Abstract
Many studies have shown that TLR4 and TLR2 deficient mice are protected from high fat diet-induced inflammation and insulin resistance, suggesting that saturated fatty acids derived from the high fat diet activate TLR-mediated proinflammatory signaling pathways and induce insulin resistance. However, evidence that palmitic acid, the major dietary saturated fatty acid, can directly activate TLR has not been demonstrated. Here we present multiple lines of evidence showing that palmitic acid directly activates TLR2, a major TLR expressed on human monocytes, by inducing heterodimerization with TLR1 in a NADPH oxidase-dependent manner. Dimerization of TLR2 with TLR1 was inhibited by the n-3 fatty acid docosahexaenoic acid. Activation of TLR2 by palmitic acid leads to expression of pro-IL-1β that is cleaved by caspase-1, which is constitutively present in monocytes, to release mature IL-1β. Our results reveal mechanistic insight about how palmitic acid activates TLR2, upregulates NALP3 expression, and induces inflammasome-mediated-IL-1β production in human monocytes which can trigger enhanced inflammation in peripheral tissues, and suggest that these processes are dynamically modulated by the types of dietary fat we consume.
Introduction
Chronic inflammation is one of the key etiological conditions for the development and progression of many chronic diseases. Low-grade chronic inflammation (LGC inflammation) characterized by elevated circulating concentrations of proinflammatory cytokines, acute-phase proteins and adhesion molecules is known to be associated with obesity and insulin resistance. What causes and mediates LGC inflammation and how it can be suppressed through dietary means which can provide preventive efficacy for inflammatory chronic disease are challenging questions.
Blood monocytes are sentinel immune effector cells that detect and respond to invading pathogens and dietary components absorbed from the gut. Activated blood monocytes can extravasate into peripheral tissues and become resident macrophages or dendritic cells (DCs) that can trigger inflammatory signals in response to exogenous and endogenous stimuli (1, 2). Recent animal studies have shown that a high fat diet increases infiltration of macrophages and dentritic cells, that originate from circulating blood monocytes, in adipose tissue where they play a central role in the development of chronic inflammation and insulin resistance (3). Therefore, activation of proinflammatory pathways in circulating blood monocytes is the gateway toward enhanced inflammation in various tissues. IL-1β is a major cytokine involved in the activation of blood monocyte and proinflammatory signaling pathways in peripheral tissues. Unlike other proinflammatory cytokines, IL-1β production is tightly regulated by a unique two-signal mechanism. The primary signals induce the expression of pro-IL-1β mediated in part through the activation of Toll-like receptors (TLRs). The secondary signals activate the nucleotide-binding oligomerization domain-like receptor (NLR) pyrin domain-containing 3 (NALP3) inflammasome, an intracellular signaling complex composed of NALP3, pro-caspase-1, and the adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC). Formation of the inflammasome complex results in proteolytic cleavage of pro-caspase-1 to yield active caspase-1 which in turn cleaves pro-IL-1β producing mature and active IL-1β. Mature IL-1β released extracellularly binds its cognate receptor in a paracrine manner resulting in amplification of proinflammatory responses (4–7). However, recent findings suggest that the two-signal model for NALP3 inflammasome-mediated IL-1β secretion may not be applicable to all IL-1β secreting cells including blood monocytes and dendritic cells. Tissue macrophages require both primary and secondary signals for NALP3 inflammasome-mediated IL-1β secretion. In contrast, blood monocytes have constitutively active NALP3 inflammasome; therefore, TLR stimulation alone is sufficient to induce inflammasome-mediated IL-1β production in monocytes (8–10). Similarly, both bone marrow-derived and splenic dendritic cells secrete substantial amounts of inflammasome-mediated IL-1β upon TLR activation in the absence of secondary signals (3, 11). Therefore, regulation of NALP3 inflammasome-mediated IL-1β production in mononuclear phagocytes appears to be cell-type specific.
Pattern recognition receptors (PRRs) induce innate immune responses by recognizing invariant pathogen-associated molecular patterns (PAMPs) leading to activation of downstream signaling pathways and the expression of a diverse array of proinflammatory gene products that are required for host defense against invading pathogens. In addition, PRRs are activated by endogenous molecules derived from tissue injury and elicit sterile inflammation to initiate wound-healing processes (12, 13). Activation of PRRs can also be modulated by dietary components and their metabolites bridging immune responses to metabolic homeostasis (3, 14–19).
Our previous studies first revealed that a medium chain saturated fatty acid, lauric acid can activate TLR4, TLR2, and NOD2-mediated signaling pathways; whereas, the n-3 fatty acid, docosahexaenoic acid (DHA) inhibits lauric acid-induced activation of TLRs or NOD-mediated signaling pathways (15, 20–26). Many studies with animal models also showed that TLR4 or TLR2 deletion or mutant mice were protected from high fat diet-induced inflammation and insulin resistance (3, 14, 16, 27–31) suggesting that high saturated fat diet activates TLR-mediated proinflammatory signaling pathways and induces insulin resistance. However, evidence that saturated fatty acids derived from high fat diet can directly activate TLRs in humans has not been demonstrated. Here, we present multiple lines of evidence that palmitic acid, the major dietary saturated fatty acid, directly activates TLR2, a predominant TLR expressed on blood monocytes, by inducing heterodimerization with TLR1. The dimerization of TLR2 with TLR1 as assessed by time resolved-fluorescence resonance energy transfer (TR-FRET) was inhibited by docosahexaenoic acid (DHA). Activation of TLR2 leads to expression of pro-IL-1β that is cleaved by caspase-1, which is activated by constitutively active inflammasome, to release mature IL-1β in blood monocytes. Our results reveal mechanistic insight about how the saturated fatty acid, palmitic acid, induces inflammasome-mediated-IL-1β production and suggest that inflammasome-mediated IL-1β production in human blood monocytes, which can trigger enhanced inflammation in peripheral tissues, is dynamically modulated by the types of dietary fat we consume.
Materials and Methods
Reagents
LPS (cat #: 421) was purchased from List Biological Laboratories, Inc. (Campbell, CA). Pam3CSK4 (cat #: tlrl-pms) and TAK-242 (cat #: tlrl-cli95) were purchased from Invivogen (San Diego, CA). Bovine serum albumin (BSA, cat #: 30-AB79, Lot #: A10072001) was purchased from Fitzgerald Industries International (Acton, MA). Palmitic acid (cat #: P5585), sodium palmitate (cat #: P9767), sodium salt docosahexaenoic acid (DHA) (cat #: D8768), Lipoprotein Lipase (LPL) (cat #: L2254), endotoxin free water, polymixin B (cat #: P4932) and antibody for β-actin were purchased from Sigma (Saint Louis, MO). Apocynin (cat #: 178385) and Ac-YVAD-AOM (cat #: 400015) were purchased from EMD Chemicals Inc. (Darmstadt, Germany).
Cell Culture
THP-1 cells (human monocytic cell line, ATCC TIB-202) were cultured in RPMI-1640 medium (ATCC, cat #: 30–2001) containing 10% (v/v) Fetal Bovine Serum (FBS, Premium Select, cat #: S11550, Lot#: K0109 and K11050, Atlanta Biologicals, Lawrenceville, GA), 0.05 mM 2-mercaptoethanol, 100 units/ml penicillin, and 100 mg/ml streptomycin. Cells were maintained at 37°C in a 5% CO2/air environment. Cells were seeded at 1 × 106/ml and incubated in serum-poor medium (0.25% and 1.0% FBS) prior to treatment.
Fatty Acid Preparations
A stock solution of 100 mM sodium palmitate (C16:0) was prepared in 70% ethanol in a glass vial and heated to 60°C. Sodium palmitate was reheated to 60°C and vortexed before use to give appropriate final concentration indicated in figures. A stock solution of 10 mM DHA sodium salt was prepared in endotoxin free water, flushed with nitrogen, then sealed in air tight tube and stored at −20°C until use. Palmitic acid solubilized with BSA (C16:0-BSA) was carried out as previously described (32). Briefly, palmitic acid was dissolved in 100% ethanol (250 mM or 500 mM) then mixed with BSA in a 10:1 molar ratio in 0.25% FBS/RPMI-1640 medium. The mixture was sonicated in a water bath for 15 minutes then rocked at 55°C for 15 minutes. BSA-solubilized palmitic acid was filtered through a 0.22 µm filter before use.
We used sodium palmitate instead of BSA-solubilized palmitic acid in our studies because we initially found that many commercially available BSA preparations contain contaminants with agonist activity for certain pattern recognition receptors (PRRs) (32). We also found that sodium palmitate is able to stimulate PRR-mediated signaling pathways in most suspension cells such as THP-1 monocytes and primary human blood monocytes. However, palmitic acid needs to be solubilized with albumin (BSA) to stimulate PRR-mediated signaling pathways in adherent cells. After recently discovering a BSA preparation (Fitzgerald Industries International; cat #: 30-AB79, Lot #: A10072001) that did not significantly induce PRR target gene products, we found that BSA-solubilized palmitic acid showed similar results as sodium palmitate with regard to IL-1β production in THP-1 cells (Fig. S1).
Plasmids
Expression vectors pDisplay, pDisplay-TLR1, pDisplay-TLR2, pDisplay-TLR4, and pDisplay-TLR6 were obtained from Adeline Hajjar (University of Washington, Seattle, WA). MD2 was provided by Kensuke Miyake (Tokyo University, Japan). (2x)-NF-κB-luciferase reporter construct was provided by Frank Mercurio (Signal Pharmaceuticals, San Diego, CA). pRSV-β-galactosidase plasmid was from Jongdae Lee (University of California, San Diego, CA). The plasmid DNA from these expression vectors was prepared in large scale for transfection using the EndoFree Plasmid Maxi Kit (Qiagen, Valencia, CA).
Transfections and Luciferase Assays
Transient transfections were carried out using SuperFect transfection reagent (Qiagen) according to the manufacturer’s recommendations. HEK293T cells were seeded at 2 × 105 per well in 24-well plates and co-transfected the following day with 10 ng each of pDisplay-TLR4 and MD2, or pDisplay-TLR2 and pDisplay-TLR1, or pDisplay-TLR2 and pDisplay-TLR6, in addition to 50 ng (2x)-NF-κB-luciferase reporter and 10 ng pRSV-β-galactosidase expression vectors. Twenty nanograms (20 ng) of pDisplay empty vector was used in addition to the above amounts of (2x)-NF-κB and pRSV-β-galactosidase for transfection as controls. Twenty-four hours after transfection the cells were serum starved in 0.25% FBS/DMEM medium for 6 hours followed by 12 hour treatment with BSA-solubilized palmitic acid in the same low serum medium. The cells were lysed. Luciferase and β-galactosidase enzyme activities were determined from the lysate supernatants using the luciferase and β-galactosidase enzyme assay system (Promega, Madison, WI) according to the manufacturer’s instructions. Luciferase activity was normalized by β-galactosidase activity to correct differences in transfection efficiency among samples. Each experiment was repeated at least three times.
Immunoblot and ELISA Assays
Immunoblotting was performed as previously described (17). Briefly, THP-1 cells were lysed by sonication in cell lysis buffer (Cell Signaling Technology, Danvers, MA) containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, and 1 µg/ml leupeptin plus 1 mM phenylmethylsulfonyl fluoride. Lysate supernatants were collected by centrifugation and subjected to 10% or 10–20% SDS-PAGE followed by protein transfer to polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). The membrane was blocked in TBST buffer [20 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 0.05% (v/v) Tween 20] containing 5% nonfat milk or BSA. The membrane was probed with primary antibody for 1 hour at room temperature or overnight at 4°C followed by incubation with horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ) for 1 hour at room temperature. Proteins were detected by ECL Western blot detection reagents (Amersham Biosciences) followed by exposure to autoradiography film (BioExpress, Kaysville, UT). Anti-IL-1β (3ZD) antibody was obtained from the National Cancer Institute Preclinical Repository, anti-caspase-1 p10 (C-20) and anti-IκBα (C-21) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-NALP3 (cat #: ALX-804–881) antibody was purchased from Enzo Life Science (Plymouth Meeting, PA). Anti-JNK (cat #: 9252), anti-phospho-JNK (cat #: 9251), anti-ERK (cat #: 9102), anti-phospho-ERK (cat #: 9101), anti-phospho-IκBα (cat #: 2859), anti-NF-κB p65 (cat #: 3034), anti-phospho-NF-κB p65 (cat #: 3033), and anti-MyD88 (cat #: 4283) antibodies were purchased from Cell Signaling Technology. Anti-Flotillin-1 (cat #: 610820) and anti-p47phox (cat #: 610355) antibodies were purchased from BD Biosciences (San Jose, CA). Anti-Transferrin Receptor (cat #: 13–6890) antibody was purchased from Life Technologies Corporation (Grand Island, NY). Cell culture supernatants were analyzed for IL-1β using BD OptEIA Human IL-1β Enzyme-linked immunosorbent assay (ELISA) using a Synergy 2 plate reader (BioTek, Winooski, VT) according to the manufacturer’s instructions. To verify that secreted IL-1β in cell culture supernatants was the 17-kDa bioactive form, we concentrated the supernatants using Amicon Ultra Centrifugal Filters (Millipore, Billerica, MA) according to the manufacturer’s instructions then performed immunoblot by which pro-IL-1β and bioactive IL-1β can be separated by size difference. The immunoblots showed only 17-kDa IL-1β indicating that IL-1β in the supernatant is mostly bioactive IL-1β.
Confocal Microscopy
ROS was examined with a Zeiss LSM 510 confocal microscope. THP-1 cells were serum starved in 0.25% FBS-RPMI-1640 for 12 hours then treated with CM-H2DCFDA (10 µM) in pre-warmed PBS for 30 min at 37°C. Cells were washed three times with warm PBS then treated with indicated concentration of sodium palmitate for 20 minutes in 0.25% FBS-RPMI-1640. Cells were then washed with ice cold PBS, fixed in 10% formalin for 30 minutes at 4°C, and washed again with ice cold PBS. The cells were mounted on glass slides and analyzed with a 40 × 1.3 oil objective lens using laser excitation at 488 nm.
TLR2 siRNA Transfection Assays
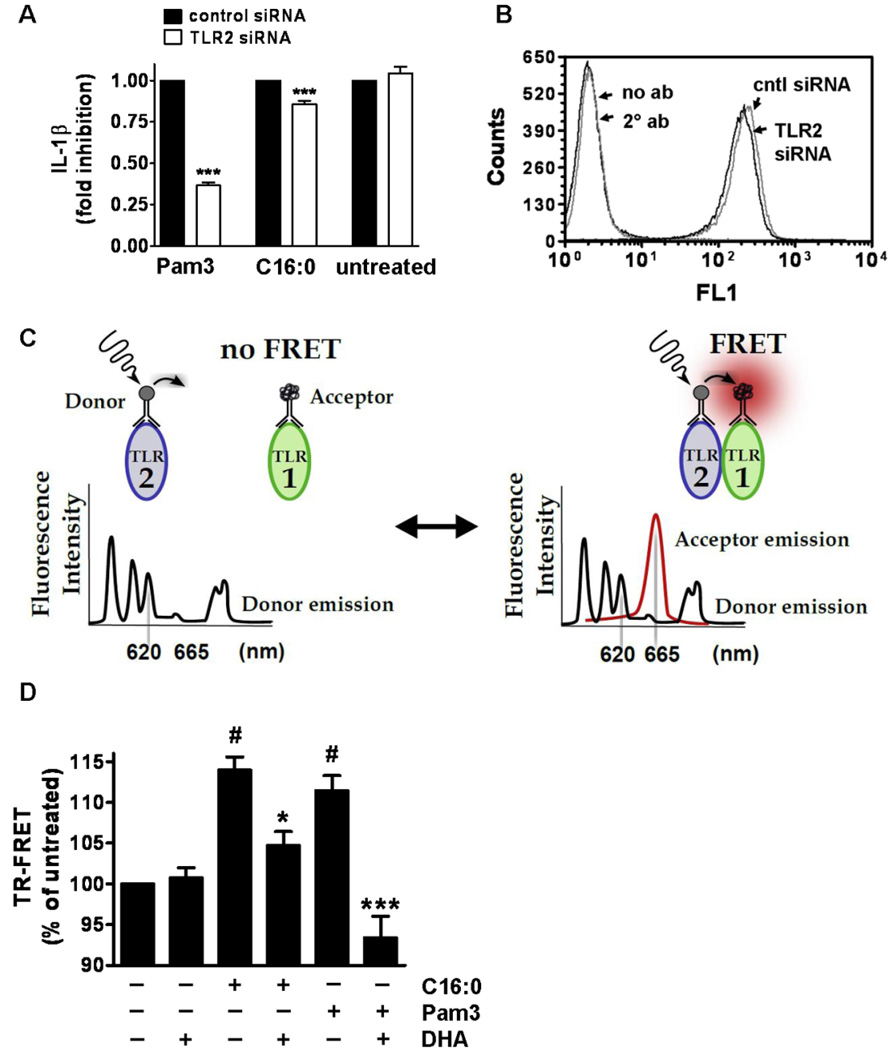
Silencer Select pre-designed and pre-validated negative control and TLR2 siRNAs (cat #: 4390843 and 4392420, respectively) were purchased from Life Technologies Corporation. THP-1 cells were transiently transfected with siRNAs (25 nM) using Lipofectamine RNAiMAX (cat #: 13778) reagent following manufacturer’s instructions. Cell surface TLR2 expression was analyzed by flow cytometry 60 hours after transfection then cells were treated with sodium palmitate (150 µM) and Pam3CSK4 (10 ng/ml) for 24 hours. Cell culture supernatants were collected and analyzed for IL-1β by ELISA. For cell surface expression of TLR2 by flow cytometry, transfected THP-1 cells were stained for 30 minutes on ice with anti-TLR2 antibody (clone TL2.1) then analyzed with a FACScaliber flow cytometer by collecting 50,000 events in the FL1 channel.
Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) Assays to Determine TLR2/1 Dimerization
Anti-TLR2 (clone TL2.1, cat #: 16–9922–82) and anti-TLR1 (clone GD2.F4, cat #: 16–9911–82) antibodies extensively characterized in traditional FRET experiments (33–36) were purchased from eBioscience (San Diego, CA) and custom labeled with europium cryptate (donor) and proprietary d2 dye (acceptor), respectively by CisBio Bioassays (Bedford, MA). THP-1 cells (106 cells/ml) were serum starved in 0.25% FBS-RPMI-1640 with 44.6 ng/ml TLR2-europium cryptate and 220 ng/ml of TLR1-d2 antibodies (donor:acceptor ratio of 1:5) for 12 hours. Cells were washed in PBS to remove unbound antibody then resuspended in 0.25% FBS-RPMI-1640. Cells were pretreated for 1 hour if applicable then treated for 10 minutes. Cells were washed in PBS then fixed in 1% paraformaldehyde on ice for 20 minutes. Cells were washed again in PBS then placed in incubation buffer (calcium and magnesium-free DPBS with 400 mM potassium fluoride and 0.1% BSA) at a concentration of 3×106/ml. Fifty microliters per well was loaded into a half-area white 96 well plate and analyzed on a Synergy 2 plate reader (BioTek) configured with a 330/80 nm excitation filter and 620/10 and 665/8 emission filters. Fluorescence of the europium cryptate donor and d2 acceptor were measured respectively at 620 and 665 nm (100 µsec time delay, 300 µsec integration) upon 330 nm excitation. The 620 and 665 nm sample emissions were corrected for background by subtracting the fluorescence of incubation buffer at the respective wavelengths. The energy transfer ratio for each individual well was calculated using the formula: (signal 665 nm/signal 620 nm) x 104. Of the two sequential measurements carried out, the time resolved fluorescence emission measured at 620 nm is used as an internal reference correlated with cell surface TLR2 expression levels. At the same time, the d2 emission measured at 665 nm upon 620 nm excitation signifies energy transfer through proximity of the labeled TLR1 and TLR2 antibodies. The principle of TR-FRET is illustrated in Figure 5C. All experimental samples were run in triplicate.
Figure 5. Palmitic acid-induced IL-1β secretion in THP-1 cells is mediated through activation of TLR2.
(A) THP-1 cells were transfected with negative control or TLR2 siRNA. After 48 hours cells were serum starved in 0.25% FBS-RPMI-1640 for 12 hours then treated with C16:0 (150 µM) or Pam3CSK4 (10 ng/ml) for 24 hours. Supernatants were analyzed for IL-1β by ELISA. Data are presented as mean percentages of control siRNA treated cells ± SEM and were calculated from three independent experiments. (***P < 0.001) Significance was determined by two-tailed, unpaired t-test. (B) TLR2 cell surface expression on THP-1 cells was measured 60 hours after transient transfection with siRNAs by flow cytometry. Data is representative of at least 3 independent experiments. (C) Schematic of the TR-FRET assays performed to detect dimerization of TLR2 with TLR1 using anti-TLR2 antibodies labeled with europium cryptate as the donor fluorophore and anti-TLR1 antibodies labeled with d2 as the acceptor fluorophore. (D) THP-1 cells were serum starved in 0.25% FBS-RPMI-1640 for 12 hours with TLR2 and TLR1 antibodies then incubated with DHA (10 µM) for one hour and treated with C16:0 (150 µM) or Pam3CSK4 (100 ng/ml) for 10 minutes. TR-FRET data are presented as mean percentages of untreated cells ± SEM and were calculated from at least five independent experiments. Significance was determined by ANOVA (#P < 0.001 significantly different from untreated, *P < 0.05, ***P < 0.001 significantly different from C16:0 and Pam3, respectively).
Isolation of Lipid Raft Fractions using Sucrose Gradient Ultracentrifugation
THP-1 cells (5×107) stimulated with palmitic acid and Pam3CSK4 were used to isolate lipid rafts. Cells were incubated in serum-poor RPMI-1640 (1.0% FBS) for 12 hours prior to treatment. Lipid rafts were isolated by lysing cells in 750 µl of TNE lysis buffer (25mM Tris-HCl, pH 7.5, 150 mM NaCl, and 5 mM EDTA, with 1% Triton X-100, 1 mM sodium orthovanadate, 5 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, and 10 µg/ml each of aprotinin and leupeptin) for 30 minutes on ice followed by passage through a 25 gauge needle. Lysates were mixed with an equal volume of 80% sucrose in TNE buffer and placed in an ultracentrifuge tube. Two ml of 30% sucrose in TNE buffer was then overlaid followed by 1 ml of 5% sucrose in TNE buffer. Centrifuge tubes were placed in an SW60 rotor in a Beckman L-70 Ultracentrifuge and spun for 16 hours at 40,000 rpm at 4°C using maximum acceleration and no brake conditions. Following fractionation eight 500-µl fractions were collected from top down. For immunoblotting, fractionated samples were mixed with sample buffer and subjected to electrophoresis.
Preparation of Triglyceride-Rich Lipoproteins (TGRL) and TGRL Lypolysis Products
Postprandial blood samples were obtained from healthy male and female volunteers 3.5 hours after consumption of a standard moderately high-fat meal (40% calories from fat). This time point has been shown to correlate with elevated circulating TGRL (37, 38). All procedures were conducted under a protocol approved by the Human Subjects Institutional Review Committee at the University of California Davis. Written informed consent was obtained from all study subjects before participating. Blood was collected in 10 mL Vacutainer tubes containing K2-EDTA (BD Biosciences). TGRL isolated as previously described (39–41) contained ≤0.5 endotoxin unit/mL according to a Pyrochrome kit from Associates of Cape Cod (Falmouth, MA). Samples were stored over nitrogen gas in sealed tubes at 4°C. For preparation of TGRL lipolysis products, TGRL (50 mg/dL triglycerides) was subjected to enzymatic lipolysis with bovine lipoprotein lipase (LPL) (5 units/ml) in 0.25% (for THP-1 cell treatments) or 2% (for primary monocyte treatments) HI-FBS RPMI-1640 for 60 minutes at 37°C. The conditioned media was immediately used for incubations with cells in culture for indicated times.
Isolation of Primary Human Monocytes and Treatment with Fatty Acids
Whole blood was collected in 10 mL Vacutainer tubes containing sodium heparin (BD Biosciences). Blood was centrifuged at 1400 x g for 10 minutes at room temperature. Plasma was removed and buffy coats were collected, mixed with an equal volume of HBSS, then overlayed on an equal volume of Histopaque-1077 (Sigma) and centrifuged at 400 x g for 30 minutes. Peripheral blood mononuclear cells were isolated, washed twice with HBSS then resuspended at 1×106 monocytes/ml in serum-free RPMI-1640. After two hours at 37°C in a 5% CO2/air environment the medium containing nonadherent cells was aspirated, the attached monocytes were washed twice with HBSS to remove residual nonadherent cells, and then cultured in HI-FBS RPMI-1640. Monocytes were then treated with palmitic acid or TGRL lipolysis products for 24 hours. Supernatants were collected and analyzed for IL-1β by ELISA.
Treatment of Whole Blood with Lipoprotein Lipase (LPL)
To study whether endogenous saturated fatty acids derived from enzymatic hydrolysis of triglyceride-rich lipoproteins can induce inflammasome-mediated IL-1β release, blood from healthy male and female volunteers, as approved by the Human Subjects Institutional Review Committee of the University of California Davis was drawn and prepared at the Western Human Nutrition Research Center-United States Department of Agriculture (WHNRC-USDA). Written informed consent was obtained from all study subjects before participating. Fasting and 3.5 hours postprandial whole blood following consumption of a 630 calorie high fat breakfast containing 40% total fat (20% saturated fat), 16% protein, and 44% carbohydrate was collected in a 10 ml Vacutainer tube containing sodium heparin (BD Biosciences) then diluted 1:1 with serum-free RPMI-1640. If necessary, RPMI-1640 diluted whole blood was pretreated with DHA (10 µM) for 1 hour then subjected to enzymatic lipolysis with bovine lipoprotein lipase (LPL) (4 units/ml) for 24 hours at 37°C in a 5% CO2/air environment. Supernatants were collected and analyzed for IL-1β by ELISA.
Statistical Analyses
One-way ANOVA was used to determine significance of TR-FRET and IL-1β concentration differences in cell culture supernatants. Tukey’s multiple comparison test was used as a post test if any differences were noted with ANOVA. Differences in IL-1β concentrations in whole blood assays were determined using two-way repeated measures ANOVA. Factors included metabolic state (fasting and postprandial) and treatment (control, LPL, and LPL + DHA), and the interaction of metabolic state x treatment. Post hoc comparison was accomplished with Bonferroni posttests. Differences within each metabolic state of the whole blood assays were determined using repeated measures ANOVA followed by Tukey’s multiple comparison test (GraphPad Software, La Jolla, CA). A P value of < 0.05 was considered statistically significant.
Results
Palmitic acid induces the expression of pro-IL-1β and secretion of mature IL-1β in THP-1 monocytes
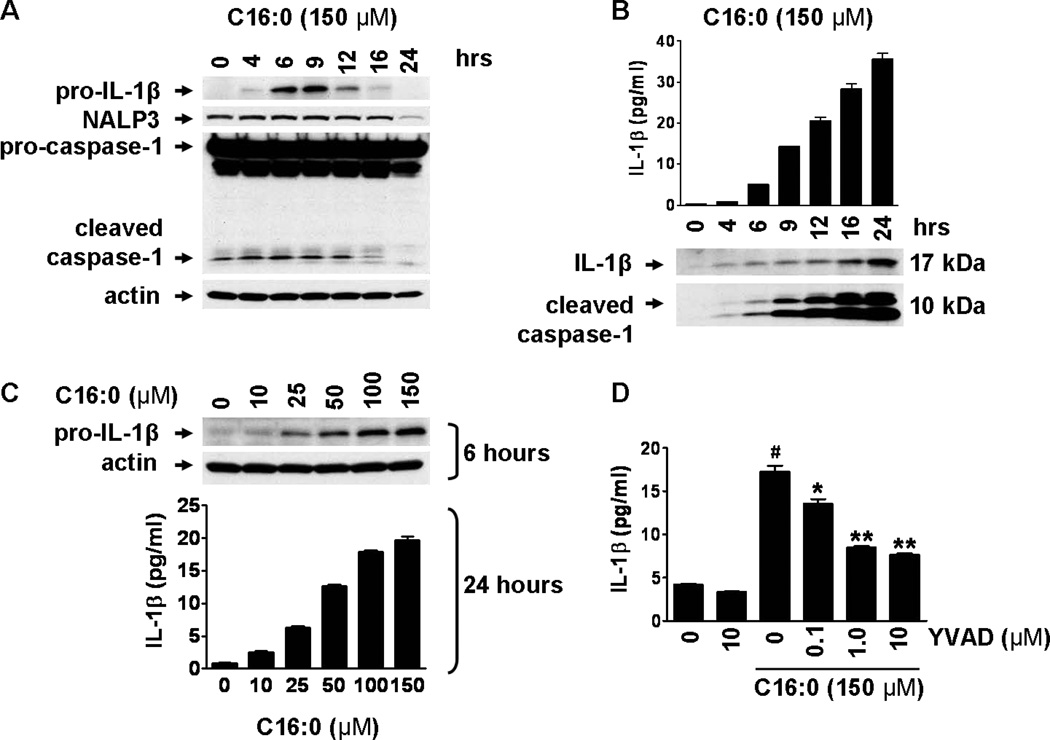
Sodium palmitate treatment led to the expression of pro-IL-1β in a time- and dose-dependent manner in THP-1 cells whereas untreated cells showed undetectable levels of pro-IL-1β (Fig. 1A,C). Sodium palmitate also induced secretion of mature IL-1β in cell culture supernatant (Fig. 1B,C), which requires caspase-1 for the cleavage of pro-IL-1β. BSA-solubilized palmitic acid also induced secretion of mature IL-1β in the supernatant in a dose-dependent manner (Fig. S1A). Inhibition of caspase-1 activity by Ac-YVAD-AOM reduced sodium palmitate-mediated secretion of IL-1β (Fig. 1D). Untreated THP-1 monocytes contain both pro-caspase-1 and inflammasome-mediated active caspase-1 (Fig. 1A). These results suggest that unstimulated monocytes possess constitutively active inflammasome. Therefore, primary signals inducing the expression of pro-IL-1β mediated by the activation of TLRs are sufficient for the production of IL-1β in monocytes. In this respect, monocytes differ from macrophages which do not contain constitutively active inflammasome, and thus require the secondary signals for the activation of inflammasome and the secretion of mature IL-1β (9–11). Untreated THP-1 cells also express NALP3 (Fig. 1A) which was enhanced by sodium palmitate treatment (Fig. S2) suggesting that palmitate not only provides the primary signal leading to the expression of pro-IL-1β but also potentiates the activation of inflammasome by upregulating the expression of NALP3.
Figure 1. Palmitic acid induces pro-IL-1β expression and inflammasome-mediated IL-1β secretion in THP-1 monocytes.
(A and B) THP-1 cells were serum starved in 0.25% FBS-RPMI-1640 for 12 hours then treated with C16:0 (150 µM) for the indicated times. (A) Cell lysates were immunoblotted for pro-IL-1β, NALP3, and caspase-1. (B) IL-1β in cell culture supernatant was analyzed by ELISA and immunoblot. Cleaved caspase-1 in supernatant was analyzed by immunoblot. (C) Serum starved THP-1 cells were treated with indicated concentrations of C16:0. After 6 hours cell lysates were immunoblotted for pro-IL-1β. After 24 hours cell culture supernatants were analyzed for IL-1β by ELISA. Data in B and C are expressed as mean ± s.d. and are representative of three independent experiments with similar results. (D) Serum starved THP-1 cells were incubated with caspase-1 inhibitor (Ac-YVAD-AOM) for 1 hour then treated with C16:0 for 24 hours. IL-1β in supernatant was analyzed by ELISA. Data are expressed as mean ± SEM of three independent experiments. Significance was determined by ANOVA (#P < 0.001 significantly different from untreated, *P < 0.05, **P < 0.01 significantly different from C16:0).
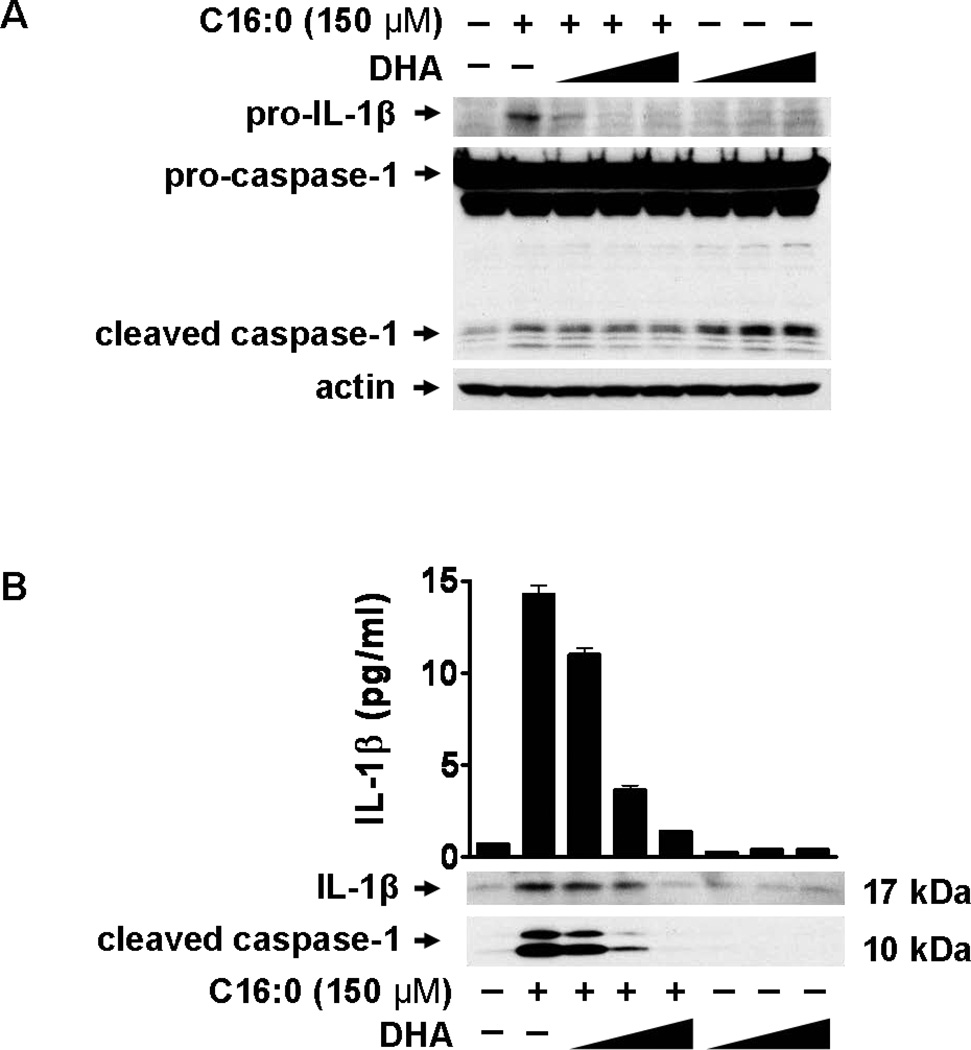
Docosahexaenoic acid (DHA) inhibits palmitic acid-induced expression of pro-IL-1β and consequent release of mature IL-1β in monocytes
Our previous studies showed that DHA is a pan inhibitor for TLR activation (20, 21, 23–25). Therefore, we determined whether the n-3 polyunsaturated fatty acid (PUFA) DHA inhibits palmitic acid-induced expression of pro-IL-1β and secretion of mature IL-1β. Pre-treating THP-1 cells with DHA dose-dependently inhibited the expression of pro-IL-1β after a 6 hour treatment with sodium palmitate (150 µM) (Fig. 2A), and the secretion of active IL-1β and caspase-1 after a 24 hour treatment (Fig. 2B). Pre-treating THP-1 cells with DHA also dose-dependently inhibited the secretion of mature IL-1β after a 24 hour treatment with BSA-solubilized palmitic acid (Fig. S1B). These results imply that DHA inhibits palmitic acid-induced activation of TLRs (primary signal) and the subsequent expression of pro-IL-1β.
Figure 2. Palmitic acid induces but Docosahexaenoic acid (DHA) inhibits the expression of pro-IL-1β and inflammasome-mediated IL-1β production.
(A and B) THP-1 cells were serum starved in 0.25% FBS-RPMI-1640 for 12 hours. Cells were incubated with DHA for 1 hour then treated with C16:0 (150 µM). (A) After 6 hours cell lysates were immunoblotted for pro-IL-1β and caspase-1. (B) After 24 hours cell culture supernatants were analyzed for IL-1β by immunoblot and ELISA. Secreted caspase-1 was analyzed by immunoblot. Data are expressed as mean ± s.d. and are representative of three independent experiments with similar results.
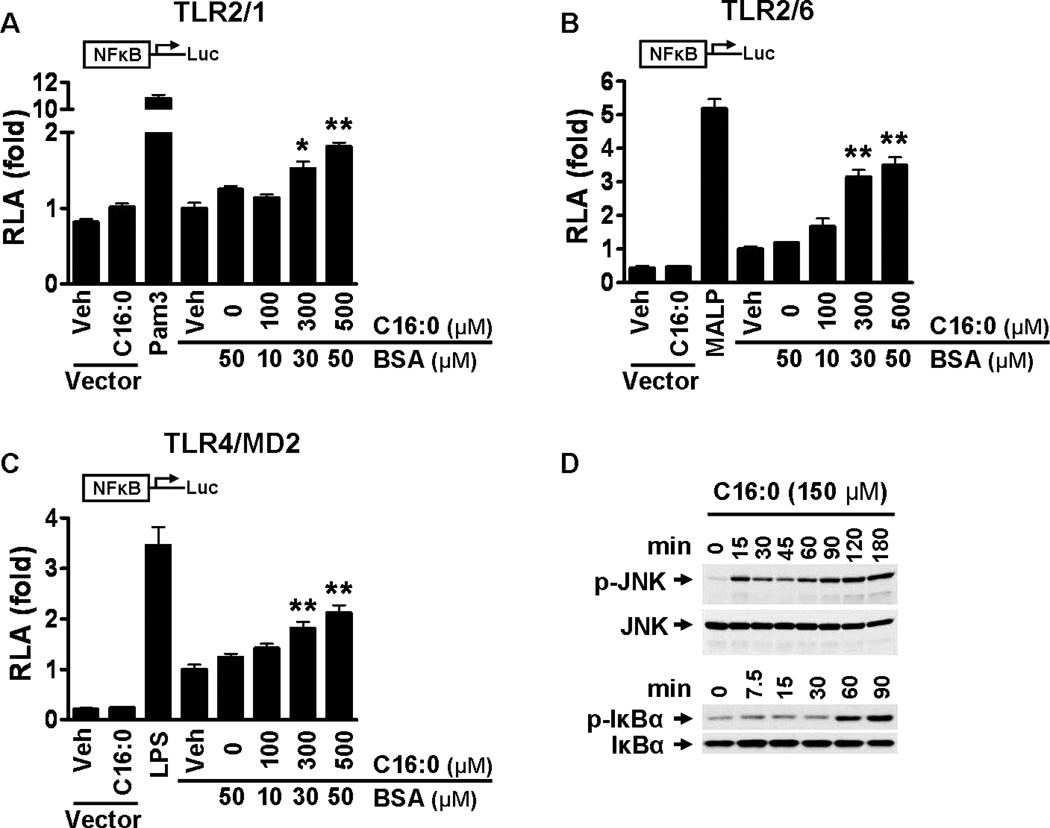
Palmitic acid induces NF-κB activation through TLR2 dimerized with TLR1 or TLR6 and through TLR4 and activates downstream signaling pathways of pattern recognition receptors (PRRs)
Pro-IL-1β is one of the TLR target gene products. To determine whether palmitic acid-induced expression of pro-IL-1β is mediated through activation of TLRs we transfected cells with expression plasmids of TLRs known to recognize ligands containing saturated lipid chains. HEK293T cells cotransfected with TLR2 and TLR1, TLR2 and TLR6, and TLR4 and MD-2 in addition to NF-κB-luciferase reporter and β-galactosidase expression vectors were treated with BSA-solubilized palmitic acid (C16:0-BSA) (Fig. 3A–C). BSA-solubilized palmitic acid dose-dependently induced NF-κB activation through all transfected TLRs indicating palmitic acid-induced pro-IL-1β is at least in part mediated through activation of TLR2 and 4. Treatment of THP-1 cells with sodium palmitate activated the downstream signaling pathways of TLRs, leading to enhanced phosphorylation of JNK and IκBα (Fig. 3D).
Figure 3. Palmitic acid induces NF-κB activation through TLR2 dimerized with TLR1 or TLR6 and through TLR4 and activates the downstream signaling pathways of pattern recognition receptors (PRRs).
(A-C) HEK293T cells were cultured in 10% FBS/DMEM medium and cotransfected with TLR2 and TLR1 (A), TLR2 and TLR6 (B), or TLR4 and MD2 (C) in addition to NF-κB-luciferase reporter and β–galactosidase expression vectors. After 24 hours the cells were serum-starved in 0.25% FBS/DMEM for 6 hours and then treated with C16:0-BSA for 12 hours. The cell lysates were assayed for luciferase and galactosidase activities. Values are expressed as RLA (relative luciferase activity). Controls: Pam3 (Pam3CSK4, TLR2/1 agonist, 10 ng/ml), MALP (MALP-2, TLR2/6 agonist, 10 ng/ml), LPS (TLR4 agonist, 50 ng/ml). pDisplay empty vector was used as a negative control. Data are expressed as mean ± SEM of three independent experiments. Significance was determined by unpaired, two-tailed, t test (*P < 0.05, **P < 0.01 significantly different from 50 µM BSA). (D) THP-1 cells were serum starved in 0.25% FBS-RPMI-1640 for 12 hours. Cells were treated with C16:0 (150 µM) for the indicated times and cell lysates were immunoblotted for phosphorylated JNK, total JNK, phosphorylated IκBα, and total IκBα.
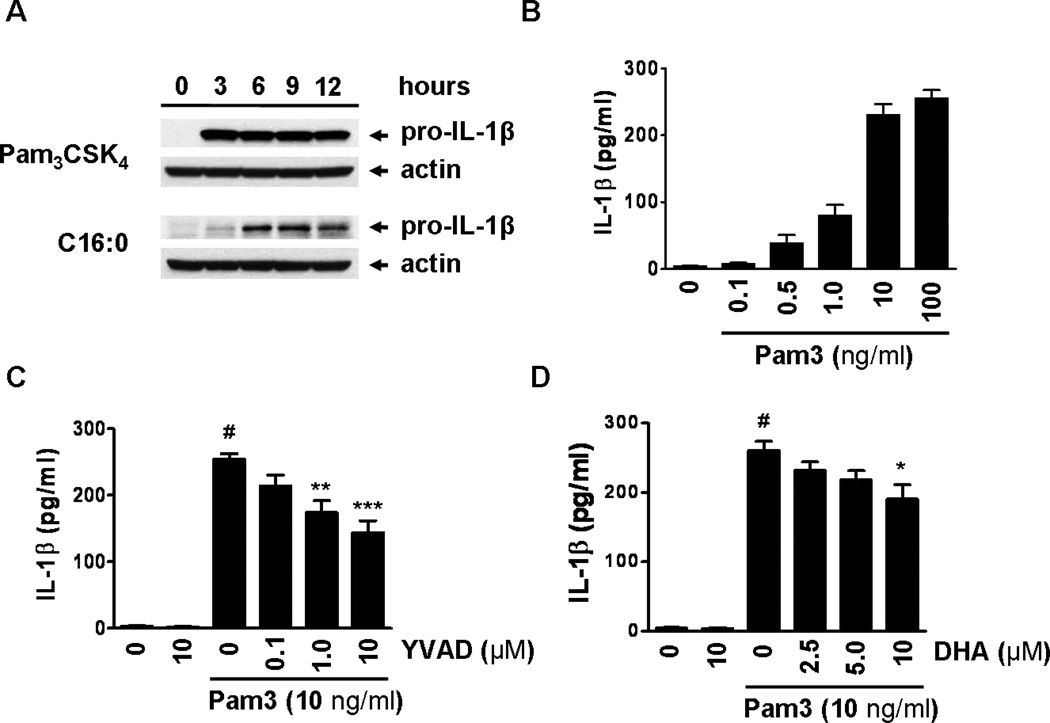
Pam3CSK4 induces but DHA inhibits the expression of pro-IL-1β and secretion of mature IL-1β in THP-1 monocytes
To confirm that pro-IL-1β expression and IL-1β secretion in THP-1 monocytes is at least in part mediated through activation of TLR2 and 1, cells were stimulated with the TLR2/1 specific tri-acylated lipopeptide ligand Pam3CSK4 (42). Treatment led to expression of pro-IL-1β in a time-dependent manner and secretion of IL-1β in a dose-dependent manner (Fig. 4A,B). Inhibition of caspase-1 activity by Ac-YVAD-AOM reduced Pam3CSK4-mediated secretion of IL-1β (Fig. 4C). Pre-treating THP-1 cells with DHA dose-dependently inhibited the secretion of mature IL-1β after a 24 hour treatment with Pam3CSK4 (Fig. 4D). Activation of TLR2 has been shown to induce apoptosis in mononuclear phagocytes, including in THP-1 cells (43, 44). Activation of inflammasome and caspase-1 also induces pyroptotic cell death (45). Therefore, we compared the cell death induced by Pam3CSK4 with that by sodium palmitate in THP-1 cells. Both treatments induced comparable levels of cell death in a time-dependent manner exhibiting a similar cellular response (Fig. S3A,B).
Figure 4. Pam3CSK4 induces pro-IL-1β expression and inflammasome-mediated IL-1β secretion in THP-1 monocytes.
(A) THP-1 cells were serum starved in 0.25% FBS-RPMI-1640 for 12 hours then treated with Pam3CSK4 (10 ng/ml) and C16:0 (150 µM) for the indicated times. Cell lysates were immunoblotted for pro-IL-1β. (B) Serum starved THP-1 cells were treated with Pam3CSK4 at indicated concentrations for 24 hours. Cell culture supernatants were analyzed for IL-1β by ELISA. Data in A and B are expressed as mean ± s.d. and are representative of two independent experiments with similar results. (C and D) Serum starved THP-1 cells were incubated with caspase-1 inhibitor (Ac-YVAD-AOM) (C) or DHA (D) for 1 hour then treated with Pam3CSK4 for 24 hours. Cell culture supernatants were analyzed for IL-1β by ELISA. Data in C and D are expressed as mean ± SEM of three independent experiments. Significance was determined by ANOVA (#P < 0.001 significantly different from untreated, *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from Pam3CSK4).
Palmitic acid-induced IL-1β secretion is mediated at least in part through activation of TLR2
Since TLR2 is predominantly expressed compared with TLR4 in monocytes as determined by flow cytometry (data not shown) and RT-PCR (26) we determined whether knocking down TLR2 expression by small interfering RNAs (siRNAs) suppresses palmitic acid-induced IL-1β secretion in THP-1 cells. Our results showed that reducing cell surface expression of TLR2 with siRNA (Fig. 5B) suppressed Pam3CSK4- and sodium palmitate-induced IL-1β 63% and 15%, respectively (Fig. 5A). Since TLR2 siRNA modestly inhibited palmitic acid-induced IL-1β, we questioned to what extent palmitic acid-induced IL-1β might be mediated through TLR4. Thus we used the TLR4-specific small molecule inhibitor TAK-242 in combination with LPS, sodium palmitate, and non-TLR4 ligands that activate PRRs which are known to be induced by SFAs. Our results showed that TAK-242 inhibited LPS-induced IL-1β by 80% but inhibited sodium palmitate-induced IL-1β by only 19% (Fig. S4). Because SFAs are also known to induce proinflammatory signaling pathways through activation of nucleotide-binding oligomerization domain-containing proteins (NODs) (26) and the unfolded protein response (UPR) (46), inhibiting both TLR2 and TLR4 is unlikely to abolish palmitic acid-induced IL-1β secretion. Therefore,,as an alternate to the siRNA approach and to determine the specificity of palmitic acid for activating TLR2 we used time resolved-fluorescence resonance energy transfer (TR-FRET) assays. Receptor dimerization is known as the proximal event in the activation of TLRs (42, 47–49). Therefore, we determined whether palmitic acid directly induces dimerization of endogenous TLR2/1. We used an anti-TLR2 antibody labeled with a lanthanide cryptate fluorophore with a long lived emission (~1 ms) as an energy donor and an anti-TLR1 antibody labeled with an acceptor fluorophore to measure energy transfer through proximity of labeled TLR1 and 2 (Fig. 5C). Our results showed that both sodium palmitate and Pam3CSK4 induced, but DHA inhibited, dimerization of TLR2 with TLR1 as determined by TR-FRET (Fig. 5D). These results further support that TLR2 is at least in part responsible for palmitic acid-induced IL-1β production.
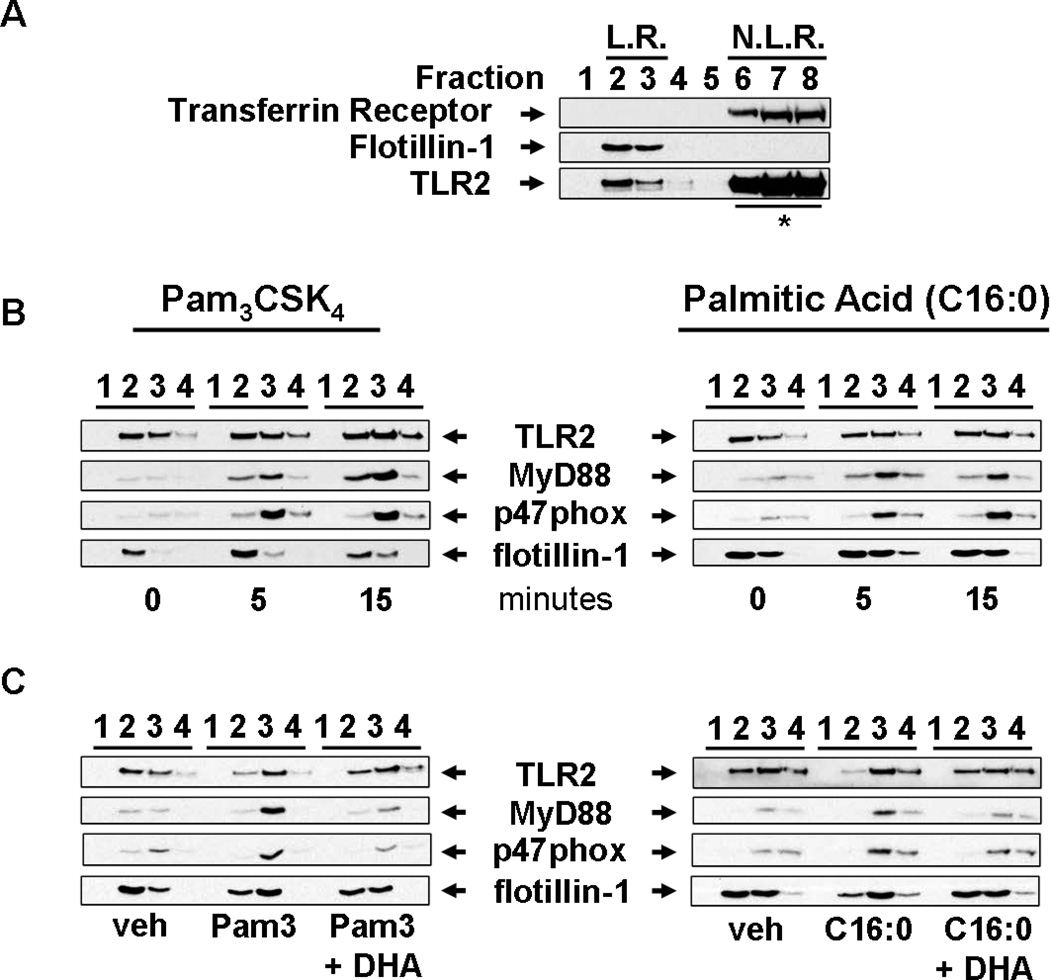
Palmitic acid induces the translocation of MyD88 and p47phox (organizer protein of NADPH oxidase 2) into lipid rafts, whereas DHA inhibits palmitic acid-induced translocation of MyD88 and p47phox
Receptor dimerization and translocation of membrane TLRs and adaptor proteins to lipid rafts upon agonist stimulation are the proximal event required for activation of downstream signaling pathways (25, 34–36, 50–55). Our previous studies showed that LPS or lauric acid induces dimerization and translocation of TLR4 into lipid rafts where it recruits downstream signaling molecules including an adaptor molecule MyD88 in a ROS-dependent manner (25). Since TLR2 activation also requires translocation and association of the adaptor molecule MyD88 and NADPH oxidase 2 (NOX2) into lipid rafts (51, 56, 57) we determined whether palmitic acid induces the translocation of TLR2, MyD88, and NOX2 into lipid rafts of THP-1 cells by isolating lipid raft fractions and examining the translocation of these proteins by immunoblotting. Our results showed that flotillin-1, which is constitutively expressed in lipid rafts (58, 59), localized to fractions 2 and 3 while transferrin receptor, which is constitutively expressed in non-lipid rafts (58), localized to fraction 6–8 (Fig. 6A). Although the majority of TLR2 resided in non-lipid raft fractions a substantial amount was expressed in lipid raft fractions isolated from unstimulated cells (Fig. 6A). Within 5 minutes of treatment, both sodium palmitate and Pam3CSK4 induced translocation of MyD88 and NOX2 organizer protein p47phox to lipid raft fractions in THP-1 monocytes (Fig. 6B). In contrast, DHA inhibited both sodium palmitate- and Pam3CSK4-induced recruitment of both MyD88 and p47phox to lipid raft fractions (Fig. 6C).
Figure 6. Palmitic acid and Pam3CSK4 induce but DHA inhibits the recruitment of MyD88 and p47phox (subunit of NADPH oxidase 2) into lipid raft fractions.
(A) To demonstrate the separation of lipid rafts (LR) and non-lipid raft (NLR) fractions of plasma membrane, cells were lysed and fractionated by sucrose-gradient ultracentrifugation. LR fractions (fractions 2 and 3) and NLR fractions (fractions 6–8) were identified by the presence of flotillin-1 (LR marker) and transferrin receptor (NLR marker), respectively. (* due to overwhelming expression only 20% of input lysate from fractions 6–8 was subjected to SDS-PAGE and immunoblotted with anti-TLR2 antibodies). (B) To determine whether palmitic acid (C16:0) or Pam3CSK4 induces recruitment of the downstream signaling components of TLR2 into LR fractions, THP-1 cells were serum starved in 1.0% FBS-RPMI-1640 for 12 hours, then treated with C16:0 (150 µM) or Pam3CSK4 (100 ng/ml) for indicated time periods. Fractions 1–4 were immunoblotted with anti-TLR2, anti-MyD88, anti-p47phox, and anti-flotillin-1 antibodies. (C) Serum starved THP-1 cells were incubated with DHA (10 µM) for 1 hour then treated with C16:0 (150 µM) or Pam3CSK4 (100 ng/ml) for 5 minutes. Cell lysate was separated by sucrose-gradient ultracentrifugation and fractions were immunoblotted.
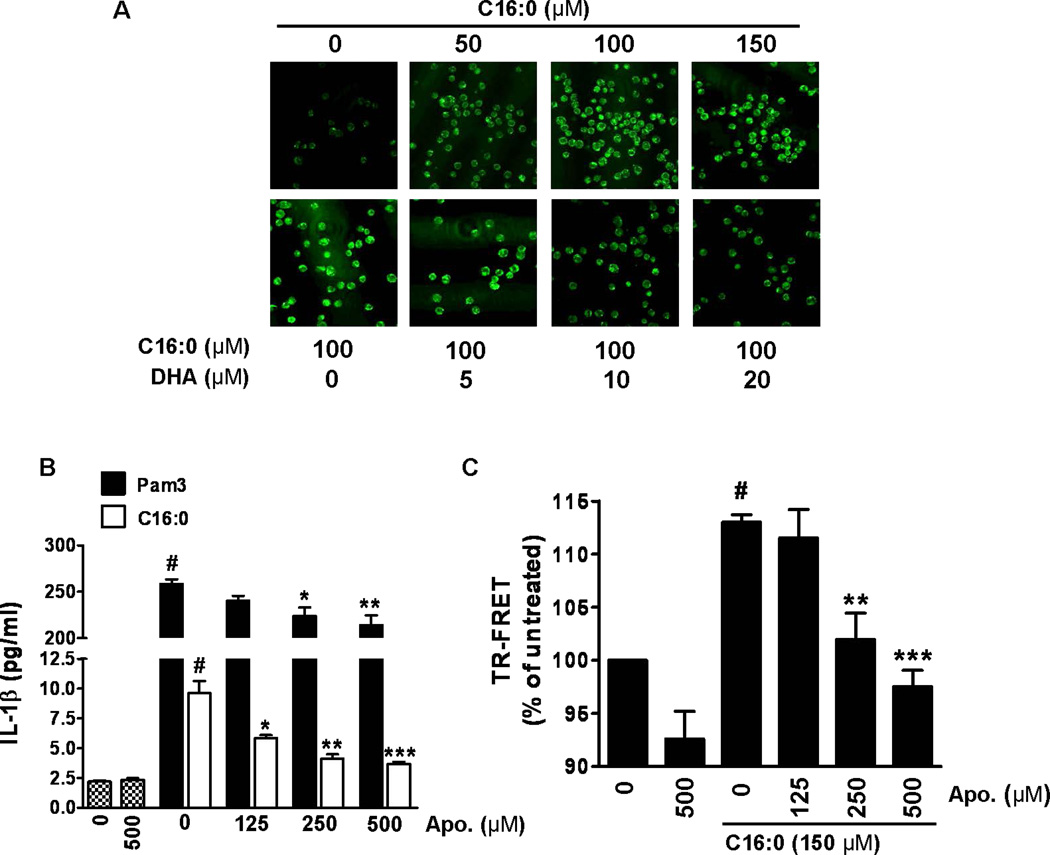
TLR2-mediated palmitic acid-induced IL-1β is dependent on NADPH oxidase activation and is inhibited by DHA
Based on our results indicating that palmitic acid induces the assembly of NADPH oxidase 2 through translocation of p47phox to lipid rafts, we determined whether palmitic acid-induced assembly of NOX2 would induce ROS. Treatment with sodium palmitate induced ROS in a dose dependent manner as assessed by confocal microscopy but was inhibited by DHA (Fig. 7A). Since NOX2 is known to be a critical component of the TLR2 signaling complex (56, 57, 60) we determined whether inhibiting NOX2 assembly would inhibit palmitic acid-induced IL-1β secretion. Apocycin, which inhibits the assembly and activation of NADPH oxidase 2 by interfering with the translocation of p47phox (61, 62) dose-dependently inhibited sodium palmitate- and Pam3CSK-induced IL-1β secretion (Fig. 7B). Additionally, apocynin dose-dependently inhibited sodium palmitate-induced dimerization of TLR2/1 as assessed by TR-FRET (Fig. 7C). Collectively these results reveal that palmitic acid and DHA reciprocally modulate activation of TLR2 by modulating the dimerization of TLR2/1 and that dimerization is dependent on NOX2 activation.
Figure 7. Palmitic acid induces but DHA inhibits ROS production.
(A) THP-1 cells were serum starved in 0.25% FBS-RPMI-1640 for 12 hours. Cells were incubated with CM-H2DCFDA (10 uM) for 30 minutes then pretreated with or without DHA for 1 hour prior to treatment with C16:0 for 20 minutes. Cells were fixed and imaged by confocal microscopy. (B) Serum starved THP-1 cells were incubated with apocynin for 1 hour then treated with C16:0 (150 µM) or Pam3CSK4 (10 ng/ml) for 24 hours. Cell culture supernatants were analyzed for IL-1β by ELISA. Data are expressed as mean ± SEM of three independent experiments. Significance was determined by ANOVA (#P < 0.001 significantly different from untreated, *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from C16:0 or Pam3). (C) THP-1 cells were serum starved with fluorophore-labeled TLR2 and TLR1 antibodies for 12 hours. Cells were incubated with apocynin (125, 250, 500 µM) for one hour then treated with C16:0 (150 µM) for 10 minutes. TR-FRET data are presented as mean percentages of untreated cells ± SEM and were calculated from at least three independent experiments. Significance was determined by ANOVA (#P < 0.001 significantly different from untreated, **P < 0.01, ***P < 0.001 significantly different from C16:0).
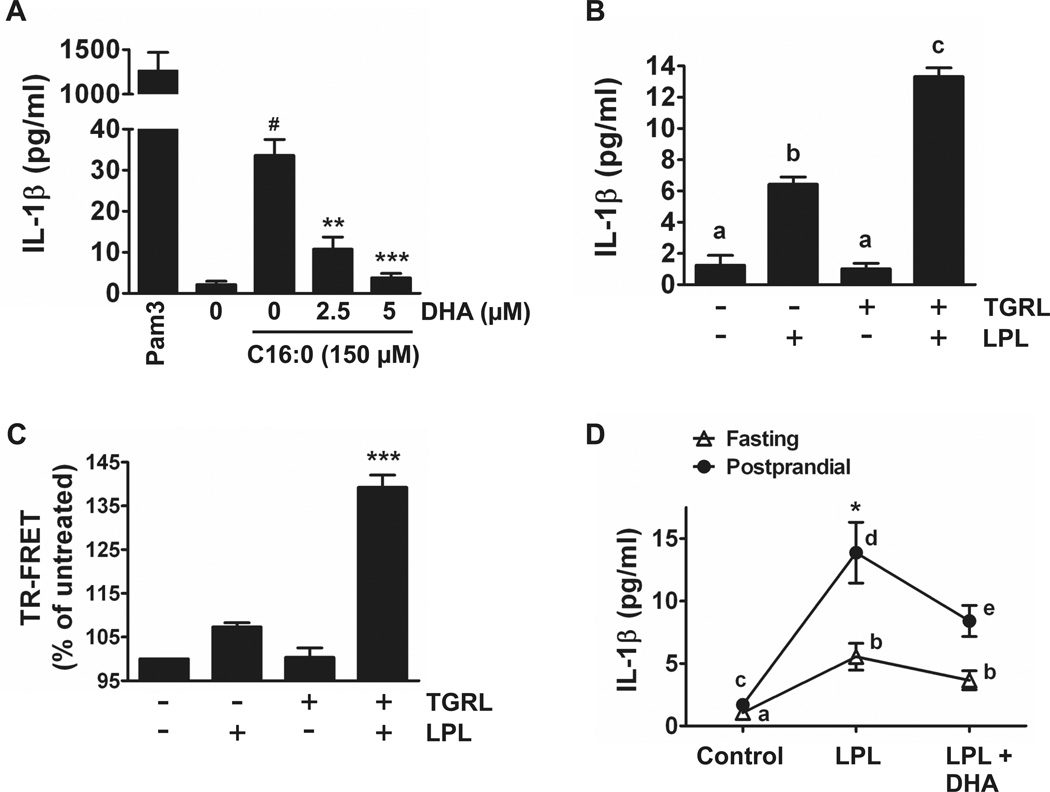
Both exogenous palmitic acid and endogenous fatty acids derived from the lipolysis products of triglyceride-rich lipoproteins (TGRL) induce but DHA inhibits IL-1β production in human peripheral blood monocytes and whole blood
Next, we determined whether palmitic acid also induces IL-1β production in primary human monocytes. Sodium palmitate induced but DHA inhibited IL-1β production in primary monocytes cultured in 1% HI-FBS RPMI-1640 (Fig. 8A) similar to the results obtained from the THP-1 monocytic cell line. Next, we determined whether a physiological source of dietary saturated fatty acids recapitulates the effects of palmitic acid. Postprandial lipemia is characterized by transient accumulation of triglyceride-rich lipoproteins (TGRL) in the blood following ingestion of a fatty meal. In vivo, TGRLs are hydrolyzed by lipoprotein lipase (LPL), an enzyme anchored to endothelial cells, releasing free fatty acids into the blood in immediate proximity to blood monocytes. Our previous studies showed that palmitic acid is the major saturated fatty acid found in the lipolysis products (free fatty acids) of these TGRLs isolated from subjects consuming the high fat meal (40). Therefore, we determined whether the lipolysis products of TGRLs also induce IL-1β production in primary monocytes or in whole blood. The lipolysis products of TGRLs induced IL-1β production in primary monocytes (Fig. 8B). Increased IL-1β production by the treatment of primary monocytes with conditioned media prepared with lipoprotein lipase without added TGRL is likely due to release of free fatty acids from lipids present in FBS (2% final concentration in media). Lipolysis products of TGRLs also increased heterodimerization of TLR2 with TLR1 as assessed by TR-FRET in THP-1 monocytes treated in 0.25% FBS (Fig. 8C). Finally, direct treatment of fasting and postprandial whole blood with lipoprotein lipase induced robust production of IL-1β. Treatment of postprandial whole blood with LPL induced significantly more IL-1β compared to fasting whole blood. Furthermore, concomitant treatment of both LPL-treated fasting and postprandial whole blood samples with DHA inhibited the IL-1β production (Fig. 8D). These results suggest that inflammasome-mediated IL-1β production in blood monocytes can be dynamically modulated by the types of dietary fat we consume.
Figure 8. Palmitic acid and endogenous fatty acids derived from the lipolysis products of triglyceride-rich lipoproteins (TGRL) induce but DHA inhibits IL-1β production in human blood monocytes or whole blood.
(A) Primary monocytes cultured in 0.25% HI-FBS-RPMI-1640 were incubated with or without DHA for one hour then treated with C16:0 (150 µM) for 24 hours. Cell culture supernatants were analyzed for IL-1β by ELISA. Data are expressed as mean ± SEM of three independent experiments. Significance was determined by ANOVA (#P < 0.001 significantly different from untreated, **P < 0.01, ***P < 0.001 significantly different from C16:0). Pam3 (50 ng/ml) was used as a positive control. (B) Primary monocytes cultured in 2% HI-FBS-RPMI-1640 were treated with LPL-, TGRL-, or LPL + TGRL-conditioned media for 24 hours and supernatants were analyzed for IL-1β by ELISA. Bars not sharing a common superscript are significantly different (P < 0.05) as determined by ANOVA. (C) THP-1 cells were serum starved in 0.25% FBS-RPMI-1640 for 12 hours with fluorophore-labeled TLR2 and TLR1 antibodies then treated with LPL-, TGRL-, or LPL + TGRL-conditioned media for 10 minutes. TR-FRET data are presented as mean percentages of untreated cells ± SEM and were calculated from four independent experiments. Significance was determined by ANOVA (***P < 0.001 significantly different from all treatments). (D) Fasting and postprandial whole blood was incubated with DHA (10 µM) for 1 hour then treated with LPL for 24 hours. Supernatants were analyzed for IL-1β by ELISA. Data are presented as mean ± SEM. Two-way repeated measures ANOVA was performed to test for the effects of metabolic state, treatment, and metabolic state x treatment. There was a significant effect of metabolic state (P = 0.0001), treatment (P = 0.0002), and metabolic state x treatment (P = 0.0079). Means for each of the 3 treatments were compared by Bonferroni posttests. Fasting LPL was significantly different from postprandial LPL (*P < 0.001). Within each metabolic state, points with different superscripts are significantly different (P < 0.05), n = 12 for fasting and n = 15 for postprandial.
Discussion
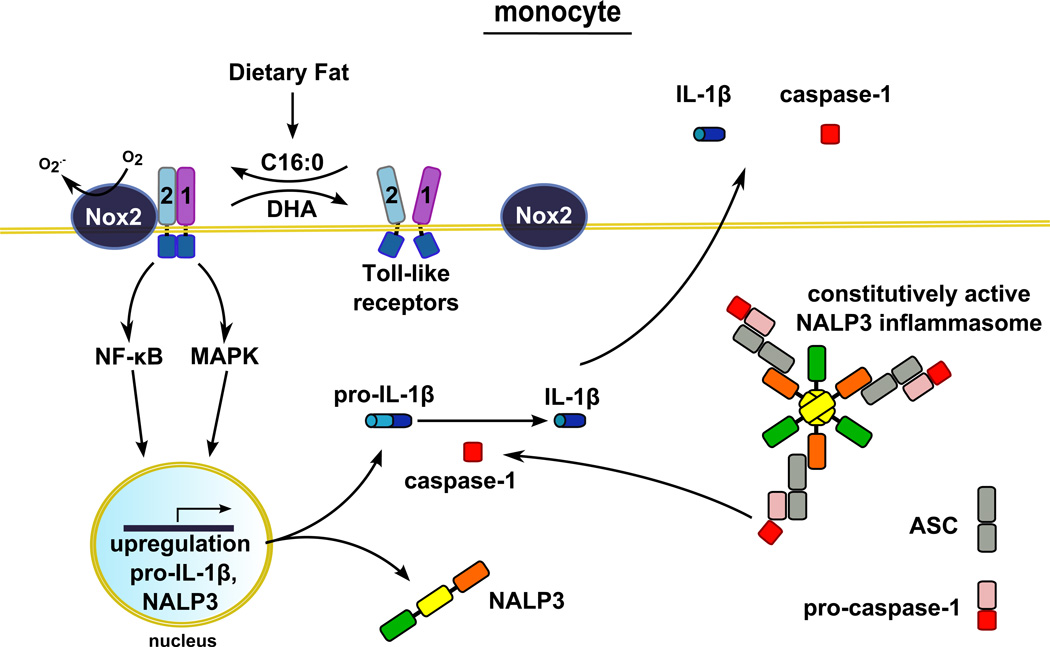
Our results demonstrate that palmitic acid, a predominant dietary saturated fatty acid, induces whereas, the n-3 fatty acid DHA inhibits the expression of pro-IL-1β and subsequent release of mature IL-1β in both THP-1 monocytes and primary human blood monocytes. These results further support the findings that blood monocytes contain active caspase-1 due to presence of constitutively active NALP3 inflammasome (8–10). Therefore, primary signals inducing the expression of pro-IL-1β are sufficient for inflammasome-mediated IL-1β production in blood monocytes. Pro-IL-1β is one of the target gene products derived from the activation of TLRs expressed in monocytes. Therefore, any agonists that activate TLRs should be able to induce inflammasome-mediated IL-1β production in human blood monocytes without secondary signals required for the activation of inflammasome as depicted in Figure 9.
Figure 9. Illustration depicting the modulation of the production of NALP3 inflammasome-mediated IL-1β in human monocytes by dietary fatty acids.
The major dietary saturated fatty acid palmitic acid (C16:0) induces, but the n-3 fatty acid docosahexaenoic acid (DHA) inhibits, heterodimerization of TLR2 with TLR1. The heterodimerization leads to the activation of downstream intracellular signaling pathways that culminates in the expression of pro-IL-1β which is cleaved by constitutively activated caspase-1 to secrete mature IL-1β. Therefore, the primary signals to induce the expression of pro-IL-1β are sufficient for the production of mature IL-1β in human monocytes. Palmitic acid also upregulates the expression of NALP3 which could potentiate NALP3 inflammasome activity.
Animal studies showed that palmitic acid can activate NALP3 inflammasome in bone marrow derived macrophages (BMDMs) pretreated with LPS; however, palmitic acid alone did not induce pro-IL-1β expression in BMDMs suggesting that palmitic acid can provide the secondary signal to activate inflammasome but not the primary signals to induce the expression of pro-IL-1β in BMDMs (19). Thus, for palmitic acid to induce inflammasome-mediated IL-1β secretion in macrophages, the expression of pro-IL-1β by the primary signals (e.g., LPS) would be required. In contrast to BMDMs, bone marrow-derived dendritic cells (BMDCs) were shown to secrete inflammasome-mediated IL-1β upon TLR activation by free fatty acids or LPS alone suggesting that the primary signal is sufficient to induce NALP3 inflammasome-mediated IL-1β secretion in BMDCs (3, 11). The different ability of mononuclear phagocytes to secrete NALP3 inflammasome-mediated IL-1β may be explained at least in part by the differential levels of expression of TLRs and NALP3 in steady-state conditions. Monocytes constitutively express NALP3 and active caspase-1 in steady-state conditions (Fig. 1A). NALP3 is detectable in unstimulated BMDCs but not BMDMs (11). Furthermore, BMDCs were shown to express more TLR2 and TLR4 compared with BMDMs (3). Consequently, BMDCs expressed higher amounts of both pro-IL-1β and NALP3 proteins in response to TLR ligands than did BMDMs (11). Therefore, the inability of palmitic acid alone to induce NALP3 inflammasome-mediated IL-1β production in macrophages may be due to the lower expression levels of TLR2 and TLR4 compared to monocytes or dendritic cells. As a result, palmitic acid may be unable to sufficiently activate TLR signaling pathways to induce adequate levels of pro-IL-1β and NALP3 expression despite the ability of palmitic acid to provide the secondary signals activating the inflammasome.
How saturated fatty acids can provide the secondary signals for inflammasome activation is an intriguing question. A recent animal study showed that C2 ceramide can activate NALP3 inflammasome in BMDMs (18). Another animal study showed that high fat diet stimulates ceramide synthesis in a TLR4 dependent manner and suggested that ceramide mediates high fat diet induced insulin resistance (14). These results suggest that the degree to which fatty acids modulate NALP3 inflammasome-mediated IL-1β production is cell type-specific. Therefore, saturated fatty acids derived from high fat diet, in vivo can render both primary and secondary signals in tissues containing infiltrating monocytes, dendritic cells and macrophages through activation of TLRs leading to the expression of pro-IL-1β and by stimulating synthesis of endogenous molecules (e.g., ceramide) that can activate the inflammasome.
Next we determined whether endogenous saturated fatty acids derived from the high fat diet also induce inflammasome-mediated IL-1β release in human blood monocytes. Upon digestion and absorption dietary saturated fatty acids are incorporated primarily into triglyceride-rich lipoproteins (TGRL). Our previous human studies showed that palmitic acid is the major saturated fatty acid released after the treatment of postprandial TGRL with lipoprotein lipase (LPL) (40). Therefore, we determined whether the lipolysis products of TGRL containing dietary palmitic acid can also induce inflammasome-mediated IL-1β. Our results showed that lipolysis products of TGRL isolated from postprandial blood derived from human subjects consuming the high fat meal are sufficient to induce inflammasome-mediated IL-1β production in blood monocytes. Treatment of whole blood samples with lipoprotein lipase also induced IL-1β release; IL-1β release from the postprandial whole blood was greater than that of fasting whole blood. LPL-induced IL-1β secretion in both fasting and postprandial whole blood was suppressed by DHA. Together, these results are significant in view of the fact that blood monocytes are sentinel immune cells in constant surveillance of invading pathogens, tissue injury, and metabolic fluctuations. Our results suggest that the propensity of monocyte activation in response to such stimuli can be greatly modulated by types of plasma fatty acids which in turn are altered by types of dietary fat consumed.
How saturated fatty acids induce but DHA inhibits TLR activation that leads to pro-IL-1β expression is an important question. Both TLR4 and TLR2 ligands LPS and tri-acylated lipopeptide Pam3CSK4, respectively, are acylated by saturated fatty acids. No ligands for TLRs other than TLR4 and TLR2 so far identified are known to be acylated by fatty acids. If these saturated fatty acids are removed from LPS or Pam3CSK4, the ligands completely lose ability to activate their respective receptor demonstrating that these saturated fatty acids are required for their ligand activity (48, 63–68). Lipid A acylated by unsaturated fatty acids instead of saturated fatty acids is nontoxic and acts as an antagonist against the wild type endotoxin (64, 69, 70). Indeed, many studies have suggested that saturated fatty acids but not unsaturated fatty acids can activate TLR4 and TLR2 in both in vitro and in vivo systems (3, 16, 20, 21, 23, 24, 30, 31, 71–77). However, direct evidence that palmitic acid can activate TLRs has not been reported.
The X-ray crystallographic structure for the TLR2-TLR1 heterodimer revealed that two ester-bound fatty acyl (palmitic acid) chains of Pam3CSK4 are inserted into the hydrophobic lipid binding pocket in TLR2, while the amide-bound fatty acyl group is inserted into a hydrophobic channel in TLR1 (42). Thus, binding of fatty acyl groups of Pam3CSK4 into TLR2 induces heterodimerization of TLR2 with TLR1. Structure-function analysis for the lipopeptides revealed that the two ester-bound fatty acids are an essential determinant for its ligand activity for TLR2, and that the acyl chain with 16 carbons (palmitic acid) provides optimal stimulatory activity compared with ester-bound fatty acyl chains of different length (63). It is an interesting question whether palmitic acid itself without the peptide moiety can interact with the hydrophobic lipid binding sites in TLR2 or TLR1 and promote the dimerization of the receptors leading to recruitment of the immediate downstream signaling molecules including MyD88 and NOX2. Although the activation of downstream signaling molecules such as NF-κB or target gene expression are often used as surrogate markers of TLR activation, they do not necessarily reflect direct activation of TLRs since many receptors other than TLRs also induce NF-κB activation and similar target gene expression. Dimerization of TLRs is the most proximal event for the receptor activation required for the activation of downstream signaling pathways (25, 35, 51, 54, 78). Therefore, dimerization of TLRs would be a faithful readout for the direct activation of TLRs. So far, no direct evidence that palmitic acid can induce TLR dimerization has ever been presented.
Dimerization allows for the proper orientation of the TIR domains of TLRs to induce the recruitment and interaction of adaptor proteins in lipid rafts. Several TLRs exist as inactive preformed hetero- and homodimers in absence of ligand (42, 79). TLR2 has been shown to exist as an inactive loosely bound heterodimer with either TLR1 or TLR6 in absence of ligand (34, 80). Upon lipopeptide binding, preformed TLR2/1 dimers undergo rearrangement subsequently bringing TLR2 and TLR1 in much closer proximity (42). This ligand-induced conformational rearrangement results in the immediate recruitment of signaling molecules including MyD88 and the cytoplasmic NOX2 organizer protein p47phox (56, 57, 60). Therefore, we next determined whether palmitic acid induces heterodimerization of TLR2 with TLR1 in close proximity that can be detected by time resolved-fluorescence resonance energy transfer (TR-FRET). Since FRET occurs only when two receptors are within close proximity, it can be used as a direct readout for receptor dimerization. In addition, TR-FRET allows for detection of the dimerization process of native TLR1 and 2 in their biological context. Our results showed that palmitic acid or Pam3CSK4 increased TLR2/1-dependent TR-FRET within 10 minutes of incubation time, whereas DHA attenuates both palmitic acid- and Pam3CSK4-induced TR-FRET in THP-1 cells. To our knowledge, these results are the first demonstrating that the dietary saturated fatty acid palmitic acid directly induces while DHA inhibits the dimerization of TLR2 and TLR1. While our manuscript was in review, Yan et al. (81) reported that DHA inhibits inflammasome activation in murine BMDMs. These results together with our results demonstrate that DHA can inhibit both TLR-mediated pro-IL-1β expression (primary signal) and NALP3 inflammasome activation (secondary signal) in a cell-type specific manner.
Dimerized TLR2 recruits MyD88 and p47phox into lipid raft fractions, which can be an additional readout of TLR activation. Concomitant recruitment of MyD88 and p47phox into lipid raft fractions (Fig. 6B,C), and corresponding increase in ROS generation by palmitic acid (Fig. 7A) strongly suggests that palmitic acid induces recruitment of downstream signaling molecules of TLRs including MyD88 and p47phox. Recruitment of cytoplasmic p47phox to the plasma membrane allows for the association with gp91phox and p22phox, and activation of the NOX2 enzyme complex (82). Recently, several studies have demonstrated recruitment and activation of NOX2 by agonist induced activation of TLR4 and TLR2 (25, 51, 56, 57, 60, 83, 84). Our results showing that NOX2 inhibitor apocynin dose-dependently inhibits palmitic acid induced dimerization of TLR2 (Fig. 7C) and IL-1β release (Fig. 7B) further support that palmitic acid induced activation of TLR2 is also dependent on NOX2 activation. These results suggest that the downstream component of TLRs can exert positive feedback for activation of TLRs. Such a positive feedback mechanism can render rapid burst of ROS production upon microbial infection for killing invading pathogens. However, excessive production of ROS resulting from the activation of TLRs induced by endogenous molecules can trigger harmful effects leading to chronic sterile inflammation. How NOX-induced ROS can promote the dimerization of TLR4 or TLR2 is an intriguing question. One possibility is that NOX-induced ROS may promote the formation of disulfide bond between TLR2 and TLR1 as an analogy to many redox-sensitive proteins (85–88). Dimerization of TLR2 involves formation of a disulfide bond (89) which may be sensitively regulated by the immediate redox status of cell’s micro-environment. Significant corollary to these results is that dietary components and physiological or metabolic processes that affect cellular redox status can directly modulate TLR-mediated inflammatory responses and their subsequent consequences.
We next determined whether endogenous saturated fatty acids derived from dietary fat recapitulate the effects of palmitic acid on dimerization of TLR2 with TLR1 in monocytes. The results showed that the lipolysis product of TGRL induced the dimerization of TLR2 with TLR1 as does palmitic acid (Fig. 8C). Recruitment of MyD88 by dimerized TLR2 led to activation of further downstream signaling molecules including NF-κB and MAPKs and eventually the expression of target gene products (i.e., pro-IL-1β) (Fig. 1A, 3D) and release of IL-1β in primary monocytes (Fig. 8B).
Collectively, our results reveal that saturated fatty acids derived from high saturated fat meals can induce inflammasome-mediated IL-1β production in human monocytes by inducing the dimerization of TLR2 with TLR1. To our knowledge, this is the first report demonstrating compelling evidence that palmitic acid can directly activate TLR2 by inducing receptor dimerization leading to the activation of downstream signaling pathways and target gene expression. These results further suggest that inflammasome-mediated IL-1β production in blood monocytes is dynamically modulated by the types of dietary fat we consume.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by grants from the National Institutes of Health R01 (DK064007, DK41868, DK078328S) and program funds from the Western Human Nutrition Research Center/ARS/USDA (5306-51530-017-00D). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies. USDA is an equal opportunity employer and provider.
Abbreviations used in this article
- DHA
docosahexaenoic acid
- NLR
nucleotide-binding oligomerization domain-like receptor
- NALP3
NLR pyrin domain-containing 3
- NOX2
NADPH oxidase 2
- Pam3CSK4
Pam3CysSerLys4
- PRR
pattern recognition receptor
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SFA
saturated fatty acid
- TLR
Toll-like receptor
References
- 1.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 2.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 4.Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455–464. doi: 10.1016/j.tcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Lamkanfi M, Dixit VM. The inflammasomes. PLoS Pathog. 2009;5:e1000510. doi: 10.1371/journal.ppat.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 7.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 8.Carta S, Tassi S, Pettinati I, Delfino L, Dinarello CA, Rubartelli A. The rate of interleukin-1beta secretion in different myeloid cells varies with the extent of redox response to Toll-like receptor triggering. J Biol Chem. 2011;286:27069–27080. doi: 10.1074/jbc.M110.203398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y, Franchi L, Nunez G. TLR Agonists Stimulate Nlrp3-Dependent IL-1beta Production Independently of the Purinergic P2×7 Receptor in Dendritic Cells and In Vivo. J Immunol. 2013;190:334–339. doi: 10.4049/jimmunol.1202737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010 doi: 10.1155/2010/672395. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through toll-like receptor 4-derived signaling pathways. FASEB J. 2001;15:2556–2564. doi: 10.1096/fj.01-0432com. [DOI] [PubMed] [Google Scholar]
- 16.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PC, Joosten LA, Netea MG, Kanneganti TD. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 23.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 24.Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 25.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L, Kwon MJ, Huang S, Lee JY, Fukase K, Inohara N, Hwang DH. Differential modulation of Nods signaling pathways by fatty acids in human colonic epithelial HCT116 cells. J Biol Chem. 2007;282:11618–11628. doi: 10.1074/jbc.M608644200. [DOI] [PubMed] [Google Scholar]
- 27.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, Van Lommel L, Pospisilik JA, Tschopp O, Schultze SM, Malipiero U, Esterbauer H, Ellingsgaard H, Rutti S, Schuit FC, Lutz TA, Boni-Schnetzler M, Konrad D, Donath MY. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia. 2010;53:1795–1806. doi: 10.1007/s00125-010-1747-3. [DOI] [PubMed] [Google Scholar]
- 28.Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010;24:731–739. doi: 10.1096/fj.09-141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochemical and biophysical research communications. 2007;354:45–49. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- 30.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 31.Suganami T, Yuan X, Shimoda Y, Uchio-Yamada K, Nakagawa N, Shirakawa I, Usami T, Tsukahara T, Nakayama K, Miyamoto Y, Yasuda K, Matsuda J, Kamei Y, Kitajima S, Ogawa Y. Activating transcription factor 3 constitutes a negative feedback mechanism that attenuates saturated Fatty acid/toll-like receptor 4 signaling and macrophage activation in obese adipose tissue. Circ Res. 2009;105:25–32. doi: 10.1161/CIRCRESAHA.109.196261. [DOI] [PubMed] [Google Scholar]
- 32.Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang S, Wang M, Triantafilou K, Triantafilou M, Nawar HF, Russell MW, Connell TD, Hajishengallis G. The A subunit of type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J Immunol. 2007;178:4811–4819. doi: 10.4049/jimmunol.178.8.4811. [DOI] [PubMed] [Google Scholar]
- 34.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 35.Triantafilou M, Gamper FG, Lepper PM, Mouratis MA, Schumann C, Harokopakis E, Schifferle RE, Hajishengallis G, Triantafilou K. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell Microbiol. 2007;9:2030–2039. doi: 10.1111/j.1462-5822.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- 36.Triantafilou M, Manukyan M, Mackie A, Morath S, Hartung T, Heine H, Triantafilou K. Lipoteichoic acid and toll-like receptor 2 internalization and targeting to the Golgi are lipid raft-dependent. J Biol Chem. 2004;279:40882–40889. doi: 10.1074/jbc.M400466200. [DOI] [PubMed] [Google Scholar]
- 37.Chan JW, Motton D, Rutledge JC, Keim NL, Huser T. Raman spectroscopic analysis of biochemical changes in individual triglyceride-rich lipoproteins in the pre- and postprandial state. Anal Chem. 2005;77:5870–5876. doi: 10.1021/ac050692f. [DOI] [PubMed] [Google Scholar]
- 38.Hyson DA, Paglieroni TG, Wun T, Rutledge JC. Postprandial lipemia is associated with platelet and monocyte activation and increased monocyte cytokine expression in normolipemic men. Clin Appl Thromb Hemost. 2002;8:147–155. doi: 10.1177/107602960200800211. [DOI] [PubMed] [Google Scholar]
- 39.Eiselein L, Wilson DW, Lame MW, Rutledge JC. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am J Physiol Heart Circ Physiol. 2007;292:H2745–H2753. doi: 10.1152/ajpheart.00686.2006. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50:204–213. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YI, Schulze J, Raymond N, Tomita T, Tam K, Simon SI, Passerini AG. Endothelial inflammation correlates with subject triglycerides and waist size after a high-fat meal. Am J Physiol Heart Circ Physiol. 2011;300:H784–H791. doi: 10.1152/ajpheart.01036.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 44.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. The EMBO journal. 2000;19:3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunological reviews. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins KA, Mansell A. TIR-containing adaptors in Toll-like receptor signalling. Cytokine. 2010;49:237–244. doi: 10.1016/j.cyto.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity. 2008;29:182–191. doi: 10.1016/j.immuni.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 49.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Lizarbe S, Pascual M, Gascon MS, Blanco A, Guerri C. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Mol Immunol. 2008;45:2007–2016. doi: 10.1016/j.molimm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olsson S, Sundler R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol Immunol. 2006;43:607–612. doi: 10.1016/j.molimm.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Shin DM, Yang CS, Lee JY, Lee SJ, Choi HH, Lee HM, Yuk JM, Harding CV, Jo EK. Mycobacterium tuberculosis lipoprotein-induced association of TLR2 with protein kinase C zeta in lipid rafts contributes to reactive oxygen species-dependent inflammatory signalling in macrophages. Cell Microbiol. 2008;10:1893–1905. doi: 10.1111/j.1462-5822.2008.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 55.Triantafilou M, Morath S, Mackie A, Hartung T, Triantafilou K. Lateral diffusion of Toll-like receptors reveals that they are transiently confined within lipid rafts on the plasma membrane. J Cell Sci. 2004;117:4007–4014. doi: 10.1242/jcs.01270. [DOI] [PubMed] [Google Scholar]
- 56.Ogier-Denis E, Mkaddem SB, Vandewalle A. NOX enzymes and Toll-like receptor signaling. Semin Immunopathol. 2008;30:291–300. doi: 10.1007/s00281-008-0120-9. [DOI] [PubMed] [Google Scholar]
- 57.Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, Bae YS, Jo EK. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009;182:3696–3705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- 58.Dhungana S, Merrick BA, Tomer KB, Fessler MB. Quantitative proteomics analysis of macrophage rafts reveals compartmentalized activation of the proteasome and of proteasome-mediated ERK activation in response to lipopolysaccharide. Mol Cell Proteomics. 2009;8:201–213. doi: 10.1074/mcp.M800286-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li N, Mak A, Richards DP, Naber C, Keller BO, Li L, Shaw AR. Monocyte lipid rafts contain proteins implicated in vesicular trafficking and phagosome formation. Proteomics. 2003;3:536–548. doi: 10.1002/pmic.200390067. [DOI] [PubMed] [Google Scholar]
- 60.Yang CS, Shin DM, Lee HM, Son JW, Lee SJ, Akira S, Gougerot-Pocidalo MA, El-Benna J, Ichijo H, Jo EK. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell Microbiol. 2008;10:741–754. doi: 10.1111/j.1462-5822.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 61.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 62.Wedgwood S, Lakshminrusimha S, Farrow KN, Czech L, Gugino SF, Soares F, Russell JA, Steinhorn RH. Apocynin improves oxygenation and increases eNOS in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2012;302:L616–L626. doi: 10.1152/ajplung.00064.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Ulmer AJ. Lipopeptide structure determines TLR2 dependent cell activation level. FEBS J. 2005;272:6354–6364. doi: 10.1111/j.1742-4658.2005.05029.x. [DOI] [PubMed] [Google Scholar]
- 64.Kitchens RL, Ulevitch RJ, Munford RS. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992;176:485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumazawa Y, Nakatsuka M, Takimoto H, Furuya T, Nagumo T, Yamamoto A, Homma Y, Inada K, Yoshida M, Kiso M, et al. Importance of fatty acid substituents of chemically synthesized lipid A-subunit analogs in the expression of immunopharmacological activity. Infect Immun. 1988;56:149–155. doi: 10.1128/iai.56.1.149-155.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munford RS, Hall CL. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234:203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- 67.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, Seydel U, Di Padova F, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 68.Schletter J, Heine H, Ulmer AJ, Rietschel ET. Molecular mechanisms of endotoxin activity. Arch Microbiol. 1995;164:383–389. doi: 10.1007/BF02529735. [DOI] [PubMed] [Google Scholar]
- 69.Krauss JH, Seydel U, Weckesser J, Mayer H. Structural analysis of the nontoxic lipid A of Rhodobacter capsulatus 37b4. Eur J Biochem. 1989;180:519–526. doi: 10.1111/j.1432-1033.1989.tb14677.x. [DOI] [PubMed] [Google Scholar]
- 70.Qureshi N, Takayama K, Kurtz R. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect Immun. 1991;59:441–444. doi: 10.1128/iai.59.1.441-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–533. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 72.Joosten LA, Netea MG, Mylona E, Koenders MI, Malireddi RK, Oosting M, Stienstra R, van de Veerdonk FL, Stalenhoef AF, Giamarellos-Bourboulis EJ, Kanneganti TD, van der Meer JW. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1beta production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 2010;62:3237–3248. doi: 10.1002/art.27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 74.Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz EA, Zhang WY, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol. 2010;30:802–808. doi: 10.1161/ATVBAHA.109.201681. [DOI] [PubMed] [Google Scholar]
- 76.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281:26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- 77.Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009;284:26533–26546. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Triantafilou M, Lepper PM, Olden R, Dias IS, Triantafilou K. Location, location, location: is membrane partitioning everything when it comes to innate immune activation? Mediators Inflamm. 2011:186093. doi: 10.1155/2011/186093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, Espevik T, Golenbock DT. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- 80.Tapping RI, Tobias PS. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res. 2003;9:264–268. doi: 10.1179/096805103225001477. [DOI] [PubMed] [Google Scholar]
- 81.Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity. 2013;38:1154–1163. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 82.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 83.DeLeo FR, Renee J, McCormick S, Nakamura M, Apicella M, Weiss JP, Nauseef WM. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simon F, Fernandez R. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. J Hypertens. 2009;27:1202–1216. doi: 10.1097/HJH.0b013e328329e31c. [DOI] [PubMed] [Google Scholar]
- 85.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 86.Cumming RC, Andon NL, Haynes PA, Park M, Fischer WH, Schubert D. Protein disulfide bond formation in the cytoplasm during oxidative stress. J Biol Chem. 2004;279:21749–21758. doi: 10.1074/jbc.M312267200. [DOI] [PubMed] [Google Scholar]
- 87.Miki H, Funato Y. Regulation of intracellular signalling through cysteine oxidation by reactive oxygen species. J Biochem. 2012;151:255–261. doi: 10.1093/jb/mvs006. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y, Song Y, Loscalzo J. Regulation of the protein disulfide proteome by mitochondria in mammalian cells. Proc Natl Acad Sci U S A. 2007;104:10813–10817. doi: 10.1073/pnas.0702027104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tao X, Xu Y, Zheng Y, Beg AA, Tong L. An extensively associated dimer in the structure of the C713S mutant of the TIR domain of human TLR2. Biochemical and biophysical research communications. 2002;299:216–221. doi: 10.1016/s0006-291x(02)02581-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.