus the reliability of the responses cannot be verified, and the results may not be generalizable. This study reports the experience of regular, long-term anatabine users with joint pain, not the larger set of all users. However, the anonymity afforded by this type of research methodology eliminates the potential for investigator bias that could affect responses, and should allow for truthful reporting. Finally, the study design provides no objective measure of supplement efficacy, although the survey results do provide useful information that could be used to plan a controlled trial to assess the efficacy of anatabine supplementation for joint pain conditions.
In conclusion, the results of this online survey study provide evidence that anatabine supplementation can lead to substantial improvement of musculoskeletal aches, pains, and stiffness, and can provide benefits for a wide range of medical conditions at multiple joint locations. Data from preclinical studies showing substantial anti-inflammatory effects of anatabine in vivo and in vitro, and data from epidemiological studies showing inverse relationships between both tobacco use and the consumption of Solanaceae vegetables with the incidence of disorders characterized by excessive inflammation, suggest that anatabine may be the dietary ingredient responsible for these effects. The results of the current study show that anatabine was very helpful for musculoskeletal issues for a large proportion of those who reported using the product for several months, and suggest that a randomized, controlled clinical evaluation of the safety and effects of anatabine in subjects with joint pain conditions may be warranted.
Footnotes
Author Contributions
Conceived and designed the experiments: RL, KG, AC, MV. Analyzed the data: KG. Wrote the first draft of the manuscript: RL. Contributed to the writing of the manuscript: RL, KG, AC, MV. Agree with manuscript results and conclusions: RL, KG, AC, MV. Jointly developed the structure and arguments for the paper: RL, KG, AC, MV. Made critical revisions and approved final version: RL, KG, AC, MV. All authors reviewed and approved of the final manuscript.
Competing Interests
All authors are employees of Rock Creek Pharmaceuticals, Inc.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
This study was funded by Rock Creek Pharmaceuticals, Inc., Gloucester, MA. The study authors are all employees of Rock Creek.
References
- 1.United States Centers for Disease Control, National Center for Health Statistics. Summary Health Statistics for U.S. Adults: National Health Interview Survey. 2 2009. (Vital and Health Statistics Series 10). [Google Scholar]
- 2.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 3.Walker JS, Sheather-Reid RB, Carmody JJ, Vial JH, Day RO. Nonsteroidal antiinflammatory drugs in rheumatoid arthritis and osteoarthritis: support for the concept of “responders” and “nonresponders”. Arthritis Rheum. 1997;40(11):1944–54. doi: 10.1002/art.1780401105. [DOI] [PubMed] [Google Scholar]
- 4.van der Goes MC, Jacobs JW, Boers M, et al. Monitoring adverse events of low-dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann Rheum Dis. 2010;69:1913–9. doi: 10.1136/ard.2009.124958. [DOI] [PubMed] [Google Scholar]
- 5.Whittle SL, Colebatch AN, Buchbinder R, et al. Multinational evidence-based recommendations for pain management by pharmacotherapy in inflammatory arthritis: integrating systematic literature research and expert opinion of a broad panel of rheumatologists in the 3e Initiative. Rheumatology (Oxford) 2012;51(8):1416–25. doi: 10.1093/rheumatology/kes032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akhtar Nahid, Tariq M. Current nutraceuticals in the management of osteoarthritis: a review. Ther Adv Musculoskel Dis. 2012;4(3):181–207. doi: 10.1177/1759720X11436238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paris D, Beaulieu-Abdelahad D, Bachmeier C, et al. Anatabine lowers Alzheimer’s Abeta production in vitro and in vivo. Eur J Pharmacol. 2011;670(2–3):384–91. doi: 10.1016/j.ejphar.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Paris D, Beaulieu-Abdelahad D, Mullan M, et al. Amelioration of experimental autoimmune encephalomyelitis by anatabine. PLoS One. 2013;8(1):e55392. doi: 10.1371/journal.pone.0055392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paris D, Beaulieu-Abdelahad D, Abdullah L, et al. Anti-inflammatory activity of anatabine via inhibition of STAT3 phosphorylation. Eur J Pharmacol. 2013;698(1–3):145–53. doi: 10.1016/j.ejphar.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Caturegli P, De Remigis A, Ferlito M, et al. Anatabine ameliorates experimental autoimmune thyroiditis. Endocrinology. 2012;153(9):4580–7. doi: 10.1210/en.2012-1452. [DOI] [PubMed] [Google Scholar]
- 11.Rasch EK, Hirsch R, Paulose-Ram R, Hochberg MC. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum. 2003;48(4):917–26. doi: 10.1002/art.10897. [DOI] [PubMed] [Google Scholar]
- 12.Walker-Bone KE, Palmer KT, Reading I, Cooper C. Soft-tissue rheumatic disorders of the neck and upper limb: prevalence and risk factors. Semin Arthritis Rheum. 2003;33(3):185–203. doi: 10.1016/s0049-0172(03)00128-8. [DOI] [PubMed] [Google Scholar]
- 13.Andersson C, Wennström P, Gry J. Nicotine alkaloids in Solanaceous food plants. Copenhagen: TemaNord. 2003;531:1–37. [Google Scholar]
- 14.Jacob P, III, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am J Public Health. 1999;89(5):731–6. doi: 10.2105/ajph.89.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob P, III, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1668–73. [PubMed] [Google Scholar]
- 16.Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2004;89:6077–86. doi: 10.1210/jc.2004-0431. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen IB, Laurberg P, Knudsen N, et al. Smoking is negatively associated with the presence of thyroglobulin autoantibody and to a lesser degree with thyroid peroxidase autoantibody in serum: a population study. Eur J Endocrinol. 2008;158(3):367–73. doi: 10.1530/EJE-07-0595. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi LM, Khalili H, Chan AT, Richter JM, Bousvaros A, Fuchs CS. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol. 2012;107:1399–406. doi: 10.1038/ajg.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasouli B, Grill V, Midthjell K, Ahlbom A, Andersson T, Carlsson S. Smoking is associated with reduced risk of autoimmune diabetes in adults contrasting with increased risk in overweight men with type 2 diabetes: a 22-year follow-up of the HUNT study. Diabetes Care. 2013;36:604–10. doi: 10.2337/dc12-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Huang X, Guo X, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74(11):878–84. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40(4):728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 22.Szoeke CE, Cicuttini FM, Guthrie JR, Clark MS, Dennerstein L. Factors affecting the prevalence of osteoarthritis in healthy middle-aged women: Data from the longitudinal Melbourne Women’s Midlife Health Project. Bone. 2006;39(5):1149–55. doi: 10.1016/j.bone.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 23.United States Centers for Disease Control, Division of Adult and Community Health, National Center for Chronic Disease Prevention and Health Promotion. [Accessed Aug 1, 2013]. http://www.cdc.gov/arthritis/basics/osteoarthritis.htm.
- 24.Hui M, Doherty M, Zhang W. Does smoking protect against osteoarthritis? Meta-analysis of observational studies. Ann Rheum Dis. 2011;70(7):1231–7. doi: 10.1136/ard.2010.142323. [DOI] [PubMed] [Google Scholar]
- 25.Pearce F, Hui M, Ding C, Doherty M, Zhang W. Does smoking reduce the progression of osteoarthritis? Meta-analysis of observational studies. Arthritis Care Res (Hoboken) 2013 Jul;65(7):1026–33. doi: 10.1002/acr.21954. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen SS, Franklin GM, Longstreth WT, Swanson PD, Checkoway H. Nicotine from edible Solanaceae and risk of Parkinson disease. Ann Neurol. 2013 doi: 10.1002/ana.23884. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012 Jul;27(8):947–57. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green JT, Thomas GA, Rhodes J, et al. Nicotine enemas for active ulcerative colitis—a pilot study. Aliment Pharmacol Ther. 1997;11:859–63. doi: 10.1046/j.1365-2036.1997.00220.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Zhao B. Nicotine attenuates beta-amyloid peptide-induced neurotoxicity, free radical and calcium accumulation in hippocampal neuronal cultures. Br J Pharmacol. 2004;141(4):746–54. doi: 10.1038/sj.bjp.0705653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newhouse P, Kellar K, Aisen P, et al. Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology. 2012;78(2):91–101. doi: 10.1212/WNL.0b013e31823efcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schep LJ, Slaughter RJ, Beasley DM. Nicotinic plant poisoning. Clin Toxicol (Phila) 2009;47:771–81. doi: 10.1080/15563650903252186. [DOI] [PubMed] [Google Scholar]
- 32.Benowitz NL. Nicotine addiction. New Engl J Med. 2010;362:2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto I, Tomizawa M, Saito T, Miyamoto T, Walcott EC, Sumikawa K. Structural factors contributing to insecticidal and selective actions of neonicotinoids. Arch Insect Biochem Physiol. 1998;37:24–32. doi: 10.1002/(SICI)1520-6327(1998)37:1<24::AID-ARCH4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 34.Calder PC, Yaqoob P. Understanding omega-3 polyunsaturated fatty acids. Postgrad Med. 2009;121(6):148–57. doi: 10.3810/pgm.2009.11.2083. [DOI] [PubMed] [Google Scholar]
- 35.Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808. doi: 10.1056/NEJMoa052771. [DOI] [PubMed] [Google Scholar]
- 36.Bencherif M, Lippiello PM, Lucas R, Marrero MB. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68(6):931–49. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265(6):663–79. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]