Abstract
Cyclophosphamide, within well-defined therapeutic regimens, increases the antineoplastic effects of immunotherapy. We have recently identified multiple factors and mechanisms that underlie the paradoxical synergy between these two treatment modalities. In particular, we found that cyclophosphamide stimulates anticancer immune responses upon the perception by the immune system of inflammatory danger signals associated with the death of leukocytes, via p53 and type I interferon-related mechanisms.
Keywords: Type 1 interferon, alkylating agents, cellular response to anticancer drugs, chemoimmunotherapy, gene expression profiling, immunomodulation, immunotherapy, sterile inflammation
Although cyclophosphamide was traditionally regarded as an immunosuppressant, owing to its robust cytotoxicity for rapidly proliferating cells (including leukocytes), several studies have demonstrated that this drug can also exert immunostimulatory properties, which can be taken advantage of to enhance the efficacy of anticancer immunotherapy.1 The mechanisms that have been proposed to underlie this paradoxical phenomenon are related to the ability of cyclophosphamide to (1) establish a myelo-leukodepleted state, providing space for adoptively transferred immune effector cells,1 (2) suppress the activity of regulatory T cells,1 (3) induce homeostatic proliferation among B and T cells, (4) facilitate tumor infiltration by lymphocytes and (5) trigger a cytokine storm during the recovery phase that follows leukodepletion.2 Studies in mice have shown that type I interferon (IFN) is an important mediator of the immunomodulatory effects of cyclophosphamide, accounting for the expansion of memory CD4+ and CD8+ T cells,3 as well as of dendritic cells (DCs).4 Genomic and proteomic analyses have demonstrated that cyclophosphamide alters the expression of ~1000 genes in the bone marrow and spleen, as well as the levels of a high number of cytokines/chemokines in the plasma and bone marrow of tumor-bearing mice.5 However, in spite of a multiplicity of studies (reviewed in ref. 1), the specific mechanisms whereby cyclophosphamide exerts so many immunomodulatory effects are still matter of debate. Moreover, these mechanisms have most often been investigated in murine models.
In order to identify the mediators and mechanisms through which cyclophosphamide potentiate anticancer immune responses in humans, we analyzed the systemic effects of a single administration of high-dose cyclophosphamide (3–4 g/m2) in patients affected by hematologic malignancies. In particular, we analyzed the transcriptional profile of peripheral blood mononuclear cells (PBMCs) before and at different time points (1, 2 and 5 d) after treatment.6 The transcriptional modulation induced by cyclophosphamide turned out to be broad (890 distinct genes were differentially expressed), rapid (it manifested 1–2 d post-treatment) and transient (most transcripts altered by cyclophosphamide returned to pre-treatment levels by day 5).
We then functionally classified the genes that were differentially expressed in response to cyclophosphamide by gene ontology (GO) and pathway analyses. Among downregulated genes, the most significantly enriched GO biological processes terms included “metabolic,” “cell cycle,” “ribonucleoprotein complex biogenesis” and “biosynthetic” processes, as expected for a cytotoxic treatment. Remarkably, the most significantly enriched biological process among the genes that were upregulated by cyclophosphamide was “immune response,” along with “response to stress” and “cell death.” In addition, upregulated genes were associated with the pathways “lysosome,” “p53 signaling,” “inflammation mediated by chemokine and cytokine signaling” and “B cell activation.”
“Immune response”-related transcripts included many coding for scavenger receptors (CD68, MARCO, CD163L1 SCARB2) and C1R, encoding a component of the supramolecular complex C1, which is involved in the recognition of stressed and dying cells. Along similar lines, the levels of transcripts coding for factors implicated in antigen processing and presentation (CIITA, CD68, CTSC, CTSL1, CTSZ, GLA, GAA, TPP1, NEU1, SLC11A, LAMP-2) or receptors for inflammatory and anti-inflammatory cytokines (IL8R, IL10R, IL17R) were increased. Also genes encoding receptors of the tumor necrosis factor (TNF) superfamily, such as TNFRSF1A (best known as TNFR1), which triggers either inflammation or apoptosis upon TNFα binding, TNFSF13B (BAFF), which plays a critical role in B-cell expansion/migration, and TNFRSF4 (OX40), which is important for antigen-specific T-cell expansion/survival, were upregulated. Of note, several among cyclophosphamide-induced genes (OAS1, CXCL10, TNFSF13B, IFITM2, IFI6, IRF5, IRF7, STAT2, UBE2L6, UNC93B1, ISG20L1, TYK2) are known to be transactivated in response to type I IFN, and are indicative of a type I IFN signature in different settings.7
Alongside, the administration of high-dose cyclophosphamide altered the expression of numerous genes associated with stress, cell death, DNA damage repair, apoptosis, autophagy and chemotherapy resistance, including genes whose products are related to the “p53 signaling pathway” (CDKN1A, CCND3, BAX, BBC3, BID, DDB2, SESN2). Since type I IFN signaling can stimulate p53 responses to stress, and vice versa, p53 can activate the IFN signal transduction pathway,8 it is tempting to speculate that p53 and type I IFN might cooperate not only to trigger cyclophosphamide-mediated apoptosis, but also to potently stimulate innate immune cells and promote the secretion of pro-inflammatory cytokines, most likely via the upregulation of IRF7 and IRF5.
The administration of high-dose cyclophosphamide also increased the circulating levels of type I IFN-induced cytokines (CXCL10, TNFSF13B) and inflammatory mediators (CCL2, IL-8). The latter have been shown by others to be secreted in response to apoptotic bodies in the course of sterile inflammatory responses.9 Of interest, cytofluorometric analyses revealed that high-dose cyclophosphamide induces a state of leukodepletion affecting both monocytes and lymphocytes, yet does so in a rather selective manner, with specific cell subpopulations undergoing differential quantitative alterations. Indeed, while we found total monocytes to be depleted, we observed increased percentages of a specific monocyte subset (namely, CD14+CD16+ cells) that is characterized by macrophage-like morphology, robust endocytic activity, elevated antigen presentation capacity and intense secretion of pro-inflammatory cytokines.10 Moreover, cyclophosphamide appeared to elevate the percentage of MARCO- and IL8R-expressing monocytes as that of MARCO-expressing circulating DCs, while increasing the expression levels of HLA-DR on both these types of antigen-presenting cells (APCs). Of note, following the administration of high-dose cyclophosphamide, along with the expected lymphodepletion, we observed the expansion of lymphocytes expressing the activation marker CD69, the co-stimulatory molecule OX40 and IL8R.
In conclusion, our findings suggest that the cyclophosphamide-induced death of leukocytes is perceived by surrounding cells as a danger signal and promote the establishment of a systemic sterile inflammatory response characterized by increased concentrations of pro-inflammatory cytokines and the expansion of activated APCs and lymphocytes (Fig. 1). Such a sterile inflammatory response may engender an immunogenic milieu that boosts the efficacy immunotherapeutic interventions. Furthermore, our data point to a critical role for type I IFN and p53 in the regulation of cyclophosphamide-induced sterile inflammation.
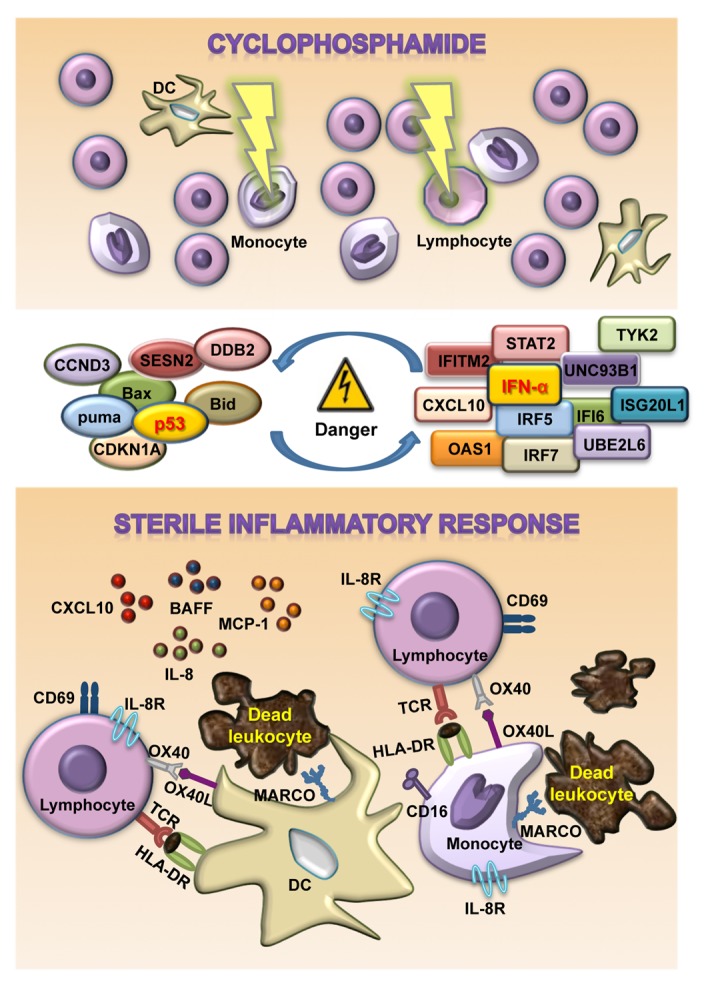
Figure 1. Sterile inflammatory response induced by cyclophosphamide. The administration of cyclophosphamide results in the death of highly proliferating cells (including lymphocytes and monocytes) along with the activation of the p53 signaling pathway and type I interferon (IFN)-regulated genes, favoring the elicitation of the innate immune responses. Dead cells indeed emit danger-associated molecular patterns (DAMPs) that can be recognized by pattern recognition receptors on antigen presenting cells, such monocytes and dendritic cells (DCs). These DAMPs generally stimulate the phagocytosis of apoptotic corpses, exacerbate antigen presentation and promote the secretion of pro-inflammatory cytokines, leading to the homeostatic proliferation and activation of T cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- APC
antigen presenting cells
- DAMP
danger-associated molecular pattern
- DC
dendritic cell
- GO
gene ontology
- IFN
interferon
- PBMC
peripheral blood mononuclear cell
- TNF
tumor necrosis factor
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25789
References
- 1.Proietti E, Moschella F, Capone I, Belardelli F. Exploitation of the propulsive force of chemotherapy for improving the response to cancer immunotherapy. Mol Oncol. 2012;6:1–14. doi: 10.1016/j.molonc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13:644–53. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 3.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, et al. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–30. [PubMed] [Google Scholar]
- 4.Salem ML, Díaz-Montero CM, Al-Khami AA, El-Naggar SA, Naga O, Montero AJ, et al. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C) J Immunol. 2009;182:2030–40. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moschella F, Valentini M, Aricò E, Macchia I, Sestili P, D’Urso MT, et al. Unraveling cancer chemoimmunotherapy mechanisms by gene and protein expression profiling of responses to cyclophosphamide. Cancer Res. 2011;71:3528–39. doi: 10.1158/0008-5472.CAN-10-4523. [DOI] [PubMed] [Google Scholar]
- 6.Moschella F, Torelli GF, Valentini M, Urbani F, Buccione C, Petrucci MT, et al. Cyclophosphamide induces a type I interferon-associated sterile inflammatory response signature in cancer patients’ blood cells: implications for cancer chemoimmunotherapy. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-3666. [DOI] [PubMed] [Google Scholar]
- 7.Aricò E, Belardelli F. Interferon-α as antiviral and antitumor vaccine adjuvants: mechanisms of action and response signature. J Interferon Cytokine Res. 2012;32:235–47. doi: 10.1089/jir.2011.0077. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz-Fontela C, Macip S, Martínez-Sobrido L, Brown L, Ashour J, García-Sastre A, et al. Transcriptional role of p53 in interferon-mediated antiviral immunity. J Exp Med. 2008;205:1929–38. doi: 10.1084/jem.20080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CA, et al. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1α. Proc Natl Acad Sci U S A. 2011;108:20684–9. doi: 10.1073/pnas.1116848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]


