Abstract
Objective
Circulating testosterone, estradiol, and estrone concentrations vary considerably between men. Though a substantial proportion of this variation may be attributed to morbidity and behavioral factors, these cannot account for its entirety, suggesting genetic inheritance as a potential additional determinant. The analysis described here was intended to estimate the heritability of male circulating total testosterone (TT), calculated free testosterone (cFT), estrone (E1), estradiol (E2), and sex hormone-binding globulin (SHBG), along with the genetic correlation between these factors.
Design
Cross-sectional, observational analysis of data from male members of the Offspring and Generation Three cohorts of the Framingham Heart Study. Data were collected in the years 1998–2005.
Participants
A total of 3,367 community-dwelling men contributed to the analysis, including 1,066 father/son and 1,284 brother pairs among other family relationships.
Measurements
Serum sex steroids (TT, E1 and E2) by liquid chromatography-tandem mass spectrometry, SHBG by immunofluorometric assay, cFT by mass action equation. Heritability was obtained using variance components analysis with adjustment for covariates including age, diabetes mellitus, body mass index, and smoking status.
Results
Age-adjusted heritability estimates were 0.19, 0.40, 0.40, 0.30, and 0.41 for cFT, TT, E1, E2, and SHBG, respectively. Adjustment for covariates did not substantially attenuate these estimates; SHBG-adjusted TT results were similar to those obtained for cFT. Genetic correlation coefficients (ρG) indicated substantial genetic association between TT and cFT (ρG=0.68), between TT and SHBG (pG = 0.87), between E1 and E2 (ρG = 0.46), and between TT and E2 (ρG = 0.48).
Conclusion
Circulating testosterone, estradiol, and estrone concentrations exhibit substantial heritability in adult men. Significant genetic association between testosterone and estrogen levels suggests shared genetic pathways.
Key Terms: Testosterone, Estrone, Estradiol, Sex Hormone-Binding Globulin, Heritability
INTRODUCTION
Circulating sex steroid concentrations exhibit tremendous inter- and intra-individual variation in population-based studies of men.1 Though numerous comorbid and behavioral factors have been associated with sex steroid concentrations in adults,2–4 it is thought that some proportion of this variation may be determined by genetic inheritance.5–16 In order to establish the degree to which genetic factors regulate male reproductive function in adulthood, it is important to estimate how much variation in sex steroid concentrations is attributable to genetic variation, as distinct from environmental causes.
Existing data, derived primarily from classical twin and family studies, have estimated the heritability of testosterone in adult men to be between approximately 0.2 and 0.7,6–8,10,11,15 while indicating the proportion of variation in sex steroid levels under genetic control may vary tremendously across the male life course, presumably as the relative import of environmental influences waxes and wanes with age.8,9,12,16 There are fewer population-based estimates of the heritability of estradiol and estrone concentrations – important for the maintenance of bone mass and suppression of fracture risk in men17–19 – and few existing investigations have produced estimates of the genetic correlation between testosterone and estradiol.14
Because testosterone and - to a lesser extent - estradiol are bound to sex hormone-blinding globulin (SHBG) in circulation, SHBG concentrations influence the bioavailability of these hormones.20 Interindividual variation in SHBG levels is partially under genetic control,21,22 and polymorphisms of the SHBG gene may have influence on its binding to testosterone as well as downstream biomedical outcomes.23–25 Population-based estimates of the genetic correlation between circulating testosterone and SHBG are therefore of great interest.
To provide estimates of the heritability of and genetic correlation between sex steroids in men from extended families, we analyzed data from the 3,367 men who are members of the second and third generation cohorts enrolled in the Framingham Heart Study (FHS). These men have had state-of-the-art measurement of total testosterone (TT) and calculated free testosterone (cFT), estrone (E1) and estradiol (E2), and SHBG, from which heritability estimates for each of the sex steroids were obtained. Estimates of genetic correlation between testosterone fractions, TT and SHBG, estrone and estradiol, and between TT and E2 were also generated.
METHODS
The FHS design and study cohorts have been previously described.26 An initial sample of 5,209 adult male and female residents of Framingham, MA was initially recruited in 1948. The sample was predominantly white and of European ancestry. In 1971, children of the initial cohort and their spouses were recruited as a second generation (the Offspring cohort). Recruitment of a third generation (the Generation 3 cohort) of selected children of the Offspring cohort was performed in 2002. All participants executed written informed consent approved by the institutional review board at Boston University Medical Center.
For analyses described here, the total of 3,367 men of the Offspring cohort who had sex steroid measurements obtained at Offspring Examination 7, occurring between 1998 and 2001, and of the Generation 3 cohort, who had sex steroid measurements between 2002 and 2005, were eligible. Data on covariates were obtained in clinic visits concurrent to hormone measurement.
Sex Steroid Measurements
Blood samples were drawn in the supine position in the early morning after an overnight fast. Sera were aliquoted and immediately stored at −80° C, remaining frozen until the time of assay. Serum TT levels were measured by liquid chromatography tandem mass-spectrometry (LC-MS/MS) as previously described.2 The functional sensitivity of the TT assay was 0.07 nmol/L and the interassay coefficient of variation was 15.8%, at 0.42 nmol/L, 10.6%, at 0.82 nmol/L, 7.9%, at 1.7 nmol/L, 7.7% at 8.4 nmol/L, 4.4% at 18.5 nmol/L, and 3.3% at 35.3 nmol/L, respectively. As part of the Centers for Disease Control’s (CDC) Testosterone Assay Harmonization Initiative, quality control samples provided by the CDC were run every three months; the bias in quality control samples with testosterone concentrations in 3.5 to 35 nmol/L range was consistently less than 6%. Serum estradiol and estrone levels were measured simultaneously using LC-MS/MS after derivatization with dansyl chloride.27 The limit of quantitation for both hormones was 2 pg/mL. Interassay CVs for estrone were 4.5%, 7.7%, and 6.9% at estrone concentrations of 29.6, 285, and 773 pmol/L, respectively, and for estradiol 6.9%, 7.0%, and 4.8% at estradiol concentrations of 29.3, 283, and 756 pmol/L, respectively.
SHBG was measured using a two-site directed immunofluorometric assay that had a sensitivity of 0.5 nM (Delphia-Wallac, Inc., Turku, Finland); cFT was calculated from total testosterone, SHBG, E1 and E2 using an iterative law-of-mass-action solution.28 Protocol for the maintenance of stored samples in FHS has previously been described.29
Covariate Measurement
Height and weight were measured using a standardized protocol. Type 2 diabetes mellitus (T2DM) was defined as a fasting glucose > 6.94 mmol/L and/or participant self-reported use of diabetes medications. Participants were classified as being a ‘current smoker’ if they reported smoking at least one cigarette per day over the preceding year.
Statistical Analysis
Descriptive analyses were performed using SAS v9.2 (SAS, Inc, Cary, NC). Analyses of heritability decomposes total phenotypic variation in sex steroid and SHBG levels into components attributable to genetic and environmental causes via a variance components analysis that assumes a normal distribution in the traits (i.e. hormone measurements). Accordingly, serum hormones were transformed by rank-normalization to minimize the influence of skew and residual kurtosis. Following age-adjustment, estimates were re-calculated after adjusting for BMI, smoking status, and T2DM. For testosterone heritability estimates, we also adjusted for SHBG; for estradiol heritability, results were adjusted for TT and SHBG as well. The SOLAR (Sequential Oligogenic Linkage Analysis Routines) statistical software30 was used to estimate heritability (h2), defined as the total proportion of phenotypic variation in sex steroid and SHBG levels attributable to genetic variation, as opposed to shared or unique environmental influences. Joint analyses of pairs of hormones were used to examine the genetic correlation between circulating hormones and were similarly adjusted for the above covariates.
RESULTS
A total of 3,367 men contributed to the analysis, representing 543 pedigrees; these were comprised of 1,066 father/son pairs, 1,284 brother pairs, and additional extended family relationships. Participants had mean (Standard Deviation) age 49 (14) years and BMI 28 (5) kg/m2; 16% were current smokers and 7.5% had T2DM. The distributions of hormones and SHBG are described in Table 1. Aside from being older, subjects from the Offspring cohort exhibited greater rates of T2DM (14% vs. 3%) and included a slightly lower percentage of current smokers (13% vs. 16%) than their Generation 3 counterparts, while BMI, at 29 (5) vs. 28 (5), was similar in the two cohorts.
Table 1. Serum sex steroid and SHBG levels.
Descriptive statistics and estimates of heritability and pairwise association.
| Descriptive statistics, and estimates of heritability (adjusted for age) | |||||||
|
TT (nmol/L) |
cFT (pmol/L) |
E1 (pmol/L) |
E2 (pmol/L) |
SHBG (nmol/L) |
|||
| N | 3367 | 3367 | 3270 | 3318 | 3367 | ||
| Mean (SD) | 21.5 (8.4) | 382 (18) | 159 (63) | 158 (66) | 49 (26) | ||
| h2 (SE) | 0.40 (0.05) | 0.19 (0.04) | 0.40 (0.05) | 0.30 (0.05) | 0.41 (0.05) | ||
| p-value | 1.4×10−21 | 3.0×10−7 | 8.9×10−23 | 1.3×10−12 | 1.2×10−20 | ||
| Estimates of pairwise genetic (ρG) and environmental (ρE) correlation (adjusted for age, BMI, smoking status, and T2DM) | |||||||
| TT, cFT | E1, E2 | TT, E2 | TT, SHBG | cFT, SHBG | E2, SHBG | ||
| N | 3363 | 3315 | 3363 | 3363 | 3363 | 3312 | |
| ρG (SE) | 0.68 (0.07) | 0.48 (0.07) | 0.38 (0.09) | 0.87 (0.05) | 0.24 (0.14) | 0.26 (0.092) | |
| H: ρG=0 p-value | 7.5×10−6 | 4.1×10−6 | 4.9×10−4 | 6.9×10−23 | 0.066 | 8.75×10−3 | |
| H: ρG=1 p-value | 4.1×10−4 | 6.1×10−13 | 4.3×10−13 | 0.002 | 6.1×10−4 | 1.17×10−12 | |
| ρE (SE) | 0.78 (0.02) | 0.65 (0.03) | 0.41 (0.04) | 0.25 (0.05) | 0.38 (0.04) | 0.16 (0.05) | |
| H: ρE=0 p-value | 3.1×10−54 | 1.4×10−31 | 5.4×10−16 | 1.2 ×10−5 | 1.2 ×10−16 | 1.2 ×10−3 | |
TT, total testosterone; cFT, calculated free testosterone; E1, estrone; E2, estradiol; SHBG, sex hormone-binding globulin; BMI, body mass index; T2DM, type-2 diabetes mellitus; SD, standard deviation; SE, standard error; h2, heritability estimate. To convert testosterone to nmol/L, multiply by 0.0347. To convert estradiol to pmol/L, multiply by 3.671. To convert estrone to pmol/L, multiply by 3.699.
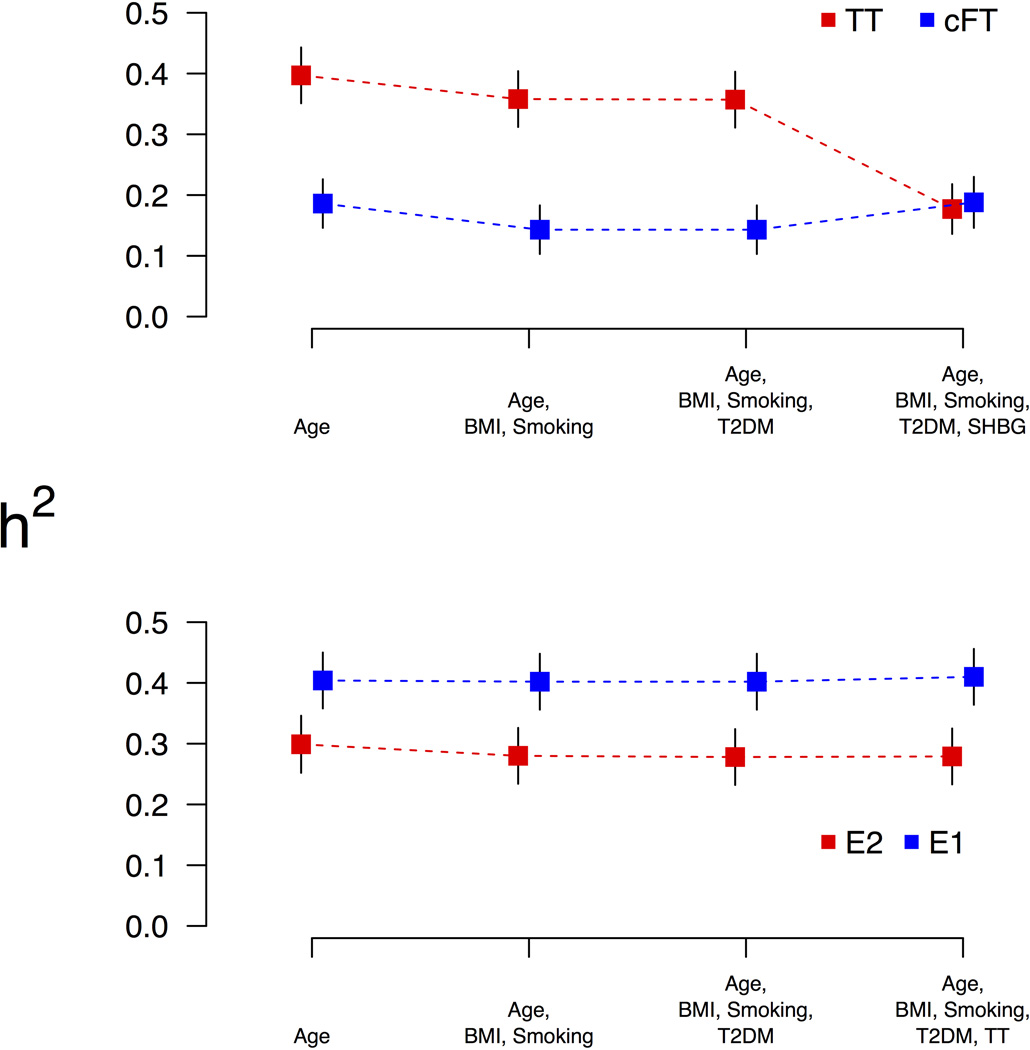
Estimates of age-adjusted heritability are presented in Table 1. In age-adjusted analyses, TT, E1 and SHBG exhibited substantial heritability – estimated h2 (Standard Error; SE) was approximately 0.40 (0.5) in each case, whereas E2 and cFT had estimated h2 (SE) 0.30 (0.05) and 0.19 (0.04), respectively. Additional adjustment for BMI, smoking, and T2DM produced no substantial reduction in estimates of heritability (Figure 1). For TT, however, further adjustment for SHBG produced a substantial reduction in estimated heritability, h2 = 0.18 (0.04), such that the SHBG-adjusted TT heritability estimated was similar to the heritability estimate for cFT, 0.19 (0.04). For E1 and E2, by contrast, further adjustment for SHBG produced no meaningful difference in results. For E2, adjustment for age, BMI, smoking and T2DM yielded estimated h2 = 0.28 (0.05); the addition of SHBG yielded estimated h2=0.26 (0.05).
Figure 1.
Change in estimates of heritability (h2) with successive adjustment for covariates. Moving left to right, each independent analysis adds new covariates as indicated along the horizontal axis. When total testosterone is adjusted for SHBG (along with all other covariates), the heritability estimate is substantially reduced, so that it is similar to that exhibited by free testosterone.
Paired analyses of hormones and SHBG adjusted for covariates demonstrated significant genetic correlation between TT and cFT (ρG = 0.68), between TT and SHBG (ρG = 0.87), between E2 and E1 (ρG = 0.48), and between TT and E2 (ρG = 0.38). There was somewhat lesser genetic correlation between cFT and SHBG (ρG = 0.24) and between E2 and SHBG (ρG=0.26).
The estimated environmental correlation (ρE) between each pair of measurements was generally comparable to its genetic counterpart, with the exception of the TT and SHBG pairing, where ρE (0.28) was substantially lower than ρG.
DISCUSSION
These analyses provide evidence of heritability (h2 approximately 0.40) of total testosterone and SHBG among men of predominantly European ancestry, and similarly high heritability for circulating estrone and estradiol in men (h2 0.30 to 0.40). Additionally these analyses demonstrate strong genetic correlation between TT and SHBG (ρG = 0.87), suggesting that a specific gene or set of genes may have pleiotropic effects on both. It may be that genetic effects on SHBG may affect SHBG concentrations or its binding to testosterone and thus influence circulating testosterone concentrations. This observation is supported by our finding that the estimated heritability of TT is greater than that of cFT or of TT when adjusted for SHBG - the latter two of which are essentially identical - suggesting that a large proportion of the heritability of TT is due to that of SHBG. The heritability of both total and free testosterone levels – and in addition E1 and E2 levels - may in addition be in part determined by the heritability of luteinizing hormone, with which unbound testosterone levels are expected to be in reciprocal feedback,15 or of enzymes regulating sex steroid production and metabolism.11,31
Estimates of E2 heritability were largely unaffected by adjustment for SHBG, a result in line with the more modest finding of genetic correlation between E2 and SHBG (ρG = 0.26). These analyses indicate a similarly modest genetic association between TT and E2 (ρG = 0.38),which may be consistent with indirect control of E2 via genes influencing TT concentrations, given that estradiol is derived from aromatization of testosterone. Because SHBG is more strongly associated with testosterone than estradiol, our finding that genetic correlations between testosterone and SHBG are greater than those between estradiol and SHBG are not unexpected.
To our knowledge these are among the first estimates of genetic correlation between circulating testosterone and estradiol derived using the referent LC-MS/MS assay methods.
Population-based studies indicate that testosterone levels decline in men at a rate of 0.5–2.0% per year beginning in the third decade of life.2,4,32,33 Though these changes have been associated with diverse morbidity and mortality, it remains unclear to what degree these age-related changes in testosterone levels contribute directly to various health outcomes.34,35 If a substantial proportion of between-person variation in sex steroids can be attributed to clustering within families, then identification of genetic determinants of testosterone levels24 may be useful in predicting their influence on age-related health outcomes.
Our observed levels of heritability are lower than those previously reported in some investigations; for instance, the recent report by Bogaert and colleagues14 on brother pairs (age 25–45 years) living in and around Ghent produced age- and BMI-adjusted h2 estimates of 0.65 and 0.44 for total testosterone and estradiol, respectively, which are higher than those provided here (0.4 and 0.3, respectively). Likewise, the estimate of heritability of TT obtained by Hong and colleagues10 in the HERITAGE family study (~0.70), and that obtained by Ring and coworkers11 using data from the NHLBI twin study (~0.55) were greater than that reported here. It is possible that that differences in study design at least partly explains the differences in the proportion of phenotypic variation attributed to genetic causes in these reports. In particular, while twin studies maintain powerful advantages in estimation of genetic influences on phenotypic variation, it has been pointed out that twin studies may underestimate the influence of shared environment. At the same time, it seems likely that the dramatic variations between study population – specifically in age, but also in other factors – may exercise considerable influence over the degree to which sex steroid levels are subject to genetic control under specific circumstances and points within the life course.
As others have noted,11 testosterone heritability estimates obtained in various populations differing in age display striking variation, with essentially no heritability observed at birth9,16 and moderate to high heritability in adolescence and adulthood. It is therefore possible that the relative age range of the respective cohorts may partially explain between-study variation in results, though the mechanism by which this would occur are not clear. Just as age-specific sex steroid concentrations vary dramatically across the life course, so too may the relative contributions of genetic and environmental influences.
At birth, the influence of intrauterine environment on sex steroid levels is known to be profound, though genetic control over testosterone levels may be greater than that over estrogens.9 It may be that the influence of intrauterine factors at the time of birth eclipses that of environmental influences in young to mid-adulthood; the influence of genetic factors might therefore appear greater in the latter instance than the former. In adults, we expect that the observed relationship between age and sex steroids capture not only the direct influence of the former on the latter but also some portion of the influence of environmental influences, illnesses and behaviors that track with age. Certain environmental exposures may have a cumulative influence on sex steroids with time, thus becoming more evident in older age.
Though estimates of heritability for single sex steroids were lower here than in some other studies, the estimates of genetic correlation between hormones provided here are for the most part similar to those previously observed by Bogaert and colleagues, with one exception: the multiply-adjusted genetic correlation between E2 and SHBG in FHS was 0.26, which substantially exceeded the age- and BMI-adjusted associations observed in the Ghent cohort (0.09).
Taken together these investigations provide evidence of significant genetic correlation between circulating testosterone levels and both estradiol and SHBG in men; by comparison, the evidence of genetic correlation between the estradiol and SHBG is not as strong.
Several epidemiologic studies have linked serum testosterone or SHBG concentrations to obesity, diabetes mellitus and metabolic syndrome in men. It has been debated whether the observed association of total testosterone with diabetes and metabolic syndrome reflects the association between testosterone and SHBG - which is a marker of insulin sensitivity, and is independently associated with diabetes and metabolic syndrome – or is rather reflective of the influence of testosterone itself.2,36–39 Our findings reinforce the observation of association between testosterone and SHBG levels, but do not elucidate their respective contributions to downstream morbidity. Though age-related changes in estradiol concentrations have been associated with loss of bone mass and increase in fracture risk,17–19 comparatively little is known concerning the genetic determination of circulating estradiol and estrone in men. The results provided here indicate that estradiol and estrone exhibit heritability comparable to that of total testosterone, and in excess of that of free testosterone, even after the influences of TT and SHBG have been subjected to statistical control.
This study has several strengths including the measurement of sex steroid concentrations by state-of-the art LC-MS/MS assays and the large, well-characterized, multigenerational Framingham Heart Study cohort. Generalizability to the broader population of men is limited by the geographic and socioeconomic homogeneity of the FHS cohort and by cross-sectional assessment of circulating sex steroid concentrations. Since the Framingham men were almost all of European ancestry, these results may not be generalizable to men of other race/ethnicities, although evidence that age-specific sex steroid levels differ by race and ethnicity40 is not definitive,41 and SHBG in some other populations exhibits similar heritability to that we observe in FHS.42 At this time, the hypothesis that broad racial and ethnic groups exhibit a level of genetic control of sex steroids at variance with that among other groups is speculative. In addition, the genetic determination of between and within-person variation in other hormones (e.g. DHEAS and DHT) that themselves play a profound role in male reproductive aging is of great interest, but is not captured here.
SUMMARY
These results provide evidence of heritability of circulating testosterone, estradiol and estrone, and SHBG concentrations among adult men. They suggest that specific polymorphisms may contribute to population-level variation in these hormones. Close study of genetic influences, such as genome-wide association scans, on circulating sex steroid levels in men is needed given their hypothesized role in the pathogenesis of age-related conditions and potentially in early mortality.
Acknowledgments
Grant Support: This project was supported primarily by NIH grant 1RO1AG31206. Additional support was provided by the Boston Claude D. Pepper Older Americans Independence Center grant 5P30AG031679 from the National Institute on Aging and by a grant from the CDC Foundation. The Framingham Heart Study is funded by National Heart, Blood, and Lung Institute (contract N01-HC-25195). Additional support for this manuscript was provided by the National Institute on Aging (R21 AG032598). DPK effort and some of the support for sex steroid measures were funded by the National Institute of Arthritis, Musculoskeletal and Skin Diseases and the National Institute on Aging (R01 AR/AG 41398). SB effort was supported by an unrestricted grant to Boston University by Abbott Laboratories.
Footnotes
Disclosure Summary: There are no additional disclosures.
References
- 1.Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007;67(6):853–862. doi: 10.1111/j.1365-2265.2007.02976.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J. Clin. Endocrinol. Metab. 2011;96(8):2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeBlanc ES, Wang PY, Lee CG, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J. Clin. Endocrinol. Metab. 2011;96(12):3855–3863. doi: 10.1210/jc.2011-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J. Clin. Endocrinol. Metab. 2007;92(2):549–555. doi: 10.1210/jc.2006-1859. [DOI] [PubMed] [Google Scholar]
- 5.Meikle AW, Stringham JD, Bishop DT, West DW. Quantitating genetic and nongenetic factors influencing androgen production and clearance rates in men. J. Clin. Endocrinol. Metab. 1988;67(1):104–109. doi: 10.1210/jcem-67-1-104. [DOI] [PubMed] [Google Scholar]
- 6.Meikle AW, Bishop DT, Stringham JD, West DW. Quantitating genetic and nongenetic factors that determine plasma sex steroid variation in normal male twins. Metab. Clin. Exp. 1986;35(12):1090–1095. doi: 10.1016/0026-0495(86)90020-x. [DOI] [PubMed] [Google Scholar]
- 7.Bishop DT, Meikle AW, Slattery ML, Stringham JD, Ford MH, West DW. The effect of nutritional factors on sex hormone levels in male twins. Genet. Epidemiol. 1988;5(1):43–59. doi: 10.1002/gepi.1370050105. [DOI] [PubMed] [Google Scholar]
- 8.Harris JA, Vernon PA, Boomsma DI. The heritability of testosterone: a study of Dutch adolescent twins and their parents. Behav. Genet. 1998;28(3):165–171. doi: 10.1023/a:1021466929053. [DOI] [PubMed] [Google Scholar]
- 9.Sakai LM, Baker LA, Jacklin CN, Shulman I. Sex steroids at birth: genetic and environmental variation and covariation. Dev Psychobiol. 1991;24(8):559–570. doi: 10.1002/dev.420240804. [DOI] [PubMed] [Google Scholar]
- 10.Hong Y, Gagnon J, Rice T, et al. Familial resemblance for free androgens and androgen glucuronides in sedentary black and white individuals: the HERITAGE Family Study. Health, Risk Factors, Exercise Training and Genetics. J. Endocrinol. 2001;170(2):485–492. doi: 10.1677/joe.0.1700485. [DOI] [PubMed] [Google Scholar]
- 11.Ring HZ, Lessov CN, Reed T, et al. Heritability of plasma sex hormones and hormone binding globulin in adult male twins. J. Clin. Endocrinol. Metab. 2005;90(6):3653–3658. doi: 10.1210/jc.2004-1025. [DOI] [PubMed] [Google Scholar]
- 12.Hoekstra RA, Bartels M, Boomsma DI. Heritability of testosterone levels in 12-year-old twins and its relation to pubertal development. Twin Res Hum Genet. 2006;9(4):558–565. doi: 10.1375/183242706778025071. [DOI] [PubMed] [Google Scholar]
- 13.Crabbe P, Bogaert V, De Bacquer D, Goemaere S, Zmierczak H, Kaufman JM. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J. Clin. Endocrinol. Metab. 2007;92(9):3604–3610. doi: 10.1210/jc.2007-0117. [DOI] [PubMed] [Google Scholar]
- 14.Bogaert V, Taes Y, Konings P, et al. Heritability of blood concentrations of sex-steroids in relation to body composition in young adult male siblings. Clin. Endocrinol. (Oxf) 2008;69(1):129–135. doi: 10.1111/j.1365-2265.2008.03173.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuijper EAM, Lambalk CB, Boomsma DI, et al. Heritability of reproductive hormones in adult male twins. Hum. Reprod. 2007;22(8):2153–2159. doi: 10.1093/humrep/dem145. [DOI] [PubMed] [Google Scholar]
- 16.Caramaschi D, Booij L, Petitclerc A, Boivin M, Tremblay RE. Genetic and environmental contributions to saliva testosterone levels in male and female infant twins. [Accessed January 3, 2013];Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.04.008. Available at: http://www.sciencedirect.com/science/article/pii/S0306453012001588. [DOI] [PubMed] [Google Scholar]
- 17.Fink HA, Ewing SK, Ensrud KE, et al. Association of Testosterone and Estradiol Deficiency with Osteoporosis and Rapid Bone Loss in Older Men. Journal of Clinical Endocrinology & Metabolism. 2006;91(10):3908–3915. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 18.Cauley JA, Ewing SK, Taylor BC, et al. Sex steroid hormones in older men: longitudinal associations with 4.5-year change in hip bone mineral density--the osteoporotic fractures in men study. J. Clin. Endocrinol. Metab. 2010;95(9):4314–4323. doi: 10.1210/jc.2009-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin S, Zhang Y, Felson DT, et al. Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. Am. J. Med. 2006;119(5):426–433. doi: 10.1016/j.amjmed.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Anderson DC. Sex-hormone-binding globulin. Clin. Endocrinol. (Oxf) 1974;3(1):69–96. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 21.Ahrentsen OD, Jensen HK, Johnsen SG. Sex-hormone-binding globulin deficiency. Lancet. 1982;2(8294):377. doi: 10.1016/s0140-6736(82)90560-8. [DOI] [PubMed] [Google Scholar]
- 22.Meikle AW, Stanish WM, Taylor N, Edwards CQ, Bishop CT. Familial effects on plasma sex-steroid content in man: testosterone, estradiol and Sex-hormone-binding globulin. Metab. Clin. Exp. 1982;31(1):6–9. [PubMed] [Google Scholar]
- 23.Xita N, Tsatsoulis A. Genetic variants of sex hormone-binding globulin and their biological consequences. Mol. Cell. Endocrinol. 2010;316(1):60–65. doi: 10.1016/j.mce.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Ohlsson C, Wallaschofski H, Lunetta KL, et al. Genetic determinants of serum testosterone concentrations in men. PLoS Genet. 2011;7(10):e1002313. doi: 10.1371/journal.pgen.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanbillemont G, Bogaert V, De Bacquer D, et al. Polymorphisms of the SHBG gene contribute to the interindividual variation of sex steroid hormone blood levels in young, middle-aged and elderly men. Clin. Endocrinol. (Oxf) 2009;70(2):303–310. doi: 10.1111/j.1365-2265.2008.03365.x. [DOI] [PubMed] [Google Scholar]
- 26.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am. J. Epidemiol. 2007;165(11):1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 27.Kushnir MM, Rockwood AL, Yue B, Meikle AW. High sensitivity measurement of estrone and estradiol in serum and plasma using LC-MS/MS. Methods Mol. Biol. 2010;603:219–228. doi: 10.1007/978-1-60761-459-3_20. [DOI] [PubMed] [Google Scholar]
- 28.Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74(6):512–519. doi: 10.1016/j.steroids.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Ingelsson E, Massaro JM, Sutherland P, et al. Contemporary trends in dyslipidemia in the Framingham Heart Study. Arch. Intern. Med. 2009;169(3):279–286. doi: 10.1001/archinternmed.2008.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams JT, Van Eerdewegh P, Almasy L, Blangero J. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. I. Likelihood formulation and simulation results. Am. J. Hum. Genet. 1999;65(4):1134–1147. doi: 10.1086/302570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarrabeitia MT, Hernandez JL, Valero C, et al. Adiposity, estradiol, and genetic variants of steroid-metabolizing enzymes as determinants of bone mineral density. Eur. J. Endocrinol. 2007;156(1):117–122. doi: 10.1530/eje.1.02318. [DOI] [PubMed] [Google Scholar]
- 32.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 33.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002;87(2):589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 34.Yeap BB. Are declining testosterone levels a major risk factor for ill-health in aging men? Int. J. Impot. Res. 2009;21(1):24–36. doi: 10.1038/ijir.2008.60. [DOI] [PubMed] [Google Scholar]
- 35.Yeap BB. Testosterone and ill-health in aging men. Nat Clin Pract Endocrinol Metab. 2009;5(2):113–121. doi: 10.1038/ncpendmet1050. [DOI] [PubMed] [Google Scholar]
- 36.Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone– binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes care. 2004;27(5):1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 37.Chubb SA, Hyde Z, Almeida OP, et al. Lower sex hormone-binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the Health in Men Study. European journal of endocrinology. 2008;158(6):785–792. doi: 10.1530/EJE-07-0893. [DOI] [PubMed] [Google Scholar]
- 38.Lakshman KM, Bhasin S, Araujo AB. Sex hormone-binding globulin as an independent predictor of incident type 2 diabetes mellitus in men. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65(5):503–509. doi: 10.1093/gerona/glq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhasin S, Jasjua GK, Pencina M, et al. Sex hormone-binding globulin, but not testosterone, is associated prospectively and independently with incident metabolic syndrome in men: the framingham heart study. Diabetes Care. 2011;34(11):2464–2470. doi: 10.2337/dc11-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orwoll ES, Nielson CM, Labrie F, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J. Clin. Endocrinol. Metab. 2010;95(10):E151–E160. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB. Serum androgen levels in black, Hispanic, and white men. J. Clin. Endocrinol. Metab. 2006;91(11):4326–4334. doi: 10.1210/jc.2006-0037. [DOI] [PubMed] [Google Scholar]
- 42.Jaquish CE, Blangero J, Haffner SM, Stern MP, MacCluer JW. Quantitative genetics of serum sex hormone-binding globulin levels in participants in the San Antonio Family Heart Study. Metab. Clin. Exp. 1997;46(9):988–991. doi: 10.1016/s0026-0495(97)90266-3. [DOI] [PubMed] [Google Scholar]