Abstract
The prognostic relevance of innate immune cells infiltrating colorectal carcinoma lesions is highly debated. By evaluating the expression of myeloperoxidase (MPO) as a marker of neutrophil granulocytes in a large cohort of colorectal carcinoma specimens, we have observed that robust tumor-infiltration by MPO+ cells correlates with improved patient survival independently of other histopathological parameters, including disease stage.
Keywords: CD15, human colorectal cancer, mismatch repair status, myeloperoxidase, prognosis, tissue microarray
The clinical course of colorectal carcinoma (CRC) critically depends on cancer cell-intrinsic features, including specific mutations, microsatellite instability, and epigenetic alterations, as well as on the tumor microenvironment, as shaped by the interaction of malignant and non-transformed cells. Tumor infiltration by cellular components of the adaptive immune system has been shown to predict the survival of CRC patients more efficiently than the tumor-node-metastasis (TNM) staging.1 However, the role of the innate immune system in CRC progression remains matter of debate.
We observed that the infiltration of CRC lesions by natural killer (NK) cells is infrequent and devoid of prognostic significance.2 In contrast, tumor infiltration by CD33+HLA-DR-CD16+ myeloid cells was associated with improved patient survival, independently of TNM stage.3 Functionally active neutrophil granulocytes (NGs) express high amounts of CD16 (Fcγ receptor IIIB, FCGR3B), which decrease along with a progressive functional decline that precedes apoptosis.4 Based on these premises, we have recently addressed the prognostic significance of NG infiltration in CRC.5
NGs are the most abundant circulating white cells and are the most prominent component of the first-line mechanism of defense against infection. Nonetheless, NGs have been long neglected by tumor immunologists. Notably, high amounts of intratumoral myeloid cells are generally thought to promote tumor progression and hence to correlate with poor disease outcome. In particular, CRC infiltration by CD66B+ granulocytes has been proposed as a marker of adverse prognosis.6
Recent studies, mostly based on preclinical tumor models, have promoted a resurgence in the interest of tumor immunologists for the role of NGs in cancer immunobiology.7 In particular, it has been suggested that, similar to macrophages, NGs may undergo cytokine-driven differentiation toward an N1 and an N2 phenotype, which are associated with anti- and pro-tumor effects, respectively.7
Myeloperoxidase (MPO), a heme protein that generates cytotoxic oxidants from hydrogen peroxide, chloride anion and tyrosine, is abundantly expressed by NGs and has been proposed to serve as an autocrine regulator of their activation.8 Therefore, MPO might represent a valuable biomarker for the identification and quantification of functionally active NGs in clinical specimens. Conversely, the use of CD15 may be associated with a limited specificity, as this protein is expressed not only on mature neutrophils but also on a variety of malignant cells.
By using a tissue microarray (TMA) including a large number (> 1400) of clinically annotated specimens, we observed a significantly higher amount of infiltration by MPO+ and CD15+ cells in CRC lesions than in the normal colonic mucosa. A strong (R = 0.75) correlation between MPO+ and CD15+ cell infiltration was detectable at the tumor site. Moreover, univariate Cox regression analyses revealed that a high density of MPO+ or CD15+ infiltrating cells, detectable in 14.5% and 10.8% of CRC specimens, respectively, was significantly associated with early tumor stage (pT1–2), absence of local recurrence and increased 5-y survival rate. After adjusting for several known prognostic factors including age, sex, T stage, N stage, tumor grade, vascular invasion, tumor border configuration, and microsatellite stability, only the abundance of tumor-infiltrating MPO+ cells retained a prognostic significance. Ex vivo analyses of MPO+ cells infiltrating CRC lesions and the normal mucosa showed that a large majority of these cells also expressed CD15, CD16 and CD66B, consistent with the phenotype of the granulocytic lineage. However, a substantial percentage of CD66B+ CRC-infiltrating cells was MPO-.6
To the best of our knowledge, we were the first to document a positive prognostic impact for tumor infiltration by NGs among CRC patients. Our analysis involved a large number of cases with an extensive clinical annotation, further increasing its value. Future research is warranted to gain additional insights into the molecular mechanisms underlying our observations.
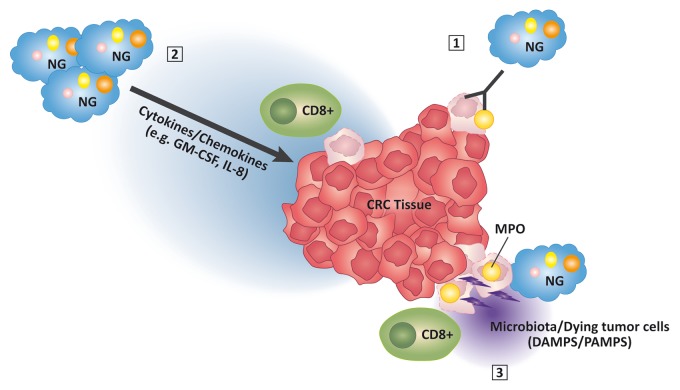
CRC-infiltrating NGs might exert direct antitumor effects, perhaps upon the release of cytokines, chlorinated oxidants or enzymes including MPO (Fig. 1). However, such a direct antitumor activity has rarely been ascribed to human NGs, and was near-to-invariably associated with the presence of tumor-specific opsonizing monoclonal antibodies.9 Alternatively, NG infiltration might constitute an epiphenomenon of the release of chemokines and cytokines such as interleukin-8 (IL-8) and granulocyte macrophage colony-stimulating factor (GM-CSF) by malignant cells or tumor-infiltrating lymphocytes.10 Thus, while devoid of intrinsic antitumor functions, NGs might constitute a marker of favorable microenvironmental features (Fig. 1).
Figure 1. Molecular mechanisms potentially underlying the favorable effects of myeloperoxidase-expressing neutrophil granulocytes in colorectal carcinoma. Myeloperoxidase (MPO)-expressing neutrophil granulocytes (NGs) might exert direct antitumor effects on opsonized cancer cells (1), or they might be recruited to neoplastic lesions by the secretion of immunostimulatory cytokines including interleukin-8 (IL-8) and granulocyte macrophage colony-stimulating factor (GM-CSF) (2). The activation of MPO+ NGs by danger-associated molecular patterns (DAMPs) released by dying tumor cells or by microbiota-derived pathogen-associated molecular patterns (PAMPs) might further promote the antitumor activity of these cells (3).
These explanations are not necessarily mutually exclusive. NGs could be recruited to neoplastic lesions and activated by chemokines and lymphokines secreted by cells of the adaptive immune system in the context of their interaction with cancer cells.1 It is tempting to speculate that products of commensal microorganisms might also play a role in the recruitment and activation of immune cells in the peculiar CRC microenvironment. Within this framework, it would be important to specifically investigate the functions of tumor-infiltrating NGs (Fig. 1).
In summary, the mobilization of granulocytes and their sustained activation at the tumor site might be of benefit for CRC patients, both as a natural process and in the context of monoclonal antibody-based immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Hirt C, Eppenberger-Castori S, Sconocchia G, Iezzi G, Tornillo L, Terracciano L, Spagnoli G, Droeser R. Colorectal cancer infiltration by myeloperoxidase positive neutrophil granulocytes is associated with favorable prognosis. OncoImmunology 2013; 2:e25990;
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25990
References
- 1.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 2.Sconocchia G, Arriga R, Tornillo L, Terracciano L, Ferrone S, Spagnoli GC. Melanoma cells inhibit NK cell functions. Cancer Res. 2012;72:5428–9. doi: 10.1158/0008-5472.CAN-12-1181. author reply 5430. [DOI] [PubMed] [Google Scholar]
- 3.Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M, Tornillo L, et al. Tumor infiltration by FcγRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer. 2011;128:2663–72. doi: 10.1002/ijc.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moulding DA, Hart CA, Edwards SW. Regulation of neutrophil FcgammaRIIIb (CD16) surface expression following delayed apoptosis in response to GM-CSF and sodium butyrate. J Leukoc Biol. 1999;65:875–82. doi: 10.1002/jlb.65.6.875. [DOI] [PubMed] [Google Scholar]
- 5.Droeser RA, Hirt C, Eppenberger-Castori S, Zlobec I, Viehl CT, Frey DM, Nebiker CA, Rosso R, Zuber M, Amicarella F, et al. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS One. 2013;8:e64814. doi: 10.1371/journal.pone.0064814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao H-L, Chen J-W, Li M, Xiao Y-B, Fu J, Zeng Y-X, Cai M-Y, Xie D. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS One. 2012;7:e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 8.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brümmer J, Rudolph V, Münzel T, Heitzer T, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci U S A. 2005;102:431–6. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valerius T, Repp R, de Wit TP, Berthold S, Platzer E, Kalden JR, Gramatzki M, van de Winkel JG. Involvement of the high-affinity receptor for IgG (Fc gamma RI; CD64) in enhanced tumor cell cytotoxicity of neutrophils during granulocyte colony-stimulating factor therapy. Blood. 1993;82:931–9. [PubMed] [Google Scholar]
- 10.De Larco JE, Wuertz BRK, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:4895–900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]