Abstract
Cancer treatment generally relies on tumor ablative techniques that can lead to major functional or disfiguring defects. These post-therapy impairments require the development of safe regenerative therapy strategies during cancer remission. Many current tissue repair approaches exploit paracrine (immunomodulatory, pro-angiogenic, anti-apoptotic and pro-survival effects) or restoring (functional or structural tissue repair) properties of mesenchymal stem/stromal cells (MSC). Yet, a major concern in the application of regenerative therapies during cancer remission remains the possible triggering of cancer recurrence. Tumor relapse implies the persistence of rare subsets of tumor-initiating cancer cells which can escape anti-cancer therapies and lie dormant in specific niches awaiting reactivation via unknown stimuli. Many of the components required for successful regenerative therapy (revascularization, immunosuppression, cellular homing, tissue growth promotion) are also critical for tumor progression and metastasis. While bidirectional crosstalk between tumorigenic cells (especially aggressive cancer cell lines) and MSC (including tumor stroma-resident populations) has been demonstrated in a variety of cancers, the effects of local or systemic MSC delivery for regenerative purposes on persisting cancer cells during remission remain controversial. Both pro- and anti-tumorigenic effects of MSC have been reported in the literature. Our own data using breast cancer clinical isolates have suggested that dormant-like tumor-initiating cells do not respond to MSC signals, unlike actively dividing cancer cells which benefited from the presence of supportive MSC. The secretome of MSC isolated from various tissues may partially diverge, but it includes a core of cytokines (i.e. CCL2, CCL5, IL-6, TGFβ, VEGF), which have been implicated in tumor growth and/or metastasis. This article reviews published models for studying interactions between MSC and cancer cells with a focus on the impact of MSC secretome on cancer cell activity, and discusses the implications for regenerative therapy after cancer.
Keywords: Mesenchymal stem/stromal cells, regenerative therapy after cancer, cancer recurrence, tumor-initiating cells
1. Introduction
Cancer treatment often relies on non-selective tumor ablative techniques that can result into severe functional impairments or disfiguring damages. Cellular therapy using hematopoietic stem cells (HSC) is already well established to rescue the bone marrow from the massive cytotoxic effects associated with dose-intensive treatment of hematologic malignancies. The emergence of regenerative medicine strategies using non-HSC populations offers comparable alternatives to restore other organ functions and rebuild excised tissues after cancer surgery. Mesenchymal stem/stromal cells (MSC) exhibit a set of pro-regenerative features (multi-lineage differentiation capacity, homing to sites of injury and inflammation, and paracrine immunomodulatory, pro-angiogenic, anti-apoptotic and pro-proliferative effects, Figure 1) that make them an attractive candidate for modulation of immune disorders and regenerative therapy approaches [1–3]. Unfortunately, the tumor and wound microenvironments share a lot of similarities [4] and MSC have been shown to similarly respond to tumor-associated inflammatory signals and home to malignant sites [5]. While this MSC tumor tropism has been encouragingly exploited to develop tumor targeting strategies [6], it also indicates that caution is required when delivering MSC to cancer-surviving patients for regenerative purposes [7–9]. A number of studies have stressed the in vivo recruitment of MSC by pre- or co-injected cancer cell lines in a variety of animal models and the subsequent promotion (or inhibition) of either tumor growth or metastasis (Table 1). This review outlines the conflicting data currently available in the literature from in vitro and in vivo models of cancer cell-MSC interactions with an emphasis on MSC-secreted factors and their role on tumor development. We discuss the potential impact of these interactions under regenerating conditions.
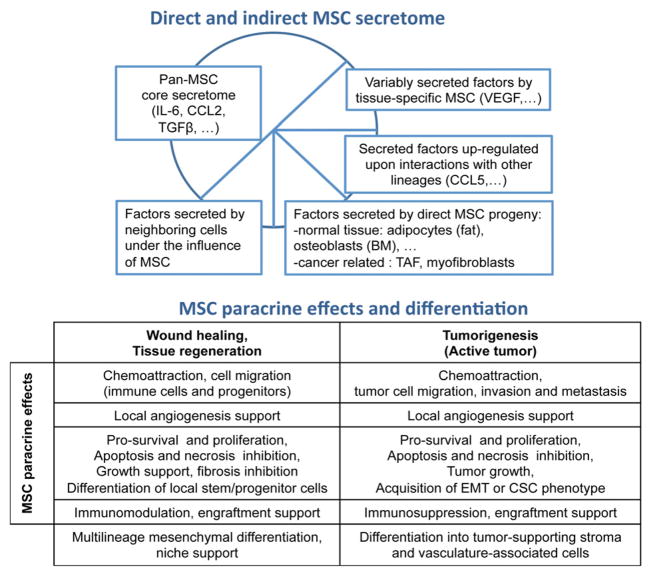
Figure 1.
MSC paracrine activity and incidence on oncogenesis. MSC exert paracrine interactions by a combination of direct (MSC-secreted) and indirect (released by MSC differentiated progeny or neighboring cells) secreted factors. MSC can secrete a large array of cytokines, chemokines and growth factors natively or upon interactions with other cell types. According to the MSC tissue of isolation, levels of MSC secreted factors may vary. MSC secretome shares similar activities during wound healing and interactions with active tumor, including pro-migratory, pro-angiogenic, pro-proliferative, anti-apoptotic and immunosuppressive effects. MSC can also affect the cellular fate of surrounding cells (including tumor cells) and their state of differentiation. Upon interactions with cancer cells, MSC may promote acquisition of pro-tumorigenic CSC activity, or pro-invasion epithelial-to-mesenchymal transition. While MSC multilineage differentiation capacity is a great advantage for regenerative purposes, MSC may also directly support tumor progression by replenishing the local stroma (tumor-associated fibroblasts) or supporting the development of the tumor vasculature (pericytes/myofibroblasts). While the effects of MSC on active tumor seems to mimic wound healing activities, interactions with resting tumor-initiating cells involved during delayed cancer relapse is still poorly characterized.
Table 1.
In vitro and in vivo studies of interactions between MSC and cancer cells.
| cancer | cell lines or clinical isolates | MSC source | in vitro | in vivo | references | |
|---|---|---|---|---|---|---|
| Epithelial | bladder cancer | VX2 | rabbit BM | pro-growth, pro-angiogenic | [155] | |
| breast cancer | 4t1, Eo771, HMLER, MCF7, MDA-MB-231, MDA-MB-435, SK-Br-3, SUM149, SUM159, T47D, spontaneous tumor, clinical isolates | human BM, human SA, human BA, human UC, mouse BM, mouse SA, rat BM | tumor tropism, anti- or pro-proliferation or no effect, pro-apoptotic or no effect on survival, anti- or pro-migration or no effect, pro-invasion, pro-CSC or EMT phenotype | tumor tropism, pro-engraftment or no effect, anti- or pro-growth, anti- or pro-proliferation or no effect, pro-apoptotic, pro-angiogenic, source of TAF, pro-invasion, anti- or pro-metastasis or no effect, pro-CSC phenotype | [32–34, 51, 68, 71–73, 85, 88, 100, 112, 115, 116, 144, 156–159] | |
| cervical cancer | Hela | human SA | no effect on survival | [34] | ||
| colon cancer | Caco-2, COLO 320DM (CC3), H1D2, KM12SM, MC38, SW480 | human BM, human SA, mouse BM, rat BM MCP1cE line | tumor tropism, anti-proliferation or no effect, anti-survival, pro-angiogenic, pro-migration, pro-invasion | tumor tropism, pro-engraftment, anti- and pro-growth, pro-proliferation, anti-apoptotic, immunomodulation, pro-angiogenic, pro- invasion, pro-metastasis, pro-necrosis | [34, 38, 41, 160–163] | |
| endometrial cancer | Hec1a | human BM, human OA, human SA | tumor tropism, anti- or pro-proliferation or no effect | tumor tropism, pro-growth or no effect, pro- proliferation, pro-angiogenic, ECM remodeling, anti-necrosis | [54] | |
| gastric cancer | SGC-7901, spontaneous tumor | human BM, human foreskin, mouse BM | anti-proliferation or no effect, pro-apoptotic, pro-angiogenic, pro-migration, pro-EMT | pro-engraftment, anti- and pro-growth, anti- and pro-proliferation, pro-apoptotic, pro-angiogenic | [37, 38, 89] | |
| liver cancer | H7402, HepG2, HuH7, MHCC97L, H22 | human SA, human fetal derm Z3 line, human liver cancer, mouse BM | anti- or pro-proliferation, pro-apoptotic, anti-survival, pro-invasion | anti- or pro-growth, pro-metastatic | [34, 74, 164, 165] | |
| insulinoma | INS-1 | mouse BM | anti-proliferation | [165] | ||
| lung cancer | A549, H460, Lewis, UCH10 (small cell lung cancer) | human BM, human SA, mouse SA, mouse C3H10T1 line | anti- and pro-proliferation or no effect | anti- or pro-growth, anti-apoptotic, pro-angiogenic, anti-metastasis | [30, 38–41, 108, 112] | |
| melanoma | A375SM, B16 | human BM, mouse BM, mouse SA, mouse C3H10T1 line | anti- or pro-proliferation, immunosuppression | tumor tropism, pro-growth, pro-engraftment, anti-metastasis | [39, 40, 71, 166, 167] | |
| ovarian cancer | A2780, Hey1, ID8, IGROV-1, OVCAR3, SKOV3, primary cultures | human BM human SA, human ovarian cancer, mouse BM, mouse C3H10T1 line | pro-proliferation or no effect, pro- angiogenic, source of TAF, increase CSC phenotype, pro-migration, pro-invasion | pro-growth, pro-angiogenic, pro-invasion, pro-metastasis, source of TAF | [39, 45, 68, 69, 75, 82, 120] | |
| pancreatic cancer | BxPC-3, Capan-1, Capan-2, Colo-357, MIA-Pa-Ca2, Panc-1 | human BM, human SA | tumor tropism, anti-proliferation, anti-survival, pro-necrosis, no effect on apoptosis | tumor tropism, anti-growth, anti-proliferation, pro-angiogenic, pro-apoptotic | [34, 84, 168] | |
| prostate cancer | LNCaP, MDA-Pca-118b, PC3, TRAMP-C2 | human BM, human SA, mouse BM, mouse C3H10T1 line | tumor tropism, anti- and pro-survival, pro-proliferation or no effect, pro-migration, pro-invasion, ECM remodeling, chemoresistance | tumor tropism, anti- or pro-growth or no effect, pro-angiogenesis, anti-metastasis, pro-necrosis | [34–36, 39, 86, 106, 119, 169, 170] | |
| renal cancer | Renca | mouse C3H10T1 line | no effect on proliferation | pro-engraftment, pro-proliferation, immunomodulation, no effect on metastasis | [39] | |
| Hematopoietic | AML | HL60, KG1a | human BM, human SA | anti-proliferation | [33, 41] | |
| CML | BV173 | human BM | anti-proliferation, anti-apoptotic | pro-growth | [41] | |
| Erythroleukemia | K562 | human BM, human SA | anti-proliferation | anti-proliferation | [33, 41] | |
| transformed B cell line | wS9-B-LCL | human BM | anti-proliferation | [41] | ||
| lymphoma | BJAB, Daudi, EL-4, Raji, SKW6.4, U937, YAC-1 | human BM, mouse BM, mouse C3H10T1 line | anti- or pro-proliferation, anti- or pro- apoptotic or no effect, pro-angiogenic | pro-engraftment, anti- or pro-growth, pro- proliferation, pro-angiogenic, pro-necrosis | [38, 39, 105, 165, 171, 172] | |
| Multiple Myeloma | 5T33MM, ARP1, H929, HLE, MM5.1, RPMI8226, U266, clinical isolates | human BM, human placenta, mouse BM, mouse C3H10T1 line | tumor tropism, anti- or pro-proliferation or no effect, anti- or pro-apoptotic | tumor tropism, anti- or pro-growth, anti- apoptotic | [26, 39, 43] | |
| T cell leukemia | Jurkat | human BM | [41] | |||
| Nervous system | glioma - glioblastoma | C6, Gli36, U87MG | human BM, human SA, human UC, mouse C3H10T1 line | pro-proliferation or no effect, anti-survival | pro-growth or no effect, anti-apoptotic | [30, 31, 39, 173] |
| neuroblastoma | CHLA-255, NB-19 | human BM | pro-proliferation or no effect, anti-apoptotic or no effect | pro-growth | [117] | |
| Non-epithelial | Kaposi’s sarcoma | KSIMM | human BM | anti-proliferation | tumor tropism, anti-growth | [106] |
| osteosarcoma | COS1NR, Saos-2 | human BM, rat BM | tumor tropism, pro-proliferation, pro-migration | tumor tropism, pro-growth, pro-metastasis | [44, 87, 118] | |
| fetal MSC-derived | F6 | human fetal, adult BM | pro-engraftment, pro-growth, pro-invasion, pro-angiogenic, pro-necrosis | [160] |
BA= breast adipose, BM = bone marrow, CSC = cancer stem cell, EMT = epithelial-mesenchymal transition, ECM = extracellular matrix, OA = omental adipose, SA = subcutaneous adipose, TAF = tumor-associated fibroblast, UC = umbilical cord.
2. MSC and regenerative therapy after cancer
The attractiveness of MSC for cell-based regenerative therapies relies not only on their capacity to differentiate into multiple mesenchymal lineages [10], but also on the delivery of various paracrine signals responsible for chemoattractant, immunomodulatory, angiogenic, anti-apoptotic, anti-scarring, and pro-survival effects [11]. Yet, the same MSC-secreted factors that accompany tissue regeneration and revascularization have also been linked to the promotion of cancer growth and metastasis (Figure 1) [7]. The safety of bone marrow (BM)-derived MSC (BM-MSC) was assessed in clinical trials in 1995 [12] and MSC-based strategies were subsequently introduced for regenerative trials for bone [13, 14] and cartilage [15] defects, or immunomodulation of graft versus host disease [16, 17], autoimmune disease [18] and stroke [19]. HSC transplantation was widely used in the 1990s to rescue the hematopoietic system of breast cancer patients undergoing intensive chemotherapy [20]. This strategy was ultimately abandoned because no significant therapeutic effect could be demonstrated over conventional therapies. However, the co-administration of MSC and HSC in breast cancer patients significantly accelerated the restoration of the hematopoietic compartment [21]. Several studies have investigated the effects of BM-MSC and HSC co-transplantation to facilitate engraftment or reduce graft-versus-host disease into patients treated for hematopoietic malignancies [16, 22, 23]. Autologous BM-MSC were also delivered in a fibrin spray to accelerate wound healing in patients with acute wounds including skin cancer surgery-induced lesions [24], and our group has recently validated in vitro an analogous strategy using unpassaged adipose-derived MSC [25]. Intrabone and systemic delivery of MSC has been tested in a multiple myeloma animal model for simultaneous inhibition of tumor growth and regeneration of bone lesions [26].
Another MSC-based approach currently under consideration for regenerative therapy after cancer is cell-assisted soft tissue reconstruction for patients treated for head and neck or breast cancer [7]. Cosmetic restoration after disfiguring surgical tumor excision remains an important part of the treatment. Soft tissue reconstruction after breast cancer was pioneered in late 19th century by Czerny [27] and could provide satisfactory short-term cosmetic results, but remained flawed mainly due to poor long term volume retention [28, 29]. Recently, MSC-assisted autologous fat transfer approaches for soft tissue reconstruction have been developed and have been shown to enhance graft survival and local angiogenesis to sustain stable, functional and natural appearance [7].
3. Models of MSC-tumor cell interactions
A list of currently published studies examining interactions between MSC and cancer cells is summarized in Table 1. Most investigators relied on established cancer cell lines rather than clinical isolates to mimic tumor behavior in epithelial, hematopoietic and mesenchymal cancers. These studies exposed a variety of cell-cell and paracrine interactions (including both pro- and anti-tumor activities) relying primarily on breast cancer cell lines and MSC isolated mostly from human BM and adipose (Table 1). These studies are sometimes contradictory, and MSC can be shown to either promote or inhibit tumor progression within the same cancer model (Table 1), occasionally using identical cancer cell lines. For example, human adipose-derived MSC support proliferation of the glioma cell line U87MG in vitro and tumor growth in vivo [30], while human umbilical cord-derived MSC were shown to be cytotoxic to the same line in a separate publication [31]. Such discrepancies are even more evident in studies of MSC interactions with epithelial cancers. MSC interactions can vary tremendously depending on numerous factors, including MSC tissue of origin, cancer type and model, pre-treatment of MSC using cytokines or small molecules, and a variety of in vitro and in vivo system-related discrepancies, including the relative number of both MSC and cancer cells, simultaneous or individual injection of MSC and cancer cells, local versus systemic MSC delivery or the kinetics of tumorigenesis. Human BM- and adipose-derived MSC were demonstrated to respectively promote and inhibit the in vitro proliferation of the breast cancer cell line MCF7, as well as the in vitro survival or in vivo growth of the PC3 prostate cancer line [32–36]. BM-MSC and foreskin-derived MSC respectively promoted and inhibited SGC-7901 gastric cancer growth in vivo [37, 38]. Lung cancer models using the identical cancer cell line (A549) or similar Lewis tumors revealed diverging effects of MSC on either tumor in vitro proliferation or in vivo growth [38–41]. These inconsistencies can even be detected using both the same source of MSC and cancer cell line (BM-MSC pro-and anti-proliferative effects on breast cancer MDA-MB-231 line [32, 42] or pro- and anti-tumor growth in vivo with the prostate cancer PC3 line [35, 36]). Some authors preferred using immortalized MSC lines, which could also affect the outcomes, as mouse BM-MSC had no effect on the proliferation of the multiple myeloma cell line RPMI8226, whereas the mouse C3H10T1/2 line exerted potent inhibitory activity [39, 43]. Co-implantation of rat BM-MSC with COS1NR osteosarcoma cells accelerated early onset of tumor growth, but not metastasis, whereas intravenous MSC injection did increase the number of metastatic nodules without affecting tumor growth [44]. Finally, some authors emphasized aberrant behavior of MSC isolated from cancer clinical isolates, compared with healthy BM- or adipose-derived MSC [45].
3.1. How to model regenerative therapy after cancer?
MSC selection techniques can vary in the literature, but plastic adherence is typical and considered axiomatic [46]. This crude selection method does not exclude heterogeneity of MSC sources within a single tissue (e.g. adipose) [47–49] or persistence of hematopoietic lineages at early passages (e.g. macrophages) [50, 51]. Although all MSC populations share basic similarities immunophenotypically and functionally, differences can be demonstrated using high resolution techniques [52, 53] and are reflected in variability within their secretome [7, 54]. A growing number of studies have developed models to study MSC-tumor interactions (Table 1). Only a few groups have investigated these interactions using clinical isolates [26, 45, 51] (including ours) which may be more relevant to the in vivo tumor heterogeneity than homogeneous cancer cell lines. The source of MSC in these studies can vary tremendously, including differences of species (human, mouse, rat, rabbit) and tissue of origin (i.e. normal bone marrow, umbilical cord, placenta, subcutaneous, omental and breast adipose, or cancer tissue). Some authors relied on immortalized MSC lines (mouse C3H10T1, human fetal derm Z3 and rat MCP1cE), but most studies employed the two most prevalent MSC currently used in clinical practice: human BM and subcutaneous adipose (SA) –derived MSC. Dissimilarities between BM-MSC and adipose-derived MSC (termed adipose-derived stem/stromal cells or ASC), have already been reviewed in [55].
3.1.1. MSC variability
Multipotent MSC were originally isolated from bone marrow [10] and have been defined as a plastic-adherent fibroblastic cell population, exhibiting a defined immunophenotype (e.g. expression of CD73, CD90, CD105 and lack of expression of hematopoietic/endothelial markers), and capable of clonal differentiation towards mesenchymal lineages (e.g., adipogenic, osteogenic and chondrogenic lineages) [46]. Similar mesenchymogenic populations have been isolated from the connective tissue of multiple tissues [56], including adipose [57]. Recent studies have unraveled transcriptomic, proteomic or epigenomic [53, 58–60] disparities between tissue-specific MSC, which may mark some degree of niche-associated bias. The inherent heterogeneity of the pool of mesenchymogenic progenitors participating in the MSC activity of each tissue can be reflected by some disparities measured at the secretome level [7, 54]. Yet, it seems that shared sources of MSC, such as the ubiquitous pericytes, retain functionality across discrete niches. CD146+ perivascular cells, or pericytes, represent a ubiquitous source of MSC throughout various organs [61, 62], whereas other more specialized progenitor populations may contribute to MSC activity in tissues such as fat [47–49]. CD146+ BM-resident subendothelial cells are in vivo precursors of BM-MSC and can organize the hematopoietic niche via their secretome (i.e. release of Angiopoietin-1) and support adult HSC [63]. This presumably BM-specific function is retained by non-medullar sources of MSC such as adipose [64], although this activity seems to be restricted to the CD146+ pericytic source of ASC [65]. Inversely, ASC secrete adipose-specific factors, such as leptin and adipsine [7], which are not shared with BM-MSC, and may reflect heterogeneity and/or specialization within the pool of adipose progenitors [66]. The bulk of MSC-secreted factors comprises a common core, independently of their tissue of origin, including an overlapping set of anti-apoptotic, immunomodulatory, anti-scarring, supportive, angiogenic and chemoattractant factors such as interleukin-6 (IL6), chemokine C-C motif ligand 2 (CCL2), PAI-1, transforming growth factor-beta1 (TGFβ1), CD106 and vascular endothelial growth factor (VEGF) [11, 67]. A few studies have compared the effects of distinct MSC populations in cancer models. Both BM-MSC and adipose-resident cells have been shown to be recruited to sites of ovarian tumors, where BM-MSC preferentially give rise to tumor-associated fibroblasts (TAF) while the adipose stroma contributes to vascular/fibrovascular lineages [68]. Yet, both BM-MSC and subcutaneous adipose-derived MSC can acquire a TAF phenotype in presence of ovarian cancer cells [69]. Breast-derived ASC are potent activator of basal-type breast cancer progression and invasiveness [70] via secretion of specific factors (Matrix metalloproteinase-1 (MMP1), MMP3) not expressed by BM-MSC. Adipose tissue is distributed in multiple depots, which may have distinct developmental origins and visceral fat possesses potent inflammatory activity. Klopp et al. compared the behavior of BM-MSC, visceral and subcutaneous ASC [54] in both in vitro and in vivo studies of endometrial cancer. Both BM-MSC and omental-ASC displayed robust tumor-homing, pro-angiogenic (including higher pericyte coverage), extra-cellular matrix (ECM)-remodeling and pro-proliferative activities in vivo. While the tumor proliferation enhancing effects of omental ASC were confirmed in vitro, BM-MSC seemed to display an opposite behavior in co-culture experiments. Surprisingly, subcutaneous ASC did not display any significant effect for all pro-tumoral activities [54]. Omental ASC were also the only MSC population to protect cancer cells from necrosis in vivo. Szebeni et al analyzed mouse BM-MSC and subcutaneous ASC interactions with breast cancer and melanoma in vivo models [71], but did not report any divergent effects on tumor growth, vascularity and metastasis support. In another study, human cord blood-derived MSC and breast-derived ASC exhibited a similar behavior when injected intravenously in a breast cancer model, including tumor tropism and inhibition of both tumor growth and metastasis [72]. A multiple myeloma model revealed minor differences between mouse and human BM-MSC in the presence of tumor cells, although both populations contributed to tumor growth augmentation [43]. Both mouse and human ASC have been shown to support the growth of breast cancer cell lines [73] and any direct distinction between species remains to be investigated.
3.1.2. Tumor effects on MSC
Some authors have also analyzed the effects of tumor-derived MSC populations [45, 74, 75] on cancer progression. Evidence has been accumulating concerning the existence of deranged tumor-resident MSC isolated from several cancers including multiple myeloma [76–80], breast cancer [81], liver cancer [74] and ovarian cancer [45, 75, 82]. The tumor-supporting properties of MSC (immunomodulation, angiogenesis, cell survival or migration) often seem to be enhanced in tumor-derived MSC populations [45, 74, 75]. For instance, human ovarian cancer-derived MSC showed higher pro-tumor growth activity than normal human BM-MSC and ASC, promoting the acquisition of a phenotype resembling putative cancer stem cells (CSC) [45], in support of local tumor-MSC crosstalk leading to specialized tumor-resident MSC populations. Both BM- and adipose-derived MSC display tumor tropism due to various tumor-released chemotactic factors including CCL25 [43], C-X-C motif chemokine-1 (CXCL1) [54], epidermal growth factor (EGF) [83, 84], hepatoma-derived growth factor (HDGF) [85], IL-8 [54], platelet-derived growth factor (PDGF) [84], stromal cell-derived factor-1 (SDF1, a.k.a. CXCL12) [86, 87], TGFβ [88], and VEGF [84]. Recruited tumor-resident MSC populations or their direct progeny (i.e. TAF, myofibroblasts) often possess augmented ability to promote tumor growth [45, 75, 77, 82] and invasion [74, 75] compared with healthy donor MSC via superior angiogenesis [75, 77, 80, 82, 89], or abnormal immunomodulation [76, 79, 81], resulting in increased release of cytokines/growth factors including hepatocyte growth factor (HGF) [80, 82], IL-6 [76, 77, 79, 82], IL-10 [81], fibroblast-specific protein-1 (FSP1, a.k.a. S100A4) [74], TGFβ [79, 81] and VEGF [75, 80, 82] by tumor-resident MSC.
3.1.3. Tumor-initiating cells
Hematopoietic rescue by autologous blood or bone marrow transplantation following high dose chemotherapy is possibly the most exploited strategy to treat hematologic malignancies and can achieve significant clinical responses, but does not invariably prelude future cancer relapse. Similarly, therapy for epithelial cancers such as breast cancer is rarely curative and cancer recurrence remains a significant cause of mortality after induction of successful and often durable remissions. Late recurrence is well documented and provides direct evidence for the persistence of tumor-initiating cells at a subclinical level, referred as cancer dormancy. The cell cycle state (quiescent or homeostatic) of dormant cancer cells and their reactivation after a symptom-free interval remain both poorly understood. Tumor-initiating cells, often referred as CSC, are restricted to specific tumor subsets within the heterogeneous bulk of malignant cells in several cancers, and are characterized by the expression of markers originally associated with normal stem/progenitor cells. These include CD44, CD90, CD117, and CD133 [90]. While cancer dormancy depends on the sub-clinical persistence of rare tumor-initiating cells, the CSC paradigm might offer clues to how tumor subsets may escape anti-cancer therapies [91, 92]. In vitro cellular models of cancer dormancy [93–96], CSC [97], or other tumor-initiating cells possibly involved during cancer relapse remain poorly established and only rare studies rely on the purification of resting subsets of tumorigenic cells akin to the cells involved during relapse [51]. Dormant and active breast cancer cells possess distinct genome-wide expression signature, especially angiogenesis-related genes [96] and our own published work support that resting and active tumor-initiating breast cancer cells respond differently to MSC signals [51]. Our in vivo xenograft approach relied on an animal model utilizing unpassaged sort-purified breast cancer clinical isolates injected in limited number and resulting in small (5–10mm3) tumors developing 6 or more months after injection. Breast tumor-initiating activity is enriched in the CD44+CD24-CD326+ fraction of breast cancer cells, which was originally shown to contain both quiescent and actively proliferating cells [98]. We previously refined the breast cancer tumor-initiating activity to a CD90+ subset of CD44+ cells [99], which is localized at the invasive front in breast cancer tumors [90]. Small (low light scatter) resting CD90+ breast cancer cells give rise to tumors with high efficiency (<100 cells/injection) [90, 99], independently of supportive stroma/ASC [51]. Large (high light scatter) CD90+ tumor-initiating cells include a large number of dividing/aneuploid cells and are only tumorigenic at higher dose (>600 cells) [90, 99], although co-injection with ASC can rescue their tumorigenic potential at lower dose (100 cells) [51]. Importantly, our in vivo mouse model displayed tumor growth kinetics and incidence similar to dormant cancer cell line models [93–96], in contrast to studies relying on aggressive cancer cell lines and resulting often into >100mm3 tumors less than a month after implantation [7]. Models using aggressive cell lines have little relevance to regenerative therapy after cancer, but may be more appropriate for evaluating potential suppressive effects of MSC on rapidly growing high-grade therapy unresponsive tumors.
4. The MSC secretome and cancer cells
MSC can be mobilized and recruited to active tumor sites, where they can incorporate into the tumor’s microenvironment [5, 68, 100–103]. There they can potentiate further tumorigenesis via differentiation into tumor-nurturing stroma (TAF, myofibroblasts) [82, 104], direct cell contact interaction with cancer cells [105, 106] or release of paracrine factors (Table 2). Tumor-MSC interactions studies have revealed MSC tumor-supporting paracrine activities (local immunosuppression and angiogenesis, promotion of tumor growth and invasion (i.e. acquisition of epithelial-mesenchymal transition (EMT)/CSC phenotype or ECM remodeling), inhibition of tumor apoptosis or necrosis) in a large spectrum of cancers (Table 1). Table 2 summarizes published MSC-secreted factors that have been identified during MSC-cancer cell interactions and their reported effect on cancer cells. Several cytokines usually involved during MSC-mediated tissue regeneration (e.g. IL-6, TGFβ, VEGF) are secreted at elevated levels by MSC upon recruitment by cancer cells and support actively growth or invasion of cancer cells. As mentioned previously, the exact role(s) that MSC play in the modulation of tumor cell growth remains controversial [7–9] and release of some factors such as DKK1 can inhibit the proliferation of hematopoietic cancer cells [33, 43, 77]. Pro-tumorigenic effects of MSC can be inhibited by pretreatment of MSC with imatinib (PDGF-receptor inhibition) [107], gefitinib (EGFR inhibition) [83] or interferon-gamma (INFγ) [108] while some preconditioning treatment (hypoxia, irradiation, genetic engineering) enhance MSC migratory and pro-tumoral activities [32, 109–111]. Obesity may also accelerate tumor growth, via an increased endogenous ASC reservoir, which directly contribute to sustain the tumor microenvironment [112]. IL-6 is an MSC-secreted inflammatory cytokine displaying pro-survival, pro-growth and pro-angiogenic activities [11], which has been implicated in tumor progression of various cancers including breast cancer [113, 114]. Secretion of elevated levels of IL-6 by MSC has been detected upon interaction with malignant cells in several epithelial, hematopoietic and mesenchymal cancers (Table 2) [43, 69, 76, 77, 82, 115–119]. In these studies, MSC-released IL-6 supported tumor growth by stimulating cancer cell proliferation and survival or protecting from apoptosis. BM-MSC and ASC could also potentiate cancer cell migration, invasion and metastasis via the release of IL-6 in the tumor microenvironment [116, 120]. BM-MSC and ASC can also secrete a combination of anti-apoptotic and angiogenic factors [121], including HGF, SDF-1/CXCL12, CD106 (sVCAM) and VEGF which can promote tumor growth, local angiogenesis and metastasis [42, 84, 122–127]. Secretion levels of some cytokines, such as VEGF, can vary depending on the tissue from which MSC are derived. Subcutaneous adipose-derived MSC populations seem to secrete lower level of VEGF than BM-MSC [7, 54] or visceral ASC [54]. The monocyte chemoattractant protein-1 (MCP1) or CCL2 is commonly detected among MSC secreted cytokines/chemokines [7, 128]. Although not reported in direct tumor cell-MSC interaction studies (Table 2), MCP1 can be secreted by stromal [129] or tumor cells (to recruit MSC [130] and macrophages). MCP1 is a critical chemoattractant responsible for the recruitment of macrophages into tumor and for angiogenesis in breast cancer [131, 132], and may contribute to indirect crosstalk between MSC and cancer cells via recruitment of tumor-resident macrophages. The immunosuppressive activity of MCP1 has been implicated in the progression and metastasis of cancer in animal models of skin papilloma [133], colon carcinoma [134], prostate cancer [135], breast cancer [136, 137] and lung cancer [138]. MSC-mediated immunosuppression activity has been shown to be modulated via tumor necrosis factor-alpha (TNFα) [139]. MSC have also been shown to release elevated levels of TGFβ upon interaction with breast and prostate cancer [32, 35, 81], resulting into stimulation of the proliferative and migratory capacities of the cancer cells. The implication of TGFβ signaling in promotion of tumor invasion and metastasis [140] via EMT [141] is well established. Another MSC-secreted pro-metastasis cytokine, CCL5 (RANTES), can be secreted upon interaction with cancer cells and is associated with tumor progression and invasion in various cancers [73, 87, 100, 142–144]. CCL5 can be secreted by both BM-MSC and ASC [100, 144] and displays pro-proliferative activities on breast cancer cell lines [145, 146]. Other MSC-secreted factors upregulated during interactions with cancer cells and exhibiting potent effect on tumor cells include BMP2, CXCL1, CXCL5, CXCL6, CXCL7, EGF, IL4, IL8, IL10, IL17b or S100A4.
Table 2.
MSC-secreted factors detected during studies of cancer cell-MSC interactions.
| MSC-secreted factors | MSC source | cancer | reported MSC paracrine activities on cancer cells | references |
|---|---|---|---|---|
| IL-6 | human BM, human SA, human BA, mouse BM | breast cancer, ovarian cancer, prostate cancer neuroblastoma, multiple myeloma, osteosarcoma | tumor tropism, pro-growth, pro-survival, pro-proliferation, anti-apoptosis, pro-migration, pro-invasion, pro-metastasis | [43, 69, 76, 77, 82, 115–120] |
| VEGF | human BM, human OA, human SA, ovarian cancer, mouse BM | endometrial cancer, gastric cancer, ovarian cancer, pancreas cancer, prostate cancer, multiple myeloma | tumor tropism, pro-angiogenesis | [43, 54, 69, 75, 80, 82, 84, 86, 89] |
| CCL5 | human BM, human SA | breast cancer, osteosarcoma | pro-migration, pro-invasion, pro-metastasis | [73, 87, 100, 144] |
| TGFβ | human BM, human BA | breast cancer, prostate cancer | pro-survival, pro-proliferation, pro-migration, pro-invasion | [32, 35, 81] |
| DKK1 | human BM, human SA, mouse BM | erythroleukemia, multiple myeloma | anti-proliferation | [33, 43, 77] |
| IL-17b | human BM | breast cancer | pro-migration, pro-metastasis | [88] |
| S100A4 | human liver cancer | hepatocellular carcinoma | pro-proliferation, pro-invasion, pro-metastasis | [74] |
| BMP2 (BMP4, BMP6) | human BM, human SA, ovarian cancer | ovarian cancer | pro-growth, pro-proliferation, pro-CSC phenotype | [45] |
| IGF1 | human BM, mouse BM | prostate cancer, multiple myeloma, osteosarcoma | pro-survival | [43, 118, 119] |
| SDF1 | human BM, human SA, mouse SA | endometrial cancer, ovarian cancer | pro-migration, pro-metastasis | [54, 69, 73] |
| EGF | human BM | ovarian cancer | pro-proliferation | [82] |
| HGF | human BM | ovarian cancer, multiple myeloma | - | [80, 82] |
| IL-10 | human BM, human BA, mouse BM | breast cancer, multiple myeloma | - | [43, 81] |
| IL-4 | human breast cancer | breast cancer | - | [81] |
| IL-8, CXCL5, CXCL6, CXCL7, CXCL1 | human BM | breast cancer | - | [115] |
| FGF | human BM, human OA, human SA | endometrial cancer, ovarian cancer, multiple myeloma | - | [54, 80, 82] |
| TSP1, TnC, SL1 | human BM | ovarian cancer | - | [82] |
| Non-specified paracrine factors | human BM, human SA, human UC, human foreskin, mouse C3H10T1 line | breast cancer, gastric cancer, lung cancer, pancreatic cancer, prostate cancer, renal cancer | pro-engraftment, anti- or pro-growth, anti- or pro-proliferation or no effect, pro-apoptosis, pro-angiogenesis pro-EMT phenotype | [34, 36–40, 156, 157, 159] |
5. Summary and conclusions
Early cancer recurrence following hematopoietic or epithelial cancer treatment is often characterized by very aggressive active disease [7], a clear contraindication to regenerative reconstructive therapy. On the other hand, patients with responsive disease who enter clinical remission are nonetheless at risk for late relapse, implying the persistence of a distinct population of dormant cancer-initiating cells. While bi-directional cross-talk between MSC and aggressive cancer cells is well documented, specific interactions between MSC and dormant-like tumor-initiating cells remain poorly established. A non-obvious parallel comes from our experience in cellular reprogramming of myeloid progenitors to pluripotency [147]. Many of the same reprogramming elements are shared between pluripotency and tumorigenicity [148] and the most commonly used reprogramming factors for induced pluripotent stem cell (iPSC) technology are known oncogenes (MYC) or have been directly linked to tumorigenicity in a variety of human cancers (NANOG, SOX2, OCT4) [148]. Indeed, non-tumorigenic epithelial mammary cells have been shown to be induced with CSC activity via cellular reprogramming [149]. Interestingly, hematopoietic progenitors seem to be more amenable to cellular reprogramming than conventional stem cells [150] and we have demonstrated that MSC co-cultured with actively dividing myeloid progenitor cells facilitate their acquisition of induced pluripotency, via both cell-cell contacts and release of multiple cytokines and growth factors [147]. These studies illustrate differential reprogramming behavior of progenitor and stem cell populations and confirm that MSC cross-talk with progenitor populations can potentiate their cellular fate.
Cancer cells can display fluctuating levels of stem-like activities [151]. In fact, MSC may exert distinct effects on tumor-initiating cell populations according to their degree of stemness. This may result into promotion of a pro-resting CSC niche [152, 153] for the most therapy-resistant stem-like cells, or recruitment and promotion of tumorigenesis for more active progenitor cells. Our previously published in vivo breast cancer model provides the only available data on the interaction of adipose-derived MSC with tumor cell subsets sort-purified from unpassaged clinical isolates. A basic comparison of the major cytokines, chemokines and growth factors secreted by ASC revealed a close correspondence to the secretome of BM-MSC, including the major cytokines implicated in promotion of tumor growth, such as IL-6. Although levels of VEGF secreted by ASC were moderate, we could still detect the development of human blood vessels within tumor xenografts coinjected with human ASC. The effects of a few secreted factors unique to adipose derived MSC, such as leptin and adipsin, remain unclear, although, leptin has been associated with tumor progression in breast cancer [154]. Engraftment and tumorigenesis of active tumor cells significantly benefited from the coinjection of ASC. Yet, resting cells were not responsive to local ASC signals, although they were consistently able to generate tumors from a limited number of injected cells. We could not detect differences (size, histology) between tumors generated by active and resting tumor-initiating cells.
Taken together, the secretome of MSC exert potent tissue remodeling effects. The results from multiple laboratories suggest that the effects of MSC on tumor cells are multiple and may depend on the state of the tumor cell, the properties of specific MSC populations, and interactions with other cell types, such as tumor infiltrating immune cells..
Highlights.
MSC regenerative potential relies in part on paracrine activities.
MSC secretome can interact with tumor-initiating cancer cells.
Same MSC signals are involved in regenerative and pro-cancer activities.
Dormant cancer may not respond to the same signals as active malignant tumor.
Acknowledgments
Drs. Albert and Vera Donnenberg were supported by grants BC032981 and BC044784 from the Department of Defense, grant R01CA 114246 from the NIH, grant R01-HL-085819 from the National Heart, Lung, and Blood Institute, the Hillman Foundation, the Glimmer of Hope Foundation, the Commonwealth of Pennsylvania, through the McGowan Institute of Regenerative Medicine, the NHLBI (Production Assistance for Cellular Therapy (PACT) N01-HB-37165), and the Department of Defense Biomedical Translational Initiative (W911QY-09-C-0209). Drs. Donnenberg would also like to thank Diana Napper from The Glimmer of Hope Foundation for her support. Dr. Zambidis and Dr. Park were supported by grants from NIH 1U01HL099775 and U01HL100397 (ETZ) and the Maryland Stem Cell Research Fund: 2011-MS CRF II-0008-00 and 2007-MSCRF II-0379-00 (ETZ), and the Maryland Stem Cell Research Fund (MSCFR) Postdoctoral Fellowship grant 2009-MSCRF III-106570 (TSP).
Abbreviations
- ASC
adipose-derived stem/stromal cells
- BA
breast adipose
- BM
bone marrow
- CCL
chemokine C-C motif ligand
- CSC
cancer stem cells
- CXCL
C-X-C motif chemokine
- ECM
extra-cellular matrix
- EGF
epidermal growth factor
- EMT
epithelial-mesenchymal transition
- FSP1
fibroblast-specific protein-1
- HDGF
hepatoma-derived growth factor
- HGF
Hepatocyte growth factor
- HSC
hematopoietic stem cells
- IL-6
interleukin 6
- INFγ
interferon-gamma
- IPSC
induced pluripotent stem cell
- MCP1
monocyte chemoattractant protein-1
- MMP
matrix metalloproteinases
- MSC
mesenchymal stromal/stem cells
- OA
omental adipose
- PDGF
platelet-derived growth factor
- SA
subcutaneous adipose
- SDF1
stromal cell-derived factor-1
- TAF
tumor-associated fibroblasts
- TGFβ
transforming growth factor-beta
- TNFα
Tumor necrosis factor-alpha
- UC
umbilical cord
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of Interest
Authors do not have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 3.Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3:25–33. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 5.Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene therapy. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 6.Fritz V, Jorgensen C. Mesenchymal stem cells: an emerging tool for cancer targeting and therapy. Curr Stem Cell Res Ther. 2008;3:32–42. doi: 10.2174/157488808783489462. [DOI] [PubMed] [Google Scholar]
- 7.Donnenberg VS, Zimmerlin L, Rubin JP, Donnenberg AD. Regenerative therapy after cancer: what are the risks? Tissue Eng Part B Rev. 2010;16:567–575. doi: 10.1089/ten.teb.2010.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., 3rd Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B, Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. 2008;10:657–667. doi: 10.1080/14653240802486517. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Meirelles da SL, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- 13.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. [PubMed] [Google Scholar]
- 16.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR, Jr, Moseley AB, Bacigalupo A. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 18.Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann N Y Acad Sci. 2012;1266:107–117. doi: 10.1111/j.1749-6632.2012.06667.x. [DOI] [PubMed] [Google Scholar]
- 19.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ G. Canadian Critical Care Trials, . Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PloS one. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadtmauer EA, O’Neill A, Goldstein LJ, Crilley PA, Mangan KF, Ingle JN, Brodsky I, Martino S, Lazarus HM, Erban JK, Sickles C, Glick JH. Conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer. Philadelphia Bone Marrow Transplant Group. N Engl J Med. 2000;342:1069–1076. doi: 10.1056/NEJM200004133421501. [DOI] [PubMed] [Google Scholar]
- 21.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 22.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, Chen Y, Zeng Y, Xu L, Liu D, Chen H, Zhang X, Han W, Wang Y, Zhao T, Wang J, Wang J, Han Q, Zhao C, Huang X. Coinfusion of mesenchymal stromal cells facilitates platelet recovery without increasing leukemia recurrence in haploidentical hematopoietic stem cell transplantation: a randomized, controlled clinical study. Stem Cells Dev. 2011;20:1679–1685. doi: 10.1089/scd.2010.0447. [DOI] [PubMed] [Google Scholar]
- 24.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerlin L, Rubin JP, Pfeifer ME, Moore LR, Donnenberg VS, Donnenberg AD. Human adipose stromal vascular cell delivery in a fibrin spray. Cytotherapy. 2012;15:102–108. doi: 10.1016/j.jcyt.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Ling W, Pennisi A, Wang Y, Khan S, Heidaran M, Pal A, Zhang X, He S, Zeitlin A, Abbot S, Faleck H, Hariri R, Shaughnessy JD, Jr, van Rhee F, Nair B, Barlogie B, Epstein J, Yaccoby S. Human placenta-derived adherent cells prevent bone loss, stimulate bone formation, and suppress growth of multiple myeloma in bone. Stem Cells. 2011;29:263–273. doi: 10.1002/stem.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czerny V. Plastischer Ersatz der Brustdruse durch ein Lipom. Chir Kong Verhandl. 1895;2:216. [Google Scholar]
- 28.Niechajev I, Sevcuk O. Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg. 1994;94:496–506. doi: 10.1097/00006534-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Peer LA. Loss of Weight and Volume in Human Fat Grafts: With Postulation of A “Cell Survival Theory”. Plastic and Reconstructive Surgery. 1950;5:217–230. [Google Scholar]
- 30.Yu JM, Jun ES, Bae YC, Jung JS. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17:463–473. doi: 10.1089/scd.2007.0181. [DOI] [PubMed] [Google Scholar]
- 31.Kang SG, Jeun SS, Lim JY, Kim SM, Yang YS, Oh WI, Huh PW, Park CK. Cytotoxicity of human umbilical cord blood-derived mesenchymal stem cells against human malignant glioma cells. Childs Nerv Syst. 2008;24:293–302. doi: 10.1007/s00381-007-0515-2. [DOI] [PubMed] [Google Scholar]
- 32.Hung SP, Yang MH, Tseng KF, Lee OK. Hypoxia-induced Secretion of TGF-beta 1 in Mesenchymal Stem Cell Promotes Breast Cancer Cell Progression. Cell Transplant. 2012 doi: 10.3727/096368912X657954. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, Li J, Yan X, Liu Y, Shao C, Zhao RC. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009;23:925–933. doi: 10.1038/leu.2008.384. [DOI] [PubMed] [Google Scholar]
- 34.Cousin B, Ravet E, Poglio S, De Toni F, Bertuzzi M, Lulka H, Touil I, Andre M, Grolleau JL, Peron JM, Chavoin JP, Bourin P, Penicaud L, Casteilla L, Buscail L, Cordelier P. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One. 2009;4:e6278. doi: 10.1371/journal.pone.0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye H, Cheng J, Tang Y, Liu Z, Xu C, Liu Y, Sun Y. Human bone marrow-derived mesenchymal stem cells produced TGFbeta contributes to progression and metastasis of prostate cancer. Cancer Invest. 2012;30:513–518. doi: 10.3109/07357907.2012.692171. [DOI] [PubMed] [Google Scholar]
- 36.Chanda D, Isayeva T, Kumar S, Hensel JA, Sawant A, Ramaswamy G, Siegal GP, Beatty MS, Ponnazhagan S. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in prostate cancer bone metastasis. Clin Cancer Res. 2009;15:7175–7185. doi: 10.1158/1078-0432.CCR-09-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zhao Y, Cheng Z, Zhan J, Sun X, Qian H, Zhu W, Xu W. Mesenchymal stem cell-like cells from children foreskin inhibit the growth of SGC-7901 gastric cancer cells. Exp Mol Pathol. 2013;94:430–437. doi: 10.1016/j.yexmp.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Zhu W, Huang L, Li Y, Qian H, Shan X, Yan Y, Mao F, Wu X, Xu WR. Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle. 2011;10:3198–3207. doi: 10.4161/cc.10.18.17638. [DOI] [PubMed] [Google Scholar]
- 39.Djouad F, Bony C, Apparailly F, Louis-Plence P, Jorgensen C, Noel D. Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation. 2006;82:1060–1066. doi: 10.1097/01.tp.0000236098.13804.0b. [DOI] [PubMed] [Google Scholar]
- 40.Maestroni GJ, Hertens E, Galli P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cellular and molecular life sciences: CMLS. 1999;55:663–667. doi: 10.1007/s000180050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 42.Martin TA, Parr C, Davies G, Watkins G, Lane J, Matsumoto K, Nakamura T, Mansel RE, Jiang WG. Growth and angiogenesis of human breast cancer in a nude mouse tumour model is reduced by NK4, a HGF/SF antagonist. Carcinogenesis. 2003;24:1317–1323. doi: 10.1093/carcin/bgg072. [DOI] [PubMed] [Google Scholar]
- 43.Xu S, Menu E, De Becker A, Van Camp B, Vanderkerken K, Van Riet I. Bone marrow-derived mesenchymal stromal cells are attracted by multiple myeloma cell-produced chemokine CCL25 and favor myeloma cell growth in vitro and in vivo. Stem Cells. 2012;30:266–279. doi: 10.1002/stem.787. [DOI] [PubMed] [Google Scholar]
- 44.Tsukamoto S, Honoki K, Fujii H, Tohma Y, Kido A, Mori T, Tsujiuchi T, Tanaka Y. Mesenchymal stem cells promote tumor engraftment and metastatic colonization in rat osteosarcoma model. Int J Oncol. 2012;40:163–169. doi: 10.3892/ijo.2011.1220. [DOI] [PubMed] [Google Scholar]
- 45.McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, Cabrera L, Keller E, McCauley L, Cho KR, Buckanovich RJ. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121:3206–3219. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerlin L, Donnenberg VS, Rubin JP, Donnenberg AD. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 2013;83:134–140. doi: 10.1002/cyto.a.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The Tunica Adventitia of Human Arteries and Veins as a Source of Mesenchymal Stem Cells. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 51.Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jansen BJ, Gilissen C, Roelofs H, Schaap-Oziemlak A, Veltman JA, Raymakers RA, Jansen JH, Kogler G, Figdor CG, Torensma R, Adema GJ. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010;19:481–490. doi: 10.1089/scd.2009.0288. [DOI] [PubMed] [Google Scholar]
- 53.Noël D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, Jorgensen C, Cousin B. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Experimental Cell Research. 2008;314:1575–1584. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 54.Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, Debeb B, Woodward W, Schmandt R, Broaddus R, Lu K, Kolonin MG. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18:771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 56.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 57.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ragni E, Montemurro T, Montelatici E, Lavazza C, Vigano M, Rebulla P, Giordano R, Lazzari L. Differential microRNA signature of human mesenchymal stem cells from different sources reveals an “environmental-niche memory” for bone marrow stem cells. Exp Cell Res. 2013 doi: 10.1016/j.yexcr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park HW, Shin JS, Kim CW. Proteome of mesenchymal stem cells. Proteomics. 2007;7:2881–2894. doi: 10.1002/pmic.200700089. [DOI] [PubMed] [Google Scholar]
- 61.Covas DT, Panepucci RA, Fontes AM, Silva WA, Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146(+) perivascular cells and fibroblasts. Exp Hematol. 2008 doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 62.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 64.De Toni F, Poglio S, Youcef AB, Cousin B, Pflumio F, Bourin P, Casteilla L, Laharrague P. Human adipose-derived stromal cells efficiently support hematopoiesis in vitro and in vivo: a key step for therapeutic studies. Stem Cells Dev. 2011;20:2127–2138. doi: 10.1089/scd.2011.0044. [DOI] [PubMed] [Google Scholar]
- 65.Corselli M, Chin CJ, Parekh C, Sahaghian A, Wang W, Ge S, Evseenko D, Wang X, Montelatici E, Lazzari L, Crooks GM, Peault B. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013;121:2891–2901. doi: 10.1182/blood-2012-08-451864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YD, Cheatham B, Storms RW, Gimble JM. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 68.Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, Andreeff M, Marini FC. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7:e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ko SY, Barengo N, Ladanyi A, Lee JS, Marini F, Lengyel E, Naora H. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122:3603–3617. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao M, Sachs PC, Wang X, Dumur CI, Idowu MO, Robila V, Francis MP, Ware J, Beckman M, Rizki A, Holt SE, Elmore LW. Mesenchymal stem cells in mammary adipose tissue stimulate progression of breast cancer resembling the basal-type. Cancer Biol Ther. 2012;13:782–792. doi: 10.4161/cbt.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szebeni GJ, Kriston-Pal E, Blazso P, Katona RL, Novak J, Szabo E, Czibula A, Fajka-Boja R, Hegyi B, Uher F, Krenacs L, Joo G, Monostori E. Identification of galectin-1 as a critical factor in function of mouse mesenchymal stromal cell-mediated tumor promotion. PLoS One. 2012;7:e41372. doi: 10.1371/journal.pone.0041372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, Kang SK, Lee YS, Kang KS. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289–298. 281. doi: 10.1080/14653240902807026. following 298. [DOI] [PubMed] [Google Scholar]
- 73.Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM, Devarajan E, Liu W, Arlinghaus RB, Alt EU. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589–597. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 74.Yan XL, Jia YL, Chen L, Zeng Q, Zhou JN, Fu CJ, Chen HX, Yuan HF, Li ZW, Shi L, Xu YC, Wang JX, Zhang XM, He LJ, Zhai C, Yue W, Pei XT. Hepatocellular carcinoma-associated mesenchymal stem cells promote hepatocarcinoma progression: Role of the S100A4-miR155-SOCS1-MMP9 axis. Hepatology. 2013 doi: 10.1002/hep.26257. [DOI] [PubMed] [Google Scholar]
- 75.Pasquet M, Golzio M, Mery E, Rafii A, Benabbou N, Mirshahi P, Hennebelle I, Bourin P, Allal B, Teissie J, Mirshahi M, Couderc B. Hospicells (ascites-derived stromal cells) promote tumorigenicity and angiogenesis. Int J Cancer. 2010;126:2090–2101. doi: 10.1002/ijc.24886. [DOI] [PubMed] [Google Scholar]
- 76.Arnulf B, Lecourt S, Soulier J, Ternaux B, Lacassagne MN, Crinquette A, Dessoly J, Sciaini AK, Benbunan M, Chomienne C, Fermand JP, Marolleau JP, Larghero J. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia. 2007;21:158–163. doi: 10.1038/sj.leu.2404466. [DOI] [PubMed] [Google Scholar]
- 77.Corre J, Mahtouk K, Attal M, Gadelorge M, Huynh A, Fleury-Cappellesso S, Danho C, Laharrague P, Klein B, Reme T, Bourin P. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21:1079–1088. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garayoa M, Garcia JL, Santamaria C, Garcia-Gomez A, Blanco JF, Pandiella A, Hernandez JM, Sanchez-Guijo FM, del Canizo MC, Gutierrez NC, San Miguel JF. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia. 2009;23:1515–1527. doi: 10.1038/leu.2009.65. [DOI] [PubMed] [Google Scholar]
- 79.Li B, Fu J, Chen P, Zhuang W. Impairment in immunomodulatory function of mesenchymal stem cells from multiple myeloma patients. Archives of medical research. 2010;41:623–633. doi: 10.1016/j.arcmed.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Zhang Z, Yao C. Angiogenic activity of mesenchymal stem cells in multiple myeloma. Cancer Invest. 2011;29:37–41. doi: 10.3109/07357907.2010.496758. [DOI] [PubMed] [Google Scholar]
- 81.Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-beta1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011;266:116–122. doi: 10.1016/j.cellimm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 82.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iivanainen E, Lauttia S, Zhang N, Tvorogov D, Kulmala J, Grenman R, Salven P, Elenius K. The EGFR inhibitor gefitinib suppresses recruitment of pericytes and bone marrow-derived perivascular cells into tumor vessels. Microvasc Res. 2009;78:278–285. doi: 10.1016/j.mvr.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 84.Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner W, Diehlmann A, Saffrich R, Schubert M, Ho AD, Giese N, Buchler MW, Friess H, Buchler P, Herr I. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin SY, Yang J, Everett AD, Clevenger CV, Koneru M, Mishra PJ, Kamen B, Banerjee D, Glod J. The isolation of novel mesenchymal stromal cell chemotactic factors from the conditioned medium of tumor cells. Exp Cell Res. 2008;314:3107–3117. doi: 10.1016/j.yexcr.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin G, Yang R, Banie L, Wang G, Ning H, Li LC, Lue TF, Lin CS. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010 doi: 10.1002/pros.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu WT, Bian ZY, Fan QM, Li G, Tang TT. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009;281:32–41. doi: 10.1016/j.canlet.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 88.Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010;70:10044–10050. doi: 10.1158/0008-5472.CAN-10-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem. 2008;283:19864–19871. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- 90.Donnenberg VS, Donnenberg AD, Zimmerlin L, Landreneau RJ, Bhargava R, Wetzel RA, Basse P, Brufsky AM. Localization of CD44 and CD90 positive cells to the invasive front of breast tumors. Cytometry B Clin Cytom. 2010;78:287–301. doi: 10.1002/cyto.b.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45:872–877. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- 92.Donnenberg V, Zimmerlin L, Donnenberg A. The Role of ABC Transporters in Cancer Stem Cell Drug Resistance. In: Allan AL, editor. Cancer Stem Cells in Solid Tumors. Humana Press; 2011. pp. 361–379. [Google Scholar]
- 93.Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn E, Watnick RS, Straume O, Akslen LA, Folkman J, Almog N. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006;98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- 94.Goodison S, Kawai K, Hihara J, Jiang P, Yang M, Urquidi V, Hoffman RM, Tarin D. Prolonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from nonmetastatic breast tumors as revealed by labeling with green fluorescent protein. Clin Cancer Res. 2003;9:3808–3814. [PubMed] [Google Scholar]
- 95.Suzuki M, Mose ES, Montel V, Tarin D. Dormant cancer cells retrieved from metastasis-free organs regain tumorigenic and metastatic potency. Am J Pathol. 2006;169:673–681. doi: 10.2353/ajpath.2006.060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Almog N, Ma L, Raychowdhury R, Schwager C, Erber R, Short S, Hlatky L, Vajkoczy P, Huber PE, Folkman J, Abdollahi A. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69:836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- 97.Engelmann K, Shen H, Finn OJ. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Research. 2008;68:2419–2426. doi: 10.1158/0008-5472.CAN-07-2249. [DOI] [PubMed] [Google Scholar]
- 98.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donnenberg VS, Luketich JD, Landreneau RJ, DeLoia JA, Basse P, Donnenberg AD. Tumorigenic epithelial stem cells and their normal counterparts. Ernst Schering Found Symp Proc. 2006:245–263. doi: 10.1007/2789_2007_054. [DOI] [PubMed] [Google Scholar]
- 100.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 101.Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, Endoh Y, Okumura C, Okuhara Y, Magae J, Emura M, Ochiya T, Ochiai A. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309:232–240. doi: 10.1016/s0006-291x(03)01544-4. [DOI] [PubMed] [Google Scholar]
- 102.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, Lin RJ, Yang DM, Chang CW, Chen WH, Wei HJ, Gelovani JG. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- 104.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 105.Roorda BD, Elst A, Boer TG, Kamps WA, de Bont ES. Mesenchymal stem cells contribute to tumor cell proliferation by direct cell-cell contact interactions. Cancer Invest. 2010;28:526–534. doi: 10.3109/07357900903179625. [DOI] [PubMed] [Google Scholar]
- 106.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Onoyama M, Ohnishi M, Ohara E, Higashi Y, Tanaka S, Yasui W, Chayama K. Stroma-directed imatinib therapy impairs the tumor-promoting effect of bone marrow-derived mesenchymal stem cells in an orthotopic transplantation model of colon cancer. Int J Cancer. 2013;132:813–823. doi: 10.1002/ijc.27735. [DOI] [PubMed] [Google Scholar]
- 108.Du J, Zhou L, Chen X, Yan S, Ke M, Lu X, Wang Z, Yu W, Xiang AP. IFN-gamma-primed human bone marrow mesenchymal stem cells induce tumor cell apoptosis in vitro via tumor necrosis factor-related apoptosis-inducing ligand. Int J Biochem Cell Biol. 2012;44:1305–1314. doi: 10.1016/j.biocel.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 109.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim SM, Oh JH, Park SA, Ryu CH, Lim JY, Kim DS, Chang JW, Oh W, Jeun SS. Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells. 2010;28:2217–2228. doi: 10.1002/stem.543. [DOI] [PubMed] [Google Scholar]
- 111.Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE, Dzau VJ. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. 2010;106:1753–1762. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72:5198–5208. doi: 10.1158/0008-5472.CAN-12-0294. [DOI] [PubMed] [Google Scholar]
- 113.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21:3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 114.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafe M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, Dontu G, Taichman R, Wicha MS. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS, Groshen SG, Seeger RC, DeClerck YA. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009;69:329–337. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bian ZY, Fan QM, Li G, Xu WT, Tang TT. Human mesenchymal stem cells promote growth of osteosarcoma: involvement of interleukin-6 in the interaction between human mesenchymal stem cells and Saos-2. Cancer Sci. 2010;101:2554–2560. doi: 10.1111/j.1349-7006.2010.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lescarbeau RM, Seib FP, Prewitz M, Werner C, Kaplan DL. In vitro model of metastasis to bone marrow mediates prostate cancer castration resistant growth through paracrine and extracellular matrix factors. PLoS One. 2012;7:e40372. doi: 10.1371/journal.pone.0040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Touboul C, Lis R, Al Farsi H, Raynaud CM, Warfa M, Althawadi H, Mery E, Mirshahi M, Rafii A. Mesenchymal stem cells enhance ovarian cancer cell infiltration through IL6 secretion in an amniochorionic membrane based 3D model. J Transl Med. 2013;11:28. doi: 10.1186/1479-5876-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 122.Pasquet M, Golzio M, Mery E, Rafii A, Benabbou N, Mirshahi P, Hennebelle I, Bourin P, Allal B, Teissie J, Mirshahi M, Couderc B. Hospicells (ascites-derived stromal cells) promote tumorigenicity and angiogenesis. International journal of cancer Journal international du cancer. 2010;126:2090–2101. doi: 10.1002/ijc.24886. [DOI] [PubMed] [Google Scholar]
- 123.Sun B, Zhang S, Ni C, Zhang D, Liu Y, Zhang W, Zhao X, Zhao C, Shi M. Correlation between melanoma angiogenesis and the mesenchymal stem cells and endothelial progenitor cells derived from bone marrow. Stem Cells Dev. 2005;14:292–298. doi: 10.1089/scd.2005.14.292. [DOI] [PubMed] [Google Scholar]
- 124.Zhang K, Shi B, Chen J, Zhang D, Zhu Y, Zhou C, Zhao H, Jiang X, Xu Z. Bone marrow mesenchymal stem cells induce angiogenesis and promote bladder cancer growth in a rabbit model. Urol Int. 2010;84:94–99. doi: 10.1159/000273474. [DOI] [PubMed] [Google Scholar]
- 125.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 126.Jedeszko C, Victor BC, Podgorski I, Sloane BF. Fibroblast hepatocyte growth factor promotes invasion of human mammary ductal carcinoma in situ. Cancer Res. 2009;69:9148–9155. doi: 10.1158/0008-5472.CAN-09-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Edakuni G, Sasatomi E, Satoh T, Tokunaga O, Miyazaki K. Expression of the hepatocyte growth factor/c-Met pathway is increased at the cancer front in breast carcinoma. Pathol Int. 2001;51:172–178. doi: 10.1046/j.1440-1827.2001.01182.x. [DOI] [PubMed] [Google Scholar]
- 128.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, Ochiai A. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276–1284. doi: 10.1002/ijc.24378. [DOI] [PubMed] [Google Scholar]
- 130.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O’Brien T, Kerin MJ. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 131.Neumark E, Sagi-Assif O, Shalmon B, Ben-Baruch A, Witz IP. Progression of mouse mammary tumors: MCP-1-TNFalpha cross-regulatory pathway and clonal expression of promalignancy and antimalignancy factors. Int J Cancer. 2003;106:879–886. doi: 10.1002/ijc.11337. [DOI] [PubMed] [Google Scholar]
- 132.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–3289. [PubMed] [Google Scholar]
- 133.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, Kollias G, Balkwill F. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 134.Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, Egashira K, Mukaida N. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69:7884–7892. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- 135.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–562. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu X, Kang Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem. 2009;284:29087–29096. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yoshimura T, Howard OM, Ito T, Kuwabara M, Matsukawa A, Chen K, Liu Y, Liu M, Oppenheim JJ, Wang JM. Monocyte Chemoattractant Protein-1/CCL2 Produced by Stromal Cells Promotes Lung Metastasis of 4T1 Murine Breast Cancer Cells. PLoS One. 2013;8:e58791. doi: 10.1371/journal.pone.0058791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fridlender ZG, Kapoor V, Buchlis G, Cheng G, Sun J, Wang LC, Singhal S, Snyder LA, Albelda SM. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. American journal of respiratory cell and molecular biology. 2011;44:230–237. doi: 10.1165/rcmb.2010-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, Jorgensen C, Noel D. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595–1603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 140.Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, Peng XH, Chirgwin JM, Guise TA. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aldinucci D, Lorenzon D, Cattaruzza L, Pinto A, Gloghini A, Carbone A, Colombatti A. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int J Cancer. 2008;122:769–776. doi: 10.1002/ijc.23119. [DOI] [PubMed] [Google Scholar]
- 143.Schaffler A, Furst A, Buchler C, Paul G, Rogler G, Scholmerich J, Herfarth H. Secretion of RANTES (CCL5) and interleukin-10 from mesenteric adipose tissue and from creeping fat in Crohn’s disease: regulation by steroid treatment. J Gastroenterol Hepatol. 2006;21:1412–1418. doi: 10.1111/j.1440-1746.2006.04300.x. [DOI] [PubMed] [Google Scholar]
- 144.Pinilla S, Alt E, Abdul Khalek FJ, Jotzu C, Muehlberg F, Beckmann C, Song YH. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett. 2009;284:80–85. doi: 10.1016/j.canlet.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Y, Yao F, Yao X, Yi C, Tan C, Wei L, Sun S. Role of CCL5 in invasion, proliferation and proportion of CD44+/CD24− phenotype of MCF-7 cells and correlation of CCL5 and CCR5 expression with breast cancer progression. Oncol Rep. 2009;21:1113–1121. [PubMed] [Google Scholar]
- 146.Murooka TT, Rahbar R, Fish EN. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem Biophys Res Commun. 2009;387:381–386. doi: 10.1016/j.bbrc.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 147.Park TS, Huo JS, Peters A, Talbot CC, Jr, Verma K, Zimmerlin L, Kaplan IM, Zambidis ET. Growth factor-activated stem cell circuits and stromal signals cooperatively accelerate non-integrated iPSC reprogramming of human myeloid progenitors. PLoS One. 2012;7:e42838. doi: 10.1371/journal.pone.0042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nishi M, Sakai Y, Akutsu H, Nagashima Y, Quinn G, Masui S, Kimura H, Perrem K, Umezawa A, Yamamoto N, Lee SW, Ryo A. Induction of cells with cancer stem cell properties from nontumorigenic human mammary epithelial cells by defined reprogramming factors. Oncogene. 2013 doi: 10.1038/onc.2012.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Eminli S, Foudi A, Stadtfeld M, Maherali N, Ahfeldt T, Mostoslavsky G, Hock H, Hochedlinger K. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nature genetics. 2009;41:968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Donnenberg AD, Hicks JB, Wigler M, Donnenberg VS. The cancer stem cell: cell type or cell state? Cytometry A. 2013;83:5–7. doi: 10.1002/cyto.a.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nie D. Cancer stem cell and niche. Frontiers in bioscience. 2010;2:184–193. doi: 10.2741/s56. [DOI] [PubMed] [Google Scholar]
- 153.Borovski T, De Sousa EMF, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 154.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased Expression of Leptin and the Leptin Receptor as a Marker of Breast Cancer Progression: Possible Role of Obesity-Related Stimuli. Clinical Cancer Research. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 155.Zhang K, Shi B, Chen J, Zhang D, Zhu Y, Zhou C, Zhao H, Jiang X, Xu Z. Bone marrow mesenchymal stem cells induce angiogenesis and promote bladder cancer growth in a rabbit model. Urologia internationalis. 2010;84:94–99. doi: 10.1159/000273474. [DOI] [PubMed] [Google Scholar]
- 156.Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C, Miller N, Hennessy E, Dockery P, Barry FP, O’Brien T, Kerin MJ. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT) Breast Cancer Res Treat. 2010;124:317–326. doi: 10.1007/s10549-010-0734-1. [DOI] [PubMed] [Google Scholar]
- 157.Chao KC, Yang HT, Chen MW. Human umbilical cord mesenchymal stem cells suppress breast cancer tumourigenesis through direct cell-cell contact and internalization. J Cell Mol Med. 2012;16:1803–1815. doi: 10.1111/j.1582-4934.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Galie M, Konstantinidou G, Peroni D, Scambi I, Marchini C, Lisi V, Krampera M, Magnani P, Merigo F, Montani M, Boschi F, Marzola P, Orru R, Farace P, Sbarbati A, Amici A. Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene. 2008;27:2542–2551. doi: 10.1038/sj.onc.1210920. [DOI] [PubMed] [Google Scholar]
- 159.Klopp AH, Lacerda L, Gupta A, Debeb BG, Solley T, Li L, Spaeth E, Xu W, Zhang X, Lewis MT, Reuben JM, Krishnamurthy S, Ferrari M, Gaspar R, Buchholz TA, Cristofanilli M, Marini F, Andreeff M, Woodward WA. Mesenchymal stem cells promote mammosphere formation and decrease E-cadherin in normal and malignant breast cells. PLoS One. 2010;5:e12180. doi: 10.1371/journal.pone.0012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 161.Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, Tanaka S, Yasui W, Chayama K. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer. 2010;127:2323–2333. doi: 10.1002/ijc.25440. [DOI] [PubMed] [Google Scholar]
- 162.Ishii S, Tsuji S, Tsujii M, Kanazawa Y, Nishida T, Iijima H, Yasumaru M, Irie T, Yamamoto K, Tsutsui S, Eguchi H, Kawano S, Hayashi N. Involvement of bone marrow-derived stromal cells in gastrointestinal cancer development and metastasis. J Gastroenterol Hepatol. 2008;23(Suppl 2):S242–249. doi: 10.1111/j.1440-1746.2008.05446.x. [DOI] [PubMed] [Google Scholar]
- 163.Ohlsson LB, Varas L, Kjellman C, Edvardsen K, Lindvall M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol. 2003;75:248–255. doi: 10.1016/j.yexmp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 164.Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L, Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 165.Lu YR, Yuan Y, Wang XJ, Wei LL, Chen YN, Cong C, Li SF, Long D, Tan WD, Mao YQ, Zhang J, Li YP, Cheng JQ. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 2008;7:245–251. doi: 10.4161/cbt.7.2.5296. [DOI] [PubMed] [Google Scholar]
- 166.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 167.Han Z, Tian Z, Lv G, Zhang L, Jiang G, Sun K, Wang C, Bu X, Li R, Shi Y, Wu M, Wei L. Immunosuppressive effect of bone marrow-derived mesenchymal stem cells in inflammatory microenvironment favours the growth of B16 melanoma cells. J Cell Mol Med. 2011;15:2343–2352. doi: 10.1111/j.1582-4934.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, Shi Y, Abbruzzese J, Konopleva M, Andreeff M, Marini FC. Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12:615–625. doi: 10.3109/14653241003631815. [DOI] [PubMed] [Google Scholar]
- 169.Prantl L, Muehlberg F, Navone NM, Song YH, Vykoukal J, Logothetis CJ, Alt EU. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate. 2010;70:1709–1715. doi: 10.1002/pros.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ren C, Kumar S, Chanda D, Kallman L, Chen J, Mountz JD, Ponnazhagan S. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene therapy. 2008;15:1446–1453. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kyriakou CA, Yong KL, Benjamin R, Pizzey A, Dogan A, Singh N, Davidoff AM, Nathwani AC. Human mesenchymal stem cells (hMSCs) expressing truncated soluble vascular endothelial growth factor receptor (tsFlk-1) following lentiviral-mediated gene transfer inhibit growth of Burkitt’s lymphoma in a murine model. The journal of gene medicine. 2006;8:253–264. doi: 10.1002/jgm.840. [DOI] [PubMed] [Google Scholar]
- 172.Secchiero P, Zorzet S, Tripodo C, Corallini F, Melloni E, Caruso L, Bosco R, Ingrao S, Zavan B, Zauli G. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin’s lymphoma xenografts. PLoS One. 2010;5:e11140. doi: 10.1371/journal.pone.0011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]