Abstract
Meat-eating was a game changer for human evolution. We suggest that the limiting factors for expanding brains earlier were scarcities of nicotinamide and tryptophan. In humans and some other omnivores, lack of meat causes these deficiencies. Nicotinamide adenine dinucleotide (NADH) is necessary to synthesize adenosine triphosphate (ATP) via either glycolysis or via the mitochondrial respiratory chain. NAD consumption is also necessary for developmental and repair circuits. Inadequate supplies result in “de-evolutionary” brain atrophy, as seen with pellagra. If trophic nicotinamide/tryptophan was a “prime mover” in building bigger brains, back-up mechanisms should have evolved. One strategy may be to recruit extra gut symbionts that produce NADH precursors or export nicotinamide (though this may cause diarrhea). We propose a novel supplier TB that co-evolved early, which did not originally and does not now inevitably cause disease. TB has highly paradoxical immunology for a pathogen, and secretes and is inhibited by nicotinamide and its analogue, isoniazid. Sharp declines in TB and diarrhea correlated with increased meat intake in the past, suggesting that dietary vitamin B3 and tryptophan deficiencies (also associated with poor cognition and decreased lifespans) are still common where meat is unaffordable.
Keywords: dementia, diarrhea, cancer, hypervitaminosis B3, pellagra, Parkinson’s, serotonin
Introduction
Among primates as a whole, meat-eating is limited, being most common only among chimpanzees and baboons.1,2 By contrast, omnivory and considerable meat-eating, or at least a meat-hunger and a meat-culture (characterized by many cattle-related phrases in all languages and frequent depictions of hunting in paleolithic art), is universal among modern humans, and has a deep history within our lineage. Meat intake was high among the Neanderthals (Homo neanderthalensis) and other archaic humans (H. heidelbergensis and allies),3 and was important for anatomically and behaviorally modern humans (H. sapiens).4,5 Sustaining an optimal supply must have been a recurrent problem and, in cases of poverty, continues to be—although there is, equally, a risk of “too much of a good thing,” which is evident now in cases of economic affluence.
Brains are expensive to run, as there is a constant need to balance energy budgets that are tight given the high nicotinamide adenine dinucleotide (NADH) costs incurred during the processes of mitochondrial oxidative phosphorylation that are necessary to propagate action potentials and process information. Recently evolved circuits, whether in the neocortex or striatum, are on an “edge,” as they have exceptionally complex wiring with synaptic connections and long axons that “die back” if the energy/NAD supply fails. These connections may also “commit suicide” if they are not used, given that there are many neuronal “mouths to feed” that, in effect, compete throughout life over the flow of NAD.6,7 Notably, these neuronal connections include prefrontal top–down executive and inhibitory social pathways, attention/working memory circuits in the hippocampus and pulvinar (which are affected in the dementias),8 and bottom–up “Go” pathways in the striatum (such as those affected in Parkinson’s disease).9,10 These diseases may reflect the concept of “dissolution” of recently evolved circuits, first suggested by the neurologist Hughlings- Jackson (1835–1911).11
Meat (especially when cooked) is a high-energy food but, as a source of calories, is risky relative to plants.12,13 The chance of a kill is variable, and this activity requires the immediate expenditure of energy and stored capital in learned cooperative hunting and communication skills (acquired during a now prolonged adolescence), attended by substantial risk of injury or death. In fact, this very danger implies that the conversion from prey to a top predator in order to obtain meat was a major agent of selection relative to danger from microbes. The added value from meat, over and above calories, may be in terms of a micronutrient; we propose that nicotinamide, the precursor to NAD—which is largely unavailable from plants—alongside animal proteins with their high tryptophan content had hitherto constrained brain size, internal connectivity, and hence the behavioral flexibility required to cope with stress, to innovate, and to take on challenges. These may in turn have restricted the construction of social and domestic networks, as well as a heritable ecological, energetic, and informatic niche capable of ratcheting-up the NAD supply.14,15
Meat intake across the globe varies at least eightfold, typically averaging 20–40 g or less per day in sub-Saharan Africa and 300 g per day in the US,16 with a recommended dose of 50–100 g per day to satisfy the protein, vitamin B12, iron, and zinc requirements that are known to have beneficial effects on brain and body development.17 Most of the concerns that have been expressed in this respect have focused on the ills of excessive meat consumption. Among these disadvantages are obesity, heart disease, diabetes, and cancers (whose frequencies all correlate positively with meat consumption, although the mechanism for this is not understood).18,19 In contrast, the consequences of too little meat/too much grain consumption are rarely mentioned, even though its variance across the world must now be higher than earlier in our evolution, when meat intake was generally high and shortages were probably short-lived rather than chronic. We shall argue that the evidence suggests that even mild and intermittent shortages of meat have adverse consequences for energy and micronutrient-sensitive tissues, like the brain, that require “food for thought.” Sometimes, out of necessity, building brains on the cheap drives intraspecific variation but by reducing physiological capital impairs the ability to handle a second hit (such as brain trauma, hypoxia or further nutritional deprivation), thereby affecting an individual’s long-term cognition and survival. This is compatible with evidence that meat-eating, and associated nicotinamide and tryptophan content, improves cognition (including literacy and numeracy), social behavior, and motor development (such as speech and bipedalism), and later reduces the incidence of dementia.20–22 We suggest that these pressures led to the acquisition of specialist “hedge” mutualists as back-up sources of nicotinamide (and, therefore, NAD), and that one of these, initially nonantagonistic, symbionts was Mycobacterium tuberculosis.
To make a case for this hypothesis, we first discuss pellagra as an extreme, but archetypal, example of the “de-evolutionary” non-ephemeral transgenerational consequences of meat shortages, and review the biochemical pathways involved; we then make the case for the acquisition of TB as a solution to the problems created by mild meat shortages. Lastly, we will touch upon whether nicotinamide dosage can overshoot as the trade-off for guaranteeing early supplies and, for some, becomes a longer-term toxin.
Pellagra and the Consequences of Meat Shortage and Cereal-Dependence
The epidemic of pellagra in the southeastern USA during the early 1900s was largely triggered by a socioeconomic collapse in the cotton industry. Poverty induced a monophagic diet that relied on maize (corn) and little or no meat.23 Maize is easily grown, with record yields when compared to other cereals. Although cultivated varieties need human input in order to reproduce, they require minimal effort and little technology—hence its popularity and the temptation to overlook its dangers. Maize is naturally low in nicotinamide and tryptophan (which is somewhat alleviated by careful culturally-learned cooking)—as, to a lesser extent, are all other cereals when compared with animal sources of protein. Ingestion of maize protein (zein) even decreases plasma tryptophan levels and brain serotonin, and wheat protein (gluten) does little better, whereas bovine lactalbumin causes significant increases.24,25 So, when combined with little meat, maize was (and still is in Africa) a diet with adverse consequences severe enough to have driven epidemics and emigrations in the past. At the other extreme, most cases of niacin/tryptophan deficiency (which may amount to many millions of the poorest children and adults) remain undiagnosed and untreated, making the form of pellagra that presents without the classic and diagnostic dermatitis (pellagra sine pellagra) potentially the most overlooked, yet treatable, diagnosis in the world—as we shall attempt to demonstrate.26,27
The clinical phenotype of pellagra includes poor cognition, illiteracy, and acalculia from poor brain development, later presenting with progressive dementia—often with Parkinsonism and even motor neuron disease, multiple sclerosis, and prion disease mimics—alongside apathy and little will-power or self-control, and many other neuro-psychiatric and sociopathic features.28 Pellagrins are prone to chronic infections, especially gut infections and colitis causing diarrhea, and, at postmortem, tuberculosis (TB).28 Patients suffer from a range of metabolic disorders and some cancers. The diagnosis relies on a characteristic photosensitive dermatitis (Casal’s necklace): (nicotinamide protects against ultraviolet radiationinduced deoxyribonucleic acid (DNA) damage),29 though this was not always present and may be less noticeable and much less likely to be expressed in those with light-protected pigmented skin (as it happens, those currently most at risk—though not in the American and European epidemics where mostly poor, white folk were affected). Sufferers cope poorly with other stresses, whether instigated by an acute infection, a physical trauma, a chemical toxin, or a social challenge. Addictions were common, and a particular craving for tobacco was observed, perhaps because it not only contains nicotine, but it also contains niacin. An “honor” culture of violence and homicide was present, beginning with cattle thieves.30 Familial cases were frequently observed due to common environments, though heritable epigenetic and other factors may well have played a part in creating a transgenerational downward (de-evolutionary) spiral. Notably, despite the very wide clinical phenotype, pellagrins show no signs of autoimmune or allergic disease, as they are, for instance, hyporesponsive to histamine.28
In the US cotton belt, mortality rates were high and there were clinical and pathological signs of multiorgan degeneration, inflammation, and premature aging. Many, if not most, cases were undiagnosed despite “pellagra sine pellagra” being well recognized (it manifested as poor brain development producing poor scholars)—something that might explain the well-documented low rates of individuals who successfully passed the intellectual tests that were demanded for military entrance (and “corny” sense of humor!) when hailing from badly affected states.23 Chromatolysis and death of the large pyramidal cells in the cortex was characteristic of pellagra; signs of protein mishandling with prominent amyloidosis, and of oxidative stress with lipofuscinosis, were also present. The cause of this was found by Elvehjem in 1937 (following detective work by Goldberger) to be a deficit in vitamin B3 (nicotinamide) and the essential amino-acid, tryptophan, which gets converted by a de novo synthetic pathway to NAD, which is in turn activated to compensate for an inadequate dietary supply of nicotinamide or nicotinamide–riboside that are normally the predominant sources of NAD via the synthetic/salvage pathway.31,32
Famous earlier commentators, such as Lombroso, described pellagrins as atavistic “wrecks of humanity” and evolutionary throwbacks with a systems degeneration that also wrecked their social (and we argue, symbiotic) webs and networks. Although the most spectacular examples of pellagra are from the southeastern US around 1910, and from 18th century Europe (where it was officially described by Casal, though it was long known to and named by the polenta-eating peasantry), there is historical evidence of pellagra-like conditions and “boom–bust” demographics with a poor constitution, especially as far as imported infections were concerned, in earlier maize-worshipping, low meat pre-Columbian cultures in meso-America. The consequences of a scarcity of meat were clearly noted by the first European arrivals (eg, Columbus, who may have developed this condition on his voyages and subsequently diagnosed himself), making niacin deficiency an old issue. Furthermore, this condition may have also been described in ancient Egyptian writings on the “Steles of Famine.”33
The Biochemical Pathway
Nicotinamide (vitamin B3) is essential to the production of NAD, the electron carrier that feeds mitochondria and is oxidized to produce proton-motive forces to form adenosine triphosphate (ATP), which in turn drives household cellular activities, defends against microbes, and facilitates a wealth of metabolic transformations. NAD is often limiting and is the key regulator within NAD/NADH redox ratios. NAD forms NADH which, in turn, acts as the cosubstrate for various redox reactions and dehydrogenases (such as of alcohol and lactate), thereby playing a pivotal role in the regulation of ion channels, and converts to NADPH and oxidant defences and synthetic anabolism.34,35 A good diet and nicotinamide supply would be expected both to improve robustness against acute microbial attack and to optimize brain function. In addition it is increasingly recognized that NAD(H)-based metabolism is the “engine” that not only sustains all cellular activities but can also “steer” and control cellular processes including differentiation. 36 Recently described effects on the survival and programming of stem cells by nicotinamide and tryptophan/ serotonin put them in a position to benefit brain development and maintenance, and enable the initial evolution of big brains as a single unit. Specifically, only a few extra cycles of cortical neurogenesis affect the proliferation of cells migrating to the prefrontal and parietal cortex, with enhanced differentiation of post-mitotic offspring (such as large cortical pyramidal neurons, which are affected in pellagra). These cycles could lead to a bigger neocortex and, after a series of environmentally-sensitive selective culls during development, could produce plastic and adapted cortico-basal ganglia, forming the better connected and neurochemically modulated mosaic brain that ultimately shapes our actions and minds.37–42
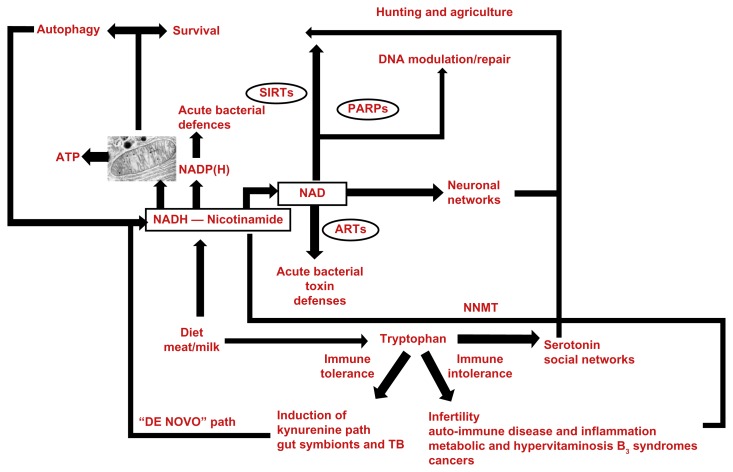
NAD serves as a precursor of adenosine diphosphate (ADP)–ribose-containing messenger molecules, as well as deacetylation messenger molecules that respond to stress and drive many cell and social processes (Fig. 1). NAD consumer hubs involve sirtuins (SIRTs) and poly(ADP-ribose) polymerases (PARPs), that are inhibited by nicotinamide and have natural diet-derived agonists such as resveratrol (used by plants to cope with stress). These integrate a number of external interactions such as foraging, exercise, social interactions, and learning. They are also involved in internal regulatory roles that have pervasive effects on the immune-system, cell fates, and cycles such as differentiation, growth, autophagy, chromatin regulation with epigenetic gene silencing, microtubule organization, somatic mutation rate, and DNA repair.43,44 All of these link with pathophysiological stress mechanisms that are relevant to repairs, regeneration, survival, aging, and disease; there are many examples of these NAD circuits being at the nexus of metabolic homeostasis, and a “NAD or hydrogen world” has been proposed, dating from the geothermal origins of life.45–47 Indeed NAD homeostasis could, in part, replace the five factors that Cannon envisaged as the “Wisdom of the Body,” as it incorporates environmental information (initially through NAD-dependent circadian rhythms) and physiological needs with NAD(H)-powered brains to adapt to stress and prevailing circumstances in such a way as to niche construct and eco-engineer future “NAD worlds.”48
Figure 1.
The de novo pathway for the semi-vitamin nicotinamide is also the immune tolerance pathway, and is closely connected to serotonin synthesis and social signaling. This may be a homeostatic circuit that supports energy and nicotinamide/tryptophan acquisition, and responsive internal solutions such as autophagy and symbionts that supply nicotinamide and NAD(H), or, pseudo-symbionts such as cancer that sink nicotinamide excesses, as do metabolic and inflammatory syndromes, but they lead to longer term toxicity.
Abbreviations: SIRTs, sirtuins; PARPs, poly(ADP ribose polymerase); cADP, cyclic adenosine diphosphate; ARTs, T-cell ADP ribosyl-transferase; NNMT, nicotinamide-N-methyl-transferase.
This metabolic, behavioral, and immunological cross-road centers on the tryptophan to NAD de novo pathway—whose initiating and rate-limiting enzymes, indoleamine 2,3-dioxygenases (IDO-1 and IDO-2) or tryptophan 2,3 dioxygenase (TDO) are induced by cytokines—are usually affected by interferons (predominantly in professional antigen-presenting cells, or directly by infections such as human immunodeficiency virus [HIV]) that, in turn, affect immune function via kynurenines, which influence the population of T-cells (favoring regulatory T-cells over cytotoxic T-cells). Largely acting via excitotoxic and pro-oxidant quinolinic acid (picolinic acid is a neuroprotective chelating agent, but is not on the final path to NAD), the kynurenine pathway is implicated in many metabolic disorders (including insulin-resistance states), (neuro)degenerative disorders, inflammatory disorders, infectious disorders, and cancer.49–52 This tryptophan path links with protein mammalian target of rapamycin (mTOR) paths, which senses tryptophan deprivation and also plays a pivotal part in immunity and aging.53
The nicotinamide- and tryptophan-to-NAD pathway is a “tolerance” link with the immune system, allowing selected symbionts to prosper—we do not think that these links are always simply tolerated because the immune response to eliminate them would be too energetically expensive, or would involve too much collateral damage. It also interfaces with the “social” neurotransmitters (notably serotonin), allowing group activities that benefit the food supply.54 It is likely that this as the de novo pathway for NAD with synthesis from tryptophan (normally a minor contributor to the NAD pool), and all these activities work hand-in- hand over the short-term to improve the vital NAD supply—but at a longer-term cost. NAD deficiency resulting from dietary sources activating this pathway has, in recent times, been underemphasized relative to cytokine induction. Many infections (for example, Toxoplasma gondii, Leishmania donovanii, and Clamydia sp.) activate this pathway and may stimulate NAD production to the host’s benefit at times, but these infections often consume tryptophan, as it is important for their own growth; more importantly, however, they also produce toxic compounds. Therefore, it is hard to see much human benefit in these infections (except perhaps when dietary tryptophan is too high), unless the microbe simultaneously increases the supply of tryptophan or nicotinamide—as we argue happens with TB.55
Nicotinamide Switches—Recent
A switch away from the de novo pathway, which occurs when there is adequate nicotinamide via diet, could lead to less chronic symbiotic infection and degeneration, with increased longevity. However, if the nicotinamide dose rises too high, immune intolerance may develop. For example, it may cause infertility(or at least to lower numbers); it may cause allergies including to our own tissues, leading to autoimmune diseases; and it may extend to commensals, causing inflammatory disorders), perhaps in part driven by molecular mimicry.56 These conditions are now common, with many novel versions still being described, but they first emerged amongst the wealthy as “sneezes and wheezes” in the early 19th century, coinciding with reductions in many infections—sanatoria changed quickly from focusing on TB cases to offering fashionable cures for hayfever, asthma, and allergies.57,58 Nowadays, these immune intolerance syndromes are often treated with immunosuppression, but that comes with many long-term side-effects; more subtle and safer manipulations might include less dietary nicotinamide but more tryptophan (as its substrate it induces IDO).59 Molecular mimicry may be driven by antigenic overlap with “old friends” or “old foes,” and an “epidemic of absence” but on this hypothesis is driven proximally from a diet with more nicotinamide rather than better hygiene.60 Cancers from nicotinamide (vide infra) and tryptophan/serotonin toxicity (perhaps even autism, anxiety, or hypochondriasis and narcissism) may follow, as all these conditions increase in incidence with increasing affluence.61 Global maps show a mosaic pattern of disease biogeography (and corresponding changes over time) with migrants rapidly acquiring the local incidence of various diseases. These maps show striking environmental mirror images. In the north–south gradients for TB and childhood diarrhea the rates are high in the southern hemisphere (excluding wealthier Australasia) but, in contrast, autoimmune diseases, including multiple sclerosis, asthma, Crohn’s disease, diabetes and allergies, are high in the wealthier north and in the west.62 A powerful and quick-acting environmental factor (with minor input from genetic variation) has to be responsible and could be the same factor with biphasic toxicity at low and high doses and an optimal middle range.
Nicotinamide Switches—Ancient
Our argument is that if the original switch toward high dietary nicotinamide levels was a “prime mover” in our evolution because it supplied high-quality energy, hormone-like signaling properties, and the metabolic wherewithal to support big brains, then, given the inherent difficulties in guaranteeing a source of meat, strategies to cope with short-falls should have evolved.63 Egalitarian meat sharing, at least within the group (“parochial altruism”), as well as innovative hunting and butchery technology—which were all important characteristics of early man—would have helped.64 Nicotinamide occurs in milk, chiefly as a riboside (which may be particularly important for neuronal function, and may have influenced the general success of mammals), but dairy products are late “inventions,” and the capacity to ingest unprocessed dairy products in adult life is limited to those few cultures that developed husbandry, where it evolved several times independently, emphasizing its late stabilizing advantages and strong selection pressures (the adult lactose-intolerance problem with diarrhea among non-Caucasians). This concept is also supported by cultural practices, such as partly predigested milk products (like cheeses and yoghurts), and even by the co-evolution of gut symbionts that digest lactose (a demonstration of evolution finding multiple ways and means when the need is great).65,66 Autocarnivory, a feature of starvation and many degenerative diseases, is another option that can be mediated by mitochondrial and oxidant stress, or by excitotoxins on the kynurenine pathway,67 but it can only be a solution that is used in emergencies; something less threatening to survival would clearly be highly advantageous. We suggest that these were symbionts working with the de novo pathway that were able to switch to overproduce niacin for export, rather than increased use of our own pathway, thereby consuming tryptophan and constraining brain evolution by limiting protein synthesis and, more crucially, serotonin, which is developmentally and socially important as it affects personality, mood and group responses to new circumstances.68,69
Symbioses
Symbioses are widespread in the biological world. Well known examples include early unicellular players co-operating over energy-yielding reactions, followed by the endosymbiotic acquisition of mitochondria and nicotinamide ring-based energy packs (thereby allowing better delivery of electrons from hydrogen as NADH to the now available electron-acceptor oxygen, opening the doors for multicellular complexity and raised neural performance), not to mention the many gut exosymbionts found among ruminants and other mammals. Historically more recent examples include domesticates for food or older genetically modifiable microbial engines used to digest celluloses and other fibers to fatty acid metabolites (such as butyrate or propionate to NADH); these are also used as micronutrient suppliers in mutually exploitative relationships.70,71 It is notable that our (gut) symbionts are very different from those of the great apes, which may have been important in allowing us, as dietary generalists, to colonize many new habitats, as has happened with the metagenomic evolution of other invasive species. One apparent paradox is that the species hosting the “parasite” should be fit, even though the same microbe decimates related populations; some recent examples in nature include invasions of new continents, such as that of the Americas, by humans and invasions by insects.72,73 Symbiont populations are sensitive to diet (our microbiome content of Bacteroides depends on meat intake), explaining these context-dependent results: especially when coupled with their effect on brain (and gut), development and behavior that can be mind altering, as communication occurs through pheromones, the neuroendocrine system, and via transmitters (such as serotonin) and the vagus nerve. Blood-borne bacterial products (which can cross the placenta) can affect social behavior (such as kin recognition and mate choice), and these bacteria are, in turn, affected by group living arrangements that adapt to become fit for the purpose of establishing cross-infection, when advantageous.74,75 Despite the fact that this seems rather counterintuitive from a modern or medical perspective, we suggest that TB may have been one of these novel nested and co-evolved symbionts, being at times a useful member of our superorganism. We explore this possibility in more detail in the following section.
A Co-Evolutionary Role for TB?
TB co-evolved early, well before social hunting parties dispersed out of Africa 70,000 years ago.74 TB derived, not as originally thought from bovine TB (both probably evolved from a common ancestor), but from a free-living organism perhaps as many as 3 million years ago with needs that include a supply of lipids—such as cholesterol.76–80 Our large brains also evolved early, but did so in three main phases of rapid increase.81,82 The first phase (marked by a 60% increase) occurred around 1.8 million years ago with the appearance of Homo ergaster, while two later phases occurred at ~500,000 years ago (among Homo heidelbergensis, which exhibited a further 50% increase over H. ergaster) and ~200,000 years ago (among Homo sapiens, with a 16% increase over H. heidelbergensis). These phases have been closely linked with primary dietary change, enabling fast-evolving genetic adaptations (or lucky preadaptations working with standing genetic variation), thus improving brain function, symbiotic interrelationships, metabolism of substances such as starches (and we propose meat/nicotinamide), and altered regulation of gene expression (such as the enzymes involved in NAD synthesis and nicotinamide catabolism), which are completed, in part, via epigenetic differences in DNA methylation. These traits all improve mitochondrial biogenesis and maturation of developing brains and can be acted upon by natural selection.83–85 As we noted in the Introduction, our meat-based diet probably began modestly with H. ergaster, but became increasingly crucial with H. heidelbergensis and their descendents (Neanderthals and modern humans). Although the dates remain open to debate, TB could have been acquired as early as the time of the H. ergaster or as late as archaic humans, occurring alongside improvements in hunting and cooking skills (which would have released nicotinamide while most other vitamins get diluted or destroyed).
TB—the 19th century “White Plague”—might seem like a surprising choice as a symbiont. After all, it still kills up to 2 million people in Asia and sub- Saharan Africa every year and causes morbidity in as many as 8 million people, and is often considered one of the infectious “unconquerables.”86 Overall, one-third of the world’s population is infected, and this rate can rise to 100% in high risk areas and perhaps in ancient hunter-gatherer populations. However, seemingly contradictory, yet context-dependent, behavior of this kind (ie, depending on the host’s diet) is far from unknown among symbionts.87 In other words, welcomed guests can turn hostile.
Other members of the mycobiome and gut microbiome are known to synthesize micronutrients (perhaps including nicotinamide) or to harvest energy and might subserve the same function. Nicotinamide might be obtained, for example, from the normal microflora of the large intestine, as it has a specific uptake mechanism. Such symbionts are often gained from the mother, and are sustained by specific polysaccharides in her milk. However, many of these cause diarrheal disease88 or require their own supply and compete with the host (or, if they do synthesize it, the microbes may retain nicotinamide intracellularly).89 Similarly, bacilli such as Bifidobacterium, can overproduce vitamins K, B12, and other B vitamins, as well as affect choline metabolism in the microbiome of the colon.90
TB has the advantage in that (the large number of deaths notwithstanding) 90%–95% of those infected have latent or dormant TB and are asymptomatic and healthy. In addition, TB has a number of biochemical and physiological features that are puzzling for a pathogen. It excretes nicotinamide copiously enough to be useful as a diagnostic test that measures high levels in the culture medium, and is enough to elevate blood levels in patients. Evidence supporting the idea that TB causes disease does not seem to come until long after its acquisition (the earliest archaeological evidence comes from a 7,800-year-old skeleton found in Liguria, with tubercular damage to its spine, while the earliest historical records date from 4,700 BCE in China). This coincides with the decline in meat supply that was already evident just before the Neolithic agricultural revolution, as we became more dependent on cereal crops compounded by a less egalitarian social structure characterized by less meat sharing and variable success with animal husbandry.14 This decline in meat consumption would have had an interesting effect on tryptophan metabolism, as replacement with carbohydrates with a high glycemic index (that included alcohol)) enhanced insulin secretion, lowering all amino acid levels in the blood, except for tryptophan. Tryptophan would then cross the blood– brain barrier easily, as the amino-acid transporter is competitive and leads to higher brain tryptophan levels available for serotonin and NAD synthesis.91 However, the decline in meat consumption and dietary sources of nicotinamide and tryptophan may have gone too far, despite these buffers, as there was considerable evidence for a deterioration in height and health, even as populations exploded.91,92
Unlike most pathogens, TB neither contains nor exudes any toxins. Curiously, on prevailing paradigms, intravenous injection does not make laboratory animals sick; initial infections, unlike reactivations under stress, are usually completely benign in man, and stimulation of natural immunity by vaccination has little effect.93 Even more curiously, granulomas have been recently recognized as sites where the organism reproduces, easily contradicting the traditional view that these structures are there to “wall off” the pathogen.94 Phagocytosis does not kill this microbe, as there appear to be bidirectional “don’t eat me” signals. Indeed extraordinarily, TB can actively multiply in phagocytes (suggesting “grow me” signals),95 in part because it has evolved a mutation (the nuoG gene) that acts as an antiapoptosis agent and allows it to colonize macrophages without cells committing “suicide” to prevent successful invasion.96 Importantly for our idea, M. tuberculosis has been shown to synthesize nicotinamide de novo, scavenging exogenous NAD, and subsequently being able to switch between these two strategies as a function of circumstances through a DosR regulon. This allows M. tuberculosis to take niacin from the host when the host is able to supply it (though it inhibits its growth). In addition, via excretion of its waste product, and due to its often limited ability to synthesize/scavenge niacin to NAD,97M. tuberculosis supplies the host with niacin—particularly when in oxygenated sites such as the lung that is its favorite habitat—unlike most symbionts, which prefer fermentation reactions in the anoxic gut.98,99
Evidence—now largely forgotten—began to accrue during the 1940s demonstrating that nicotinamide had beneficial effects on the treatment of TB in the laboratory (as well as on the equally co-evolved mycobacterium causing leprosy).100 As is the case with subsequent antibiotics, the microbe, in fact, always survives but goes dormant, even with the unusually long courses of treatment. This fact and the fact that the bacillus itself excretes nicotinamide (which became the basis of a reliable diagnostic test for pathogenic human strains)101 led directly to the development of isoniazid, a nicotinamide analogue, as a “designer drug.”102 Other drugs such as pyrazinamide impact the same pathway (indeed, resistance correlates strongly with resistance to nicotinamide), and are still among the standard treatments used—although, ironically, killing most of the microbes can precipitate pellagra despite untreated patients having high blood levels of nicotinamide, presumably because it is supplied by the live organism.103 It is also a peculiarity of TB that exogenous nicotinamide reduces the hypersensitivity responses that are important drivers of the pathology, as measured by the skin response to injected tuberculin protein (thus providing the basis for the Mantoux response).104 Iatrogenic or HIV-related immunosuppression can be a risk factor for TB, as these immunosuppressions can mimic or compound the immune deficiency of malnutrition. (The HIV-dementia complex closely resembles pellagra at both clinical and biochemical levels.) Paradoxically, overactivity of the immune system can also be a risk factor for TB, and the organism can both inhibit and boost the host’s immune response.105 Steroids that often have to be used to suppress the immune response suppresses the kynurenine pathway, providing another link with nicotinamide metabolism.106,107 Thus, surprisingly for a pathogen but not for a symbiont, TB contains within it the seeds of its own control with a relationship that becomes such a nuanced dialogue that it looks more like a cooperation between allies dealing with a variable environment (Table 1).
Table 1.
Comparison of traits exhibited by the TB bacillus in terms of the behavior expected of a symbiont versus a pathogen.
| Trait | Symbiont | Pathogen |
|---|---|---|
| Low virulence rate with | ||
| High dormancy/persistence | √ | X |
| Nicotinamide secretion | √ | X |
| Nicotinamide role in brain growth | √ | X |
| Self-regulation to prevent host death | √ | X |
| Origin should coincide with major brain expansion | √ | X |
| Capacity to switch from NAD production to extraction | √ | X |
| Trade-off with meat consumption | √ | X |
Testing the Hypothesis: Some Pilot Data
Our hypothesis is that TB acts as a live, amplifiable, and genetically modifiable factory-farm for nicotinamide when meat is scarce. In return, hosts provide a home and nutrients, including lipids such as cholesterol, as “vitamins” for TB, perhaps lowering levels and risk of vascular disease in infected populations. Support for the hypothesis would be provided if we could show that there is a negative relationship between TB mortality rates and meat consumption.
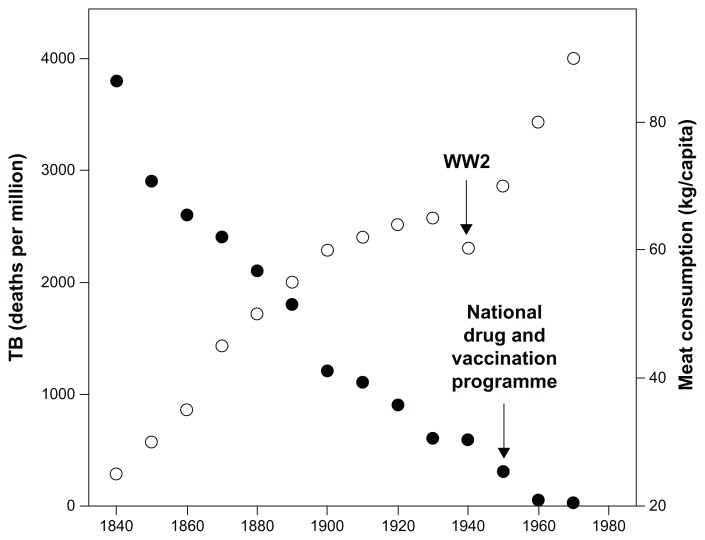
For this purpose, we focus on a natural experiment in Britain between 1850 and 1950 because there is a good epidemiological record of striking declines in TB rates that have never been adequately explained despite exhaustive debate;108,109 some hypotheses favor dietary factors, while others offer public health explanations. There is little support for genetic adaptations, as these declines happened so fast and were geographically localized. In fact, more often than resistance genes, research has found genes that increase susceptibility, both often influencing xenophagy of mycobacteria and some now showing up as risk factors for Parkinson’s. (For instance, these declines occurred much later in Japan, where red meat was banned by decree for many years). Meat consumption in Britain was changing fast over this century: meat imports (thanks to refrigeration and better transport to the UK) were 3.3 million kg in 1841, rising to more than 900 million kg in 1900—a 300-fold rise—outpacing population increase, even discounting increased home production in the aftermath of the recent agricultural revolution. Milk production per cow also rose during the 18th and 19th centuries, as did milk consumption, particularly after the building of the railways.109 In addition, focusing on this period allows us to avoid some potential confounding factors such as medical interventions, earlier/better diagnosis, and HIV co-infection. We sourced data on real incomes, money wages, food price and meat consumption from Perren.110 Mortality rates for infectious diseases were sourced from Charlton,111 the data on cancer from Logan,112 and the Parkinson’s data from Duvoisin113—all based on the Registrar General’s death registrations for England and Wales.
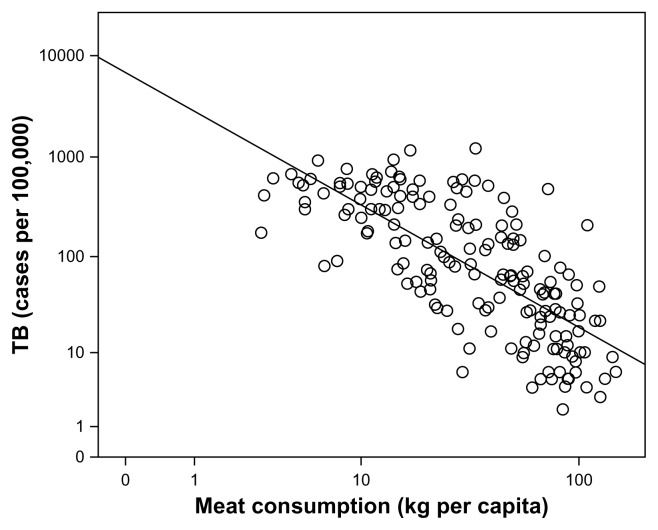
Mortality from TB decreased over the century between 1850 and 1950, while meat consumption and real incomes rose (Fig. 2). The relationship with meat consumption is highly significant (data up to 1950 only: r = −0.968; N = 12; P <0.001). This is particularly evident when analyzed by social class: the upper classes ate more meat, drank more milk, and had much lower TB rates than wage laborers.114 This relationship is not peculiar to England and Wales, but can still be seen on a country-by-country basis across the contemporary world using WHO data (Fig. 3: for log10- transformed data; r = −0.730, N = 169, P <0.001). Intriguingly, given the cognitive effects of pellagra discussed earlier, literacy (indexed as the ability to write one’s name in a marriage register rather than simply make a mark) correlated linearly with meat consumption across the nineteenth century (r = 0.980, N = 7 decades, P < 0.001).115 This may nowadays be reflected in marked variances across nations in IQ that can change quickly over time (the Flynn effect) as circumstances improve.116
Figure 2.
Meat consumption (kg per capita) plotted against the decline of TB between 1840 and 1960 in the UK.
Figure 3.
Frequency of current TB cases plotted against meat consumption for individual countries. The graph (not shown) for diarrheal illnesses looks very similar. Note the very high variances between countries in meat consumption in the contemporary world.
The raw data for TB suggest that the relationship is asymptotic, with mortality plateauing close to zero once meat consumption rises above ~60 kg/capita/ annum (around 2.5 times the internationally recommended minimum). A linear regression fitted to the normalized (standard deviates) data with meat consumption, censored to include only countries where consumption is less than 60 kg/capita/annum, yields a slope of b = −1.079, which does not differ significantly from a slope of b = − 1 (t = − 0.418, P > 0.05), suggesting a direct tradeoff between these two variables.
TB death rates also correlate significantly with the rise in real income in England and Wales during the 19th century (r = − 0.664, P < 0.001), but this could well be mediated by the increased meat consumption. The relationship between wealth and meat consumption (the Engel Effect) has been known for some time, but the link to TB morbidity and mortality has not been previously noted. Turning to other diseases, malaria death rates exhibit a significant negative correlation with meat consumption (r = − 0.730, N =14, P = 0.003). However, the onset of the decline in malaria deaths precedes the rise in meat consumption by several decades. (Others have wondered if the explanation was partly that there were more cattle around to provide an alternate blood meal for the vector, consistent with malaria’s emergence during the Neolithic revolution and the rise of cereal dependency117). Diarrheal illnesses also correlate negatively with meat consumption (r = − 0.923, P < 0.001). Rheumatic fever, cholera, smallpox, and poliomyelitis declined during this period, but were uncorrelated with meat consumption (and have alternate explanations such as cleaner water or vaccination programs) (r = − 0.361, r = −0.472, r = −0.500, and r = −0.133, respectively; all P > 0.05), while the incidence of cancer (r = 0.981, P < 0.001) and Parkinson’s disease (r = 0.846, P < 0.001) correlated positively with meat consumption, as did longevity (r = 0.832, P < 0.001).
Circumstantial evidence offers further support for our hypothesis. The North American Indian populations were decimated by TB during the 19th century, but only after they had been driven out of their natural habitats and moved to reservations where the food supply and the ability to hunt were severely restricted.118 In 1925, the Norwegian government built new spacious barracks in Trondheim as the incidence of TB was so high in naval recruits, but there was no improvement in their health until there was a radical change in their meat intake.119 Recruits to the US navy in 1949–1951 had four times the incidence of TB if they showed signs of being underfed.120 In the 1930s, the pastoralist Masai (who traditionally ate mainly milk, blood, and meat) had lower TB rates than the neighboring agricultural Kikuyu (who ate cereals and fruit).121 In one experimental manipulation, TB reinfection rates in families given supplementary vitamins were lower than in a control group.122 In a major World Health Organization review, Scrimshaw et al123 reported that TB rates increased dramatically in several European countries during the two World Wars when populations became malnourished due to food shortages or sieges, and rapidly declined again once conditions improved. Early investigators showed that poor diets could increase TB susceptibility in laboratory animals, and suggested that animal protein provided protection, while Dubos124 was impressed with the value of skim milk.
These correlational and circumstantial data provide prima facie evidence for a close relationship between meat consumption and TB. We suggest that TB (like many gut infections) can be tolerated by the host, so long as the host is not excessively challenged nutritionally. This initial toleration (or rather, we say, an immunological welcome mat) for a controlled population of TB is supported by the good health of the majority of individuals infected; indeed, in Victorian times it was recognized that individuals could be unusually creative (it became almost obligatory if one was to be taken seriously as a poet) before they became sick. Under extreme conditions a tipping point is reached, and the host is unable to control the bacillus’ population size, resulting in death as fragile cross-species homeostatic systems are ruptured.
Conclusion
Our claim is that TB co-evolved early in the human lineage as a specialist syntrophic mutualist that buffered us against periodic shortages of meat once meat’s nicotinamide and tryptophan became an essential component of the diet to fuel and grow especially large brains. We have argued that the overall behavior and the relatively slow genetic diversification of TB (until extreme meat poverty and drugs came on the scene) points to the fact that it is supporting nicotinamide metabolism; it is not a conventional pathogen in an “Arms Race” with us that became adept at evading our immune system (Table 1). Otherwise, as pointed out recently by others125 would it not have caused its and our own extinction 70,000 years ago? Furthermore, unlike most pathogens,126 it does not keep altering its surface antigens. The critical point is that TB secretes as a waste product a key micronutrient (nicotinamide, vitamin B3), which is also found in meat.
An alternative explanation for our findings might be that nicotinamide is simply an antibiotic. Although this is true (and nicotinamide also affects the production of antimicrobial peptides), we believe that its relationship with TB is more interesting because organisms normally excrete antibiotics to deter other organisms (eg, leprosy, a mycobacterial infection sensitive to nicotinamide that can spontaneously disappear when TB appears or diet improves)127 rather than themselves. Another possibility is that nicotinamide simply boosts host immunity through the NAD(P)H pathways. Again, there is support for this as many acute organisms and their toxins compete with us over the NAD supply with hormetic jolts to the system that benefit the survivors (these are akin to the effects of caloric restriction) but is often the mechanism causing cell damage or death of individuals.
We should emphasize that we are not suggesting that pellagra was the actual selection factor for TB symbiosis; rather, we offer pellagra as a tip-of-the- iceberg example of what happens when there is extreme dietary shortage of vitamin B3. Our claim is that even mild shortages of dietary nicotinamide result in deficits in neural development that have significant cognitive and social consequences, and hence any alternative strategies that buffer against dietary shortages would be advantageous. Nor are we claiming that vitamin B3 is the only factor of importance; it is one of a number of nutrient demands that must be satisfied that, among others, include a better supply of methyl donors from folate, vitamin B12, and choline—the latter being closely interlinked with nicotinamide metabolism, and similarly affects brain development and long term maintenance and epigenetic evolution.128,129 However, and this is perhaps crucial, nicotinamide is one of the few vitamins that can only be satisfied from meat or animal products, at least up until the Neolithic agricultural revolution. Of course, much later nicotinamide supplementation— in rich countries—means even strict vegetarians will obtain the 10–15 mg a day recommended dose (omnivores exceed that dose by an order of magnitude), particularly if they also consume heavily supplemented food such as “high energy” drinks. It is important to note that nicotinamide supplementation is not the same as eating more meat, as meat would also increase tryptophan intake, dampening the immune tolerance/intolerance nicotinamide switch, and increase methyl donors, ultimately facilitating nicotinamide catabolism without causing a methyl deficit. This may be an “evolutionary trap” with a misleading cue from supplementary nicotinamide if it suppresses the desire to eat meat, especially if that drives over-consumption of high-calorific (fast) foods.
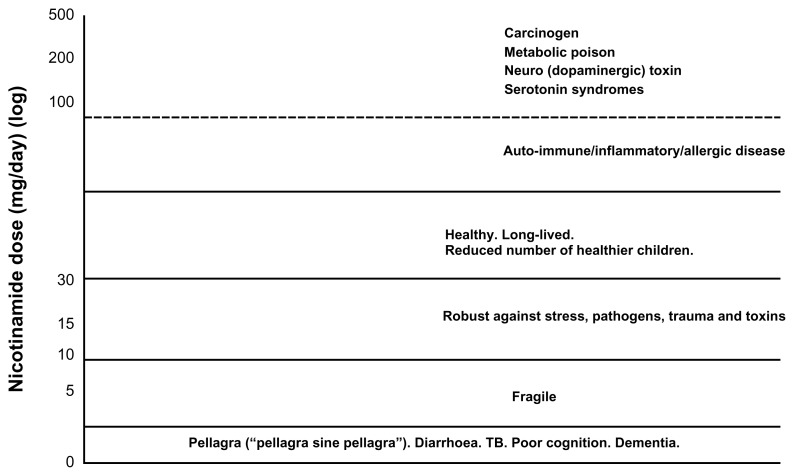
We suggested that, taken together, the biology of the TB bacillus is more consistent with its being, at first, a mutualistic symbiont and only secondarily a pathogen when dietary circumstances later changed (and changed again with antibiotics driving drug resistance in “persister” cells that may even be protected by the host protecting a misunderstood symbiont). 130 The fact that the TB bacillus can, and does, kill its host is not a counterargument to the hypothesis. Most biological processes have a U-shaped dose response, and one can point to many examples where a perfectly sound natural mechanism has adverse consequences when over-indulgence runs to excess. Symbiotic gut bacteria break down celluloses to short-chain fatty acids that act as a source of NADH for the host, or produce micronutrients that may include some nicotinamide, but pushed too far these bacteria can cause diarrhea and death (as perhaps happens in the globally common environmental enteropathies). A well known example is kwashiorkor: these conditions kill or maim physically, emotionally, and cognitively one-third of the world’s children—and surely relate more closely to “formes fruste” of pellagra than has been recently thought (Fig. 4).88,131 A dietary-driven atypical chronic gut infection profile of our “inner menagerie” can also seed amyloid or other misfolded and hyperphosphorylated proteins, and waves of prion-like neurodegeneration by inciting enteric neurons to excrete misfolded proteins that are then propagated centrally via the sympathetic and parasympathetic nervous systems.132
Figure 4.
Nicotinamide: the devil may be in the dosage. Too low and symptoms of pellagra emerge, even if rarely diagnosed as the rash may frequently not be expressed. Higher doses than usually recommended may be necessary for individuals carrying certain mutations and when under genotoxic or infectious or traumatic stresses that require temporary boosts of repair circuits. Too high a dose chronically from supplemented food-stuffs or too much meat causing nicotinamide overload (an experiment whose long-term effectiveness or toxicity has never been monitored) may express itself phenotypically in many ways covering many common diseases, as does pellagra.
Indeed, even nicotinamide seems to have a U-shaped toxicity curve (as do other NAD boosting drugs), switching, when intake is excessive, from a vitamin, prompting a downstream rash of autoimmune/ inflammatory diseases by changing symbiotic profiles upstream through the nicotinamide switch (Fig. 4). At high doses, nicotinamide might even become a poison (perhaps explaining its positive correlations with cancer, Parkinson’s disease, and other diseases of affluence). 18,19 Cancers, often related to infection, have been linked with low nicotinamide states, perhaps impairing DNA repair necessitated by damage caused by genotoxins. However, most other modern cancers are linked with inflammation, and clearing nicotinamide and tryptophan by uptake “hungers,” bipartisan “don’t eat me” signals sent to and fro with macrophages and “grow me” signals with induction of NAD synthetic pathways.133–135 NADH is then not oxidized back to NAD normally in the presence of oxygen (the unexplained Warburg paradox), compounding a runaway process that disposes naturally of excess nicotinamide. 136 In a complementary fashion, the induction of nicotinamide detoxification by NNMT in many cancers (including lung and colon, which are linked with red meat and tobacco) sinks nicotinamide, whilst consuming methyl groups demethylating the epigenome and promoting procancerous (epi)mutations or producing dopaminergic or metabolic toxins.137,138
We have shown that TB mortality correlates negatively with meat consumption—both through time within the UK, and across countries today— providing some evidence for a tradeoff between alternative sources of nicotinamide. Further tests of this hypothesis would require a more detailed biochemical study to identify nicotinamide/tryptophan deficiency (or overdosage) states. Ideally, randomized controlled trials should be conducted on patients at risk, using meat or nicotinamide/tryptophan as the intervention, while the incidence and severity of TB and other infections (notably those causing diarrhea), measures of brain development and function, and a wide range of neuro-degenerative disorders (as seen with pellagra) would serve as endpoints, with an eye to the possibility of overdosage syndromes. If results are positive, returning to our egalitarian past and redistributing meat or its components that supply NAD (avoiding both the highs and the lows between individuals and over individual lifetimes) may be more effective than subsidizing corn grain (while the increased prosperity from unlocking human potential should pay for the intervention).
Our claim is that there is sufficient evidence on the effects of meat transitions to green-light ecologicallyand evolutionary-inspired studies, particularly given concerns over poor (meat) nutrition causing poor cognition, and the severe limitations of the use of antibiotics. The situation in North Korea is a current case in point where a sevenfold rise in TB, including drug-resistant forms, is unanimously agreed to have its origin in a recent epic famine, known locally as the “Arduous March”—yet, it is the TB, not the neglect of diet, that is being described as “Public Enemy Number One.”139
Footnotes
Author Contributions
Conceived and designed the experiments: AW, RD. Analyzed the data: AW, RD. Wrote the first draft of the manuscript: AW, RD. Contributed to the writing of the manuscript: AW, RD. Agree with manuscript results and conclusions: AW, RD. Jointly developed the structure and arguments for the paper: AW, RD. Made critical revisions and approved final version: AW, RD. Both authors contributed equally for all aspects of the manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
References
- 1.Boesch C, Boesch H. Hunting behavior of wild chimpanzees in the Taï National Park. Am J Phys Anthropol. 1989;78(4):547–73. doi: 10.1002/ajpa.1330780410. [DOI] [PubMed] [Google Scholar]
- 2.Mitani JC, Watts DP. Demographic influences on the hunting behavior of chimpanzees. Am J Phys Anthropol. 1999;109(4):439–54. doi: 10.1002/(SICI)1096-8644(199908)109:4<439::AID-AJPA2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Richards MP, Trinkaus E. Isotopic evidence for the diets of European Neanderthals and early modern humans. PNAS USA. 2009;38:16034–9. doi: 10.1073/pnas.0903821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmody RN, Wrangham RW. The energetic significance of cooking. J Hum Evol. 2009;57(4):379–91. doi: 10.1016/j.jhevol.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Ungar PS, Sponheimer M. The diets of early hominins. Science. 2011;334(6053):190–3. doi: 10.1126/science.1207701. [DOI] [PubMed] [Google Scholar]
- 6.Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211(Pt 11):1792–804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- 7.Hall CN, Klein-Flügge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci. 2012;32(26):8940–51. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Feng X, Liang D, Duan Y, Lei H. Down-regulation of energy metabolism in Alzheimer’s disease is a protective response of neurons to the microenvironment. J Alzheimers Dis. 2012;28(2):389–402. doi: 10.3233/JAD-2011-111313. [DOI] [PubMed] [Google Scholar]
- 9.Surmeier DJ. Neuroscience: to go or not to go. Nature. 2013;494(7436):178–9. doi: 10.1038/nature11856. [DOI] [PubMed] [Google Scholar]
- 10.Bolam JP, Pissadaki EK. Living on the edge with too many mouths to feed: why dopamine neurons die. Mov Disord. 2012;27(12):1478–83. doi: 10.1002/mds.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlman RL. Evolution and Medicine. Oxford University Press; London: 2013. [Google Scholar]
- 12.Navarrete A, van Schaik CP, Isler K. Energetics and the evolution of human brain size. Nature. 2011;480(7375):91–3. doi: 10.1038/nature10629. [DOI] [PubMed] [Google Scholar]
- 13.Hines M. Human Evolution, Diet and Health. London, UK: Healthy Body Publishing; 2008. [Google Scholar]
- 14.Vigne JD. The origins of animal domestication and husbandry: a major change in the history of humanity and the biosphere. C R Biol. 2011;334(3):171–81. doi: 10.1016/j.crvi.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Rowley-Conwy P, Layton R. Foraging and farming as niche construction: stable and unstable adaptations. Philos Trans R Soc Lond, B, Biol Sci. 2011;366(1566):849–62. doi: 10.1098/rstb.2010.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMichael AJ, Powles JW, Butler CD, Uauy R. Food, livestock production, energy, climate change, and health. Lancet. 2007;370(9594):1253–63. doi: 10.1016/S0140-6736(07)61256-2. [DOI] [PubMed] [Google Scholar]
- 17.Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378(9799):1325–38. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- 18.Smil V. Eating meat: evolution, patterns, and consequences. Popul Dev Rev. 2002;28(4):599–639. [Google Scholar]
- 19.Rohrmann S, Overvad K, Bueno-de-Mesquita HB, et al. Meat consumption and mortality—results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013;11:63. doi: 10.1186/1741-7015-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heys M, Jiang C, Schooling CM, et al. Is childhood meat eating associated with better later adulthood cognition in a developing population? Eur J Epidemiol. 2010;25(7):507–16. doi: 10.1007/s10654-010-9466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D, Pitta M, Jiang H, et al. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol Aging. 2013;34(6):1564–80. doi: 10.1016/j.neurobiolaging.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blatt GJ, Chen JC, Rosene DL, Volicer L, Galler JR. Prenatal protein malnutrition effects on the serotonergic system in the hippocampal formation: an immunocytochemical, ligand binding, and neurochemical study. Brain Res Bull. 1994;34(5):507–18. doi: 10.1016/0361-9230(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 23.Roe D. A Plague of Corn: The Social History of Pellagra. London, UK: Cornell University Press; 1973. [Google Scholar]
- 24.Fernstrom JD, Langham KA, Marcelino LM, Irvine ZL, Fernstrom MH, Kaye WH. The ingestion of different dietary proteins by humans induces large changes in the plasma tryptophan ratio, a predictor of brain tryptophan uptake and serotonin synthesis. Clin Nutr. doi: 10.1016/j.clnu.2012.11.027. Epub Jan 23, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Orosco M, Rouch C, Beslot F, Feurte S, Regnault A, Dauge V. Alpha-lactalbumin- enriched diets enhance serotonin release and induce anxiolytic and rewarding effects in the rat. Behav Brain Res. 2004;148(1–2):1–10. doi: 10.1016/s0166-4328(03)00153-0. [DOI] [PubMed] [Google Scholar]
- 26.Crosby AW. The Columbian Exchange: Biological and Cultural Consequences of 1492. Westpoint, CT: Greenwood; 1972. [Google Scholar]
- 27.Seal AJ, Creeke PI, Dibari F, et al. Low and deficient niacin status and pellagra are endemic in postwar Angola. Am J Clin Nutr. 2007;85(1):218–24. doi: 10.1093/ajcn/85.1.218. [DOI] [PubMed] [Google Scholar]
- 28.Harris S. Clinical Pellagra. New York, NY: Mosby; 1941. [Google Scholar]
- 29.Surjana D, Halliday GM, Damian DL. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in human keratinocytes and ex vivo skin. Carcinogenesis. 2013;34(5):1144–9. doi: 10.1093/carcin/bgt017. [DOI] [PubMed] [Google Scholar]
- 30.Nisbett RE. Violence and US regional culture. Am Psychol. 1993;48(4):441–9. doi: 10.1037//0003-066x.48.4.441. [DOI] [PubMed] [Google Scholar]
- 31.Sydenstricker VP. The history of pellagra, its recognition as a disorder of nutrition and its conquest. Am J Clin Nutr. 1958;6(4):409–14. doi: 10.1093/ajcn/6.4.409. [DOI] [PubMed] [Google Scholar]
- 32.Stone TW, Forrest CM, Darlington LG. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J. 2012;279(8):1386–97. doi: 10.1111/j.1742-4658.2012.08487.x. [DOI] [PubMed] [Google Scholar]
- 33.Lichtheim M. Ancient Egyptian Literature: The Late Period. Berkeley, CA: University of California Press; 2006. [Google Scholar]
- 34.Vassilopoulos A, Fritz KS, Petersen DR, Gius D. The human sirtuin family: evolutionary divergences and functions. Hum Genomics. 2011;5(5):485–96. doi: 10.1186/1479-7364-5-5-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massudi H, Grant R, Guillemin GJ, Braidy N. NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns. Redox Rep. 2012;17(1):28–46. doi: 10.1179/1351000212Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang C, Arany Z. Sweet enticements to move. Nature. 2013;500:409–11. doi: 10.1038/nature12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son MJ, Son MY, Seol B, et al. Nicotinamide overcomes pluripotency deficits and reprogramming barriers. Stem Cells. 2013;31(6):1121–35. doi: 10.1002/stem.1368. [DOI] [PubMed] [Google Scholar]
- 38.Klempin F, Beis D, Mosienko V, Kempermann G, Bader M, Alenina N. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci. 2013;33(19):8270–5. doi: 10.1523/JNEUROSCI.5855-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23(1):75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Turlejski K. Evolutionary ancient roles of serotonin: long-lasting regulation of activity and development. Acta Neurobiol Exp (Wars) 1996;56(2):619–36. doi: 10.55782/ane-1996-1167. [DOI] [PubMed] [Google Scholar]
- 41.Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Front Integr Neurosci. 2013;7:25. doi: 10.3389/fnint.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubia K, Lee F, Cleare AJ, et al. Tryptophan depletion reduces right inferior prefrontal activation during response inhibition in fast, event-related fMRI. Psychopharmacology (Berl) 2005;179(4):791–803. doi: 10.1007/s00213-004-2116-z. [DOI] [PubMed] [Google Scholar]
- 43.Bürkle A, Virág L. Poly(ADP-ribose): PARadigms and PARadoxes. Mol Aspects Med. 2013 doi: 10.1016/j.mam.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Duan W. Sirtuins: from metabolic regulation to brain aging. Front Aging Neurosci. 2013;5:36. doi: 10.3389/fnagi.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai P, Canto C, Brunyánszki A, et al. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 2011;13(4):450–60. doi: 10.1016/j.cmet.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imai S. Dissecting systemic control of metabolism and aging in the NAD World: the importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett. 2011;585(11):1657–62. doi: 10.1016/j.febslet.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lane N, Martin WF. The origin of membrane bioenergetics. Cell. 2012;151(7):1406–16. doi: 10.1016/j.cell.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 48.Woods HA, Wilson JK. An information hypothesis for the evolution of homeostasis. Trends Ecol Evol (Amst) 2013;28(5):283–9. doi: 10.1016/j.tree.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 49.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 50.Braidy N, Guillemin GJ, Grant R. Effects of kynurenine pathway inhibition on NAD metabolism and cell viability in human primary astrocytes and neurons. Int J Tryptophan Res. 2011;4:29–37. doi: 10.4137/IJTR.S7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE. 2012;7(7):e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Combes V, Guillemin GJ, Chan-Ling T, Hunt NH, Grau GE. The crossroads of neuroinflammation in infectious diseases: endothelial cells and astrocytes. Trends Parasitol. 2012;28(8):311–9. doi: 10.1016/j.pt.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Peter C, Waldmann H, Cobbold SP. mTOR signalling and metabolic regulation of T cell differentiation. Curr Opin Immunol. 2010;22(5):655–61. doi: 10.1016/j.coi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Orabona C, Grohmann U. Indoleamine 2,3-dioxygenase and regulatory function: tryptophan starvation and beyond. Methods Mol Biol. 2011;677:269–80. doi: 10.1007/978-1-60761-869-0_19. [DOI] [PubMed] [Google Scholar]
- 55.Siddiqi SH, Hwangbo CC, Silcox V, Good RC, Snider DE, Middlebrook G. Rapid radiometric methods to detect and differentiate Mycobacterium tuberculosis/M. bovis from other mycobacterial species. Am Rev Respir Dis. 1984;130(4):634–40. doi: 10.1164/arrd.1984.130.4.634. [DOI] [PubMed] [Google Scholar]
- 56.Gowthaman U, Eswarakumar V. Molecular mimicry: Good artists copy, great artists steal. Virulence. 2013;4(6) doi: 10.4161/viru.25780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baïz N, Annesi-Maesano I. Is the asthma epidemic still ascending? Clin Chest Med. 2012;33(3):419–29. doi: 10.1016/j.ccm.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Linneberg A. The increase in allergy and extended challenges. Allergy. 2011;66(Suppl 95):1–3. doi: 10.1111/j.1398-9995.2011.02619.x. [DOI] [PubMed] [Google Scholar]
- 59.Harden JL, Egilmez NK. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest. 2012;41(6–7):738–64. doi: 10.3109/08820139.2012.676122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Bubnoff D, Bieber T. The indoleamine 2,3-dioxygenase (IDO) pathway controls allergy. Allergy. 2012;67(6):718–25. doi: 10.1111/j.1398-9995.2012.02830.x. [DOI] [PubMed] [Google Scholar]
- 61.Ezzati M, Vander Hoorn S, Lawes CM, et al. Rethinking the diseases of affluence paradigm: global patterns of nutritional risks in relation to economic development. PLoS Med. 2005;2(5):e133. doi: 10.1371/journal.pmed.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 63.Thompson JN. Coevolution: the geographic mosaic of coevolutionary arms races. Curr Biol. 2005;15(24):R992–4. doi: 10.1016/j.cub.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 64.Bernhard H, Fischbacher U, Fehr E. Parochial altruism in humans. Nature. 2006;442(7105):912–5. doi: 10.1038/nature04981. [DOI] [PubMed] [Google Scholar]
- 65.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–30. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 66.Ingram CJ, Mulcare CA, Itan Y, Thomas MG, Swallow DM. Lactose digestion and the evolutionary genetics of lactase persistence. Hum Genet. 2009;124(6):579–91. doi: 10.1007/s00439-008-0593-6. [DOI] [PubMed] [Google Scholar]
- 67.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–8. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young SN. The effect of raising and lowering tryptophan levels on human mood and social behaviour. Philos Trans R Soc Lond, B, Biol Sci. 2013;368(1615):20110375. doi: 10.1098/rstb.2011.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crockett MJ, Apergis-Schoute A, Herrmann B, et al. Serotonin modulates striatal responses to fairness and retaliation in humans. J Neurosci. 2013;33(8):3505–13. doi: 10.1523/JNEUROSCI.2761-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robles Alonso V, Guarner F. Linking the gut microbiota to human health. Br J Nutr. 2013;109(Suppl 2):S21–6. doi: 10.1017/S0007114512005235. [DOI] [PubMed] [Google Scholar]
- 71.Singh Y, Ahmad J, Musarrat J, Ehtesham NZ, Hasnain SE. Emerging importance of holobionts in evolution and in probiotics. Gut Pathog. 2013;5(1):12. doi: 10.1186/1757-4749-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diamond JM. Guns, Germs and Steel: The Fates of Human Societies. New York, NY: Norton & Company Incorporated; 1997. [Google Scholar]
- 73.Vilcinskas A, Stoecker K, Schmidtberg H, Röhrich CR, Vogel H. Invasive harlequin ladybird carries biological weapons against native competitors. Science. 2013;340(6134):862–3. doi: 10.1126/science.1234032. [DOI] [PubMed] [Google Scholar]
- 74.Flint HJ. The impact of nutrition on the human microbiome. Nutr Rev. 2012;70(Suppl 1):S10–3. doi: 10.1111/j.1753-4887.2012.00499.x. [DOI] [PubMed] [Google Scholar]
- 75.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–12. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 76.Daniel TM. The bioarchaeology of tuberculosis: a global review on a reemerging disease. Am J Trop Med Hyg. 2005;73(3):649–50. [Google Scholar]
- 77.Wilbur AK, Farnbach AW, Knudson KJ, Buikstra JE. Diet, tuberculosis, and the paleopathological record. Curr Anthropol. 2008;49(6):963–77. doi: 10.1086/592434. discussion 977–91. [DOI] [PubMed] [Google Scholar]
- 78.Hershberg R, Lipatov M, Small PM, et al. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 2008;6(12):e311. doi: 10.1371/journal.pbio.0060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith NH, Hewinson RG, Kremer K, Brosch R, Gordon SV. Myths and misconceptions: the origin and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol. 2009;7(7):537–44. doi: 10.1038/nrmicro2165. [DOI] [PubMed] [Google Scholar]
- 80.Holloway KL, Henneberg RJ, de Barros Lopes M, Henneberg M. Evolution of human tuberculosis: a systematic review and meta-analysis of paleopathological evidence. Homo. 2011;62(6):402–58. doi: 10.1016/j.jchb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 81.Kubo D, Kono RT, Kaifu Y. Brain size of Homo floresiensis and its evolutionary implications. Proc Biol Sci. 2013;280(1760):20130338. doi: 10.1098/rspb.2013.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shultz S, Nelson E, Dunbar RI. Hominin cognitive evolution: identifying patterns and processes in the fossil and archaeological record. Philos Trans R Soc Lond, B, Biol Sci. 2012;367(1599):2130–40. doi: 10.1098/rstb.2012.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Enard W, Fassbender A, Model F, Adorján P, Pääbo S, Olek A. Differences in DNA methylation patterns between humans and chimpanzees. Curr Biol. 2004;14(4):R148–9. [PubMed] [Google Scholar]
- 84.Fisher SE, Ridley M. Evolution. Culture, genes, and the human revolution. Science. 2013;340(6135):929–30. doi: 10.1126/science.1236171. [DOI] [PubMed] [Google Scholar]
- 85.Gabel HW, Greenberg ME. The Maturing Brain Methylome. Science. 2013;341:626–7. doi: 10.1126/science.1242671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dormandy T. The White Death: A History of Tuberculosis. London, UK: Hambledon Press; 1999. [Google Scholar]
- 87.Daskin JH, Alford RA. Context-dependent symbioses and their potential roles in wildlife diseases. Proc Biol Sci. 2012;279(1733):1457–65. doi: 10.1098/rspb.2011.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ley RE. Nutrition: when guests turn hostile. Nature. 2013;494(7438):437–8. doi: 10.1038/494437a. [DOI] [PubMed] [Google Scholar]
- 89.Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437(3):357–72. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turroni F, Ribbera A, Foroni E, van Sinderen D, Ventura M. Human gut microbiota and bifidobacteria: from composition to functionality. Antonie Van Leeuwenhoek. 2008;94(1):35–50. doi: 10.1007/s10482-008-9232-4. [DOI] [PubMed] [Google Scholar]
- 91.Cohen MN. Health and the Rise of Civilization. New Haven, CT: Yale University Press; 1989. [Google Scholar]
- 92.Donoghue HD. Insights gained from palaeomicrobiology into ancient and modern tuberculosis. Clin Microbiol Infect. 2011;17(6):821–9. doi: 10.1111/j.1469-0691.2011.03554.x. [DOI] [PubMed] [Google Scholar]
- 93.Dye C. Making wider use of the world’s most widely used vaccine: Bacille Calmette-Guerin revaccination reconsidered. J R Soc Interface. 2013;10(87):20130365. doi: 10.1098/rsif.2013.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shaler CR, Horvath CN, Jeyanathan M, Xing Z. Within the Enemy’s Camp: contribution of the granuloma to the dissemination, persistence and transmission of Mycobacterium tuberculosis. Front Immunol. 2013;4:30. doi: 10.3389/fimmu.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gordon SV, Bottai D, Simeone R, Stinear TP, Brosch R. Pathogenicity in the tubercle bacillus: molecular and evolutionary determinants. Bioessays. 2009;31(4):378–88. doi: 10.1002/bies.200800191. [DOI] [PubMed] [Google Scholar]
- 96.Miller JL, Velmurugan K, Cowan MJ, Briken V. The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-alpha-mediated host cell apoptosis. PLoS Pathog. 2010;6(4):e1000864. doi: 10.1371/journal.ppat.1000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seiner DR, Hegde SS, Blanchard JS. Kinetics and inhibition of nicotinamidase from Mycobacterium tuberculosis. Biochemistry. 2010;49(44):9613–9. doi: 10.1021/bi1011157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boshoff HI, Xu X, Tahlan K, et al. Biosynthesis and recycling of nicotinamide cofactors in mycobacterium tuberculosis. An essential role for NAD in nonreplicating bacilli. J Biol Chem. 2008;283(28):19329–41. doi: 10.1074/jbc.M800694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leistikow RL, Morton RA, Bartek IL, Frimpong I, Wagner K, Voskuil MI. The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J Bacteriol. 2010;192(6):1662–70. doi: 10.1128/JB.00926-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chorine V. Action of nicotinamide on bacilli of the species Mycobacterium. Rend Acad Sci. 1945;220:150–1. [Google Scholar]
- 101.Soares MB, Silva CV, Bastos TM, et al. Anti-Trypanosoma cruzi activity of nicotinamide. Acta Trop. 2012;122(2):224–9. doi: 10.1016/j.actatropica.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 102.McDermott W. The story of INH. J Infect Dis. 1969;119(6):678–83. doi: 10.1093/infdis/119.6.678. [DOI] [PubMed] [Google Scholar]
- 103.Shibata K, Fukuwatari T, Sugimoto E. Effects of dietary pyrazinamide, an antituberculosis agent, on the metabolism of tryptophan to niacin and of tryptophan to serotonin in rats. Biosci Biotechnol Biochem. 2001;65(6):1339–46. doi: 10.1271/bbb.65.1339. [DOI] [PubMed] [Google Scholar]
- 104.Yiasemides E, Sivapirabu G, Halliday GM, Park J, Damian DL. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30(1):101–5. doi: 10.1093/carcin/bgn248. [DOI] [PubMed] [Google Scholar]
- 105.Murray MF. Insights into therapy: tryptophan oxidation and HIV infection. Sci Transl Med. 2010;2(32):32ps23. doi: 10.1126/scitranslmed.3001082. [DOI] [PubMed] [Google Scholar]
- 106.Suzuki Y, Suda T, Asada K, et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin Vaccine Immunol. 2012;19(3):436–42. doi: 10.1128/CVI.05402-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grohmann U, Volpi C, Fallarino F, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13(5):579–86. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 108.Gage TB. Are modern environments really bad for us? Revisiting the demographic and epidemiologic transitions. Am J Phys Anthropol. 2005;(Suppl 41):96–117. doi: 10.1002/ajpa.20353. [DOI] [PubMed] [Google Scholar]
- 109.McKeown T. The Role of Medicine: Dream, Mirage, or Nemesis? London: Nuffield Provincial Hospitals Trust; 1976. [Google Scholar]
- 110.Perren R. The Meat Trade in Britain, 1840–1914. London, UK: Routledge Kegan Paul; 1978. [Google Scholar]
- 111.Charlton J, Murphy M. The Health of Adult Britain 1841–1994, volumes I, II. London, UK: Office of National Statistics; 1997. [Google Scholar]
- 112.Logan WPD. Mortality in England and Wales from 1848 to 1947. Popul Stud (Camb) 1950;4(2):132–78. [Google Scholar]
- 113.Duvoisin RC, Schweitzer MD. Paralysis agitans mortality in England and Wales, 1855–962. Br J Prev Soc Med. 1966;20(1):27–33. [Google Scholar]
- 114.Farr W. Annual Report of the Registrar General. London: HMSO; 1948. [Google Scholar]
- 115.Greenwood M. Some British Pioneers of Social Medicine. Oxford; Oxford University Press; 1948. [Google Scholar]
- 116.Lynn R, Meisenberg G, Mikk J, Williams A. National IQs predict differences in scholoastic achievement in 67 countries. J Biosoc Sci. 2007;39(06):861–74. doi: 10.1017/S0021932007001964. [DOI] [PubMed] [Google Scholar]
- 117.Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev. 2002;15(4):564–94. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pope AS, Gordon JE. The impact of tuberculosis on human populations. Am J Med Sci. 1955;230(3):317–53. doi: 10.1097/00000441-195509000-00011. [DOI] [PubMed] [Google Scholar]
- 119.Munro WT, Leitch I. Diet and tuberculosis. Proc Nutr Soc. 1945;3:155–64. doi: 10.1079/pns19450006. [DOI] [PubMed] [Google Scholar]
- 120.Palmer CE, Jablon S, Edwards PQ. Tuberculosis morbidity of young men in relation to tuberculin sensitivity and body build. Am Rev Tuberc. 1957;76(4):517–39. doi: 10.1164/artpd.1957.76.4.517. [DOI] [PubMed] [Google Scholar]
- 121.Price WA. Nutrition and Physical Degeneration A Comparison of Primitive and Modern Diets and Their Effects. 6th ed. Chicago, IL: McGraw-Hill; 2003. [Google Scholar]
- 122.Downes J. An experiment in the control of tuberculosis among Negroes. Milbank Mem Fund Q. 1950;28(2):127–59. [PubMed] [Google Scholar]
- 123.Scrimshaw NS, Taylor CE, Gordon JE. Interactions of Nutrition and Infection. Geneva, Switzerland: World Health Organization; 1968. [PubMed] [Google Scholar]
- 124.Dubos RJ. Effect of metabolic factors on the susceptibility of albino mice to experimental tuberculosis. J Exp Med. 1955;101(1):59–84. doi: 10.1084/jem.101.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013 Sep 1; doi: 10.1038/ng.2744. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lehrman S. The Diabolical Genius of an Ancient Scourge. Scientific American. 2013 Jul;:69–73. doi: 10.1038/scientificamerican0713-80. [DOI] [PubMed] [Google Scholar]
- 127.Trindade MÂ Miyamoto D, Benard G, Sakai-Valente NY, de Vasconcelos DM, Naafs B. Leprosy and tuberculosis co-infection: clinical and immunological report of two cases and review of the literature. Am J Trop Med Hyg. 2013;88(2):236–40. doi: 10.4269/ajtmh.2012.12-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zeisel SH. Diet-gene interactions underlie metabolic individuality and influence brain development: implications for clinical practice derived from studies on choline metabolism. Ann Nutr Metab. 2012;60(Suppl 3):19–25. doi: 10.1159/000337310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lister R, Mukamel EA, Nery JR, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341(6146):1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pontali E, Matteelli A, Migliori GB. Drug-resistant tuberculosis. Curr Opin Pulm Med. 2013;19(3):266–72. doi: 10.1097/MCP.0b013e32835f1bf3. [DOI] [PubMed] [Google Scholar]
- 131.Kumar JS, Subramanian VS, Kapadia R, Kashyap ML, Said HM. Mammalian colonocytes possess a carrier-mediated mechanism for uptake of vitamin B3 (niacin): studies utilizing human and mouse colonic preparations. Am J Physiol Gastrointest Liver Physiol. 2013;305(3):G207–13. doi: 10.1152/ajpgi.00148.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grathwohl SA, Steiner JA, Britschgi M, Brundin P. Mind the gut: secretion of α-synuclein by enteric neurons. J Neurochem. 2013;125(4):487–90. doi: 10.1111/jnc.12191. [DOI] [PubMed] [Google Scholar]
- 133.Ulanovskaya OA, Zuhl AM, Cravatt BF. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat Chem Biol. 2013;9(5):300–6. doi: 10.1038/nchembio.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tan B, Young DA, Lu ZH, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, in human cancer cells: metabolic basis and potential clinical implications. J Biol Chem. 2013;288(5):3500–11. doi: 10.1074/jbc.M112.394510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zitron IM, Kamson DO, Kiousis S, Juhász C, Mittal S. In vivo metabolism of tryptophan in meningiomas is mediated by indoleamine 2,3-dioxygenase 1. Cancer Biol Ther. 2013;14(4):333–9. doi: 10.4161/cbt.23624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104(3):275–81. doi: 10.1111/cas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sartini D, Morganti S, Guidi E, et al. Nicotinamide N-methyltransferase in non-small cell lung Cell Lung Cancer: Promising Results for Targeted Anti-cancer: promising results for targeted anti-cancer therapy. Cell Biochem Biophys. doi: 10.1007/s12013-013-9574-z. Epub Mar 27, 2013. [DOI] [PubMed] [Google Scholar]
- 138.Li D, Luo N, Ma Q, et al. Excessive nictonic acid increases methyl consumption and hydrogen peroxide generation in rats. Pharm Biol. 2013;51(1):8–12. doi: 10.3109/13880209.2012.697175. [DOI] [PubMed] [Google Scholar]
- 139.Stone R. Public enemy number one. Science. 2013;340(6131):422–5. doi: 10.1126/science.340.6131.422. [DOI] [PubMed] [Google Scholar]