Abstract
Cognitive behavioral therapy (CBT) is a well-established treatment for anxiety disorders, and evidence is accruing for the effectiveness of acceptance and commitment therapy (ACT). Little is known about factors that relate to treatment outcome overall (predictors), or who will thrive in each treatment (moderators). The goal of the current project was to test attentional bias and negative emotional reactivity as moderators and predictors of treatment outcome in a randomized controlled trial comparing CBT and ACT for social phobia. Forty-six patients received 12 sessions of CBT or ACT and were assessed for self-reported and clinician-rated symptoms at baseline, post treatment, 6, and 12 months. Attentional bias significantly moderated the relationship between treatment group and outcome with patients slow to disengage from threatening stimuli showing greater clinician-rated symptom reduction in CBT than in ACT. Negative emotional reactivity, but not positive emotional reactivity, was a significant overall predictor with patients high in negative emotional reactivity showing the greatest self-reported symptom reduction.
Keywords: Social anxiety disorder, Attentional bias, Emotional reactivity, Cognitive behavioral therapy, Acceptance and commitment therapy
The efficacy of Cognitive Behavioral Therapy (CBT) for treatment of anxiety disorders is well established (Butler, Chapman, Forman, & Beck, 2006; Norton & Price, 2007; Tolin, 2010). Other behavioral treatments, such as Acceptance and Commitment therapy (ACT; Hayes, Strosahl, & Wilson, 1999) are garnering support as well (Arch et al., 2012; Craske et al., 2013; Meuret, Twohig, Rosenfield, Hayes, & Craske, 2012). However, many patients do not respond to behavioral treatments, drop out of treatment or show a return of symptoms at follow-up (Loerinc, Meuret, Twohig, Rosenfield, & Craske, 2013). The National Institute of Mental Health has called for a focus on personalized medicine to identify which treatment under what conditions will be most effective (National Institute of Mental Health, 2010). The goal of the current study was to examine attentional bias and emotional reactivity as predictors of response to behavioral treatment and differential moderators of response to CBT and ACT for patients with social phobia.
Attentional biases and emotional reactivity each have been implicated as important factors in the development and maintenance of social phobia (Campbell-Sills & Barlow, 2007; Clark & Wells, 1995; Rapee & Heimberg, 1997). In particular, findings from meta-analyses and review papers show that patients with social phobia are more likely to attend to social stimuli that are indicative of external threat (e.g. angry faces, social rejection words) than non-anxious controls (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Heinrichs & Hofmann, 2001). Cognitive theories of social phobia posit that selectively attending to external social threats increases anxiety, promotes maladaptive thinking, and maintains ineffective social behavior in social situations (Clark & Wells, 1995). In terms of emotional reactivity, individuals with social phobia report more negative affect when viewing negative images from the international affective picture system (Goldin, Manber, Hakimi, Canli, & Gross, 2009), and show more bilateral amygdala and insula activity (areas associated with emotional processing) than control participants while viewing these negative images (Brühl et al., 2011; Shah, Klumpp, Angstadt, Nathan, & Phan, 2009). Furthermore, proneness to emotional reactivity is not only characteristic of social phobia (Brown, Chorpita, & Barlow, 1998; Prenoveau et al., 2010) but has been shown to predict the onset of anxiety in general (Hayward, Killen, Kraemer, & Taylor, 2000; Krueger, Caspi, Moffitt, Silva, & McGee, 1996; Watson, Gamez, & Simms, 2005). Although social phobia has been linked to low positive affect (Brown et al., 1998; Watson, Clark, & Carey, 1988), there is no evidence for differential amygdala or insula activation in response to positive images in patients with social phobia compared to controls (Shah et al., 2009). Attentional bias and negative emotional reactivity are strongly related since induction of negative affect in the form of anxiety enhances attentional bias to threat (Chen, 1996; MacLeod & Mathews, 1988; Mogg, Bradley, & Hallowell, 1994). Also, training attentional bias towards negative stimuli increases self-reported distress to a laboratory stressor (MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002), and training attention away from threatening stimuli lowers self-reported anxiety to a naturalistic stressor (MacLeod & Bridle, 2009). However, very few studies have examined attentional bias and emotional reactivity as predictors of treatment response.
Waters, Mogg, and Bradley (2012) assessed attentional bias for threatening faces as a predictor of response to CBT in 35 children with generalized anxiety disorder or social phobia. Although metaanalyses show that anxious individuals are vigilant to threat, some studies have found that socially anxious individuals avoid threatening stimuli (Chen, Ehlers, Clark, & Mansell, 2002; Mansell, Clark, Ehlers, & Chen, 1999). Therefore, Waters and colleagues assessed attentional bias using the dot probe task (MacLeod, Mathews, & Tata, 1986), which allows separation of individuals into vigilant (those who attend toward threat) and avoidant (those who attend away from threat) bias types. Children who were vigilant to threat responded more favorably to CBT than those who were avoidant of threat. Using a similar method, Price, Tone, and Anderson (2011) found that adults with social phobia who were vigilant to threat responded more favorably to CBT than those who were avoidant of threat. Price and colleagues speculated that those who are more avoidant of threat engage less in exposure practice, which limits corrective learning.
Other researchers have aimed to precisely define the nature of bias within the vigilant group. In the original dot probe task (MacLeod et al., 1986), one neutral face and one threatening face are presented on the screen side by side. A probe then appears in place of one of the faces, and the participant identifies the location of the probe. Anxious individuals tend to take longer to identify probes that appear in place of neutral faces, suggesting that their attention is on the threatening face. However, this task does not differentiate between faster initial orienting toward threatening stimuli and delayed disengagement from threatening stimuli. Using the Spatial Cueing paradigm, Fox, Russo, Bowles, and Dutton (2001) identified that the attentional bias in anxiety is more likely explained by difficulty disengaging from threat rather than faster orienting toward threat. A number of studies have since supported this hypothesis (Amir, Elias, Klumpp, & Przeworski, 2003; Fox, Russo, & Dutton, 2002; Georgiou et al., 2005). Therefore, speed of disengagement from threat may be a more sensitive predictor of treatment response than vigilant versus avoidant subtypes. Thus, we elected to measure speed of disengagement as a potential predictor and moderator of treatment outcome.
In attentional bias tasks, many studies have used angry faces, even though the primary concern in social phobia is rejection by others. More relevant stimuli may be faces that appear disapproving or rejecting and indicate negative evaluation. In a recent study, Burklund, Eisenberger, and Lieberman (2007) found that individuals high in rejection sensitivity showed greater dorsal anterior cingulate cortex activity (an area activated in response to social distress) while viewing disapproving facial expressions compared to angry or disgusted expressions. The authors suggest that disapproving faces pose a distinct type of threat and should be tested in studies examining response to social threat. Therefore, we evaluated attentional bias to both angry and disapproving faces.
In terms of emotional reactivity as a predictor, one study evaluated neural activity to emotional stimuli as a predictor of social phobia treatment response (Doehrmann, 2012) but found no evidence for a relationship between amygdala activity and treatment outcome. To our knowledge, no studies have examined subjective report of positive or negative affect in response to positive and negative images respectively as predictors of treatment response.
The primary goal of the current study was to evaluate attentional bias to external threat and self reported emotional reactivity as predictors of response to behavioral treatment for social phobia. Based on previous research, we hypothesized that patients with social phobia who demonstrated slower disengagement from threatening facial stimuli (i.e., more vigilance to threat) would respond most favorably to treatment. We also hypothesized that greater self reported negative emotional reactivity to negative stimuli would predict better treatment response based on research showing a causal link between attentional bias and emotional reactivity (MacLeod et al., 2002). Further, we assessed speed of disengagement and emotional reactivity as moderators of response to two types of behavioral treatment, CBT and ACT, to determine whether these constructs indicated who would respond most favorably to each treatment. Because the question of moderation by attentional bias and emotional reactivity had not been previously been examined, we had no a priori hypotheses.
Method
Participants
Social phobia
Sixty-two participants who met DSM-IV criteria for a principal or co-principal diagnosis of social phobia, generalized type were randomized to ACT (n = 29) or CBT (n = 33). Participants were screened using the Anxiety Disorders Interview Schedule IV (Brown, Di Nardo, & Barlow, 1994) and had a clinical severity rating of 4 or greater. See below for a description of this interview and the clinical severity rating. Analysis of baseline data included all participants who were randomized. Analysis of follow-up data included only participants who completed treatment (n = 24 ACT, n = 22 CBT).2 See Craske et al. (2013) for participant flow of the full sample. A revised chart summarizing flow of participants for the current sample is depicted in Fig. 1 and demographics are reported in Table 1. Participants were recruited from the Los Angeles area in response to local flyers, Craigslist and local newspaper advertisements, and referrals. The study took place at the Anxiety Disorders Research Center at the University of California Los Angeles, Department of Psychology.
Fig. 1.
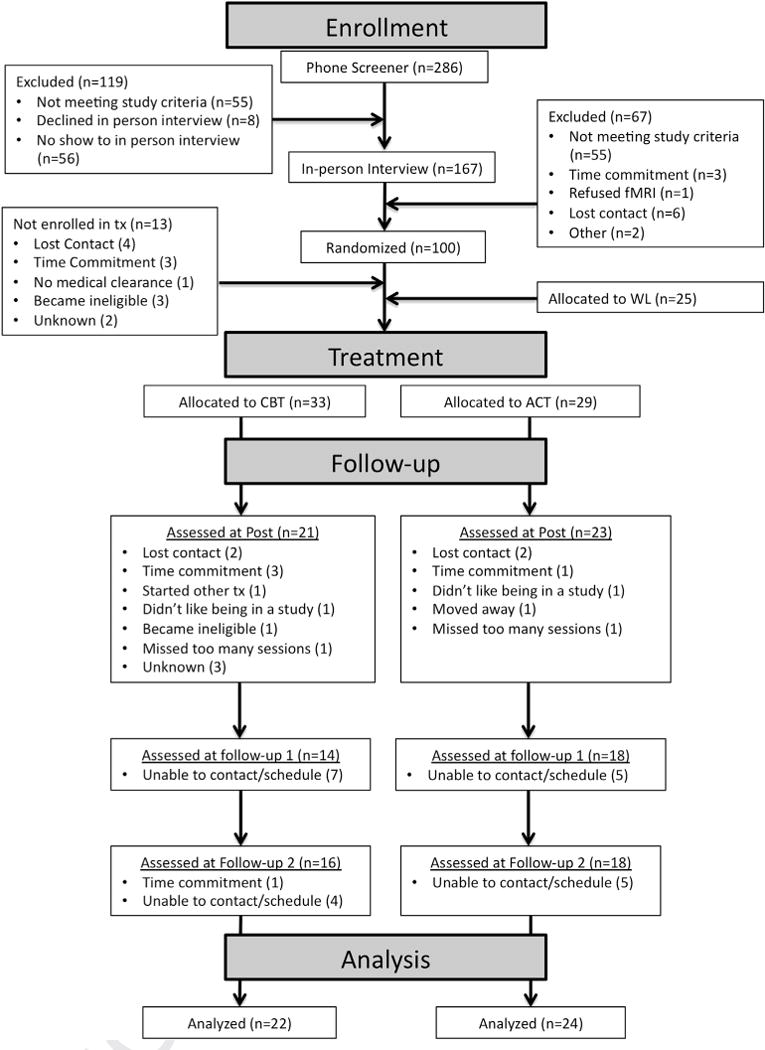
Patient flow chart.
Table 1.
Demographic and clinical characteristics of social phobia treatment completers and healthy controls.
| Characteristic | Total | CBT | ACT | Healthy controls |
|---|---|---|---|---|
| Gender (Female) | 47.69% (31/65) | 42.45% (10/22) | 41.67% (10/24) | 57.89% (11/19) |
| Reported race/ethnicitya | ||||
| White | 55.38% (36/65) | 59.09% (13/22) | 54.17% (13/24) | 52.63% (10/19) |
| Hispanic/Latino/a | 12.31% (8/65) | 9.09% (2/22) | 20.83% (5/24) | 5.26% (1/19) |
| Asian–American/Pacific Islander | 21.54% (14/65) | 13.64% (3/22) | 16.67% (4/24) | 36.84% (7/19) |
| Age, in yearsb | 28.07 (6.49) Range: 18–42 |
29.05 (7.18) | 27.68 (5.73) | 27.47 (6.81) |
| Education, in years | 15.26 (1.87) Range: 10–19 |
15.77 (1.97) | 15.04 (1.92) | 14.95 (1.65) |
| Marital status | ||||
| Married/Cohabiting | 10.77% (7/65) | 18.18% (4/22) | 4.17% (1/24) | 10.53% (2/19) |
| Single | 83.08% (54/65) | 77.27% (17/22) | 87.50% (22/24) | 84.21% (16/19) |
| Other | 6.15% (4/65) | 4.55% (1/22) | 8.33% (2/24) | 5.26% (1/19) |
| Children (1+) | 7.69% (5/65) | 9.09% (2/22) | 4.17% (1/24) | 10.53% (2/19) |
| Currently on psychotropic medication | 26.09% (12/46) | 22.73% (5/22) | 29.17% (7/24) | n/a |
| Comorbid anxiety disorder (1+)c | 23.91% (11/46) | 18.18% (4/22) | 29.17% (7/24) | n/a |
| Comorbid depressive disorderc | 13.04% (6/46) | 4.55% (1/22) | 20.83% (5/24) | n/a |
| Social phobia clinical severity rating (mean) | 5.57 (.93) Range: 4–7 |
5.73 (.83) | 5.42 (1.02) | n/a |
For race/ethnicity, analyses assessed group differences in minority versus white status.
Demographic data was missing for 1 P.
Comorbidity was defined as a clinical severity rating of 4 or above on the ADIS.
Participants were either medication-free or stabilized on psychotropic medications for a minimum length of time (1 month for benzodiazepines and beta blockers, 3 months for SSRIs/SNRIs, heterocyclics, and MAO inhibitors). Also, participants were psychotherapy-free or stabilized on alternative psychotherapies (other than cognitive or behavioral therapies) that were not focused on their anxiety disorder for at least 6 months prior to study entry. Exclusion criteria included active suicidal ideation, severe depression (clinical severity rating > 6, see below), or a history of bipolar disorder or psychosis. Participants with substance abuse or dependence within the last 6 months, or who had been diagnosed with respiratory, cardiovascular, pulmonary, neurological, muscular-skeletal diseases or pregnancy were excluded. Patients with asthma, high blood pressure or thyroid diseases were included only if they were currently receiving treatment and were stabilized for these conditions. In the case of uncertainty regarding medical conditions, confirmation was received from the participant’s physician. Because our study included neuroimaging (results reported elsewhere) additional exclusion criteria were left handedness, metal implants, and claustrophobia. Participants were financially compensated for post and follow-up assessments. The study was fully approved by the UCLA Human Subjects Protection Committee; full informed consent was obtained from all participants, including for video and audio-recordings.
Healthy controls
Nineteen age and gender-matched healthy control participants were recruited through advertising on UCLA campus and surrounding areas. This group served as a validation of the clinical relevance of our predictor variables. Healthy control participants did not meet diagnostic or NOS criteria for any anxiety or mood disorder as assessed by the ADIS-IV, and the same exclusion criteria applied to the control participants as to the social phobia participants.
Design
Patients with social phobia were assessed at four time-points: pre-treatment (Pre), post-treatment (Post), and 6 months (6MFU) and 12 months (12MFU) after Pre. 6MFU refers to approximately 3 months after treatment completion and 12MFU refers to approximately 9 months after treatment completion. Healthy control participants were assessed once. Assessments included a diagnostic interview, self-report questionnaires, and a laboratory assessment that included the emotional reactivity and attentional bias tasks (the laboratory assessment was not conducted at 6MFU). The current paper includes moderator analyses of baseline data collected during the laboratory assessment only. See Craske et al. (Craske et al., 2013) for additional moderator results from self-report questionnaires, diagnostic information, and demographics.
Treatments
Participants in CBT or ACT received twelve weekly, reduced-cost, one-hour, individual therapy sessions based on detailed treatment manuals.3 ACT and CBT were matched on number of sessions devoted to exposure but differed in framing of the intent of exposure. Following the 12 sessions, therapists conducted follow-up booster phone calls (20–35 min) once per month for 6 months to reinforce progress consistent with the assigned therapy condition.
Cognitive behavioral therapy
CBT for social phobia was derived largely from standard CBT protocols (e.g. Hope, Heimberg, Juster, & Turk, 2004). Session 1 focused on assessment, self-monitoring, and psychoeducation. Sessions 2–4 emphasized cognitive restructuring errors of over-estimation and catastrophizing regarding negative evaluation, combined with hypothesis testing, self-monitoring, and breathing retraining. Exposure to feared social cues (including in-vivo, imaginal, and interoceptive exposure combined with in-vivo exposure) was introduced in Session 5, and emphasized strongly in Sessions 6–11. Session 12 focused on relapse prevention.
Acceptance and commitment therapy
ACT for anxiety disorders largely followed a manual authored by Eifert and Forsyth (2005).4 Session 1 focused on psychoeducation, experiential exercises, and discussion of acceptance and valued action. Sessions 2–3 explored creative hopelessness or whether efforts to manage and control anxiety had “worked” and how such efforts had led to the reduction or elimination of valued life activities. Sessions 4 and 5 emphasized mindfulness, acceptance and cognitive defusion or the process of experiencing anxiety-related language (e.g. thoughts, self-talk, etc.) as part of the broader, ongoing stream of present experience rather than getting stuck in responding to its literal meaning. Sessions 6–11 continued to hone acceptance, mindfulness, and defusion, and added values exploration and clarification with the goal of increasing willingness to pursue valued life activities. Behavioral exposures (e.g. interoceptive, in-vivo, imaginal) were employed to provide opportunities to practice mindfully observing and accepting anxiety and to practice engaging in valued activities while experiencing anxiety. Session 12 reviewed what worked and how to continue moving forward.
Therapists
Advanced clinical psychology doctoral students at UCLA served as study therapists (see Craske et al., 2013 for more details). Therapists completed intensive 2-day workshops, led by Dr. Craske for CBT and Dr. Hayes (University of Nevada) for ACT, prior to treating participants. Therapists were assigned to ACT, CBT, or both (i.e., treated in both CBT and ACT, though never at the same time).
Weekly, hour-long group supervision for study therapists was led separately by Dr. Craske and advanced therapists from UCLA and from Dr. Hayes’ laboratory at the University of Nevada, Reno, where ACT was originally developed.
Predictor and moderator variables
For the current analyses, attentional bias and emotional reactivity at baseline were assessed as predictors and moderators of treatment outcome.
Spatial cueing attentional bias task
Images
Photographs were taken of individuals displaying angry, neutral, and disapproving facial expressions. Three photographs of the same individual making each type of expression were selected. Images of eight different individuals were used. In addition, images of household objects from the international affective picture system (IAPS) (Lang, Bradley, & Cuthbert, 1999) were used as non-social control stimuli. Photographs measured 197 pixels wide and 227 pixels high.
Disapproving facial expressions were operationally defined as raising one side of the upper lip, lowering the inner corners of the brow such as might be displayed in a “confused” expression, and slightly tilting or pulling the head backward (Burklund et al., 2007). Examples are shown in Fig. 2. The expressions were viewed and rated by UCLA undergraduates (n = 43), who selected which emotion was represented from a list of emotions. The average percentage of raters who identified the images as angry, disapproving, neutral, confused, disgusted, and sad was calculated. Accuracy rates for angry, disapproving, and neutral faces were 68.9%, 44.2%, and 80.0% respectively (see Table 2). Disapproving faces were rated as confused 28.7% of the time. The undergraduates also rated the valence and arousal of each face (0 = neutral/not at all arousing and 8 = extremely negative/extremely arousing). There was a significant effect of face type on valence (F(2,84) = 255.62, p < .001) with angry faces rated as more negative than disapproving faces and neutral faces, and disapproving faces rated as more negative than neutral faces (ps < .001) (see Table 2). There was also a significant effect of face type on arousal (F(2,84) = 72.69, p < .001) with angry faces rated as more arousing than disapproving and neutral faces, and disapproving faces rated as more arousing than neutral faces (ps < .001).
Fig. 2.

Examples of disapproving facial expressions.
Table 2.
Average percentage of raters selecting target and non-target labels for images of emotional faces used in the spatial cueing task.
| Label | Photograph
|
||
|---|---|---|---|
| Anger | Disapproval | Neutral | |
| Anger | 68.9 | 2.0 | 1.2 |
| Disapproval | 6.1 | 44.2 | 3.8 |
| Neutral | .3 | 2.3 | 80.0 |
| Confusion | 7.0 | 26.7 | 1.2 |
| Sadness | 5.2 | 1.7 | 9.3 |
| Disgust | 8.7 | 11.9 | .9 |
| No ratinga | 3.8 | 11.0 | 4.1 |
| Valence m (sd) | 5.1 (1.4) | 3.5 (1.3) | .7 (.7) |
| Arousal m (sd) | 3.5 (1.9) | 2.6 (1.6) | .8 (.8) |
Note. Target emotion in bold.
Participant selected N/A or left item blank.
Procedure
The procedure followed that of Georgiou et al. (2005). Participants were seated approximately 50 cm from the computer in a quiet 2 m by 6 m room. The stimuli were presented on a computer screen and participants responded using the computer keyboard. Two keys at equal distance from the center of the keyboard were chosen to represent the two letters. The target stimuli were capital letters “X” and “P” presented on the screen in Geneva 24 pt font. The letters appeared 8 cm above (9 degrees of visual angle from the central fixation at 50 cm from the screen), below, left, or right of the centrally located image. The stimuli were presented on a Dell Inspiron 4000 laptop computer with 11.25 × 8.5 in. color screen using E-Prime software.
Participants were told that they would first see a cross, then an image would appear, and finally a letter would appear above, below, left, or right of the image. They were asked to identify the letter using the keys on the keyboard while keeping attention focused on the central image. Between trials, they were to keep their eyes focused on the central cross and to respond as quickly and accurately as possible.
The cross was presented for 1000 ms, the image then appeared by itself in the center of the screen for 600 ms. Then the target letter appeared at 1 of 4 locations for 50 ms, and participants categorized the letter as X or P. The central image remained on the screen throughout and disappeared only after the participant had responded or 2000 ms had elapsed (whichever occurred first). There was an inter-trial interval of 500 ms before the cross reappeared.
Participants first completed a practice round of 32 trials before completing 256 trials, which were divided into 4 blocks with 64 trials each. Blocks were separated by a 30 s break. Within each block, participants saw all 4 types of images (disapproving, angry, neutral and object) an equal number of times, and therefore, each image type appeared a total of 16 times within each block. Eight different images for each image type were selected, and therefore, within each block, participants saw the same image twice. Within each block, the order of images was randomized and the randomization was different for each of the four blocks.
Disengagement scores
The amount of time that participants took to identify the letter that appeared was recorded on each trial. Because we were interested in attentional bias for threatening stimuli (e.g. angry and disapproving faces) compared to non-threatening stimuli (household objects), response times to neutral images were not included in the present analyses. Mean response times were calculated by averaging response times across all trials for each of the three image types (angry, disapproving, and household objects). Outliers were identified as response times less than 100 ms and greater than 1500 ms (Georgiou et al., 2005), and trials on which participants responded incorrectly were not included in the mean scores.
International affective picture system (IAPS) task
Images
Images used in the IAPS task were taken from the IAPS (Lang et al., 1999) image database and were selected based on valence and arousal. Valence was rated on a scale from −4 to 4 with lower numbers representing more negative valence, and arousal was rated on a scale from 0 to 9, with 9 being more arousing. Ten negative (mean valence = −2.8; mean arousal = 6.6), ten positive (mean valence = 2.5; mean arousal = 6.0), and ten neutral images (mean valence = 0.0; mean arousal = 2.8) were chosen for a total of 30 images.
Procedure
The procedure followed that of Arch and Craske (2006). Participants viewed 30 images (10 positive, 10 negative, and 10 neutral) divided into 6 blocks with 5 images in each block. Each block consisted of only one image type (negative, positive or neutral), and participants saw two blocks of each image type. All participants viewed the image blocks in the same order (neutral, negative, positive, negative, positive, neutral). Each image appeared for 6 s, and blocks lasted a total of 30 s. After each block, participants completed the state version of the 10-item Positive and Negative Affect Scale (PANAS; Mackinnon et al., 1999). A 13s ITI preceded the next block. To rule out potential order effects, negative and positive affect scores on the PANAS were compared between the first and second blocks of each image type using paired-samples t-tests. Positive and negative affect ratings did not significantly differ between blocks 1 and 2 (ps > .06).5
Emotional reactivity
Positive emotional reactivity was defined as positive affect in response to positive images, and negative emotional reactivity was defined as negative affect in response to negative images. Negative and positive affect scores from the PANAS were calculated by summing scale ratings across negative and positive items respectively. Negative and positive affect scores were then averaged across same valence image blocks for negative and positive images respectively, producing negative emotional reactivity scores for negative images and positive emotional reactivity scores for positive images. Positive and negative affect scores were also calculated for neutral images to allow comparison of reactivity to emotional stimuli to that of neutral stimuli. Therefore, each participant had four total scores: one positive emotional reactivity score, one negative emotional reactivity score, and two comparison scores for positive and negative affect in response to neutral images.
Outcome variables
We examined two outcomes that represented different modalities of assessment: symptom composite from the self-report modality, and fear and avoidance ratings from the independent clinician rating modality. Two modalities were used to test whether findings were consistent across different methods of measuring symptom severity. Outcome variables were collected at all four time-points (Pre, Post, 6MFU and 12MFU).
Self report measures
We selected three widely used and well-validated self-report measures. The Liebowitz Social Anxiety Scale – Self Report (LSAS-SR; Fresco et al., 2001) is a 24-item measure that assesses fear and avoidance of social and performance situations. Each item is rated on a scale from 0 to 3, with 0 being “no fear/never avoid” and 3 being “severe fear/usually avoid.” Scores were the sum of fear and avoidance ratings across social and performance situations. The measure shows good test-retest reliability (r = .83), internal consistency (α = .95) and convergent validity, and is sensitive to change following treatment (Baker, Heinrichs, Kim, & Hofmann, 2002). Alphas ranged from .94 to .97 across all time points. The Social Interaction Anxiety Scale (SIAS; Mattick & Clarke, 1998) is a 20-item measure that includes self-statements describing cognitive, affective or behavioral reactions to social interaction in dyads or groups. Participants respond on a Likert scale from 0 to 4, with 0 being “not at all characteristic or true of me”, and 4 being “extremely characteristic or true of me.” The scale demonstrates good internal consistency (α = .90) and correlates highly with other measures of social phobia (Osman, Gutierrez, Barrios, Kopper, & Chiros, 1998). In the current sample, as ranged from .93 to .96 across all time points. The Social Phobia Scale (SPS; Mattick & Clarke, 1998) is a 20-item measure describing situations or themes related to being observed by others. Participants rate the extent to which each item is characteristic of them on a 0 to 4 scale, with 0 being “not at all characteristic of me” and 4 being “extremely characteristic of me.” The scale demonstrates good internal consistency (α = .91) and correlates highly with other measures of social phobia (Osman et al., 1998). In the current sample, as ranged from .90 to .93 across all time points.
Symptom composite scale
To improve validity, a composite was created from the LSAS, SIAS and SPS. Z-scores were calculated for each measure at Pre and then standardization was based on Pre means and standard deviations for each subsequent assessment using the equation (time 2 score – time 1 mean)/(time 1 standard deviation). The composite score represented averages of the three measures with the exception of 6MFU at which the LSAS-SR was not included in the composite score, as this measure was not administered at 6MFU.
Independent clinician measures
Clinical diagnoses were ascertained using the Anxiety Disorders Interview Schedule-IV (ADIS-IV). Doctoral students in clinical psychology or highly trained bachelor level research assistants served as interviewers. Clinical severity ratings (CSR) were assigned to each disorder by group consensus on a 0 to 8 scale with 0 being none and 8 being extremely severe (Brown et al., 1994). A participant was eligible if he/she received a CSR rating of 4 or higher for social phobia, which indicates clinical severity based on symptoms, distress, and disablement (Craske et al., 2007). For the current analyses, we used fear and avoidance ratings (described below) rather than CSR as an outcome measure due to limited variability in CSR (range = 4–7 at Pre; range = 1–7 at Post; range = 0–7 at 6MFU and 12MFU). Digitally-recorded ADIS-IV interviews from the Anxiety Disorders Research Center were randomly selected (n = 22) for blind rating by a second interviewer. Inter-rater reliability on the principal diagnosis was 100%. Inter-rater agreement on dimensional CSR ratings across all anxiety disorders was .65 with a single-measure, one-way mixed intraclass correlation6 coefficient. For further details, see Arch et al. (2012).
Fear and avoidance ratings
As part of the ADIS-IV interview, the clinician rated fear and avoidance (0 = none and 8 = extreme anxiety or avoidance) for each of a list of 13 social situations (e.g. parties, public speaking, dating, speaking with unfamiliar people). Of the 13 situations, 10 overlap with those in the LSAS clinician administered measure, which is well validated as a clinician administered measure of social phobia (Heimberg et al., 1999). Scores were summed and ranged from 0 to 208. In the current sample, as ranged from .88 to .96 across all time points.
Statistical approach
Raw data (collapsed across time-points) were inspected graphically; outliers (± 3SD) or impossible numerical responses on computer tasks were replaced with the nearest non-outlier value based on the Winsor method (Guttman, 1973). Less than 1% of data were Winsorised. One participant’s scores on the IAPS task were dropped because responses consistently were outside the possible range. In full multi-level models, level one and two residuals were examined for normality. Residuals were normally distributed across all models.
The outcome variables were assessed at four time points – Pre, Post, 6MFU, and 12MFU. Generally, the pattern of anxiety symptom reduction assessed from pre to post treatment and through additional follow-up time points is not accurately captured by linear, quadratic or exponential curves given that the majority of change occurs directly after completion of treatment with little or no subsequent change at follow-up time points. Therefore, to circumvent this issue, we included Pre scores on the outcomes as a covariate and modeled change linearly for Post through 12MFU. In pre/post designs, pre-treatment scores can be included as covariates rather than as one of the repeated measures because including covariates more fully equates groups on baseline levels of the outcome and minimizes the variance in the outcomes (Tabachnick & Fidell, 2006). Our final model was a multi level model similar to a repeated measures analysis of covariance (ANCOVA)-like design. This statistical approach followed that of previous research examining moderators and predictors of treatment outcome (Craske et al., 2013; Wolitzky-Taylor, Arch, Rosen-field, & Craske, 2012).
Analyses were run using the xtmixed command in Stata 12. The model was a two level growth curve model. On level 1, we included Time, which consisted of the three assessments that occurred after baseline (Post, 6MFU, and 12MFU) modeled as a continuous linear predictor. On level two, we included baseline levels of symptoms or fear and avoidance ratings (as a covariate), treatment condition (CBT or ACT), and our predictors/moderators. Models were fitted using maximum likelihood and random effects of intercept and time were included in all models.
Since a moderator might interact with Group or Time, both of these interactions, and the three-way interaction between moderator, Group, and Time were included in each analysis. Further, because associations between psychological variables are often non-linear, quadratic terms for the moderator and its interaction with Group and Time were included in the model. When there was no significant quadratic relationship between the moderator and outcome, the quadratic term was dropped from the model and linear relationships were tested. Similarly, when Time did not significantly interact with the moderator and Group, Time was dropped from the model and two-way interactions were tested. When the interaction between moderator and group was not significant, the interaction was dropped from the model, and moderators were tested as both quadratic and linear predictors of the DVs.
Results
Emotional reactivity and attentional bias: social phobia vs. healthy control groups
Repeated measures ANOVAs were used to assess differences between Social Phobia and Control participants in positive and negative emotional reactivity and attentional bias. Means and standard deviations are displayed in Table 3.
Table 3.
Means and standard deviations for emotional reactivity in response to negative, neutral and positive images on the International Affective Picture System task, and for reaction time and error rate on the spatial cueing task.
| Controls m (sd) n = 19 |
Social phobia m (sd) n = 60 |
|
|---|---|---|
| IAPS emotional reactivity task | ||
| Negative affect | ||
| Negative images | 10.9 (4.9) | 13.8 (5.0) |
| Neutral images | 5.4 (.8) | 5.8 (1.3) |
| Positive affect | ||
| Positive images | 14.3 (5.5) | 13.2 (4.4) |
| Neutral images | 9.2 (3.8) | 8.1 (3.4) |
| Spatial cueing attentional bias taska | ||
| Object | ||
| Reaction time (ms) | 375 (40) | 383 (48) |
| Percent incorrect | 14.1 (2.5) | 14.9 (2.4) |
| Anger | ||
| Reaction time (ms) | 376 (41) | 384 (47) |
| Number incorrect | 14.1 (2.1) | 14.5 (2.6) |
| Disapproval | ||
| Reaction time (ms) | 376 (38) | 383 (50) |
| Number incorrect | 14.9 (2.9) | 16.0 (2.8) |
n = 18 for healthy controls and n = 53 for social phobia group due to missing data.
Positive and negative affect from the PANAS were the dependent variables for the IAPS task. To test whether positive emotional reactivity differed between Social Phobia and Control participants, we conducted a 2 (Valence: Positive, Neutral) × 2 (Group: Social Phobia, Healthy Control) repeated measures ANOVA that included Valence as the within subjects factor and Group as the between subjects factor. The dependent variable was positive affect. A significant main effect of Valence emerged, F(1, 76) = 112.19, p < .001 such that positive affect was higher for positive images (M = 13.46, 95% confidence interval (CI) = 12.89e14.03) than for neutral images (M = 8.36, CI = 7.78e8.93). To test whether negative emotional reactivity differed between Social Phobia and Control participants, we conducted a 2 (Valence: Negative, Neutral) × 2 (Group: Social Phobia, Healthy Control) repeated measures ANOVA that included Valence as the within subjects factor and Group as the between subjects factor. The dependent variable was negative affect. A significant Valence by Group interaction emerged, F(1, 77) = 4.34, p = .041. Tests of simple effects with a Bonferroni correction revealed that negative affect while viewing negative images was higher in the social phobia group than in the control group (corrected p = .005), but did not differ between groups for neutral images (corrected p = 1.0).
Error rate and average response time were the dependent variables for the Attentional Bias task. To test whether error rate and attentional bias differed between Social Phobia and Control participants, we conducted 3 (Valence: Anger, Disapproval, Object) × 2 (Group: Social Phobia, Healthy Control) repeated measures ANOVAs that included Valence as the within subjects factor and Group as the between subjects factor. For error rate, there was a significant main effect of Valence, F(2, 212) = 5.17, p = .007. Tests of simple effects with a Bonferroni correction revealed that error rate was significantly higher for disapproving faces than angry faces (corrected p < .001) and objects (corrected p = .009), but did not differ between angry faces and objects (corrected p = 1.0). For average response time, there were no significant interaction or main effects (ps > .54).
Outcome analyses
We conducted t-tests on primary outcome measures comparing those who dropped during treatment to those who completed treatment: no significant differences were found on any primary outcome variable at Pre.
To determine whether treatment outcome results differed between the completers sample and the intent-to-treat sample (Craske et al., 2013), we tested whether the self-report symptom composite and Fear and Avoidance Ratings decreased over time or differed by treatment group within the completer sample. A piecewise MLM approach was used with one segment modeling Pre to Post change and a second segment modeling change from Post through 12MFU. For further details and equations, see Craske et al. (2013). Results fully replicated those from the intent-to-treat analysis. Participants in CBT and ACT showed a significant decline in symptom composite scores from Pre to Post (ps < .001), but no significant change from Post through 12MFU (ps > .101). CBT and ACT did not differ at any time-point (ps > .539). The same pattern of results was observed for clinician-administered fear and avoidance ratings; participants in CBT and ACT showed a significant decline in fear and avoidance from Pre to Post (ps < .001), but no significant change from Post through 12MFU (ps > .092). Fear and avoidance scores at Post were marginally significantly higher in ACT than in CBT (p = .076), but did not differ between groups at 6MFU or 12MFU (ps > .193). Estimated means and confidence intervals from the model for the CBT and ACT groups at each assessment time point are displayed in Table 4.
Table 4.
Estimated means and confidence intervals (CI) of symptom composite and fear and avoidance ratings for completers in ACT and CBT by assessment occasion.
| CBT mean (95% CI) | ACT mean (95% CI) | Difference mean (95% CI) | |
|---|---|---|---|
| Self report symptom composite | |||
| Baseline | −.04 (−.40 to .32) | .01 (−.33 to .35) | .05 (−.45 to .55) |
| Post | −1.12 (−1.53 to −.70) | −1.14 (−1.55 to −.73) | −.02 (−.61 to .56) |
| 6 mo | −1.27 (−1.71 to −.83) | −1.17 (−1.59 to −.74) | .10 (−.50 to .71) |
| 12 mo | −1.43 (−1.95 to −.90) | −1.20 (−1.70 to −.70) | .23 (−.49 to .95) |
| Independent clinician fear and avoidance ratings | |||
| Baseline | 92.4 (81.3–103.4) | 94.8 (84.2–105.4) | 2.4 (−12.9–17.8) |
| Post | 57.3 (44.2–70.5) | 73.8 (61.2–86.3) | 16.4 (−1.7–34.6) |
| 6 mo | 56.8 (43.6–69.9) | 68.8 (56.3–81.3) | 12.1 (−6.1–30.2) |
| 12 mo | 56.2 (40.4–72.0) | 63.8 (48.9–78.8) | 7.7 (−14.1–29.4) |
Predictor and moderator analyses
Moderators and predictors were evaluated in terms of the symptom composite from the self-report modality, and fear and avoidance ratings from the independent clinician rating modality. Table 5 displays correlations between moderators and baseline dependent measures.
Table 5.
Correlations between moderators and dependent measures at baseline.
| Ang bias | Dis bias | Neg-Neg | Neg-Neu | Pos-Neu | Pos-Pos | Symptoms | |
|---|---|---|---|---|---|---|---|
| Dis Bias | .76*** | ||||||
| Neg-Neg | .13 | .20 | |||||
| Neg-Neu | −.20 | .01 | .53*** | ||||
| Pos-Neu | −.11 | .08 | .19 | .41** | |||
| Pos-Pos | .14 | .20 | .40 | .26 | .57*** | ||
| Symptoms | .11 | .06 | .07 | .13 | .29 | .26 | |
| FAR | −.05 | −.19 | .03 | .04 | −.10 | −.02 | .65*** |
Note:
p < .01,
p < .001;
Dis Bias = Bias for disapproving faces (reaction time to disapproving faces minus reaction time to neutral faces); Ang Bias = Bias for angry faces (reaction time to angry faces minus reaction time to neutral faces); Neg-Neg = Negative affect following negative images; Neg-Neu = Negative affect following neutral images; Pos-Neu = Positive affect following neutral images; Pos-Pos = Positive affect following positive images; Symptoms = Social anxiety symptom composite; FAR = Fear and Avoidance Ratings.
Attentional bias
When examining response time to facial expressions as a predictor or moderator of treatment outcome, response time to household objects was added as a covariate to control for overall reaction time on the task. Because no significant differences emerged in reaction time to angry and disapproving faces in socially anxious or control participants, response times to these faces were averaged to create an overall mean reaction time to negative faces. Results did not differ when angry and disapproving faces were analyzed separately.
Reaction time to negative faces significantly interacted with Group to moderate clinician fear and avoidance ratings (z = 2.83, p = .005) (see Fig. 3). Tests of simple effects revealed that slower reaction times predicted lower fear and avoidance in the CBT group (standardized beta (and avoidance in the CBT group (β) = −.70, CI = −.03 to −1.37, p = .040) whereas the relationship was not significant in the ACT group (β = .02, CI = −.56 to .53, p = .995). The CBT group was rated as less fearful and avoidant than the ACT group at reaction times greater than approximately 0.06 SD from the mean (p < .039). The same direction of effects was marginally significant for the composite of self reported symptoms (z = 1.83 p = .068). Tests of simple effects revealed that slower response time to negative faces predicted fewer symptoms after ACT (β = −.46, CI = −.01 to −.92, p = .047) as well as after CBT (β = −.84, CI = −.30 to −1.38, p = .002), but the relationship was marginally stronger in the CBT group (p = .066). Additional tests of simple effects were not significant as the groups did not differ in symptom composite scores at reactions times anywhere between 1 SD below the mean to 1 SD above the mean (ps > .129).
Fig. 3.
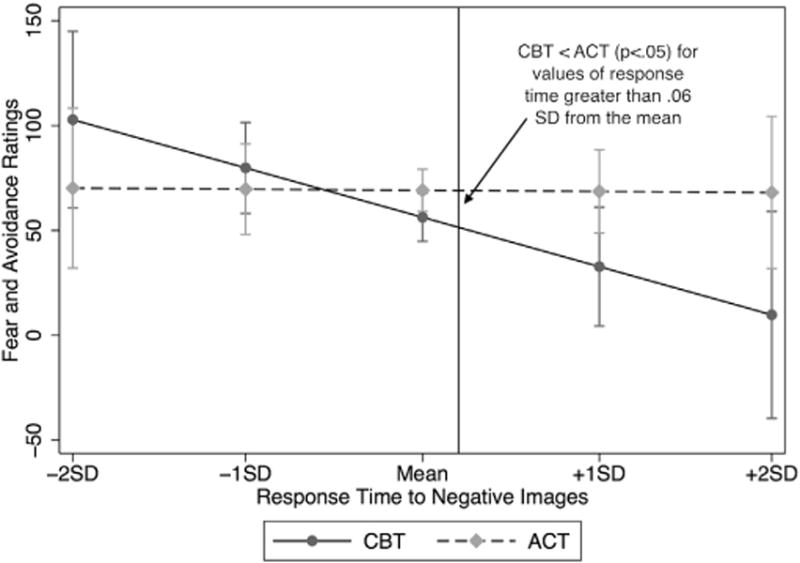
Moderation by attentional bias for negative (disapproving and angry) faces.
Emotional reactivity
Negative affect while viewing negative images did not significantly moderate either outcome measure (ps > .178), but was a significant linear predictor of the symptom composite measure (z = 3.11, p = .002), with those higher in negative affect to negative images reporting fewer symptoms (β = −.34; CI = −.55 to −.12) across groups and follow-up time points. The same direction of effect was marginally significant for fear and avoidance ratings (z = 1.79 p = .073), with those higher in negative affect while viewing negative images rated as less fearful and avoidant (β = −.20; CI = −41 to .02) across groups and follow-up time points. Negative affect while viewing neutral images did not significantly moderate or predict either outcome (ps > .088).
Positive affect while viewing positive images did not significantly moderate or predict either outcome (ps > .126). Positive affect while viewing neutral images (quadratic effect) significantly interacted with time to predict symptom outcome (z = 2.18, p = .029). However, regressions conducted separately at each time point revealed no significant simple effects (ps > .278). No other moderator or predictor effects of positive affect to neutral images were found for either outcome (ps > .068).
Discussion
The goal of the current project was to examine attentional bias towards externally threatening social stimuli and emotional reactivity as predictors and moderators of response to two behavioral treatments for social phobia: cognitive behavioral therapy (CBT) and acceptance and commitment therapy (ACT). Attentional bias emerged as a significant moderator of treatment response with those who were slower to disengage faring better in CBT than in ACT, as judged by independent clinician ratings. Negative emotional reactivity was an overall predictor, with those reporting the greatest negative affect to negative images showing the best treatment response, based on self-report of symptoms. The predictive and moderating effects of emotional reactivity and attentional bias respectively were over and above that of symptom severity at baseline, and neither attentional bias nor emotional reactivity significantly correlated with symptom severity at baseline. Therefore, these constructs were not simply indicators of disorder severity, and provided additional information about treatment response over and above that of disorder severity.
Our anxious sample reported more negative affect to negative images than healthy controls, but did not differ in reports of positive affect, which provides further support for negative emotional reactivity as a marker of anxious psychopathology. As hypothesized, greater negative emotional reactivity predicted better treatment response overall, according to self-reported symptom ratings (with similar, albeit nonsignificant, effects in independent clinician ratings). The same effect was not found for negative affect in response to neutral images, indicating that it is emotional reactivity to negative images specifically that predicts outcome. Despite evidence for lower positive affect in patients with social phobia compared to controls (Brown et al., 1998; Watson et al., 1988), no differences in positive emotional reactivity were found in the current sample. In addition, positive emotional reactivity did not predict or moderate treatment outcome.
To our knowledge, this is the first investigation of self-reported emotional reactivity as a predictor of treatment response for social phobia or any anxiety disorder. The findings parallel the evidence for elevated amygdala activation while viewing negative stimuli to predict superior outcomes from CBT for depression (Canli et al., 2005; Siegle, Carter, & Thase, 2006). Such amygdala activation was interpreted to represent deficits in emotion regulation (Siegle et al., 2006). Elevations in self reported negative emotional reactivity may similarly represent deficits in emotion regulation. Conceivably, it is the patient who shows the greatest deficits in emotion regulation who benefits most from treatments that target emotion regulation. Clearly, CBT directly targets emotion regulation through skills such as cognitive restructuring and somatic control techniques. Although ACT does not explicitly aim to regulate emotions, emotion regulation is an outcome from ACT (Arch & Craske, 2008), and both CBT and ACT increase perceived control over emotions (Arch et al., 2012). Thus, individuals with deficits in emotion regulation may benefit most from both CBT and ACT since each approach improves emotion regulation.
Another possibility is that elevated negative emotional reactivity indexes capacity to access negative emotions that are then targeted in behavioral treatment, whether by teaching emotional control strategies as in CBT or emotional acceptance strategies as in ACT. That is, emotional reactivity to generic negative images may be a marker of emotional reactivity to fear relevant stimuli within exposure therapy, a component of both CBT and ACT. Although peak fear levels during exposure do not consistently predict outcomes (Craske et al., 2008), variability in fear levels, which includes periods of elevated fear, is a positive predictor of outcome (see Craske, Liao, Brown, & Vervliet, 2012; for a review). According to theories of extinction (a purported mechanism of exposure therapy; Craske et al., 2008; Craske et al., 2012), the greater the salience of the stimulus, the more learning that occurs (Mackintosh, 1975). Models of emotional arousal and learning (Cahill, Gorski, & Le, 2003) also suggest that the greater the emotional arousal during exposure, the greater the learning. Exposure practices may have proven more salient and more arousing for patients high in negative emotional reactivity, thereby enhancing extinction learning and eventual symptom improvements.
Our patients with social phobia did not show evidence of delayed disengagement from angry or disapproving faces compared to control participants at baseline. This result is at odds with prior research using the spatial cueing paradigm with high trait-anxious individuals (Fox et al., 2001; Georgiou et al., 2005). However, evidence for attentional bias in social phobia is not unequivocal, and effect sizes are small to moderate (Bar-Haim et al., 2007). In addition, the total number of studies of attentional bias using the spatial cueing paradigm in anxiety research is small (Bar-Haim et al., 2007), and further research is necessary to identify whether evidence for delayed disengagement from threat is consistently found using the spatial cueing paradigm.
Nonetheless, socially anxious participants who were slower to disengage from threat responded more favorably to treatment within the CBT group, according to independent clinician ratings (with similar, albeit nonsignificant, effects upon self reported symptoms). Prior studies have similarly found that vigilance toward threat predicts better outcomes in CBT (Price et al., 2011; Waters et al., 2012). Conceivably, patients who attended to rather than avoided threat-relevant stimuli may have attended more to fear relevant stimuli during the exposure component of treatment, and thereby benefited more from the exposure. However, this should apply to ACT as well as CBT since both treatments employed exposure therapy, and yet attentional bias did not significantly predict outcomes from ACT. In contrast to CBT, ACT treatment specifically targets attentional processes through training in mindfulness (Semple, 2010). Conceivably, such training exerts different effects depending on attentional processes at baseline. For example, individuals with the most attentional deficit (i.e., the slowest to disengage) might benefit from training that corrects their deficit. At the same time, individuals who are already able to regulate their attention (i.e., the fastest to disengage) may also benefit from training that builds upon and strengthens their skill. Consequently, the attentional training/mindfulness component of ACT may override the moderating effects of baseline attentional bias.
Although this study has many strengths, there are some important limitations. Most importantly, the sample size was relatively small, which resulted in limited power to consistently detect significant findings. In addition, this analysis only included participants who completed treatment. Therefore, these findings do not extend to patients who begin treatment and subsequently drop out. Another limitation is that the facial images used for the spatial cueing paradigm, although validated by a small sample, have not been as extensively validated as the IAPS (Lang et al., 1999) and NimStim (Tottenham et al., 2009) image sets. Images were created for this study because disapproving facial expressions are not currently available. Although an independent sample validated the images, rates of correct identification of angry and disapproving faces (69% and 44% respectively) were low compared to validated stimuli sets such as NimStim, which may have limited our ability to detect delayed disengagement in our anxious sample. Despite lower rates of correct identification, participants rated angry and disapproving faces as significantly more negative and arousing than neutral images, suggesting that the negative images were in fact eliciting negative emotional responses in raters. Finally, effect sizes for significant effects may have been larger had we used film clips rather than still images to induce emotional reactivity, as film clips were the most effective way to induce negative emotions in a meta-analysis (Westermann, Spies, Stahl, & Hesse, 1996).
In conclusion, despite significant variability in treatment response in patients with social phobia, attempts to identify moderators and predictors have yielded few consistent results. The current study is one of the first to demonstrate that higher levels of self-reported emotional reactivity to generic negative stimuli predicts better behavioral treatment outcome in terms of self-reported symptoms and marginally in terms of clinician rated fear and avoidance. Our explanations of this finding are that greater negative emotion to generic negative stimuli represents deficits in emotion regulation that are directly targeted by behavioral treatments or is a proxy for greater fearful reactivity within exposure therapy. In addition, this study is the first to suggest that patients who are particularly slow to disengage from social stimuli may do better in CBT than in ACT. As this is one of first investigations of these research questions, replication is necessary before any conclusions can be drawn regarding prescriptive factors for socially anxious individuals receiving behavioral treatments.
Footnotes
Although multiple imputation can be used to estimate missing data, simulation studies suggest that with large amounts of missing data on the dependent variable (10–20%), multiple imputation can inflate standard errors, and therefore should not be used to replace missing values of dependent measures (Von Hippel, 2007). In the current study, the amount of missing data on the dependent variables was approximately 40%, and therefore, missing data were not imputed.
See author MGC for a copy of the CBT treatment manual; the ACT manual is published (Eifert & Forsyth, 2005).
Creative hopelessness was moved from session 1 to session 2.
Positive affect in response to neutral images was marginally significantly lower in the second block than the first (p = .06). All other comparisons between blocks were not significant (ps > .240).
This test was selected because the second interviewers included several different trained assessors who rated several tapes each.
Uncited reference
References
- Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behaviour Research and Therapy. 2003;41(11):1325–1335. doi: 10.1016/s0005-7967(03)00039-1. http://dx.doi.org/10.1016/S0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Arch JJ, Craske MG. Mechanisms of mindfulness: emotion regulation following a focused breathing induction. Behaviour Research and Therapy. 2006;44(12):1849–1858. doi: 10.1016/j.brat.2005.12.007. http://dx.doi.org/10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Arch JJ, Craske MG. Acceptance and commitment therapy and cognitive behavioral therapy for anxiety disorders: different treatments, similar mechanisms? Clinical Psychology: Science and Practice. 2008;15(4):263–279. [Google Scholar]
- Arch JJ, Eifert GH, Davies C, Vilardaga JCP, Rose RD, Craske MG. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. Journal of Consulting and Clinical Psychology. 2012;80(5):750–765. doi: 10.1037/a0028310. http://dx.doi.org/10.1037/a0028310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SL, Heinrichs N, Kim HJ, Hofmann SG. The Liebowitz social anxiety scale as a self-report instrument: a preliminary psychometric analysis. Behaviour Research and Therapy. 2002;40(6):701–715. doi: 10.1016/s0005-7967(01)00060-2. http://dx.doi.org/10.1016/S0005-7967(01)00060-2. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133(1):1. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology. 1998;107(2):179–192. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- Brown TA, Di Nardo PA, Barlow DH. The anxiety disorders interview schedule for DSM IV (ADIS IV) San Antonio, TX: Psychological Corporation/Graywind Publications Inc.; 1994. [Google Scholar]
- Brühl AB, Rufer M, Delsignore A, Kaffenberger T, Jäncke L, Herwig U. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Research. 2011;1378:72–83. doi: 10.1016/j.brainres.2010.12.084. [DOI] [PubMed] [Google Scholar]
- Burklund LJ, Eisenberger NI, Lieberman MD. The face of rejection: rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Social Neuroscience. 2007;2(3–4):238–253. doi: 10.1080/17470910701391711. http://dx.doi.org/10.1080/17470910701391711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clinical Psychology Review. 2006;26(1):17–31. doi: 10.1016/j.cpr.2005.07.003. http://dx.doi.org/10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learning & Memory. 2003;10(4):270–274. doi: 10.1101/lm.62403. http://dx.doi.org/10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. [Google Scholar]
- Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, et al. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16(12):1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- Chen E. Effects of state anxiety on selective processing of threatening information. Cognition & Emotion. 1996;10(3):225–240. http://dx.doi.org/10.1080/026999396380231. [Google Scholar]
- Chen YP, Ehlers A, Clark DM, Mansell W. Patients with generalized social phobia direct their attention away from faces. Behaviour Research and Therapy. 2002;40(6):677–687. doi: 10.1016/s0005-7967(01)00086-9. http://dx.doi.org/101016/S0005-7967(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg Richard G, Liebowitz MR, Hope D, Schneier FR., editors. Social phobia. New York, NY: Guilford Press; 1995. [Google Scholar]
- Craske MG, Farchione TJ, Allen LB, Barrios V, Stoyanova M, Rose R. Cognitive behavioral therapy for panic disorder and comorbidity: more of the same or less of more? Behaviour Research and Therapy. 2007;45(6):1095–1109. doi: 10.1016/j.brat.2006.09.006. http://dx.doi.org/101016/j.brat.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. http://dx.doi.org/10.1016/j.brat.200710.003. [DOI] [PubMed] [Google Scholar]
- Craske MG, Liao B, Brown L, Vervliet B. Role of inhibition in exposure therapy. Journal of Experimental Psychopathology. 2012;3:322–345. [Google Scholar]
- Craske MG, Niles AN, Burklund LJ, Wolitzky-Taylor K, Plumb J, Saxbe D, et al. Randomized controlled trial of cognitive behavioral therapy and acceptance and commitment therapy for social anxiety disorder: Outcomes and moderators. 2013. (submitted for publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehrmann O. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. Archives of General Psychiatry. 2012:1–11. doi: 10.1001/2013.jamapsychiatry.5. http://dx.doi.org/10.1001/2013.jamapsychiatry.5. [DOI] [PMC free article] [PubMed]
- Eifert GH, Forsyth JP. Acceptance and commitment therapy for anxiety disorders: A practitioner’s treatment guide to using mindfulness, acceptance, and values-based behavior change strategies. Oakland, CA: New Harbinger Publications, Inc.; 2005. [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130(4):681–700. [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Dutton K. Attentional bias for threat: evidence for delayed disengagement from emotional faces. Cognition & Emotion. 2002;16(3):355–379. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Leibowitz MR, Hami S, Stein MB, et al. The Liebowitz social anxiety scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine. 2001;31(6):1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Georgiou GA, Bleakley C, Hayward J, Russo R, Dutton K, Eltiti S, et al. Focusing on fear: attentional disengagement from emotional faces. Visual Cognition. 2005;12(1):145–158. doi: 10.1080/13506280444000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66(2):170–180. doi: 10.1001/archgenpsychiatry.2008.525. http://dx.doi.org/10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman I. Care and handling of univariate or multivariate outliers in detecting spuriosity: a Bayesian approach. Technometrics. 1973:723–738. [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: An experiential approach to behavior change. New York, NY: The Guilford Press; 1999. [Google Scholar]
- Hayward C, Killen JD, Kraemer HC, Taylor CB. Predictors of panic attacks in adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(2):207–214. doi: 10.1097/00004583-200002000-00021. http://dx.doi.org/10.1097/00004583-200002000-00021. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, Horner KJ, Juster HR, Safren SA, Brown EJ, Schneier FR, et al. Psychometric properties of the Liebowitz social anxiety scale. Psychological Medicine. 1999;29(01):199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]
- Heinrichs N, Hofmann SG. Information processing in social phobia: a critical review. Clinical Psychology Review. 2001;21(5):751–770. doi: 10.1016/s0272-7358(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Hope DA, Heimberg RG, Juster HA, Turk CL. Managing social anxiety: A cognitive-behavioral therapy approach client workbook. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, Silva PA, McGee R. Personality traits are differentially linked to mental disorders: a multitrait-multidiagnosis study of an adolescent birth cohort. Journal of Abnormal Psychology. 1996;105(3):299–312. doi: 10.1037//0021-843x.105.3.299. http://dx.doi.org/10.1037/0021-843X.1053.299. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Loerinc A, Meuret A, Twohig M, Rosenfield D, Craske MG. Response rates in CBT for anxiety disorders: Measurement matters. 2013. (submitted for publication). [DOI] [PubMed] [Google Scholar]
- Mackinnon A, Jorm AF, Christensen H, Korten AE, Jacomb PA, Rodgers B. A short form of the positive and negative affect schedule: evaluation of factorial validity and invariance across demographic variables in a community sample. Personality and Individual Differences. 1999;27(3):405–416. http://dx.doi.org/10.1016/S0191-8869(98)00251-7. [Google Scholar]
- Mackintosh NJ. A theory of attention: variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82(4):276–298. [Google Scholar]
- MacLeod C, Mathews A. Anxiety and the allocation of attention to threat. The Quarterly Journal of Experimental Psychology Section A. 1988;40(4):653–670. doi: 10.1080/14640748808402292. http://dx.doi.org/10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. http://dx.doi.org/10.1037/0021-843X.95.1.15. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. The Journal of Abnormal Psychology. 2002;111(1):107–123. http://dx.doi.org/10.1037/0021-843X.111.1.107. [PubMed] [Google Scholar]
- Mansell W, Clark DM, Ehlers A, Chen YP. Social anxiety and attention away from emotional faces. Cognition and Emotion. 1999;13(6):673–690. [Google Scholar]
- Mattick RP, Clarke JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy. 1998;36(4):455–470. doi: 10.1016/s0005-7967(97)10031-6. http://dx.doi.org/10.1016/S0005-7967(97)10031-6. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Twohig MP, Rosenfield D, Hayes SC, Craske MG. Brief acceptance and commitment therapy and exposure for panic disorder: a pilot study. Cognitive and Behavioral Practice. 2012;19(4):606–618. http://dx.doi.org/10.1016/j.cbpra.2012.05.004. [Google Scholar]
- Mogg K, Bradley BP, Hallowell N. Attentional bias to threat: roles of trait anxiety, stressful events, and awareness. The Quarterly Journal of Experimental Psychology Section A. 1994;47(4):841–864. doi: 10.1080/14640749408401099. http://dx.doi.org/10.1080/14640749408401099. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. From discovery to cure: Accelerating the development of new and personalized interventions for mental illness. Washington, DC: Government Printing Office; 2010. [Google Scholar]
- Norton PJ, Price EC. A meta-analytic review of adult cognitive-behavioral treatment outcome across the anxiety disorders. The Journal of Nervous and Mental Disease. 2007;195:521–531. doi: 10.1097/01.nmd.0000253843.70149.9a. http://dx.doi.org/10.1097/01.nmd.0000253843.70149.9a. [DOI] [PubMed] [Google Scholar]
- Osman A, Gutierrez PM, Barrios FX, Kopper BA, Chiros CE. The social phobia and social interaction anxiety scales: evaluation of psychometric properties. Journal of Psychopathology and Behavioral Assessment. 1998;20(3):249–264. http://dx.doi.org/10.1023/A:1023067302227. [Google Scholar]
- Prenoveau JM, Zinbarg RE, Craske MG, Mineka S, Griffith JW, Epstein AM. Testing a hierarchical model of anxiety and depression in adolescents: a tri-level model. Journal of Anxiety Disorders. 2010;24(3):334–344. doi: 10.1016/j.janxdis.2010.01.006. http://dx.doi.org/10.1016/jjanxdis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Price M, Tone EB, Anderson PL. Vigilant and avoidant attention biases as predictors of response to cognitive behavioral therapy for social phobia. Depression and Anxiety. 2011;28(4):349–353. doi: 10.1002/da.20791. http://dx.doi.org/10.1002/da.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy. 1997;35(8):741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Semple R. Does mindfulness meditation enhance attention? A randomized controlled trial. Mindfulness. 2010;1(2):121–130. http://dx.doi.org/10.1007/s12671-010-0017-2. [Google Scholar]
- Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. Journal of Psychiatry and Neuroscience. 2009;34(4):296–302. [PMC free article] [PubMed] [Google Scholar]
- Siegle PD, Carter MD, Thase MD. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163(4):735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5. Boston, MA: Pearson; 2006. [Google Scholar]
- Tolin DF. Is cognitive-behavioral therapy more effective than other therapies? A meta-analytic review. Clinical Psychology Review. 2010;30(6):710–720. doi: 10.1016/j.cpr.2010.05.003. http://dx.doi.org/10.1016/j.cpr.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hippel PT. Regression with missing Ys: an improved strategy for analyzing multiply imputed data. Sociological Methodology. 2007;37(1):83–117. [Google Scholar]
- Waters AM, Mogg K, Bradley BP. Direction of threat attention bias predicts treatment outcome in anxious children receiving cognitive-behavioural therapy. Behaviour Research and Therapy. 2012;50(6):428–434. doi: 10.1016/j.brat.2012.03.006. http://dx.doi.org/10.1016/j.brat2012.03.006. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. Journal of Abnormal Psychology. 1988;97(3):346–353. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- Watson D, Gamez W, Simms LJ. Basic dimensions of temperament and their relation to anxiety and depression: a symptom-based perspective. Journal of Research in Personality. 2005;39(1):46–66. [Google Scholar]
- Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: a meta-analysis. European Journal of Social Psychology. 1996;26(4):557–580. [Google Scholar]
- Wolitzky-Taylor KB, Arch JJ, Rosenfield D, Craske MG. Moderators and non-specific predictors of treatment outcome for anxiety disorders: a comparison of cognitive behavioral therapy to acceptance and commitment therapy. Journal of Consulting and Clinical Psychology. 2012;80(5) doi: 10.1037/a0029418. http://dx.doi.org/10.1037/a0029418. [DOI] [PubMed] [Google Scholar]


