Abstract
The adoptive transfer of T cells expressing chimeric antigen receptors (CARs) has emerged as a promising immunotherapeutic strategy against cancer. Administering CAR-expressing T cells in combination with agents that promote the expression of CAR targets or optimize T-cell function within the tumor microenvironment may further improve the therapeutic potential of this approach.
Keywords: chimeric receptor, target antigen, gangliosides, tumor microenvironment, CD19
Chimeric antigen receptors (CARs) have recently emerged as a powerful means of redirecting T-cell functions toward malignant cells. CARs consist of antibody-derived antigen-binding domains fused to components of T cell-stimulatory signaling pathways. Thus, CARs combine the ability of immunoglobulins to recognize specific antigens and of selected signaling domains to activate T cells. Genetic modification of T cells with CAR-encoding genes allows them to interact with tumor-associated antigens expressed on the surface of malignant cells independent of antigen presentation, hence overcoming various mechanisms of immune escape. Indeed, the interaction of CARs with their antigens can induce potent T-cell responses and mediate robust antitumor effects in murine tumor models.
After 15 years of preclinical and early clinical development, recent results have substantially boosted the field of CAR-based anticancer immunotherapy. Carl June’s group was the first to unequivocally demonstrate the potency of CAR-expressing T cells to eliminate human cancers. In chronic lymphocytic leukemia (CLL) patients, T cells engineered to express a CD19-specific CAR efficiently eradicated the disease. Moreover, they promoted the establishment of protective tumor-antigen specific memory responses lasting for now more than a year and resulting in durable remissions.1 Subsequent studies in acute lymphoblastic leukemia (ALL) patients have confirmed the anticancer activity of T cells expressing a CD19-specific CARs.2,3 Together, these findings underscore the clinical potential of CAR-based anticancer immunotherapy.
CAR-expressing T cells have also begun to be explored in non-hematological solid tumors. In a first-in-man clinical Phase I/II trial performed at Baylor College of Medicine (Houston, TX, USA) we demonstrated moderate antitumor effects of ganglioside GD2-specific T lymphocytes against refractory neuroblastomas that correlated with the in vivo persistence of the adoptively transferred cells.4,5 No objective responses to adoptive therapy with CAR gene-modified T cells were documented in other pilot and Phase I clinical trials in patients with solid tumors.6 Overall, solid tumors appear to be more challenging targets for CAR-expressing T cells than B-cell derived hematological malignancies.
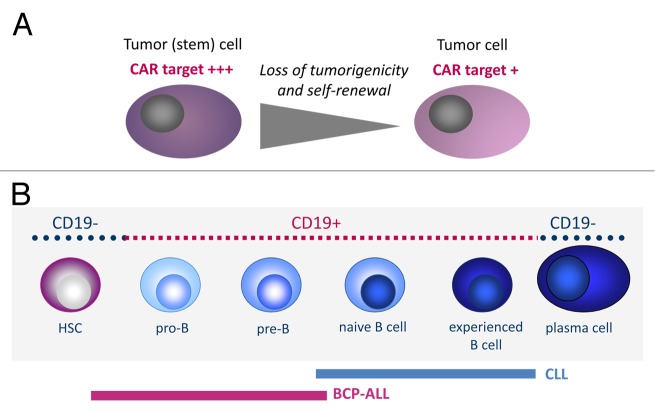
A critical factor for CAR-based immunotherapy, and a hitherto unsurmounted hurdle in most malignancies, is the availability of an adequate target antigen. Ideally, the target antigen would be reliably and exclusively expressed on the surface of all malignant cells, including highly tumorigenic and self-renewing residual cells, and be essential for cell growth and survival (Fig. 1A). The B-cell differentiation antigen CD19 fulfills at least some of these requirements. Since CLL originates from a mature B cell, the malignant cells are consistently CD19+ (Fig. 1B). Moreover, although CD19 is not a tumor-specific antigen, it is not expressed by cells that do not belong to the B-cell lineage. Thus, the elimination of CD19+ cells does not provoke on-target toxicities. Concomitant depletion of non-transformed B cells by T cells expressing CD19-specific CARs is unavoidable, but the clinical consequences of B-cell deficiency can be largely overcome by immunoglobulin substitution. Compared to CLL, CD19 is less well suited for targeting B lineage ALL, which originates from B-cell precursors. ALL patients often bear immature CD19- leukemia-propagating cell subclones that can escape CD19-directed immunotherapy7 (Fig. 1B). In fact, CD19- relapses were observed in ALL patients treated with T cells expressing a CD19-specific CAR or with CD19-targeting bispecific antibodies. Finally, CD19 appears to be functionally irrelevant for malignant growth and thus conceptually is not a good target antigen. The identification of more adequate target antigens is a critical step for extending the promise of this immunotherapeutic approach to hematological malignancies other than CLL and ALL and to solid tumors.
Figure 1. Targets for chimeric antigen receptors. (A) Ideally, targets for chimeric antigen receptors (CARs) should be expressed on all malignant cells, including immature cells with a high disease-initiating potential, to avoid the clonal escape of cancer cell subsets that do not express the CAR-targeted antigen. (B) The B-cell lineage antigen CD19 is expressed on malignant B cells or B-cell precursor cells in chronic lymphocytic leukemia (CLL) and acute lymphoblastic leukemia (ALL), respectively. In ALL patients, leukemia-propagating cells have also been found in the CD19- B-cell compartment. These cells can drive both CD19- and CD19+ relapses after the adoptive transfer of T cells expressing a CD19-specific CAR.
Candidate antigens for CAR-expressing T cells in solid tumors are the disialogangliosides GD2 and GD3. Gangliosides are glycosphingolipids anchored to the plasma membrane that are involved in various cellular functions, including signal transduction, cell proliferation, differentiation, adhesion, and cell death. Disialogangliosides are highly overexpressed in melanoma and neuroblastoma cells, reflecting the neuroectodermal tissue origin of these neoplasms . Following preliminary clinical evidence for the activity of GD2-redirected CAR-expressing T cells in neuroblastoma patients,4,5 further studies based on signal-enhanced CARs are currently ongoing at Baylor College of Medicine. Since we have recently observed that GD2 surface expression on tumor cells is also found in Ewing sarcoma,8, 9 we are now pursuing GD2-targeting by CARs to treat patients with this cancer.. Immunohistochemical studies suggest that the expression levels of GD2 may vary even within individual tumors.8 To account for the clonal heterogeneity of cancer, a detailed understanding of the characteristics of cancer cell subpopulations that express high levels of CAR-targeted antigens and the functional significance of the antigens in individual cancers is critical. Moreover, combination strategies that upregulate CAR target antigens in subsets of cancer cells that have a high capacity to reestablish the disease may be required to fully exploit the potential of CAR-based immunotherapy.
A critical barrier against the use of engineered T cells for the treatment of solid tumors is the tumor microenvironment. Whereas residual leukemia cells often remain in the bone marrow niche in close proximity to microvessels or in the circulation and hence are relatively accessible to adoptively transferred T cells, efficient targeting of solid tumors requires the recruitment of therapeutic T cells to extravascular sites. Since CAR-expressing T cells are most effective at high effector-to-target cell ratios and even relatively small lesions with a volume of approximately 1 cm3 can contain over 109 viable cancer cells, high numbers of tumor cells have to home to the tumor, or T cells have to expand within the tumor. The tumor microenvironment protects malignant cells against the antitumor activity of the immune system and promotes their growth, survival, angiogenic potential and invasive attitude. Major features of the tumor niche are a lack of the immunological danger signals that are required for the activation of immune responses, and the abundant presence of immunosuppressive factors and cells with immunoregulatory function. To become effective and exert robust antitumor effects, adoptively transferred CAR-expressing T cells must survive, remain functional within this environment and efficiently overcome local immunosuppression. Blocking immunosuppressive checkpoints has recently emerged as a potent means of breaking the immune tolerance to tumors.10 Combining this strategy with the adoptive transfer of CAR-expressing T lymphocytes may effectively prevent their exhaustion and potentiate their therapeutic effects against solid tumors.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Rossig C. Extending the chimeric receptor-based T-cell targeting strategy to solid tumors. OncoImmunology 2013; 2:e26091; 10.4161/onci.26091
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26091
References
- 1.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005930. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rehe K, Wilson K, Bomken S, Williamson D, Irving J, den Boer ML, Stanulla M, Schrappe M, Hall AG, Heidenreich O, et al. Acute B lymphoblastic leukaemia-propagating cells are present at high frequency in diverse lymphoblast populations. EMBO Mol Med. 2013;5:38–51. doi: 10.1002/emmm.201201703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kailayangiri S, Altvater B, Meltzer J, Pscherer S, Luecke A, Dierkes C, Titze U, Leuchte K, Landmeier S, Hotfilder M, et al. The ganglioside antigen G(D2) is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br J Cancer. 2012;106:1123–33. doi: 10.1038/bjc.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebsch L, Kailayangiri S, Beck L, Altvater B, Koch R, Dierkes C, Hotfilder M, Nagelmann N, Faber C, Kooijman H, et al. Ewing sarcoma dissemination and response to T-cell therapy in mice assessed by whole-body magnetic resonance imaging. Br J Cancer. 2013;109:658–66. doi: 10.1038/bjc.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]