Introduction
Unintentional electrical excitation of the facial nerve is a well-known complication of using a cochlear implant (CI) to stimulate the cochlear nerve. Facial nerve stimulation (FNS) restricts optimal use of a cochlear implant and often requires reprogramming of the stimulating strategy (1,2). In some cases, FNS results in such severe discomfort or limited stimulation range that the cochlear implant is essentially useless, which leads to explantation and reimplantation (3–5). Facial nerve stimulation includes visible facial spasm as well as milder subjective symptoms of tingling and sometimes facial pain (1,2). Kelsall et al. proposed a grading scale for FNS in which grade I is “no stimulation” and grade VI is “total stimulation” which is defined as “severe gross motion of total facial musculature and/or severe pain” (1). While the reported incidence of FNS varies between 0.9 and 14.9 %(1,4,6–9) in the general population of implanted patients, some etiologies such as temporal bone fracture, cochlear malformation and otosclerosis have been associated with a higher incidence of FNS (4,7–12). The rate of FNS in patients with otosclerosis has been reported as high as 78 % of implanted patients (2,4,13,14).
Several mechanisms have been proposed to explain the higher incidence of FNS in patients with otosclerosis. Otosclerosis is a disorder of bone metabolism affecting the enchondral bone of the otic capsule. The dysplastic bone consists of areas of resorption, vascular proliferation, cavitation and spongiosis, sclerotic bone formation and a connective tissue matrix (15,16). The otospongiosis may reduce the impedance of bone, which may facilitate a shunt of current from the electrode toward the nearby facial nerve (17,18). The positions of intracochlear electrode contacts most likely to excite the facial nerve are typically located in the upper basal turn of cochlea (mid-array contacts). The proximity of this location to the labyrinthine segment of the facial nerve suggests that pathologic involvement of the bone between the labyrinthine segment of the facial nerve and the upper basal turn of the cochlea is important in the pathophysiology of FNS(1,8,14,17). The design of the electrode array has been reported as an additional important variable with FNS being less frequent in implants with a perimodiolar electrode design than with a straight electrode (19–21).
The potential cause of FNS and why some patients with extensive otosclerosis do not have FNS while others do, remains unclear. In this temporal bone study of 11 subjects with otosclerosis who used a cochlear implant in life, we test whether specific pathologic factors are associated with FNS.
Materials and Method
All temporal bones from the collections at the Massachusetts Eye and Ear infirmary (MEEI) and the House Research Institute (HRI) which met the following criteria were included in the study: (1) implantation with a multichannel CI and (2) otosclerosis as the etiology of profound hearing loss. A total of 13 temporal bones from 11 subjects were identified.
The temporal bones were removed after death, fixed in Heidenhain Susa solution or 10% buffered formalin and decalcified in ethylene diamine tetra acetic acid (EDTA), and embedded in celloidin (22). The temporal bones were sectioned at a thickness of 20 μm in the horizontal (axial) plane, and every tenth section was stained with hematoxylin and eosin and mounted on a glass slide. Rosenthal’s canal and the cochlear duct were reconstructed in two dimensions (Figure 1) by a method described by Schuknecht (23) and Otte et al. (24). The tracks of the electrode were marked on the 2-dimensional reconstructions. Depth of insertion and the location of the tip of electrode were determined. Then the position of the electrode contacts, using the morphometric information of the electrodes published by the manufacturers, was plotted on the 2-dimensional reconstructions (Figure 1). All the slides were examined by light microscopy.
Figure 1.
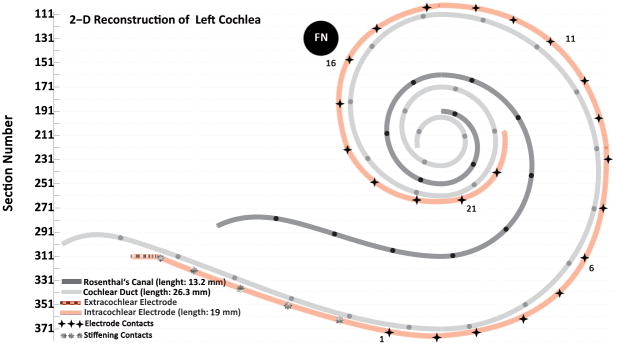
Two-dimensional reconstruction of cochlear duct, Rosenthal’s canal and the electrode track of case 11. The electrode was fully inserted and stimulation of electrode contacts 14–18 caused facial nerve stimulation. The dark stars on the intracochlear electrode track mark the estimated position of the electrode contacts. The facial nerve (FN) was very close to the upper basal turn of cochlea (Figure 4). (The black circles on the line representing Rosenthal’s canal and on the line representing the cochlear duct are 1 mm apart.)
Fisher’s exact test was used to evaluate whether FNS was significantly associated with the various pathologic findings.
Results
A total of 13 temporal bones from 11 implanted patients (8 male and 3 female) with otosclerosis were identified and studied (Table 1). The patients were middle-aged or older, and all were post-lingually deafened recipients of cochlear implants. The preoperative pure tone average in all cases was 90 dB or greater (PTA≥90 dB). The duration of use of the cochlear implant was 2 to 23 years with a mean of 11 years. There were 10 temporal bones with straight electrodes and three with perimodiolar electrodes. Although 4 electrode arrays were not fully inserted, all passed into the upper basal turn of the cochlea. Facial nerve stimulation or its equivalent (severe facial pain in case 6) occurred in 4 out of 13 cases (30.7 %). All patients with FNS had been implanted using straight electrodes (4 out of 10 with straight electrode implants). Three of the 4 had Nucleus® 22 electrode and used a bipolar stimulation strategy, and one had a Nucleus® 24M electrode and used a monopolar stimulation strategy. The number of problematic electrodes in the temporal bones ranged from 2 to 5, and all were among the mid-array electrodes (Table 1) which were located in the upper basal turn of the cochlea based on 2-dimensional reconstruction (Figure 1). In all cases, when the problematic electrodes were switched off, cochlear implant use was not compromised by FNS.
Table 1.
Patients implanted due to profound hearing loss caused by otosclerosis.
| Case | Gender | Age (yrs) | CI usage time (yrs) | Implanted ear | Device | Configuration/Strategy | Electrode shape | FNS | Electrodes causing FNS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 84 | 10 | AS | Ineraid® | MP/CIS | Straight | No | - |
| 2 | M | 77 | 11 | AD | Ineraid® | MP/CIS | Straight | No | - |
| 3 | F | 69 | 4 | AS | Nucleus® 24M | MP/ACE | Straight | Yes | 15–17 |
| 4 | F | 73 | 4 | AS | Nucleus® 24M | MP/ACE | Straight | No | - |
| 5 | F | 73 | 17 | AD | Nucleus® 22 | BP/SPEAK | Straight | No | - |
| 6 | M | 80 | 19 | AD | Nucleus® 22 | BP/SPEAK | Straight | Yes | 12,16 |
| 7 | F | 74 | 8 | AS | HF® II w/p | MP/HiRes | Perimodiolar | No | - |
| 8 | F | 74 | 8 | AD | HF® II w/p | MP/HiRes | Perimodiolar | No | - |
| 9 | M | 83 | 23 | AS | Nucleus® 22 | BP/SPEAK | Straight | Yes | 15–17 |
| 10 | M | 76 | 8 | AS | Nucleus® 24R | MP/ACE | Perimodiolar | No | - |
| 11 | M | 89 | 17 | AS | Nucleus® 22 | BP/SPEAK | Straight | Yes | 14–18 |
| 12 | M | 87 | 3 | AD | Nucleus® 22 | BP/SPEAK | Straight | No | - |
| 13 | M | 74 | 2 | AS | Nucleus® 22 | BP/SPEAK | Straight | No | - |
M: Male; F: Female; AD: Right; AS: Left; HF® II w/p: Hi Focus II with positioner; BP: Bipolar; MP: Monopolar.
All temporal bones had extensive involvement of the otic capsule by otosclerosis. The associated pathologies were grouped into six categories (see Table 2) to facilitate analysis. The criterion defining the first category was any invasion of the cochlear endosteum by otosclerotic bone. In all cases except temporal bones 4 and 5, the endosteum of the cochlea was invaded by otosclerotic bone in at least one turn. Based on the various hypothetical pathophysiologies of FNS, selected regions of the otic capsule were studied in detail to determine any association between pathologic involvement of these foci and FNS. The results of the histopathologic review of these foci are summarized in Table 2.
Table 2.
Structures altered by otosclerosis.
| Case | Categories of Pathology | |||||
|---|---|---|---|---|---|---|
| All bone between UBTC and FNC and endosteum of both UBTC and FNC | Bone adjacent to FNC and FNC endosteum | IAC | Modiolus | RW obliteration | Cochlear endosteum | |
| 1 | − | + | − | − | − | + |
| 2 | − | + | + | − | − | + |
| 3 | + | − | + | − | − | + |
| 4 | − | − | − | − | − | − |
| 5 | − | − | − | − | − | − |
| 6 | + | − | + | + | + | + |
| 7 | + | − | + | − | − | + |
| 8 | + | − | + | − | + | + |
| 9 | + | − | + | + | + | + |
| 10 | − | − | + | − | − | + |
| 11 | + | − | + | + | − | + |
| 12 | − | + | − | − | − | + |
| 13 | − | − | + | + | − | + |
UBTC: upper basal turn of the cochlea; FNC: facial nerve canal; IAC: internal auditory canal; RW: round window
The upper basal turn of the cochlea (UBTC) is closest to the facial nerve canal (FNC) especially its labyrinthine and meatal segments (17), prompting evaluation of the otosclerotic involvement of the bone separating these two structures. In 4 out of 13 temporal bones no otosclerotic changes were seen in this region. Two different patterns of otosclerotic involvement were observed in the remaining 9 bones: (1) invasion of the endosteum of both the UBTC and FNC with complete involvement of the bone between these two structures was observed in 6 temporal bones and (2) partial involvement of the bone between the UBTC and FNC (typically adjacent to facial nerve canal) with invasion of the FNC (but not the UBTC) endosteum was observed in 3 cases. Subjects with no otosclerotic changes in the UBTC region or partial involvement of this region did not experience FNS (see Tables 1 and 2), whereas 4 of 6 with otosclerotic invasion of the UBTC and FC endosteum and complete involvement of the bone between these two structures experienced FNS in life.
Table 2 also summarizes how otosclerotic involvement of the internal auditory canal (IAC), the modiolus and the round window (RW) were distributed across the subjects.
Fisher’s exact test was used to test for associations between the six categories of otosclerotic pathology (Table 2) and FNS (Table 1). As shown in Table 3, we were not able to rule out (p>0.05) a chance association of FNS with five of the six categories of otosclerotic pathology: (1) partial involvement of the bone between the UBTC and FNC and invasion of the FNC endosteum, (2) involvement of the bone of the internal auditory canal, (3) involvement of the bone of the modiolus, (4) obliteration of the round window and (5) involvement of the cochlear endosteum. The only category of pathology that showed significant association with FNS (p=0.005) was complete involvement of the bone between the UBTC and the FNC together with invasion of the endosteum of both the UBTC and of the FNC (Figure 2). Due to an insufficient n, statistical analyses were not applied to the cases with a perimodiolar electrode.
Table 3.
Results of the Fisher’s exact test (by category of pathology) applied to data from otosclerosis subjects implanted with a straight electrode. The p-value is the probability of obtaining the distribution (across subjects) of the pathology shown in Table 2 and the distribution of FNS shown in Table 1 by chance alone if there is no relationship between the pathology and FNS.
| Structures altered by otosclerosis | p-value |
|---|---|
| All bone between UBTC and FNC, and UBTC | .005 |
| endosteum and FNC endosteum | |
| Bone adjacent to FNC and FNC endosteum | .200 |
| IAC | .076 |
| Modiolus | 0.190 |
| RW obliteration | .133 |
| Cochlear endosteum | .197 |
UBTC: Upper basal turn of cochlea; FNC: Facial nerve canal; FNS: Facial nerve stimulation; IAC: Internal auditory canal
Figure 2.
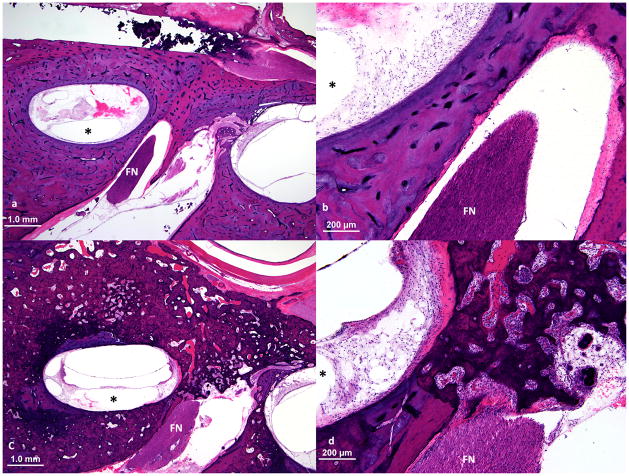
Upper basal turn of cochlea and nearby facial nerve (FN) at the junction of the labyrinthine and the meatal segments of the facial nerve. a, b. Normal bone between upper basal turn of cochlea and the facial nerve. This patient (case 4) did not experience facial nerve stimulation. c, d. This patient (case 9) had facial nerve stimulation and showed full thickness involvement by otosclerosis of the bone between upper basal turn of cochlea and the facial nerve. Although the distance between the facial nerve and upper basal turn of the cochlea was similar to that in case 4, the normal bone was replaced by spongiotic bone with soft tissue deposition and neovascularization. (The electrode track is shown by an asterisk.)
Discussion
The incidence of unintentional electrical stimulation of the facial nerve with a cochlear implant varies between 0.9 and 14.9 % (4,6–12). In this study, the incidence of FNS in the ears with otosclerosis was 30.7 % all of which occurred in 4 out of 10 (40%) cases with a straight electrode. This incidence is significantly higher than the maximum reported incidence of 14.9 % in the general population of implant recipients who were also implanted by straight electrodes (Fisher’s exact test; p = 0.049). Therefore, this study supports previous studies reporting more frequent occurrence of FNS in patients with otosclerosis ranging from 38–78% (2,4,13,14). The problematic electrodes were in the mid-array region ranging from electrode contacts 12 to 18 with the peak incidence at electrode 16 (Figure 3) and consistent with previous publications (1,8,14). In these reports as well as in the current study, it was noted that the problematic electrodes were located in the upper basal turn of cochlea (Figure 1), which is closest to the facial nerve, particularly to its labyrinthine and meatal segments (8,14,17).
Figure 3.
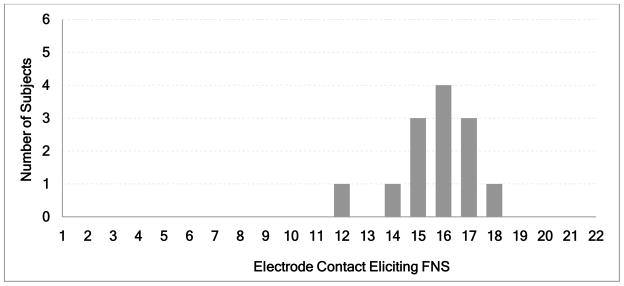
Histogram of number of subjects experiencing facial nerve stimulation (FNS) as a function of the particular electrode being stimulated. The longitudinal cochlear positions of the electrodes producing FNS correspond to the upper basal turn of the cochlea closest to the labyrinthine segment of the facial nerve.
Several theories have been proposed to explain the potential cause of FNS. It has been suggested that bony changes in otosclerosis results in lower impedance and increased conductivity in the cochlear bone which allows more current to be shunted away from the modiolus toward the facial nerve resulting in stimulation of the facial nerve (1,14,18,25,26). Mens, et al. (26) provided quantitative data to support these suggestions by measuring surface potentials (ipsilateral [re-implant] mastoid to contralateral mastoid) for each paired combination of the 22 implanted electrode contacts in 2 subjects with otosclerosis and 14 subjects without. Using relatively simple three-dimensional models of current flow (27) to predict the highly characteristic differences in the patterns of surface potentials between subjects with and without otosclerosis, Mens et al. suggested that the ratio of brain to otic-capsule resistivity is: (1) between 1:1 and 1:10 for the otosclerotic case and (2) approximately 1:100 for subjects without otosclerosis. Whiten (28), using a detailed electroanatomical model of the cochlea, demonstrated that decreasing the resistivity of the endosteal otic-capsule bone from the normal 5000 Ωcm to 600 Ωcm (similar to that measured for cancellous bone(29)) accurately predicted the characteristic patterns of surface potentials that distinguish subjects with otosclerosis from subjects without otosclerosis.
Some researchers have noted FNS caused by electrodes located in the basal segment of the cochlea, very close to the round window (7,30), and suggested the presence of a low impedance pathway at the base of the modiolus as the potential pathology underlying FNS. In the current study, no electrodes near the round window were associated with FNS, nor did we find a significant association between FNS and otosclerotic changes in the modiolus, round window or the internal auditory canal (Table 3).
Based on the higher incidence of FNS associated with mid-array electrodes located in the UBTC and in close proximity to the labyrinthine segment of the facial nerve, it has been also suggested that pathologic changes in the bone between the UBTC and FNC results in more frequent facial nerve stimulation (1,8,9,14,25). Weber et al. suggested that extensive bone resorption may cause the electrode to come into close contact with the facial nerve allowing direct stimulation of the facial nerve(18). Bigelow et al. suggested that physical pressure of a straight electrode at the lateral wall of the cochlea might erode the thin bone of the otic capsule resulting in direct stimulation of the facial nerve (14). In the current study, there was no endosteal erosion due to electrode insertion trauma or physical pressure at the upper basal turn of cochlea. Although to some extent, bone demineralization and resorption occurred in all temporal bones with positive FNS, none of the temporal bones had dehiscence of the facial nerve in the upper basal turn of cochlea.
In the current study, the only positive microscopic finding significantly associated with FNS, was full thickness replacement of normal bone by pathologic bone with spongiosis, neoangiogenesis, cavitation and soft tissue deposition with invasion of the endosteum of both the UBTC and FNC by otosclerosis (Figure 2). All cases with a straight electrode that demonstrated these changes had FNS. A review of photomicrographs published by Marsh et al. (25) in a patient with FNS using a Nucleus® 22 electrode, similar changes can be seen in the same area. There were two temporal bones in the perimodiolar group implanted with the High Focus II electrode (with positioner) that did not demonstrate FNS despite positive bony changes in that area. This finding is consistent with recently published studies demonstrating that cases implanted with perimodiolar electrodes had a significantly lower incidence of FNS (19–21). Perimodiolar electrodes, especially those with a positioner, are less prone to FNS, probably due to the closer proximity of the electrode to the modiolus which in turn reduces the current level needed to reach the same C and T levels (31–33) resulting in a decrease in the shunted current to a level below the threshold of facial nerve stimulation.
In our series as mentioned earlier, although a temporal bone with severe thinning of the bone between UBTC and FNC was seen (Figure 4), there was no temporal bone with facial nerve dehiscence at the UBTC. But as the normal bone is replaced by soft tissue deposition and spongiotic bone with cavitation (Figure 2, 4), it is possible that the intraosseous spaces create micro channels that allow spread of the current through the fluid space between UBTC and FNC. Although CT scan images are not available for comparison with our microscopic analyses to evaluate the extent to which preoperative imaging might be useful, our results suggest such imaging might be useful in recognizing changes in the bone between the UBTC and FNC (14). An example of such a case is illustrated in Figure 5 where there appeared to be a dehiscence of the facial nerve at the upper basal turn of the cochlea which could be explained by a real dehiscence of the facial nerve or by otosclerosis which resulted in thinning of the bone similar to the image illustrated in Figure 4.
Figure 4.
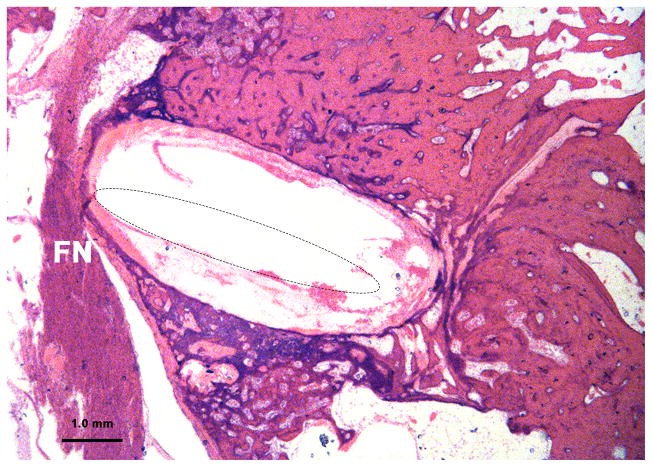
Severe resorption and spongiosis in the bone between the facial nerve and upper basal turn of cochlea due to otosclerosis in case 11. In spite of severe thinning of the bone, there was still an otosclerotic bony septum separating the upper basal turn of cochlea from the facial nerve (FN). In this case the facial nerve was stimulated unintentionally by intracochlear stimulation. (The electrode was removed prior to sectioning the temporal bone. The location of the electrode track is shown by dotted line inside the cochlea.)
Figure 5.
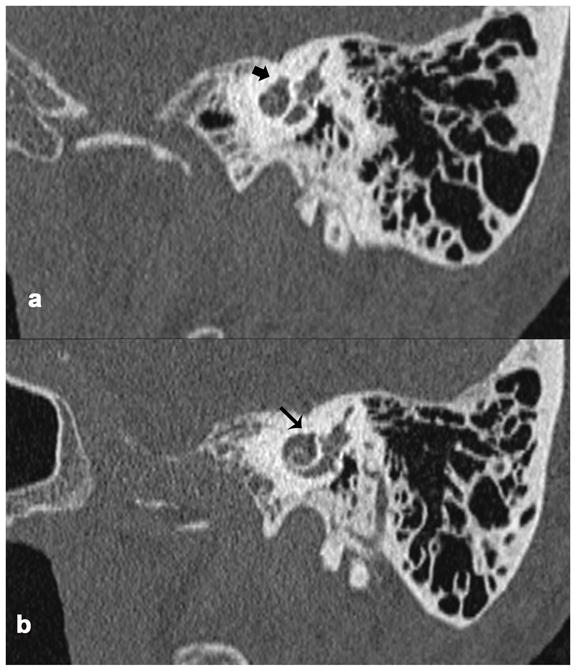
Stenvers view of the temporal bone in CT scans of a living patient with FNS and otosclerosis. a. Involvement of the otic capsule by otosclerosis (thick black arrow). b. There was no bone separating the upper basal turn of cochlea and the facial nerve canal (thin black arrow) due to otosclerosis, suggesting the presence of dehiscence of the facial nerve.
Conclusion
Patients with otosclerosis should be counseled concerning the higher incidence of facial nerve stimulation and that the use of a perimodiolar electrode is recommended especially in cases in which the preoperative CT scan suggests dysplastic bone between the facial nerve canal and the upper basal turn of cochlea. In addition, in cases with otosclerosis and bilateral profound hearing loss, cochlear implantation is recommended in the ear with less dysplastic changes in bone between the facial nerve canal and the upper basal turn of the cochlea as seen in preoperative CT scan imaging, when the candidacy of the two sides is otherwise equal.
Acknowledgments
This work was supported by grant R01-DC000152 from the National Institute of Deafness and Other Communication Disorders.
References
- 1.Kelsall DC, Shallop JK, Brammeier TG, et al. Facial nerve stimulation after Nucleus 22-channel cochlear implantation. Am J Otol. 1997;18:336–41. [PubMed] [Google Scholar]
- 2.Shea JJ, 3rd, Domico EH. Facial nerve stimulation after successful multichannel cochlear implantation. Am J Otol. 1994;15:752–6. [PubMed] [Google Scholar]
- 3.Battmer R, Pesch J, Stover T, et al. Elimination of facial nerve stimulation by reimplantation in cochlear implant subjects. Otol Neurotol. 2006;27:918–22. doi: 10.1097/01.mao.0000235374.85739.c6. [DOI] [PubMed] [Google Scholar]
- 4.Rayner MG, King T, Djalilian HR, et al. Resolution of facial stimulation in otosclerotic cochlear implants. Otolaryngol Head Neck Surg. 2003;129:475–80. doi: 10.1016/S0194-59980301444-X. [DOI] [PubMed] [Google Scholar]
- 5.Polak M, Ulubil SA, Hodges AV, et al. Revision cochlear implantation for facial nerve stimulation in otosclerosis. Arch Otolaryngol Head Neck Surg. 2006;132:398–404. doi: 10.1001/archotol.132.4.398. [DOI] [PubMed] [Google Scholar]
- 6.Cohen NL, Hoffman RA, Stroschein M. Medical or surgical complications related to the Nucleus multichannel cochlear implant. Ann Otol Rhinol Laryngol Suppl. 1988;135:8–13. doi: 10.1177/00034894880975s202. [DOI] [PubMed] [Google Scholar]
- 7.Niparko JK, Oviatt DL, Coker NJ, et al. Facial nerve stimulation with cochlear implantation. VA Cooperative Study Group on Cochlear Implantation. Otolaryngol Head Neck Surg. 1991;104:826–30. doi: 10.1177/019459989110400610. [DOI] [PubMed] [Google Scholar]
- 8.Smullen JL, Polak M, Hodges AV, et al. Facial nerve stimulation after cochlear implantation. Laryngoscope. 2005;115:977–82. doi: 10.1097/01.MLG.0000163100.37713.C6. [DOI] [PubMed] [Google Scholar]
- 9.Berrettini S, Vito de A, Bruschini L, et al. Facial nerve stimulation after cochlear implantation: our experience. Acta Otorhinolaryngol Ital. 2011;31:11–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Mylanus EA, Rotteveel LJ, Leeuw RL. Congenital malformation of the inner ear and pediatric cochlear implantation. Otol Neurotol. 2004;25:308–17. doi: 10.1097/00129492-200405000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Cushing SL, Papsin BC, Gordon KA. Incidence and characteristics of facial nerve stimulation in children with cochlear implants. The Laryngoscope. 2006;116:1787–91. doi: 10.1097/01.mlg.0000231303.85828.20. [DOI] [PubMed] [Google Scholar]
- 12.Papsin BC. Cochlear implantation in children with anomalous cochleovestibular anatomy. The Laryngoscope. 2005;115:1–26. doi: 10.1097/00005537-200501001-00001. [DOI] [PubMed] [Google Scholar]
- 13.Rotteveel LJ, Proops DW, Ramsden RT, et al. Cochlear implantation in 53 patients with otosclerosis: demographics, computed tomographic scanning, surgery, and complications. Otol Neurotol. 2004;25:943–52. doi: 10.1097/00129492-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Bigelow DC, Kay DJ, Rafter KO, et al. Facial nerve stimulation from cochlear implants. Am J Otol. 1998;19:163–9. [PubMed] [Google Scholar]
- 15.Schuknecht HF, Barber W. Histologic variants in otosclerosis. The Laryngoscope. 1985;95:1307–17. doi: 10.1288/00005537-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Schuknecht HF, Pote Merchant SN, Nadol JB. Schuknecht’s pathology of the ear. New York: McGraw-Hill Medical; 2010. pp. 716–37. [Google Scholar]
- 17.Kruschinski C, Weber BP, Pabst R. Clinical relevance of the distance between the cochlea and the facial nerve in cochlear implantation. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2003;24:823–7. doi: 10.1097/00129492-200309000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Weber BP, Lenarz T, Battmer RD, et al. Otosclerosis and facial nerve stimulation. The Annals of otology, rhinology & laryngology Supplement. 1995;166:445–7. [PubMed] [Google Scholar]
- 19.Semaan MT, Gehani NC, Tummala N, et al. Cochlear implantation outcomes in patients with far advanced otosclerosis. Am J Otolaryngol. 2012;33:608–14. doi: 10.1016/j.amjoto.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Marshall AH, Fanning N, Symons S, et al. Cochlear implantation in cochlear otosclerosis. Laryngoscope. 2005;115:1728–33. doi: 10.1097/01.mlg.0000171052.34196.ef. [DOI] [PubMed] [Google Scholar]
- 21.Matterson AG, O’Leary S, Pinder D, et al. Otosclerosis: selection of ear for cochlear implantation. Otol Neurotol. 2007;28:438–46. doi: 10.1097/MAO.0b013e31803115eb. [DOI] [PubMed] [Google Scholar]
- 22.Schuknecht H. Temporal bone removal at autopsy. Preparation and uses. Arch Otolaryngol. 1968;87:129–37. doi: 10.1001/archotol.1968.00760060131007. [DOI] [PubMed] [Google Scholar]
- 23.Schuknecht HF. Techniques for study of cochlear function and pathology in experimental animals; development of the anatomical frequency scale for the cat. AMA Arch Otolaryngol. 1953;58:377–97. doi: 10.1001/archotol.1953.00710040399001. [DOI] [PubMed] [Google Scholar]
- 24.Otte J, Schunknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope. 1978;88:1231–46. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Marsh MA, Coker NJ, Jenkins HA. Temporal bone histopathology of a patient with a nucleus 22-channel cochlear implant. Am J Otol. 1992;13:241–8. [PubMed] [Google Scholar]
- 26.Mens LH, Oostendorp T, van den Broek P. Cochlear implant generated surface potentials: current spread and side effects. Ear Hear. 1994;15:339–45. doi: 10.1097/00003446-199408000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Mens LH, Huiskamp G, Oostendorp T, et al. Modelling surface potentials from intracochlear electrical stimulation. Scand Audiol. 1999;28:249–55. doi: 10.1080/010503999424680. [DOI] [PubMed] [Google Scholar]
- 28.Whiten DM. Division of Health Sciences and Technology. Cambridge: Massachusetts Institute of Technology; 2007. Electroanatomical models of the cochlear implant; pp. 148–9. (Available at: https://myfiles.meei.harvard.edu/xythoswfs/webui/_xy-217117_1-t_B7VVmtrc. [Google Scholar]
- 29.Saha S, Williams PA. Comparison of the electrical and dielectric behavior of wet human cortical and cancellous bone tissue from the distal tibia. J Orthop Res. 1995;13:524–32. doi: 10.1002/jor.1100130407. [DOI] [PubMed] [Google Scholar]
- 30.Bredberg G, Lindstrom B. Insertion length of electrode array and its relation to speech communication performance and nonauditory side effects in multichannel-implanted patients. The Annals of otology, rhinology & laryngology Supplement. 1995;166:256–8. [PubMed] [Google Scholar]
- 31.Donaldson GS, Peters MD, Ellis MR, et al. Effects of the Clarion Electrode Positioning System on auditory thresholds and comfortable loudness levels in pediatric patients with cochlear implants. Arch Otolaryngol Head Neck Surg. 2001;127:956–60. doi: 10.1001/archotol.127.8.956. [DOI] [PubMed] [Google Scholar]
- 32.Parkinson AJ, Arcaroli J, Staller SJ, et al. The nucleus 24 contour cochlear implant system: adult clinical trial results. Ear Hear. 2002;23:41S–8S. doi: 10.1097/00003446-200202001-00005. [DOI] [PubMed] [Google Scholar]
- 33.Young NM, Grohne KM. Comparison of pediatric Clarion recipients with and without the electrode positioner. Otol Neurotol. 2001;22:195–9. doi: 10.1097/00129492-200103000-00013. [DOI] [PubMed] [Google Scholar]