Abstract
The stress-response corticotropin-releasing factor (CRF) and dynorphin systems are critically involved in alcohol drinking and “anxiety” ”-related behaviors. Selectively bred Sardinian alcohol-preferring (sP) rats display high inherent “anxiety”-related behaviors, in comparison with their alcohol-non preferring counterpart (sNP rats). The present study was undertaken to investigate: (1) if there were genetically determined differences in basal gene expression levels of CRF, CRF-R1, preprodynorphin (ppDyn) and kappa opioid receptor (KOP-r) between sP and sNP rats; specifically, mRNA levels of the above genes were measured in the central amygdala (CeA), hypothalamus and other stress responsive and mesolimbic regions of alcohol-naive sP and sNP rats; and (2) if the above mRNA levels were altered by voluntary alcohol drinking in sP rats exposed to the standard, homecage 2-bottle “alcohol vs water” choice regimen 24 hours/day for 17 days. Higher basal CRF mRNA levels were found only in CeA of alcohol-naive sP rats, compared with sNP rats; these levels were decreased after alcohol consumption. In contrast, ppDyn mRNA levels in CeA of sP rats were increased by alcohol consumption, but with no basal difference from sNP rats. Although higher basal ppDyn mRNA levels were found in hypothalamus of sP rats, compared with sNP rats, there was no alteration after alcohol drinking in sP rats. No difference for the above mRNA levels was observed in other regions, including nucleus accumbens shell or core, caudate-putamen, ventral tegmental area and medial/basolateral amygdala, between the two rat lines before or after alcohol consumption. Our results demonstrate the existence of genetically determined high basal CRF mRNA levels in CeA of sP rats. Alcohol consumption decreased CeA CRF mRNA levels with parallel increases in CeA ppDyn mRNA levels.
Keywords: corticotropin-releasing factor, preprodynorphin, central amygdala, alcohol drinking, Sardinian alcohol-preferring (sP) and -non preferring (sNP) rats, gene expression
Introduction
There is ample evidence that increased corticotropin-releasing factor (CRF) neuronal activity may represent an important step in the neurobiology of stress-related (“anxiety”-related and “depression”-like) behaviors in several rodent models. Chronic alcohol studies have demonstrated that the CRF/CRF-R1 system in the central amygdala (CeA) is involved in excessive alcohol consumption and alcohol seeking by alcohol-dependent rats [1]. CRF is expressed in the GABAergic neurons of the CeA [2], and acute alcohol administration results in an increase in the extracellular CRF levels in the CeA [3].
Alcohol consumption affects multiple neurobiological systems including the endogenous opioid systems. Specifically, activation of the dynorphin/kappa opioid receptor (KOP-r) system has been implicated in the negative reinforcement aspects of alcohol, opiate and psychostimulant addictions [4, 5]. In rats, acute administration of KOP-r agonists attenuates alcohol self-administration [6] and decreases alcohol-induced conditioned place preference [7], while selective KOP-r antagonist nor-BNI causes an increase in alcohol drinking in rats with high basal levels of alcohol consumption [8]. Recently KOP-r antagonists have also been reported to attenuate alcohol-seeking behavior induced by stress in mice [10] and to reduce alcohol consumption in alcohol-dependent rats [11]. These findings provide support for the importance of the dynorphin/KOP-r system in the process of alcohol consumption and addiction. Furthermore, microdialysis studies have found that acute alcohol administration increases the extracellular dynorphin A1–8 concentrations in the CeA and nucleus accumbens (NAc), two brain regions known to be involved in the regulation of alcohol consumption [3, 9]. Dynorphin is co-expressed with CRF in the same CeA GABAergic neurons [2], strongly suggesting a potential interaction between these two neuropeptide systems in the CeA.
Sardinian alcohol-preferring (sP) and -non preferring (sNP) rats constitute one of the few pairs of rat lines selectively bred worldwide for opposite alcohol preference and consumption [see 12, 13]. Besides differences in alcohol drinking and several other alcohol-related behaviors, sP rats display more inherent “anxiety”-related behaviors than sNP rats [14–17]. Therefore, our first research question was whether there was a genetically determined difference in CRF/CRF-R1 or dynorphin/KOP-r systems between sP and sNP rats, as reflected by basal gene expression levels of CRF, CRF-R1 receptor, ppDyn or KOP-r in different “anxiety”-related brain regions (e.g., CeA, medial/basolateral amygdala [Me/BLA] or hypothalamus). The second question of this study was to examine whether 17-day exposure to alcohol drinking altered these gene expression levels in sP rats. To answer this question, we assessed the effect of alcohol drinking on the above mRNA levels in sP rats exposed to the standard, homecage 2-bottle “alcohol (10%, v/v) vs. water” choice regimen with unlimited access for 24 hours/day and 17 consecutive days. Immediately (30 min) following the last day of drinking, specific brain regions were collected, and mRNA levels were quantified in the CeA, Me/BLA or hypothalamus, as well as several mesolimbic regions (including the NAc core and shell, caudate-putamen [CPu] and ventral tegmental area [VTA]).
Materials and Methods
1. Animals and alcohol drinking procedure
Male sP and sNP rats from the 67th generation, approximately 75 days old at the start of each study, were used. The animal facility was under an inverted 12:12 hour light-dark cycle (lights on at 09:00 pm), at a constant temperature of 22 ± 2°C and relative humidity of approximately 60%. Starting from the age of 60 days, all rats were individually housed in standard plastic cages with wood chip bedding. Standard rat chow (Mucedola, Settimo Milanese, Italy) was always available. All experimental procedures employed in the present study were in accordance with the Principles of Laboratory Animal Care (NIH Publication No 86-23, 1996), the European Communities Council Directive (86/609/EEC), and the subsequent Italian Law on the “Protection of animals used for experimental and other scientific reasons.” During all experimental procedures, the number of animals and their potential suffering were minimized.
sP rats were exposed to 10% (v/v) alcohol and water under the standard, homecage 2-bottle “alcohol vs. water” choice regimen, with unlimited access for 24 hours/day, for 17 consecutive days [12]. Bottles were refilled every day with fresh solution or water and their left-right positions interchanged at random to avoid development of position preference. Alcohol, water, and food intake were monitored by weighing both bottles and food pellets (0.1-g accuracy) once daily immediately before the start of the dark phase. Body weight was recorded once every other day. To summarize, there were three treatment groups (n=8 for each): alcohol-naive sP and sNP rats, exposed to 2-bottle “water vs. water” regimen; and alcohol-experienced sP rats, exposed to the above-mentioned 2-bottle choice regimen. All the animals in these three groups received the same procedures (including handling, weighing, etc.). All 8 sP rats exposed to the 2-bottle “alcohol vs. water” choice regimen rapidly acquired alcohol-drinking behavior, as indicated by daily alcohol intakes higher than 4 g/kg (i.e., the selection criterion adopted in the breeding program of sP rats [12]) by day 3 in each rat; subsequently, daily alcohol intake rose progressively, averaging approximately 6.5 g/kg/day throughout the 17-day period of exposure. These data were similar to those repeatedly recorded in sP rats exposed to alcohol and water under the 2-bottle choice regimen [12]. On day 18, animals were sacrificed 30 min after removal of alcohol and/or water bottles and 3 hours after lights on.
2. Rat brain dissection and preparation of RNA extracts
See SI Methods section.
3. Solution hybridization ribonuclease protection-trichloroacetic acid precipitation
The solution hybridization RNase protection-TCA precipitation protocol has been described in detail in an earlier report [18]. See SI Methods section.
4. Statistical Analyses
Group differences in mRNA levels were analyzed using one-way ANOVA for all 3 rat groups, followed by Newman-Keuls post hoc tests or Student’s t-tests when appropriate. To determine correlation between measured variables, linear regression analysis was performed. The accepted level of significance for all tests was p < 0.05. All statistical analyses were performed using Statistica (version 5.5, StatSoft Inc.).
Results
1. Genetically determined differences and effects of alcohol drinking on CRF and CRF-R1 gene expression levels
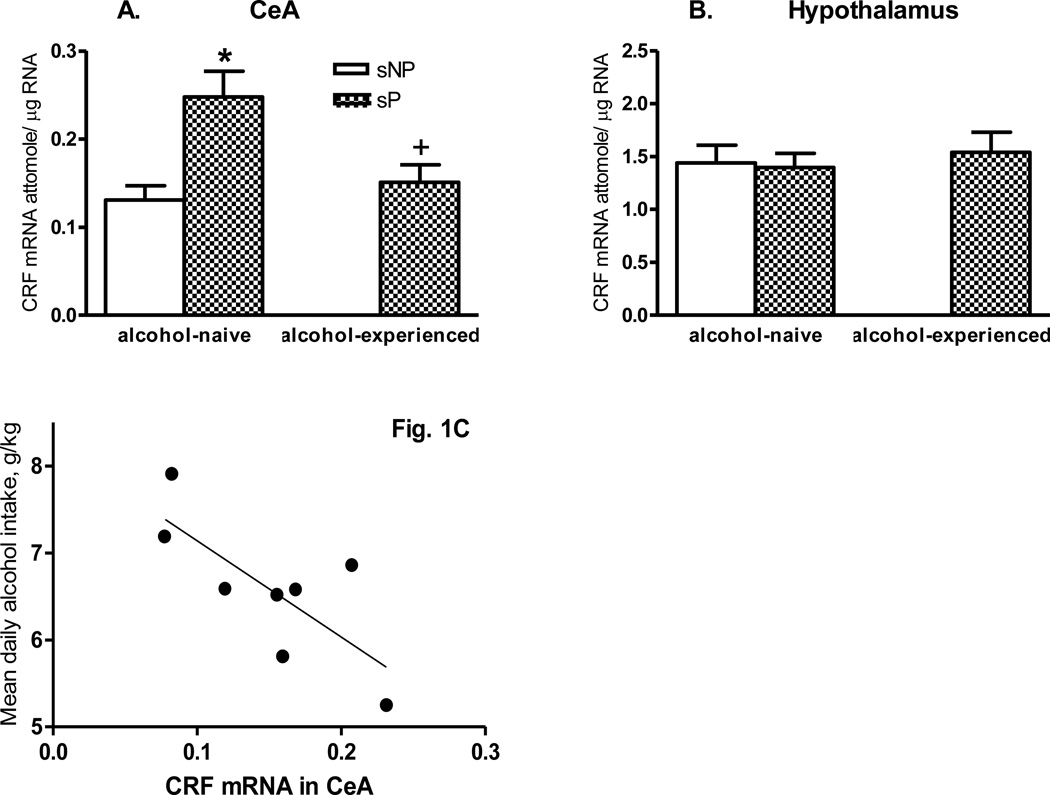
In the CeA (Fig. 1A), one-way ANOVA showed significant effects for Group (F(2,21)=5.28, p<0.05). There were significantly higher basal levels of CRF mRNA in CeA of alcohol-naive sP rats, compared with sNP rats (Newman-Keuls post hoc test, p<0.05) (t=3.34, df = 14, p<0.005). CRF mRNA levels were significantly decreased after alcohol consumption in sP rats (Newman-Keuls post hoc test, p<0.05) (t=2.26, df = 14, p<0.05). Also, sP rats after alcohol consumption displayed a significant negative correlation between CeA CRF mRNA levels and mean daily alcohol intake (p < 0.05) (Fig. 1C).
Fig. 1.
Genetically determined differences between selectively bred, Sardinian alcohol-preferring (sP) and -non preferring (sNP) rats and effects of voluntary alcohol drinking on CRF mRNA levels (attomole/µg total RNA) in the central amygdala (CeA) (A) and hypothalamus (B). sP and sNP rats were offered water as the sole fluid available (alcohol-naive sP and sNP rats) or sP rats were offered a free choice between 10% (v/v) alcohol and water for 17 consecutive days (alcohol-experienced sP rats). Rat line differences: * p<0.05; Alcohol exposure differences: + p<0.05, n=7–8. Note, different axes showing relative abundance of CRF mRNA levels in those regions. (C) Regression of CRF mRNA levels in the central amygdala (CeA) and voluntary alcohol consumption in Sardinian alcohol-preferring (sP) rats. There was a significant negative correlation between the levels of CeA CRF mRNA and mean daily alcohol intake for individual sP rats offered 10% (v/v) alcohol for 17 consecutive days (r = −0.756, d.f. = 8, p<0.05).
There was very low expression of the CRF mRNA levels in the Me/BLA of both sP and sNP rats. A pilot study conducted with two alcohol-naive rats of each line did not show significant difference in CRF mRNA levels in the Me/BLA (data not shown). Therefore, we did not further determine the effects of alcohol drinking in this brain region.
In the hypothalamus, no significant group differences were found between alcohol-naive sP and sNP rats or between alcohol-naive and -experienced sP rats (Fig. 1B).
Analysis of CRF-R1 mRNA levels in the same groups did not identify significant group differences in the CeA, hypothalamus or VTA (See SI Results section Table S1).
2. Genetically determined differences and effects of alcohol drinking on ppDyn and KOP-r gene expression levels
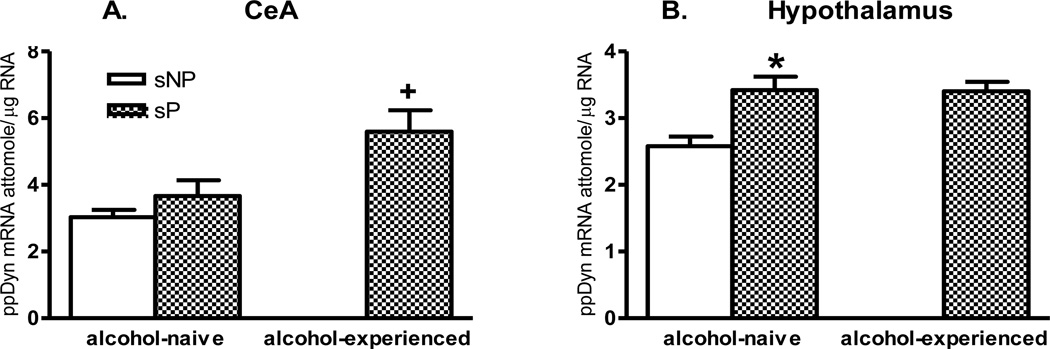
In the CeA (Fig. 2A), one-way ANOVA showed significant effects for Group (F(2,20)=7.25, p<0.005). There was no difference in basal ppDyn mRNA levels in the CeA between alcohol-naive sP and sNP rats. However, ppDyn mRNA levels were significantly increased after alcohol consumption in sP rats (Newman-Keuls post hoc test, p<0.05) (t=2.42, df = 14, p<0.05). There was no significant correlation between CeA ppDyn mRNA levels and mean daily alcohol intake in sP rats.
Fig. 2.
Genetically determined differences between selectively bred, Sardinian alcohol-preferring (sP) and -non preferring (sNP) rats and effects of voluntary alcohol drinking on preprodynorphin (ppDyn) mRNA levels (attomole/µg total RNA) in the central amygdala (CeA) (A) and hypothalamus (B). sP and sNP rats were offered water as the sole fluid available (alcohol-naive sP and sNP rats) or sP rats were offered a free choice between 10% (v/v) alcohol and water for 17 consecutive days (alcohol-experienced sP rats). Rat line differences: * p<0.05; Alcohol exposure differences: + p<0.05, n=7–8. Note, different axes showing relative abundance of ppDyn mRNA levels in those regions.
In the hypothalamus (Fig. 2B), one-way ANOVA showed significant effects for Group (F(2,21)=8.29, p<0.005. There were significantly higher basal levels of ppDyn mRNA in the hypothalamus of alcohol-naive sP rats, compared with sNP rats (Newman-Keuls post hoc test, p<0.005) (t=3.47, df = 14, p<0.005). The ppDyn mRNA levels were not altered after 17-day alcohol drinking in sP rats.
In the NAc shell, core, CPu or Me/BLA, no significant group differences were found for ppDyn mRNA levels between alcohol-naive sP and sNP rats or between alcohol-naive and - experienced sP rats (See SI Results section Table S2A).
For KOP-r mRNA levels, no significant group differences were found in the NAc shell, core or Me/BLA (See SI Results section Table S2B).
Discussion
1. CRF in the CeA
Previous lines of experimental evidence have suggested sP rats as an animal model of genetically determined “anxiety”. Indeed, alcohol-naive sP rats displayed more “anxiety”-related behaviors than alcohol-naive sNP rats when tested on the elevated plus maze [14, 15], elevated zero maze [17], and open field [16]. In the present study, our first objective was to examine whether there were genetically determined differences in gene expression levels between sP and sNP rats in the CeA or hypothalamus, two brain regions known to play important roles in “anxiety”-related and/or “depression”-like behaviors. We found sP rats had higher basal CRF mRNA levels in the CeA, compared with sNP rats. This is consistent with the results of a previous study [15] demonstrating higher basal extracellular CRF levels in CeA of alcohol-naive sP rats, compared with sNP rats. Our results may suggest a role for high basal CRF gene expression (probably a higher CRF biosynthesis rate with resultant higher peptide levels) in the genetically determined tendency of sP rats towards “anxiety”-related and “depression”-like states. These emotional states, and the search for the anxiolytic and antidepressant-like effects of alcohol, might in turn contribute to promoting the high levels of alcohol consumption and preference that characterize sP rats.
Voluntary alcohol intake reduces “anxiety”-related [14] and “depression”-like [19] behaviors in sP rats; these data suggest that sP rats may consume alcohol, at least in part, to ameliorate their high negative emotional states. To investigate whether voluntary alcohol drinking could alter CRF gene expression, we measured CRF mRNA levels in the CeA of sP rats continuously exposed to voluntary alcohol drinking. We found that 17-day consumption of relatively high amounts of alcohol (~ 6.5 g/kg/day) by sP rats was associated with decreased CRF mRNA levels in the CeA. This effect seemed to be gene-specific (no effect on CRF-R1 mRNA levels in the CeA) and region-specific (no effect on CRF mRNA levels in the hypothalamus). Reduced CRF peptide levels might be expected as a result of decreased CRF gene expression, and thus this lowered CRF may be partially responsible for the anxiolytic action of alcohol in sP rats. In support of this concept, correlation analysis between mean daily alcohol intake over the entire 17-day period and CeA CRF mRNA levels revealed that sP rats consuming relatively more alcohol showed relative lowered CeA CRF mRNA levels. In this study, however, we did not measure CRF peptide levels, and thus our findings are limited to gene expression and should be interpreted with caution.
Because increases of CRF neuronal activity are likely involved in “anxiety”-related behaviors, both previous study [15] and our results in the present study suggest that the high baseline CeA CRF expression may contribute to the “anxiety”-like emotional state of sP rats. However, lower basal CRF was found in the CeA of another line of rats selectively bred for high alcohol preference and consumption: Indiana alcohol-preferring P rats, when compared with their counterparts, alcohol-nonpreferring NP rats [20]. Together, this suggests that there are other CeA factors involved in the “anxiety”-like behaviors, like the dynorphin/KOP-r system and its potential interactions with the CRF/CRF-R1 system.
Upon alcohol withdrawal, rats and mice show increased alcohol intake, which is accompanied with increased CRF release in the CeA [21]. The enhanced CRF release and activity in the CeA after short-term withdrawal from chronic alcohol exposure could be a rebound after a decrease by chronic alcohol exposure as found by Karanikas et al [22] and present study. In parallel, short-term alcohol withdrawal increased “anxiety”-like behavior as measured by an elevated plus maze [23–25]. Also, alcohol withdrawal in rats has been associated with increased CeA CRF-R1 expression [26] and GABAergic neurotransmission [27]. CRF-R1 antagonism in the CeA abolished dependence-induced increases in GABAergic neurotransmission and alcohol intake [27]. Furthermore, it has been reported that Marchigian-Sardinian alcohol-preferring rats (a rat line derived from an early generation of sP rats) had a genetically determined up-regulation of the CRF-R1 expression in the CeA, and alcohol consumption down-regulated the CRF-R1 expression [28].
2. Dynorphin in the CeA
It was reported that ppDyn mRNA levels in the CeA were increased after 22-day voluntary alcohol drinking at 3 g/kg daily dose [29], or after acute (1-day) withdrawal from 5-day, multiple alcohol “binge” administrations (4.5 g/kg/day) [30] in Sprague-Dawley rats. The CeA is a critical brain region mediating anxiety-related behavior, and is a likely site for the interaction of the dynorphin and alcohol, although few studies have explored this interaction. The present study provides further evidence for the involvement of the CeA ppDyn system in alcohol drinking. In fact, in sP rats after alcohol drinking with large amount of consumption, there was an increase in ppDyn mRNA levels in the CeA, but not in any mesolimbic regions examined (NAc shell, core, and CPu). Therefore, high levels of alcohol consumption in sP rats activated the dynorphin/KOP-r system involved in the neuronal structure related to alcohol craving and stress responsivity (i.e., CeA), but not alcohol reward (i.e., NAc). This CeA ppDyn mRNA increase may be involved in the homeostatic adaptations of the brain after chronic alcohol exposure. In support of this concept, it is found that mice lacking dynorphin consume more alcohol, with hyper-responsivity to stress [31]. Pharmacological studies also show supportive results: the selective KOP-r antagonist nor-binaltorphimine enhances voluntary alcohol consumption in a continuous two-bottle choice paradigm, and, of particular interest, this effect occurs in rats with high, but not low, levels of alcohol consumption [8]. Most recent work has demonstrated that KOP-r inhibits GABAergic synaptic responses and aolcohl effects in the CeA [32] and bed nucleus of the stria terminalis [33], which may interact with CRF effects on GABA release.
3. Summary
Our results demonstrate the existence of genetically determined high basal levels of CRF gene expression in the CeA of selectively bred alcohol-preferring sP rats, in comparison with their alcohol-non preferring counterpart (sNP rats). Here, we further investigated the effect on CRF and ppDyn gene expression in the CeA of sP rats after voluntary alcohol drinking, as both the CRF and dynorphin/KOP-r systems are modulators of alcohol consumption. A significant decrease in CRF expression levels was associated with an increase in ppDyn expression levels in the CeA of sP rats after alcohol consumption. Because reduction of CRF neuronal activity is likely involved in anxiolytic and anti-depressive actions of alcohol, we suggest that the observed decrease in CeA CRF gene expression may contribute to the anxiolytic effect of alcohol through the CeA CRF system. This dynorphin adaptive change may be involved in the homeostatic adaptations of the brain after chronic alcohol exposure.
Supplementary Material
Highlights.
Effects of voluntary alcohol drinking on CRF and ppDyn gene expression;
Genetically determined high basal CRF mRNA levels in CeA of sP rats;
Alcohol drinking decreased CRF, but increased ppDyn mRNA levels in CeA.
Acknowledgements
The authors would like to thank Mrs. Carla Acciaro for rat breeding. The work was supported by NIH-NIDA Center Grant DA-P50-05130 (M.J.K.), the Adelson Medical Research Foundation (MJK) and CNR grant to Commessa “Neurobiologia dell’alcolismo” (G.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotrophin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchant NJ, Densmore VS, Osborne PB. Coexpression of prodynorphin and corticotropin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J. Comp. Neurol. 2007;504:702–715. doi: 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- 3.Lam MP, Gianoulakis C. Effects of corticotropin-releasing hormone receptor antagonists on the ethanol-induced increase of dynorphin A1–8 release in the rat central amygdala. Alcohol. 2011;45:621–630. doi: 10.1016/j.alcohol.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindholm S, Werme M, Brene S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav. Brain Res. 2001;120:137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- 7.Logrip ML, Janak PH, Ron D. Blockade of ethanol reward by the kappa opioid receptor agonist U50,488. Alcohol. 2009;43:359–365. doi: 10.1016/j.alcohol.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182:384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- 9.Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol. Clin. Exp. Res. 2006;30:982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- 10.Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- 11.Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo G, Lobina C, Carai MAM, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non preferring (sNP) rats. Addict. Biol. 2006;11:324–338. doi: 10.1111/j.1369-1600.2006.00031.x. (2006) [DOI] [PubMed] [Google Scholar]
- 13.Bell RL, Sable HJK, Colombo G, Hyytiä P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol. Biochem. Behav. 2012;103:119–155. doi: 10.1016/j.pbb.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, Gessa G. Sardinian alcohol-preferring rats: A genetic animal model of anxiety. Physiol. Behav. 1995;57:1181–1185. doi: 10.1016/0031-9384(94)00382-f. (1995) [DOI] [PubMed] [Google Scholar]
- 15.Richter RM, Zorrilla EP, Basso AM, Koob GF, Weiss F. Altered amygdalar CRF release and increased anxiety-like behavior in Sardinian alcohol-preferring rats: a microdialysis and behavioral study. Alcohol. Clin. Exp. Res. 2000;24:1765–1772. [PubMed] [Google Scholar]
- 16.Agabio R, Carai MAM, Lobina C, Pani M, Reali R, Vacca G, Gessa G, Colombo G G. Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol. 2001;23:123–126. doi: 10.1016/s0741-8329(00)00144-0. [DOI] [PubMed] [Google Scholar]
- 17.Cagiano R, Cassano T, Coluccia A, Gaetani S, Giustino A, Steardo L, Tattoli M, Trabace L, Cuomo V. Genetic factors involved in the effects of developmental low-level alcohol induced behavioral alterations in rats. Neuropsychopharmacology. 2002;26:191–203. doi: 10.1016/S0893-133X(01)00306-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- 19.Ciccocioppo R, Panocka I, Froldi R, Colombo G, Gessa G, Massi M. Antidepressant-like effect of ethanol revealed in forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology (Berl.) 1998;144:151–157. doi: 10.1007/s002130050988. [DOI] [PubMed] [Google Scholar]
- 20.Hwang BH, Stewart R, Zhang JK, Lumeng L, Li TK. Corticotropin-releasing factor gene expression is down-regulated in the central nucleus of the amygdala of alcohol-preferring rats which exhibit high anxiety: a comparison between rat lines selectively bred for high and low alcohol preference. Brain Res. 2004;1026:143–150. doi: 10.1016/j.brainres.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 21.Merlo Pich E, Lorang M, Yeganeh M, Rodriguez DF, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J. Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karanikas CA, Lu YL, Richardson HN. Adolescent drinking targets corticotropin-releasing factor peptide-labeled cells in the central amygdala of male and female rats. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.04.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol. Clin. Exp. Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 24.Wills TA, Knapp DJ, Overstreet DH, Breese GR. Interactions of stress and CRF in ethanol-withdrawal induced anxiety in adolescent and adult rats. Alcohol. Clin. Exp. Res. 2010;34:1603–1612. doi: 10.1111/j.1530-0277.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J. Pharmacol. Exp. Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol. Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Roberto M, Cruz MT, Gilpin NM, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol. Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Björk K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang GQ, Barson JR, Karatayev O, Chang SY, Chen YW, Leibowitz SF. Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. Alcohol. Clin. Exp. Res. 2010;34:761–770. doi: 10.1111/j.1530-0277.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Addario C, Caputi FF, Rimondini R, Gandolfi O, Del Borrello E, Candeletti S, Romualdi P. Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain. Addict. Biol. 2013;13:425–433. doi: 10.1111/j.1369-1600.2011.00326.x. [DOI] [PubMed] [Google Scholar]
- 31.Rácz I, Markert A, Mauer D, Stoffel-Wagner B, Zimmer A. Long-term ethanol effects on acute stress responses: modulation by dynorphin. Addict. Biol. 2013;18:678–688. doi: 10.1111/j.1369-1600.2012.00494.x. [DOI] [PubMed] [Google Scholar]
- 32.Park MH, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Kappa opioid receptors in the central amygdala regulate ethanol actions at presynaptic GABAergic sites. J. Pharmacol. Exp. Ther. doi: 10.1124/jpet.112.202903. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, Stuber GD, Kash TL. Presynaptic inhibition of gamma-aminobutyric acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling. Biol. Psychiatry. 2012;71:725–732. doi: 10.1016/j.biopsych.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.