Abstract
Objective
Disparities in HIV infection, with females having higher rates of HIV infection than males, have been noted among persons who inject drugs (PWID) in many countries. We examined male/female HIV disparities among PWID in Central Asia and compared these disparities with patterns worldwide.
Methods
A systematic review and meta-analyses were conducted for studies reporting HIV prevalence by gender among PWID. To be included in the analyses, reports had to contain 1) samples of PWID from Central Asian, 2) HIV data based on laboratory testing, 3) HIV prevalence reported for males and females, and 4) samples that were not recruited on the basis of HIV status.
Results
Data were abstracted from 11 studies in 5 countries in Central Asia: China, Kazakhstan, Russia, Tajikistan, and Uzbekistan; the total sample size was 12,225. The mean weighted OR for HIV prevalence among females to males was 0.913 (95% CI 0.07, 1.26), with high heterogeneity among studies (I2 = 70.0%) and a possible publication bias among studies with small sample sizes (Eggers test = -1.81, 95% CI -5.18, 0.54).
Conclusions
The non-significant higher HIV prevalence among male PWID in Central Asia contrasts with the worldwide findings which show slightly higher HIV prevalence among female PWID. This may reflect the relative recency of the HIV epidemics in Central Asia. The findings also suggest that there may be factors that protect female PWID from HIV in some settings. Further examination of transmission dynamics in Central Asia is necessary to better understand the HIV epidemic among PWID.
Keywords: People who inject drugs (PWID), Central Asia, HIV, gender, prevalence
1. INTRODUCTION
Persons who inject drugs (PWID) are at high risk for infection with human immunodeficiency virus (HIV); however, not all persons who inject drugs are at equal risk for becoming infected with the virus. There are multiple reasons why females who inject drugs may be at higher risk for becoming infected with HIV than males who inject drugs. Females may have greater difficulties in obtaining sterile injection equipment (Parker and Aggleton, 2003). Females may experience greater discrimination when they seek treatment for substance use disorders (Khalid, 2007; UNODC, 2012). Female PWID are quite likely to have male PWID as sexual partners while male PWID are likely to have females who do not inject drugs as their primary sexual partners (Go et al., 2006). Male to female sexual transmission of HIV is more efficient than female to male transmission (European Study Group on Heterosexual Transmission of HIV, 1992), so that female PWID are likely to be at higher risk for acquiring HIV through sexual transmission. There is a moderate overlap of females who are commercial sex workers and also inject drugs in certain countries, including China (Gu et al., 2009; Wang et al., 2012), Mexico (Ulibarri et al., 2010) and Russia (Decker et al., 2012; Kozlov et al., 2006), facilitating both injection related and sexual transmission of HIV. Finally, there are substantial gender inequalities in many countries (including Central Asian countries) (United Nations Development Program, 2012) that may contribute to higher HIV risk for females in general.
Many countries in Central Asia are currently experiencing injecting drug use concentrated epidemics (Mathers et al., 2008). In this report, we examine the evidence for sex disparities in HIV among PWID in countries in Central Asia, compare these disparities with sex disparities among PWID in other parts of the world, and consider the implications for the prevention and treatment of HIV among PWID in Central Asia.
2. METHODS
2.1 Search Strategies
The literature search we conducted for this review utilized PRISMA guidelines (Liberati et al., 2009). Studies were selected from several sources including PubMed, EMBASE, and Web of Science. Systematic literature searches were conducted using a comprehensive search terminology to identify potentially eligible articles from journals and government/country reports. In addition, we also searched grey literature; conference abstracts, international and regional harm reduction websites, contacted key experts and research institutions, hand searched relevant publications and emailed UNAIDS country offices regarding injecting populations in any of the countries selected for inclusion.
In order for a study to be eligible, the authors had to report HIV prevalence among PWID by gender, verified by HIV testing; the sample had to be made up of at least 90% PWID (who may or may not be currently injecting drugs). This study was restricted to central Asia, and included the following locations: Afghanistan, western China (Xinjiang, Tibet), eastern Iran, Kazakhstan, Kyrgyzstan, Mongolia, northwestern Pakistan, southwest Russia (Siberia), Tajikistan, Turkmenistan, and Uzbekistan. Studies that used self-report to assess prevalence among PWID were excluded. We did not restrict our search to any particular time period, so studies in the review included data from the earliest relevant publications available in PubMed (1982) through April 2013. We recognize that there may be considerable variation in HIV infection among PWID in different parts of the same country, particularly for large diverse countries. In these large countries, we attempted to obtain data from as many locations as possible, focusing on large cities and locations in the country where PWID are known to be located. Figure 1 gives the breakdown of terminology used to search for eligible studies. The same search terms were utilized for all databases (EMBASE, PubMed, NLM, etc.).
Figure 1. Search Terms used for Retrieval of Eligible Citations.

2.2 Data extraction and analysis
A standardized coding form was developed to abstract pertinent information for each PWID sample. Information collected included demographics of the drug using population, study design characteristics including location, HIV prevalence, and information regarding the gender makeup of the sample. Each study was assigned a reference ID number after completion of coding. A sampling of all coding forms completed by the reviewer (BP) were checked for accuracy and quality by a second reviewer (JF) before finalization. Data was extracted from each coding form and entered into a database in order to obtain odds ratios for each study.
Data on HIV prevalence by gender were abstracted from each eligible study, converted into HIV prevalence odds ratios (ORs) and then transformed into natural logarithm odds ratios (log ORs). All analyses were conducted with the log ORs. Presentation of the results used either the log ORs or conversions from log ORs back to ORs. Forest plots were used to report HIV prevalence ORs with 95% confidence intervals. Funnel plots and the Egger’s test were used to assess possible publication bias in the located studies, and I2 was used to assess heterogeneity among the log ORs. Weighting of the log ORs was done using random effects. STATA 12 (College Station, TX USA; STATA Corp, 2012) was used for analysis.
3. RESULTS
Figure 2 shows the PRISMA diagram (Liberati et al., 2009; Moher et al., 2009) for the searching and screening that led to the final number of studies included in this review. Searching identified 176 peer-reviewed articles and 2350 grey literature titles. There were 10 papers that were in languages other than English, 166 duplicates, and 11 that could not be obtained; these were eliminated from the review. We then screened 2339 abstracts against the inclusion criteria and retrieved 182 full text articles for further screening. Of the articles and reports retrieved, 11 reports met all criteria for inclusion and were coded. These 11 articles provided a total of 13 HIV prevalence odds ratio by gender from 5 different countries in Central Asia. The included studies contained a total of 12,208 subjects. The primary reasons for exclusion of abstracts and full text articles included: the sample came from an HIV medical service, i.e., all subjects were HIV positive, the HIV data were based on self report rather than laboratory testing, or the study did not report HIV prevalence by gender. When appropriate, we contacted authors of papers that did not report HIV prevalence by gender, in order to obtain this information directly from the primary author of the paper.
Figure 2. PRISMA Search Flow Chart.
In the Central Asian countries where data was available, female PWID were slightly less likely to be HIV positive when compared to male PWID (OR = 0.913, 95% CI 0.07, 1.26, p=0.583), with the lowest odds ratios documented in Kazakhstan and Western China; however, the difference in HIV prevalence did not reach statistical significance. Table 1 presents the HIV prevalence and odds ratios for PWID in Central Asian countries. Figure 3 presents a forest plot of the female:male OR values by study. The forest plots use a logarithmic scale for the x-axis so that the 95% CI will be symmetrical around the observed OR.
Table 1.
Summary of Included Studies
| Study | Country | Years | Location | N | Male | Female | HIV Prev. | HIV Prevalence Male | HIV Prevalence Female | OR Female |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhang 2007 (Zhang et al., 2007b) | China | 2002 | Xinjiang | 508 | 442 | 66 | 0.08 | 0.083 | 0.076 | 0.898 |
| Zhang 2007a (Zhang et al., 2007a) | China | 2002-2003 | Xinjiang | 781 | 698 | 83 | 0.29 | 0.309 | 0.145 | 0.377 |
| Zhang 2008 (Zhang et al., 2008) | China | 2006 | Xinjiang | 383 | 339 | 44 | 0.38 | 0.359 | 0.455 | 1.483 |
| Fang HR 2008 (Fang HR, 2008) | China | 2005-2008 | Xinjiang | 1496 | 1331 | 165 | 0.14 | 0.146 | 0.115 | 0.763 |
| Liu 2007 (Liu J.B., 2007) | China | 2005 | Xinjiang | 129 | 108 | 21 | 0.19 | 0.213 | 0.095 | 0.389 |
| Terlikbayeva 2012 (Terlikbayeva et al., 2012) | Kazakhstan | 2012 | Almaty | 580 | 351 | 229 | 0.29 | 0.174 | 0.114 | 0.609 |
| Zhussupov 2007 (Zhussupov et al., 2007) | Kazakhstan | 2002 | Karaganda | 900 | 685 | 215 | 0.023 | 0.021 | 0.029 | 1.376 |
| Zhussupov 2007 (Zhussupov et al., 2007) | Kazakhstan | 2002 | Temirtau | 899 | 662 | 237 | 0.247 | 0.21 | 0.35 | 2.028 |
| Republican AIDS Center (Ganina L., 2012) | Kazakhstan | 2011 | 22 regions (Nationwide) | 4830 | 4004 | 826 | 0.038 | 0.046 | 0.036 | 0.783 |
| Rhodes 2006 (Rhodes et al., 2006) | Russia | 2003 | Barnaul | 529 | 343 | 186 | 0.087 | 0.090 | 0.086 | 0.947 |
| Stachowiak 2006 (Stachowiak et al., 2006) | Tajikistan | 2004 | Dushanbe | 489 | 414 | 75 | 0.12 | 0.123 | 0.107 | 0.850 |
| Todd 2007 (Todd et al., 2007b) | Uzbekistan | 2003-2004 | Tasjkent | 701 | 663 | 38 | 0.31 | 0.296 | 0.342 | 1.239 |
Figure 3. Forrest Plot of Included Central Asia Studies (Weights are from random effects analysis).
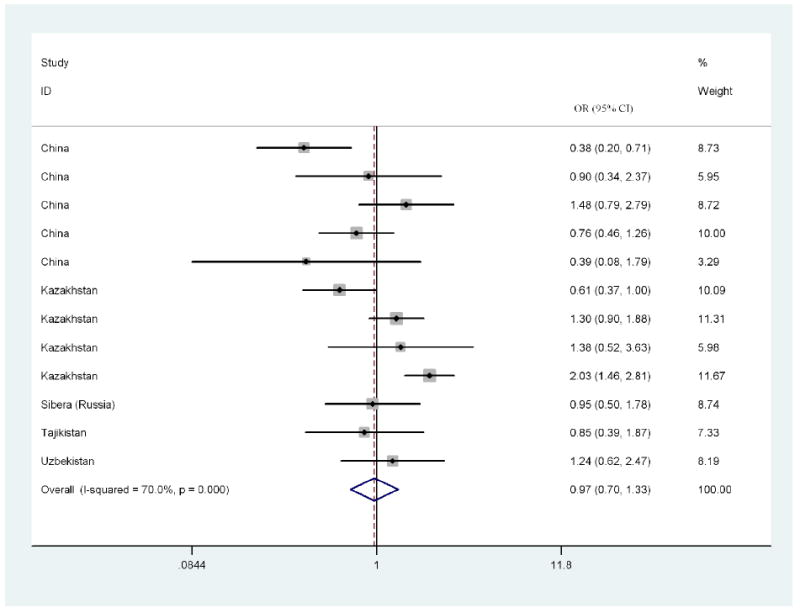
There was a great amount of heterogeneity among log ORs for female:male HIV prevalence in the studies (I2: 70.0%), Note that an I2 >50% is usually considered to be a high level of heterogeneity (Schroll et al., 2011). The range in the ORs was also quite substantial, with an absolute range of 0.39 to 2.03. The Egger’s test for publication bias was -1.81 (95% CI -5.18, 0.54), suggesting possible publication bias (bias against studies with small sample sizes.)
4. DISCUSSION
First, we must note that, despite an extensive search, we were not able to locate a large number of studies of HIV prevalence by sex for PWID in Central Asia. We did screen very large numbers of abstracts and found only a modest number of papers that met our eligibility requirements; this is similar to the results in many other systematic reviews and meta-analyses.
Despite the modest number of included studies, there are patterns in the data that need to be considered. The results from Central Asia contrast with results from international systematic reviews and meta-analyses. In an international review of sex differences in HIV prevalence among PWID, there was a very modest but statistically significant difference in HIV prevalence among female PWID compared to male PWID (summary females to males odds ratio = 1.18, 95% CI 1.10-1.26; Des Jarlais et al., 2012b). In contrast, we found a moderately lower HIV prevalence among female PWID compared to male PWID in the Central Asia (summary female to male prevalence odds ratio = 0.913, 95% CI 0.07, 1.26), though the difference in HIV prevalence in the Central Asian studies did not reach statistical significance.
There was great variation in the female/male odds ratios for the studies from Central Asia, so that one should not assume that female PWID are likely to have lower HIV prevalence than male PWID throughout the region or in any particular setting. Further research will be needed to understand this variation. The studies themselves did not discuss potential reasons for gender differences in HIV prevalence.
There are mechanisms that could potentially explain some of the disparities in HIV prevalence by gender in Central Asia. One potential mechanism for why female PWID might have lower HIV prevalence than male PWID could arise from sexual pairing of female PWID with male PWID; a female injector dependent upon her sexual partner for drugs and for injection equipment would be very likely to share injection equipment with this male partner. However, she may also be very unlikely to share injection equipment with injectors other than her sexual partner, which would protect against HIV acquisition in many epidemiological circumstances. A sentinel survey in Kazakhstan in 2011 revealed that 38.7% of female PWID were married compared to 29.7% of male PWID (Ganina L., 2012). Females who are married are less likely to inject with persons other than their sexual partners, so they would have less of chance of becoming infected with HIV (Kral et al., 2001). The protective effect for female PWID would be particularly strong if the male sexual partner avoided sharing with other PWID.
Many female PWID initiate their injection drug use later than male PWID, leading to smaller injection networks, and shorter periods of exposure, which might also contribute to lower prevalence among female PWID (Roberts, 2010). In locations where Islam predominates, women may be more limited in their social networks, and as a result, are more likely to inject with a small number of partners, or at home in a family setting. These behaviors would reduce the chance of sharing outside of the home/family setting, especially with persons who may be infected with HIV or HCV (Razani et al., 2007; Razzaghi et al., 2006).
The injecting drug use HIV epidemics in Central Asia are relatively recent compared to the injecting drug use HIV epidemics in North American and Western Europe. Female/male disparities in HIV infection among PWID tend to emerge after an epidemic has matured, injecting drug use transmission has been greatly reduced, and sexual transmission has become more important (Des Jarlais et al., 2012b). Thus, sex differences in HIV infection in Central Asia may emerge after over time as injecting related transmission is reduce and sexual transmission becomes more important.
As noted in the introduction, there are substantial gender inequalities in many Central Asian countries. These inequalities do not appear to be generating significantly higher HIV prevalence among female compared to male PWID. If the injecting drug use HIV epidemics in Central Asian countries should transition to generalized heterosexual epidemics, the existing gender inequalities may create higher risks for HIV infection among females in the general population.
4.1 Limitations
There are several limitations to this study that should be noted. First, as discussed above, it is possible that there are additional data on HIV prevalence by sex among PWID that we were not able to locate. We did, however, perform an exhaustive search of published literature and conference presentations, along with discussions with key researchers in the field. The Egger’s test indicated a moderate level of publication bias in the studies (-1.81 95% CI -5.18, 1.28, 0.54), suggesting that there was a bias against publication of studies with small samples and small differences in HIV prevalence between males and females. Inclusion of such small studies would probably move the pooled weighted OR towards 1.0 (no difference). Second, as in any systematic review and meta-analysis, we were limited by the data and analyses contained in the original reports. Many of the studies that were excluded recruited both male and female PWID, but due to the low number of females, the studies only focused on male PWID (Todd et al., 2006, 2007a), so that gender differences among those who tested HIV positive could not be established. Due to socio-cultural factors, gender inequality and the social position of women in many Central Asian countries female PWID may be hidden and as a result more difficult to recruit into studies. Among the included studies, there were diverse recruitment methods, which may have led to different non-representative samples from the different locations. However, despite these limitations, it would appear that there is great variation in the female/male HIV prevalence odds ratios in studies from Central Asia, with the weighted summary odds ratio showing a non-statistically significant, slightly lower HIV prevalence among female PWID.
4.2 Conclusion
The weighted pooled odds ratio indicates a lower, but not statistically significant, HIV prevalence among females in Central Asia. This should not be taken as a reason for failing to provide HIV prevention, care and treatment for both female and male PWID, as the difference in HIV prevalence. Even if female PWID have lower HIV prevalence in some areas, special programs may be required to reach female PWID with needed services, especially those that may be at increased risk, such as female PWID who also engage in commercial sex work.
In this review, we examined gender differences among people who inject drugs in Central Asia, documenting slightly lower (but not statistically significant) levels of HIV infection among females who inject drugs compared to males who inject drugs, which is in direct contrast to the global gender differences in HIV infection, where HIV prevalence is modestly higher among female PWID (Des Jarlais et al., 2012a). Further research and examination of factors that play a role in the differences in HIV infection by gender is required to better understand the dynamics of HIV infection among PWID in the Central Asian region and guide appropriate provision of services for both female and male PWID in the region.
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIH Grant R01 AI 083035-05; NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
D. Des Jarlais designed the study and wrote the protocol. J. Feelemyer and B. Williams managed the literature searches and summaries of previous related work. K. Arasteh undertook the statistical analysis, and authors D. Des Jarlais and Jonathan Feelemyer wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors have no conflicts of interest with respect to the submitted manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Decker MR, Wirtz AL, Baral SD, Peryshkina A, Mogilnyi V, Weber RA, Stachowiak J, Go V, Beyrer C. Injection drug use, sexual risk, violence and STI/HIV among Moscow female sex workers. Sex Transm Infect. 2012;88:278–283. doi: 10.1136/sextrans-2011-050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Feelemyer JP, Modi SN, Arasteh A, Hagan H. Are females who inject drugs at higher risk for HIV infection than males who inject drugs: an international systematic review of high seroprevalence areas. Drug Alcohol Depend. 2012a;124:95–107. doi: 10.1016/j.drugalcdep.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Feelemyer JP, Modi SN, Arasteh K, Hagan H. Are females who inject drugs at higher risk for HIV infection than males who inject drugs: an international systematic review of high seroprevalence areas. Drug Alcohol Depend. 2012b;124:95–107. doi: 10.1016/j.drugalcdep.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Study Group on Heterosexual Transmission of HIV. Comparison of female to male and male to female transmission of HIV in 563 stable couples. European Study Group on Heterosexual Transmission of HIV. BMJ. 1992;304:809–813. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang HR, M L, Liu JX. Analysis of test results of HIV and HCV in methadone maitenance treatment clinic. Chinese Community Doctors. 2008;10:137. [Google Scholar]
- Ganina L, K N, Yelizaryeva A, Zhegolko MV, Kaspirovoy AA, Sakhnova NN, Babina NI. A Review of HIV Epidemiological Situation and Results of Sentinel Surveillance Study in the Republic of Kazakhstan. National Center for the Prevention and Control of AIDS; Almaty: 2012. [Google Scholar]
- Go VF, Quan VM, Voytek C, Celentano D, Nam le V. Intra-couple communication dynamics of HIV risk behavior among injecting drug users and their sexual partners in Northern Vietnam. Drug Alcohol Depend. 2006;84:69–76. doi: 10.1016/j.drugalcdep.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Gu J, Wang R, Chen H, Lau JT, Zhang L, Hu X, Lei Z, Li Z, Cai H, Wang T, Tsui H. Prevalence of needle sharing, commercial sex behaviors and associated factors in Chinese male and female injecting drug user populations. AIDS Care. 2009;21:31–41. doi: 10.1080/09540120802068787. [DOI] [PubMed] [Google Scholar]
- Khalid A. Islam After Communism: Religion And Politics in Central Asia. University of California Press; 2007. [Google Scholar]
- Kozlov AP, Shaboltas AV, Toussova OV, Verevochkin SV, Masse BR, Perdue T, Beauchamp G, Sheldon W, Miller WC, Heimer R, Ryder RW, Hoffman IF. HIV incidence and factors associated with HIV acquisition among injection drug users in St Petersburg, Russia. AIDS. 2006;20:901–906. doi: 10.1097/01.aids.0000218555.36661.9c. [DOI] [PubMed] [Google Scholar]
- Kral AH, Bluthenthal RN, Lorvick J, Gee L, Bacchetti P, Edlin BR. Sexual transmission of HIV-1 among injection drug users in San Francisco, USA: risk-factor analysis. Lancet. 2001;357:1397–1401. doi: 10.1016/S0140-6736(00)04562-1. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Liu JB, DL XT, Li F, Zhang F, Mo LR, Jiao LD, Ai NWE, Wang JM. The effective evaluation of the methadone maintenance treatment of heroin addicts. Chinese Journal of Drug Abuse Prevention and Treatment. 2007;13 [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, Mattick RP. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman D, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PloS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Soc Sci Med. 2003;57:13–24. doi: 10.1016/s0277-9536(02)00304-0. [DOI] [PubMed] [Google Scholar]
- Razani N, Mohraz M, Kheirandish P, Malekinejad M, Malekafzali H, Mokri A, McFarland W, Rutherford G. HIV risk behavior among injection drug users in Tehran, Iran. Addiction. 2007;102:1472–1482. doi: 10.1111/j.1360-0443.2007.01914.x. [DOI] [PubMed] [Google Scholar]
- Razzaghi EM, Movaghar AR, Green TC, Khoshnood K. Profiles of risk: a qualitative study of injecting drug users in Tehran, Iran. Harm Reduct J. 2006;3:12. doi: 10.1186/1477-7517-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes T, Platt L, Maximova S, Koshkina E, Latishevskaya N, Hickman M, Renton A, Bobrova N, McDonald T, Parry JV. Prevalence of HIV, hepatitis C and syphilis among injecting drug users in Russia: a multi-city study. Addiction. 2006;101:252–266. doi: 10.1111/j.1360-0443.2006.01317.x. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Mathers BM, Degenhardt L. Women who inject drugs: a review of their risks, experiences and needs. National Drug and Alcohol Research Centre; Sydney: 2010. p. 132. [Google Scholar]
- Schroll JB, Moustgaard R, Gotzsche PC. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med Res Methodol. 2011;11:22. doi: 10.1186/1471-2288-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak JA, Tishkova FK, Strathdee SA, Stibich MA, Latypov A, Mogilnii V, Beyrer C. Marked ethnic differences in HIV prevalence and risk behaviors among injection drug users in Dushanbe, Tajikistan, 2004. Drug Alcohol Depend. 2006;82(Suppl. 1):S7–14. doi: 10.1016/s0376-8716(06)80002-5. [DOI] [PubMed] [Google Scholar]
- STATA Corp. Stata 12. College Station; Texas: 2012. [Google Scholar]
- Terlikbayeva A, El-Bassel N, Gilbert L, Rozental Y, Saltant Y, Ismailov L, Famouri L. Seek, Test and Treat Continuum Among a Cohort of Injecting Drug Users (IDUs) and Their Sexual Partners in HIV Prevention Project in Kazakhstan. The XIX International AIDS Conference Columbia University Global Health Research Center of Central Asia Almaty, Kazakhstan; Washington DC. 2012. [Google Scholar]
- Todd CS, Abed AM, Strathdee SA, Botros BA, Safi N, Earhart KC. Prevalence of HIV, Viral Hepatitis, Syphilis and Risk Behaviors Among Injection Drug Users in Kabul, Afghanistan. AIDS 2006 - XVI International AIDS Conference; Toronto. 2006. [Google Scholar]
- Todd CS, Abed AM, Strathdee SA, Scott PT, Botros BA, Safi N, Earhart KC. HIV, hepatitis C, and hepatitis B infections and associated risk behavior in injection drug users, Kabul, Afghanistan. Emerg Infect Dis. 2007a;13:1327–1331. doi: 10.3201/eid1309.070036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd CS, Earhart KC, Botros BA, Khakimov MM, Giyasova GM, Bautista CT, Carr JK, Sanchez JL. Prevalence and correlates of risky sexual behaviors among injection drug users in Tashkent, Uzbekistan. AIDS Care. 2007b;19:122–129. doi: 10.1080/09540120600852150. [DOI] [PubMed] [Google Scholar]
- Ulibarri MD, Strathdee SA, Patterson TL. Sexual and drug use behaviors associated with HIV and other sexually transmitted infections among female sex workers in the Mexico-US border region. Curr Opin Psychiatry. 2010;23:215–220. doi: 10.1097/YCO.0b013e32833864d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Development Program. Human Development Report: Gender Inequality Index (GDI) United Nations; New York: 2012. [Google Scholar]
- UNODC. HIV/AIDS prevention and care for injection drug users. Crime, U.N.O.o.D.a. Vienna International Centre; Vienna: 2012. p. 4. [Google Scholar]
- Wang H, Reilly KH, Brown K, Jin X, Xu J, Ding G, Zang C, Wang J, Wang N. HIV incidence and associated risk factors among female sex workers in a high HIV-prevalence area of China. Sex Transm Dis. 2012;39:835–841. doi: 10.1097/OLQ.0b013e318266b241. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhu J, Rui B, Zhang Y, Yin L, Ruan Y, Qian HZ, Shao Y. High HIV risk among Uigur minority ethnic drug users in northwestern China. Trop Med Int Health. 2008;13:814–817. doi: 10.1111/j.1365-3156.2008.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shan H, Trizzino J, Ruan Y, Beauchamp G, Masse B, Ma J, Gu Y, He Y, Rui B, Wang J, Poundstone K, Jiang Y, Brooks Jackson J, Shao Y. Demographic characteristics and risk behaviors associated with HIV positive injecting drug users in Xinjiang. China J Infect. 2007a;54:285–290. doi: 10.1016/j.jinf.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shan H, Trizzino J, Ruan Y, Beauchamp G, Masse B, Ma J, Rui B, Wang J, Liu M, Wang Y, He Y, Poundstone K, Jiang Y, Jackson JB, Shao Y. HIV incidence, retention rate, and baseline predictors of HIV incidence and retention in a prospective cohort study of injection drug users in Xinjiang, China. Int J Infect Dis. 2007b;11:318–323. doi: 10.1016/j.ijid.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Zhussupov B, Shakarishvilly G, Muratbayeva R, Bronzan R, Ryan C, Favorov M. Study of Behaviors Associated with HIV Infection, STI and Viral Hepatitis among Injecting Drug Users in Temirtau and Karaganda, Republic of Kazakhstan. DC/Central Asia Office; Almaty: 2007. [Google Scholar]


