Abstract
In vertebrates, chemosensitivity of nutrients occurs through activation of taste receptors coupled with G protein subunits, including α-transducin (Gαtran) and α-gustducin (Gαgust). This study was aimed at characterizing the cells expressing Gαtran-immunoreactivity throughout the mucosa of the sea bass gastrointestinal tract. Gαtran immunoreactive cells were mainly found in the stomach, and a lower number of immunopositive cells were detected in the intestine. Some Gαtran immunoreactive cells in the stomach contained Gαgust immunoreactivity. Gastric Gαtran immunoreactive cells co-expressed ghrelin, obestatin and 5-hydroxytryptamine immunoreactivity. In contrast, Gαtran immunopositive cells did not contain somatostatin, gastrin/cholecystokinin, glucagon-like peptide-1, substance P, or calcitonin gene-related peptide immunoreactivity in any investigated segments of the sea bass gastrointestinal tract. Specificity of Gαtran and Gαgust antisera was determined by Western blot analysis, which identified two bands at the theoretical molecular weight of ~45 and ~40 kDa, respectively, in sea bass gut tissue as well as in positive tissue, and by immunoblocking with the respective peptide, which prevented immunostaining. The results of the present study provide a molecular and morphological basis for a role of taste related molecules in chemosensing in the sea bass gastrointestinal tract.
Keywords: chemosensory system, gut peptides, taste receptors, teleost
Introduction
The gustatory system plays a dominant role in the detection of dietary nutrients, sodium content and the acidity of foods as well as sensing the presence of potentially harmful substances (Sternini 2007; Behrens and Meyerhof 2011). The sense of taste enables animals to adapt to specific habitats (Oike et al. 2007; Ishimaru 2009; Barreiro-Iglesias et al. 2010). In vertebrates (Chandrashekar et al. 2000; Nelson et al. 2001, 2002; Zhao et al. 2003; Behrens and Meyerhof 2011), including fish (Ishimaru et al. 2005), two families of taste receptors (TRs), T1R and T2R, which detect complex tastes, have been cloned. TRs are G-protein-coupled receptors activated by different stimuli, including sweet and bitter substances, amino acids and nucleotides, which elicit a cascade of intracellular signals (Behrens and Meyerhof 2011). In mammals, TRs are abundantly expressed in taste buds, and interact with specific G α-subunits, including α-gustducin (Gαgust), which transmit gustatory signalling from the lingual epithelium to the sensory cortex in the brain (Ming et al. 1999; Margolskee 2002; Caicedo et al. 2003; Behrens and Meyerhof 2011). In addition to Gαgust, several G protein subunits have been identified, which are associated with TR signaling, Gαi-2, Gαi-3, Gα 14, Gα 15, Gα q, Gα s, and α-transducin (Gαtran) (Ruiz-Avila et al. 1995; Kusakabe et al. 1998). TRs and signalling molecules have been reported in the human and rodent gastrointestinal mucosa and pancreas (Höfer et al. 1996; Höfer and Drenckhahn 1998; Wu et al. 2002; Rozengurt et al. 2006), supporting the concept that there is more than a “taste” function for these molecules and that taste receptor-related molecules exert non-gustatory functions outside the mouth ( Sternini 2007; Behrens and Meyerhof 2011). Gαgust and Gαtran immunoreactivities have been localised to epithelial, predominantly endocrine, cells of the stomach and intestine of rodents (Höfer et al. 1996; Wu et al. 2002; Hass et al. 2007; Sternini 2007; Sutherland et al. 2007), pigs (Clavenzani et al. 2009; Mazzoni et al. 2013) and humans (Rozengurt et al. 2006; Steinert et al. 2011), including ghrelin, somatostatin, cholecystokinin, glucagon-like peptide-1 and peptide YY positive cells (Rozengurt et al. 2006; Sutherland et al. 2007; Clavenzani et al. 2009; Fujita et al. 2009; Moran et al. 2010; Janssen et al. 2011; Steinert et al. 2011; Mazzoni et al. 2013). Endocrine cells, which are distributed throughout the gastrointestinal tract (GIT) mucosa and pancreas, control digestive functions and contribute to regulate caloric intake and metabolism (Holmgren 1985; Plisetskaya and Mommsen 1996; Palmer and Greenwood-Van Meerveld 2001; Nelson and Sheridan 2006). Since fish taste buds express similar receptors and downstream signalling molecules as mammals (Yasuoka and Abe 2009), the aim of this study was to test whether the TR gustatory signalling protein, Gαtran is expressed in the sea bass gut and characterize the types of Gαtran immunoreactive (-IR) cells.
Materials and Methods
Tissue preparation
Nine, non-sexed, 1 year-old European sea bass (Dicentrarchus labrax) were sampled from three tanks at the Laboratory of Aquaculture, Department of Veterinary Medical Science, University of Bologna, Cesenatico, Italy. The average weight and total length of the individuals were 234 ± 26 g and 26 ± 1 cm, respectively. Sea bass were sacrificed by anaesthetic overdose and segments of the GIT were harvested. The stomach, pyloric caeca and the intestine were isolated from each fish; the intestine was divided into cranial, middle and caudal segments. Tissues were either frozen for Western blot assay or fixed in 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.2) for 48 h at 4°C for immunohistochemistry. Fixed tissues were dehydrated in a graded series of ethanol and embedded in paraffin. Sections of 5 μm thickness were obtained and mounted on poly-L-lysine coated slides, and processed for immunohistochemistry.
Immunohistochemistry
Sections were processed for single and double labelling immunofluorescence. The following primary antisera (see details in Table 1) were used: Gαtran, Gαgust, ghrelin (GHR), 5-hydroxytryptamine (5-HT), obestatin (OB), somatostatin (SOM), gastrin/cholecystokinin (GAS/CCK), glucagon-like peptide-1 (GLP-1), calcitonin gene-related peptide (CGRP), and substance P (SP). Sections were deparaffinized, rehydrated and incubated in a humid chamber at room temperature with appropriate normal serum followed by the primary antibodies (2 days, at 4°C) and the appropriate secondary antibodies (1 hour at room temperature). For double labelling using antibodies raised in different species, sections were incubated with a mixture of primary antisera (e.g. Gαtran and SP or GAS/CCK) and immunoreactivities were visualized with secondary antibodies labelled with different fluorophores. Because the antibodies to Gαgust, SOM, OB, 5-HT, GLP-1 and CGRP were produced in the same species as the Gαtran antiserum, we utilised the procedure and appropriate specificity controls previously described by Takechi et al. (2008) to visualize more than one antigen. Sections were examined using a Zeiss Axioplan microscope and the images were recorded with a Polaroid DMC digital photocamera (Polaroid, Cambridge, MA, USA).
Table 1.
List of antibodies used in this study.
| Primary antisera | Antigens | Code | Dilution | Supplier |
|---|---|---|---|---|
| Polyclonal rabbit anti-α-transducin | Gαt2 of bovine origin | sc-390 | 1:50 | Santa Cruz |
| Polyclonal rabbit anti-α-gustducin | Gαgust of rat origin | sc-395 | 1:200 | Santa Cruz |
| Polyclonal rabbit anti-somatostatin | Synthetic somatostatin15–28 conjugated to keyhole limpet hemocyanin | 566 | 1:1000 | INCSTAR |
| Monoclonal mouse anti-gastrin/cholecystokinin | Human gastrin/CCK C-terminus | GAS/CCK9303 | 1:1000 | CURE Digestive Diseases Research Center, UCLA |
| Polyclonal goat anti-ghrelin | Human ghrelin | sc-10368 | 1:800 | Santa Cruz |
| Polyclonal rabbit anti-obestatin | Human obestatin | — | 1:1500 | Prof. Rindi G.** |
| Polyclonal rabbit anti-5-hydroxytryptamine | Rat hypothalamus, raphe and spinal cord | 20080 | 1:2000 | INCSTAR |
| Polyclonal rabbit anti-glucagon-like peptide-1 | Synthetic peptide: HDEFERHAEGTFTSDVSSY, corresponding to amino acids 92–110 of Human GLP 1. | Ab22625 | 1:1000 | Abcam |
| Polyclonal rabbit anti-calcitonin gene-related peptide | Synthetic rat CGRP | IHC 6006 | 1:1000 | Peninsula/Bachem |
| Monoclonal rat anti-substance P | Substance-P-BSA conjugate | 10-S15 | 1:300 | Fitzgerald |
| Secondary antisera | ||||
| FITC-conjugated goat anti-rabbit IgG | 1:600 | Calbiochem | ||
| TRITC-conjugated donkey anti-goat IgG | 1:400 | Jackson | ||
| Alexa 594-conjugated goat anti-mouse IgG | 1:400 | Mol. Probes | ||
| Alexa 594-conjugated donkey anti-rat IgG | 1:500 | Mol. Probes | ||
| Biotin-conjugated goat anti-rabbit IgG | 1:400 | Vector | ||
| Texas Red®-conjugated streptavidin | 1:2000 | Vector | ||
Kindly provided by Prof. Guido Rindi, Institute of Pathology, Catholic University of the Sacred Heart, Rome, Italy.
Antibody specificity
Specificity of Gαtran, Gαgust, GAS/CCK, GHR, OB, and GLP-1 antibodies has been assessed by Western blot and/or immunoblocking with the corresponding peptide (see details in Table 2). Specificity of the 5-HT, CGRP and SOM antibodies was previously demonstrated in the sea bass by preadsorption test (De Girolamo et al. 1999; Visus et al., 1996). The staining we obtained with the monoclonal SP antibody was completely overlapping with the immunostaining reported by Pederzoli et al. (2004) in the sea bass with a rabbit SP antibody (Cambridge Research Biochemical, U.K.), the specificity of which was verified by immunoblocking. Specificity of the secondary antibodies was assessed by omitting the primary antibodies.
Table 2.
Peptides used for absorption tests.
| Peptide | Code | Concentration | Supplier |
|---|---|---|---|
| α-transducin | sc-390 P | 20 μg peptide in 1 ml PBS | Santa Cruz Biotechnology, Santa Cruz, USA |
| α-gustducin | sc-395 P | 20 μg peptide in 1 ml PBS | Santa Cruz Biotechnology, Santa Cruz, USA |
| Ghrelin* | sc-10368 P | 20 μg peptide in 1 ml PBS | Santa Cruz Biotechnology, Santa Cruz, USA |
| Glucagon-likepeptide-1 | ab50245 | 10−5 M | Abcam, Cambridge, UK |
Ghrelin peptide was used for both anti-ghrelin and anti-obestatin antibody specificity, since obestatin belongs to the ghrelin peptide family.
Western blot
Sea bass brain, eye and stomach and mouse brain were collected, frozen in liquid nitrogen, and stored at −80°C. Tissues were homogenized into a sodium dodecyl sulfate (SDS) lysis solution (Tris-HCl 62.5 mM, pH 6.8; SDS 2%, 5% glycerol) with 0.1 mM phenylmethylsulfonylfluoride. Protein content of cellular lysates was determined by a Protein Assay Kit (TP0300; Sigma-Aldrich, St. Louis, MO). For Western blot using Gαgust and Gαtrans antibodies, 20 μg of proteins were separated on NuPage 4–12% bis-Tris Gel (Gibco-Invitrogen, Paisley, UK) for 50 minutes at 200V, then electrophoretically transferred onto a nitrocellulose membrane. For Western blot with the GAS/CCK antibody, 30 μg of proteins were separated on Novex 18% Tris-Glycine Gel (Gibco-Invitrogen, Paisley, UK) for 90 minutes at 125V, then electrophoretically transferred onto a nitrocellulose membrane. After blocking treatment, the membranes were incubated at 4°C overnight with anti-Gαgust (1:300), anti-Gαtrans (1:500) or anti-GAS/CCK antibody (1:1,000) in Tris-buffered saline-T20 (TBS-T20 20 mM Tris-HCl, pH 7.4, 500 mM NaCl, 0.1% T-20). Membranes were then washed with PBS-T20, and incubated with the secondary biotin-conjugated antibody and an anti-biotin horseradish peroxidase linked antibody (1:1,000). The blots were developed using chemiluminescent substrate (Super Signal West Pico Chemiluminescent Substrate, Pierce Biotechnology, Rockford, IL) according to the manufacturer’s instructions. The intensity of luminescent signal of the resulting bands was acquired by Fluor-STM Multimager using the Quantity One Software (Bio-Rad Laboratories, Hercules, CA).
Results
Antibody Specificity
Western blot analysis showed a major band at ~45 kDa in extracts from the sea bass gastric mucosa, brain and eye with the Gαtran antibody (theoretical molecular weight in human) and a unique band at ~40 kDa in extracts from the sea bass stomach and brain, and mouse brain (Fig. 1a, b) with the Gαgust antibody (theoretical molecular weight in human).
Fig. 1.
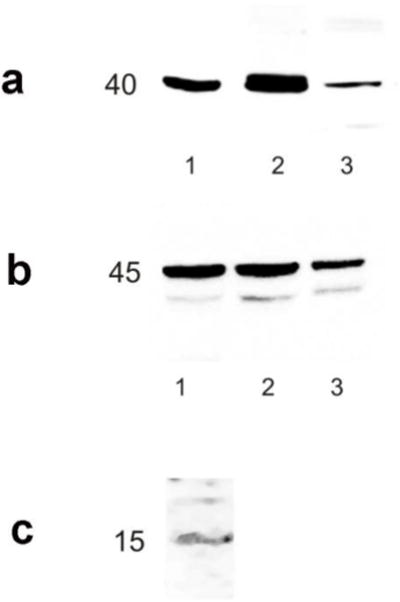
Western blot analysis showing α-gustducin (a), α-transducin (b) and cholecystokinin (c) immunoreactive bands in sea bass tissue extract. (a): α-gustducin antibody detects a single immunoreactive band near the theoretical molecular weight ~40 kDa in sea bass brain and gastric mucosa (lanes 1–2 respectively) and in mouse brain (lane 3); sea bass and mouse brain served as positive controls. (b): α-transducin antibody detects a major immunoreactive band at the theoretical molecular weight ~45 kDa in sea bass gastric mucosa, brain and eye (lanes 1, 2 and 3 respectively); the brain and eye served as positive control. (c): cholecystokinin monoclonal antibody visualizes a weak, single immunoreactive band close to the theoretical molecular weight of ~15kDa in sea bass intestinal mucosa
Different molecular forms of CCK have been described deriving from enzymatic cleavage of a precursor peptide of 115 AA (UNIPROT P06307) so the expected molecular weight of CCK is between 4 and 12 kDa. In our blot analysis, we identified a faint band near the theoretical molecular weight of ~15 kDa (Fig. 1c). We were unable to identify the smallest form probably because of the very low amount of each component present in the tissue.
Preadsorption of Gαgust, Gαtrans, GLP-1, OB and GHR antisera prevented immunostaining with each antiserum (not shown) confirming tissue staining specificity. The lack of immunostaining of sea bass retina incubated with the anti-Gαgust antibody (not shown) confirms that the Gαgust antibody used in this study does not recognize rod or cones transducins and provides additional support to the tissue specificity of this antibody.
Distribution of Gαtrans cells in the sea bass gut
In the stomach, Gαtran-IR cells were counted in 54 randomly selected fields (0.28 mm2 each) with a 40X objective lens, for a total area of 15.1 mm2. Since the intestinal mucosa differs morphologically from the stomach for the presence of folds, the number of Gαtran-IR cells in the intestine was evaluated in 200 randomly selected folds for a more accurate representation of cell density in these regions of the GIT. Values were expressed as mean ± standard error mean (SEM). The GIT of the sea bass consists of a siphonal stomach, numerous pyloric caeca and a relatively short intestine. Gαtran-IR cells were detected in the stomach and intestine, but not in the pyloric caeca. Intense immunolabelling was observed in the basal portion of the gastric gland and in the epithelial lining of the intestinal mucosal folds. Gαtran-IR cells showed homogenously labelled cytoplasm, with an unlabelled nucleus and an elongated (“bottlelike”) shape (Fig. 2a, e). These cells were characterised by two thin cytoplasmic processes, the first extending up to the endoluminal surface of the mucosa and the second projecting down to the basal lamina. These features indicate that these cells correspond to “open-type” enteroendocrine cells (EECs) (Höfer et al. 1999; Sternini 2008). The average number of Gαtran-IR cells in the stomach (Fig. 2a, c, e, g) was 15.7 ± 2.2, while in the cranial and middle-caudal portions of the intestine there were 3–4 IR cells/200 folds and 1–2 IR cells/200 folds, respectively. Furthermore, in the stomach, most Gαtran-IR cells co-expressed 5-HT (Fig. 2a, b), OB (Fig. 2c, d) or GHR (Fig. 2e, f). Only a few Gαtran-IR cells colocalized with 5-HT in the intestine. Colocalization between Gαtran- and Gαgust-IRs was observed in some cells in the gastric mucosa (Fig. 2g, h). In contrast, none of the Gαtran-IR cells contained SOM (Fig. 3g, h), GAS/CCK, CGRP, SP, and GLP-1-IR in the stomach and intestine. SOM, SP, CGRP, GAS/CCK, (Fig. 3a, b, c, d) and GLP-1 labelled cells were observed intermingled with unlabelled epithelial cells in the GIT mucosa. In addition, CGRP (Fig. 3c, e), GAS/CCK (Fig. 3f), SOM and SP antibodies labelled nerve fibres running either singly or in small fascicles in the submucosal and muscular layers, with some GAS/CCK and CGRP positive neuronal cell bodies detected only in the muscular layer (Fig. 3e, f).
Fig. 2.
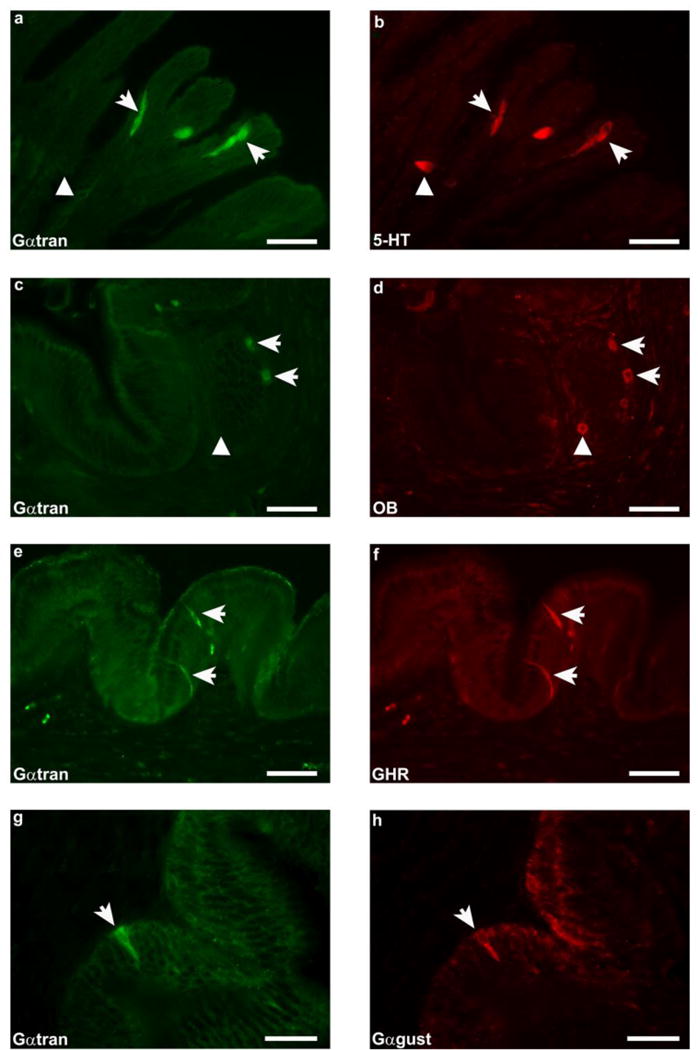
Representative images of sea bass gastric mucosa. α-transducin-immunoreactivity (Gαtran-IR) (a, c, e and g) colocalised with 5-hydroxytryptamine (5-HT) (b), obestatin (OB) (d), ghrelin (GHR) (f) and α-gustducin (Gαgust) (h) (arrows) immunoreactivity in the basal portion of the gastric gland and in the epithelial lining of the mucosal folds. Arrowheads in a, b, c and d indicate 5-HT- and OB-IR (b and d) cells negative for Gαtran (a and c). a–f scale bars = 30μm; g-h scale bars: 20μm
Fig. 3.
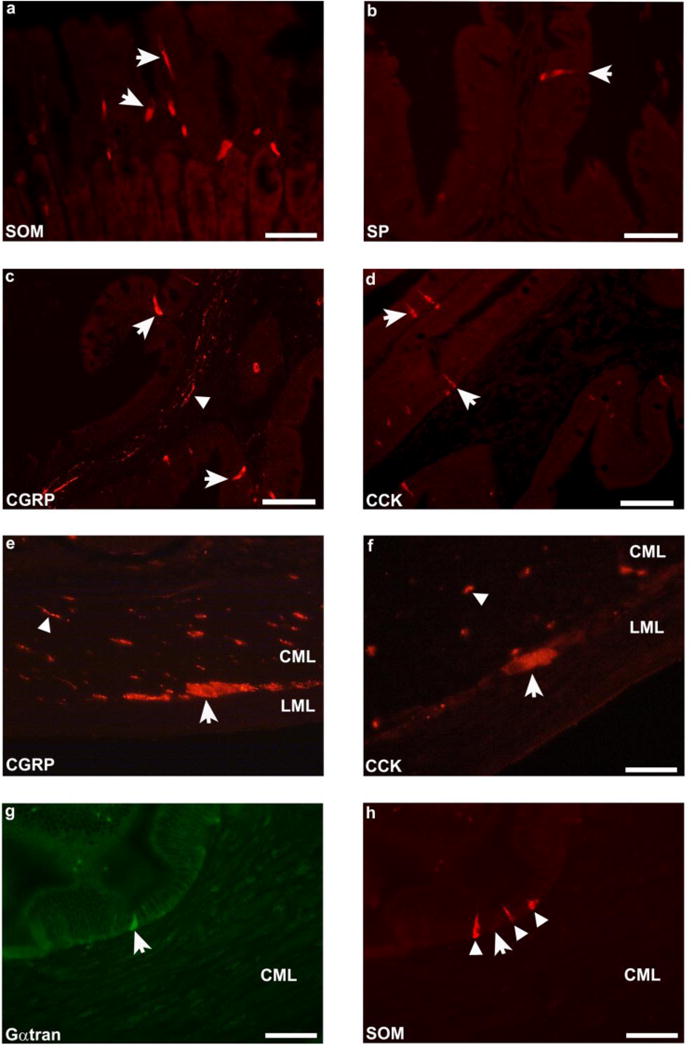
Representative images illustrating different subpopulations of EEC cells in sea bass gastric and intestinal mucosa. Somatostatin (SOM), substance P (SP), calcitonin gene-related peptide (CGRP), cholecystokinin (CCK) and α-transducin (Gαtran) (a–h) labelled cells in the stomach (a, arrows) and intestinal mucosa (b, c, d, g, arrows and h, arrowhead), respectively. CGRP and CCK immunolabelled nerve fibers (arrowheads) were observed in the stomach (e) and in the submucosal layer of the intestine (c and f). Some labelled CGRP (e) and CCK (f) neuronal cell bodies (arrows) were identified in the muscular layer. Arrow in g indicates a Gαtran-IR cell negative for SOM (h) in the intestinal mucosa and arrowheads in h point to SOM immunolabelled cells. a, b, e, and h scale bars = 30 μm; c, d scale bars = 60 μm; f scale bars = 20 μm
Discussion
Our data provide evidence for the presence of Gαtran immunolabelled cells in the sea bass GIT and their EEC nature as indicated by the co-expression with GHR, 5-HT or OB, which are markers of distinct subpopulations of EECs. Taste transduction in vertebrates is mediated by specialised receptors organised in groups of cells, which form the taste buds (Chandrashekar et al. 2000; Nelson et al. 2001; 2002; Zhao et al. 2003; Behrens and Meyerhof 2011). The molecular mechanisms through which sweet, L-amino acid (umami), and bitter tastes signal from the tongue to the sensory cortex have been clarified by the discovery of TRs (McLaughlin et al. 1992; Hoon et al. 1999; Lindemann, 2001), which activate Gα-subunits, including Gαtran and Gαgust, to transmit different tastes (Margolskee 2002; Ruiz-Avila 1995; Ming et al. 1999; Caicedo et al. 2003). Gαgust is the best characterized Gα protein associated with bitter taste transmission, however, the findings that Gαgust−/− mice retain sensitivity to bitter substances, imply that other Gα-subunits, including Gαtran, contribute to signalling bitter substances (Margolskee 2002; Ruiz-Avila et al. 1995; He et al. 2002). The discovery of Gαtran and Gαgust in the GIT of different species from the mouse (Hass et al. 2007; Sutherland et al. 2007; Wu et al. 2002), rat (Höfer et al. 1996) and pig (Clavenzani et al. 2009; Moran et al. 2010; Mazzoni et al. 2013) to human (Rozengurt et al. 2006; Steinert et al. 2011), supports the involvement of TRs in chemosensory mechanisms elicited by luminal contents in different species.
Recent studies on the fish genome failed to detect a gene encoding an ortholog of the mammalian Gαgust gene (Ohmoto et al., 2011), however, Oka and Korsching (2011) showed 80% homology between Gαgust and other G proteins, and Sarwal et al. (1996) reported a high homology of Gα gene between mammals and the puffer fish Fugu rubripes, with an identical intron/exon structure throughout the coding regions. Several lines of evidence support the tissue specificity of the Gαtran and Gαgust antibodies used in our study, including Western blot and immunoblocking experiments. Furthermore, the lack of Gαgust immunostaining in retina sections confirms that the Gαgust antibody does not detect transducins and provides further support of tissue staining specificity. In addition, Zhang et al. (2006) found Gαgust immunofluorescence in the barbells of yellow catfish (Pelteobagrus fulvidraco) with the same rabbit polyclonal Gαgust antibody we used in the present study. However, we cannot exclude the possibility that the gustducin antibody stains others G-alpha proteins in the sea bass gut, thus the term Gαgust immunolabeling should be regarded as “Gαgust like-immunoreactivity”. Finally, it should be pointed out that our transducin antibody does not differentiate between the two molecular forms of this protein, GαtranS and GαtranL, as shown by Muradov et al. (2008), who reported the expression of both these transducins in long and short photoreceptors of lamprey (Petromyzon marinus) with the same rabbit polyclonal Gαtran antibody used in our study. The observation that only some cells showed both Gαtran and Gαgust immunoreactivities in the sea bass GIT is in agreement with previous reports in mammals showing only partial colocalization of these two Gα-proteins (unpublished, personal observation) and a differential distribution in some regions (Wu et al. 2002). Gαtran and Gαgust mediate signals initiated by tastants acting at both families of TRs, the T1Rs and the T2Rs (Wong et al. 1996; Ruiz-Avila et al. 2001; He W et al 2002; Caicedo et al, 2003). Gαtran and Gαgust could serve different chemosensitive modalities depending upon the luminal content and according to the receptor subtype being stimulated, which would be consonant with the report that different T2Rs exert their function through the activation in vitro of distinct Gαi related forms (Sainz et al. 2007). Our findings that Gαtran immunoreactivity was localized to distinct subsets of EECs expand previous data in other animals species (Rozengurt et al. 2006; Sutherland et al. 2007; Moran et al. 2010; Janssen et al. 2011, Steinert et al. 2011; Mazzoni et al. 2013). EECs have been reported in the stomach and intestine of several fish species (Holmgren et al. 1982; Reinecke et al. 1997; Ku et al. 2004; Bermúdez et al. 2007; Manning et al. 2008), including the sea bass, where 5-HT-, SOM- and GHR-IR EECs have been described (Visus et al. 1996; Ferrando et al. 2009a; Terova et al. 2008). Our study has shown GHR-IR cells in the gastric mucosa, many of which contain Gαtran-IR. Our results are consistent with data from Terova et al. (2008), who observed high levels of GHR gene expression in the sea bass stomach. The colocalization of Gαtran and GHR-IRs is in line with data reported by Janssen et al. (2011) showing that 98% of the Gαtran-IR cells co-express GHR in mouse stomach, a finding of special interest as it indicates that this cell immunophenotype is conserved through evolution. GHR might have a role in regulating food intake in response to fasting and re-feeding in sea bass (Terova et al. 2008). Moreover, OB, an anorexigenic peptide derived from the GHR precursor, has been reported to counteract GHR effects on energy homeostasis and gastrointestinal function (Zhang et al. 2005). Furthermore, an OB encoding sequence has been recently identified in the black sea bream (Yeung et al. 2006) and in Atlantic halibut (Manning et al. 2008). Thus, our results, together with previous observations, suggest a morphological link between chemical sensing and food intake.
Our findings that the GIT mucosa of the sea bass contains GAS/CCK, OB, 5-HT, CGRP, SOM, GLP-1 and SP immunoreactive EECs extend previous knowledge on the distribution of bioactive messengers in the fish alimentary tract (Elbal et al. 1988; Beorlegui et al. 1992; Barrenechea et al. 1994; Groff and Youson 1997; Reinecke et al. 1997; Al-Mahrouki and Youson 1998; Domeneghini et al. 2000; Bosi et al. 2004; Ku et al. 2004; Pederzoli et al. 2004; Bosi et al. 2005a, b; Nelson and Sheridan 2006; Bermúdez et al. 2007). CGRP-, GAS/CCK-, SP- and SOM-IRs were also identified in nerve processes in the submucosal and muscular layer, and labelled neuronal cell bodies were observed within the muscular layer. Some studies have detailed peptide- and serotonin-containing innervation in fish. Bermúdez et al. (2007) have demonstrated 5-HT-, SP- and CGRP-, but not CCK-IR nerve fibers in the submucosal and muscular layers in the turbot Scophthalmus maximus; similar results were obtained by Bosi et al. (2005a) in chubs Leuciscus cephalus. Moreover, Pederzoli et al. (2004) observed SP-IR neurons in sea bass GIT. The presence of a peptidergic and serotonergic neural network, in addition to the EECs expressing the same signalling molecules, provides support for a link between chemosensory and neuronal systems, which could control GIT physiology in fish via integrated neuro-endocrine mechanisms.
In conclusion, our study demonstrates that G proteins involved in chemosensory transmission are expressed in the sea bass GIT enteroendocrine system. Nutrients may elicit the release of different bioactive messengers (mainly peptides), which directly, or via neural reflexes, contribute to the control of GIT functions and nutrient intake of this fish. Taste-related molecules might represent the initial molecular events involved in chemosensing processes. A better understanding of the mechanisms involved in luminal chemosensitivity in the fish may provide a new basis for feeding formulations to be applied in aquaculture.
Acknowledgments
This work was partially supported by grants PRIN/COFIN from the Italian Ministry of University, Research and Education 2008 and 2010 (to R. De G.); and R.F.O. funds from the University of Bologna (R. De G., R.C. and P.C.). R. De G. is the recipient of research grants from Fondazione Del Monte di Bologna e Ravenna. C.S. is supported by the National Institutes of Health DK41301 (Morphology and Cell Imaging Core) and an University of California Los Angeles (UCLA) Academic Senate Grant.
Contributor Information
Rocco Latorre, Department of Veterinary Medical Science, University of Bologna, Italy, via Tolara di Sopra, 50, 40064 - Ozzano dell’Emilia, Bologna, Italy.
Maurizio Mazzoni, Department of Veterinary Medical Science, University of Bologna, Italy, via Tolara di Sopra, 50, 40064 - Ozzano dell’Emilia, Bologna, Italy.
Roberto De Giorgio, Department of Medical and Surgical Sciences, University of Bologna, Italy, St. Orsola-Malpighi Hospital, via Massarenti, 40138 - Bologna, Italy.
Claudia Vallorani, Department of Veterinary Medical Science, University of Bologna, Italy, via Tolara di Sopra, 50, 40064 - Ozzano dell’Emilia, Bologna, Italy.
Alessio Bonaldo, Department of Veterinary Medical Science, University of Bologna, Italy, via Tolara di Sopra, 50, 40064 - Ozzano dell’Emilia, Bologna, Italy.
Pier Paolo Gatta, Department of Veterinary Medical Science, University of Bologna, Italy, via Tolara di Sopra, 50, 40064 - Ozzano dell’Emilia, Bologna, Italy.
Roberto Corinaldesi, Department of Medical and Surgical Sciences, University of Bologna, Italy, St. Orsola-Malpighi Hospital, via Massarenti, 40138 - Bologna, Italy.
Eugenio Ruggeri, Department of Medical and Surgical Sciences, University of Bologna, Italy, St. Orsola-Malpighi Hospital, via Massarenti, 40138 - Bologna, Italy.
Chiara Bernardini, Department of Veterinary Medical Science, University of Bologna, Italy, via Tolara di Sopra, 50, 40064 - Ozzano dell’Emilia, Bologna, Italy.
Roberto Chiocchetti, Department of Veterinary Medical Science, University of Bologna, Italy, via Tolara di Sopra, 50, 40064 - Ozzano dell’Emilia, Bologna, Italy.
Catia Sternini, CURE/DDRC, Division of Digestive Diseases, Departments Medicine and Neurobiology, UCLA, Los Angeles, and Veterans Administration Greater Los Angeles Health System, Bldg 115 Room 223, VAGLAHS, 11301 Wilshire Blvd, Los Angeles, CA 90073, USA, csternin@ucla.edu, Tel: +1-310-312-9477, Fax: +1-310-825-3133.
Paolo Clavenzani, Department of Veterinary Medical Science, University of Bologna, Italy, via Tolara di Sopra, 50, 40064 - Ozzano dell’Emilia, Bologna, Italy.
References
- Al-Mahrouki AA, Youson JH. Immunohistochemical studies of the endocrine cells within the gastro-entero-pancreatic system of Osteoglossomorpha, an ancient teleostean group. Gen Comp Endocrinol. 1998;110(2):125–139. doi: 10.1006/gcen.1998.7070. [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Anadon R, Rodicio MC. The gustatory system of lampreys. Brain Behav Evol. 2010;75:241–250. doi: 10.1159/000315151. [DOI] [PubMed] [Google Scholar]
- Barrenechea MA, Lopez J, Martinez A. Regulatory peptides in gastric endocrine cells of the rainbow trout Oncorhynchus mykiss: general distribution and colocalizations. Tissue Cell. 1994;26:309–321. doi: 10.1016/0040-8166(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Behrens M, Meyerhof W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol Behav. 2011;105:4–13. doi: 10.1016/j.physbeh.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Beorlegui C, Martinez A, Sesma P. Endocrine cells and nerves in the pyloric caeca and the intestine of Oncorhynchus mykiss (Teleostei): an immunocytochemical study. Gen Comp Endocrinol. 1992;86(3):483–495. doi: 10.1016/0016-6480(92)90073-S. [DOI] [PubMed] [Google Scholar]
- Bermudez R, Vigliano F, Quiroga MI, Nieto JM, Bosi G, Domeneghini C. Immunohistochemical study on the neuroendocrine system of the digestive tract of turbot, Scophthalmus maximus (L.), infected by Enteromyxum scophthalmi (Myxozoa) Fish Shellfish Immunol. 2007;22:252–263. doi: 10.1016/j.fsi.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Bosi G, Di Giancamillo A, Arrighi S, Domeneghini C. An immunohistochemical study on the neuroendocrine system in the alimentary canal of the brown trout, Salmo trutta, L., 1758. Gen Comp Endocrinol. 2004;138:166–181. doi: 10.1016/j.ygcen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Bosi G, Domeneghini C, Arrighi S, Giari L, Simoni E, Dezfuli BS. Response of the gut neuroendocrine system of Leuciscus cephalus (L.) to the presence of Pomphorhynchus laevis Muller, 1776 (Acanthocephala) Histol Histopathol. 2005a;20:509–518. doi: 10.14670/HH-20.509. [DOI] [PubMed] [Google Scholar]
- Bosi G, Shinn AP, Giari L, Simoni E, Pironi F, Dezfuli BS. Changes in the neuromodulators of the diffuse endocrine system of the alimentary canal of farmed rainbow trout, Oncorhynchus mykiss (Walbaum), naturally infected with Eubothrium crassum (Cestoda) J Fish Dis. 2005b;28:703–711. doi: 10.1111/j.1365-2761.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Pereira E, Margolskee RF, Roper SD. Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci. 2003;23(30):9947–9952. doi: 10.1523/JNEUROSCI.23-30-09947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/S0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Clavenzani P, De Giorgio R, Mazzoni M, Chiocchetti R, Barbara G, Costerbosa GL, Russo D, Sternini C. Expression of α-transducin, a chemoreceptive molecule, in endocrine and non endocrine cells of the pig gastrointestinal tract. Vet Res Commun. 2009;33:S85–S87. doi: 10.1007/s11259-009-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Girolamo P, Lucini C, Vega JA, Andreozzi G, Coppola L, Castaldo L. Co-localization of Trk neurotrophin receptors and regulatory peptides in the endocrine cells of the teleostan stomach. Anat Rec. 1999;256:219–226. doi: 10.1002/(SICI)1097-0185(19991101)256:3<219::AID-AR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Domeneghini C, Radaelli G, Arrighi S, Mascarello F, Veggetti A. Neurotransmitters and putative neuromodulators in the gut of Anguilla anguilla (L.). Localizations in the enteric nervous and endocrine systems. Eur J Histochem. 2000;44:295–306. [PubMed] [Google Scholar]
- Elbal MT, Lozano MT, Agulleiro B. The endocrine cells in the gut of Mugil saliens Risso, 1810 (Teleostei): an immunocytochemical and ultrastructural study. Gen Comp Endocrinol. 1988;70:231–246. doi: 10.1016/0016-6480(88)90144-X. [DOI] [PubMed] [Google Scholar]
- Ferrando S, Gambardella C, Bottaro M, Saroglia M, Terova G, Tagliafierro G. The compensatory growth in juveniles of sea bass gastric distributive pattern of molecules regulating metabolism. Ann N Y Acad Sci. 2009a;1163:389–393. doi: 10.1111/j.1749-6632.2009.04458.x. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Wideman RD, Speck M, Asadi A, King DS, Webber TD, Haneda M, Kieffer TJ. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab. 2009;296(3):E473–E479. doi: 10.1152/ajpendo.90636.2008. [DOI] [PubMed] [Google Scholar]
- Groff KE, Youson JH. An immunohistochemical study of the endocrine cells within the pancreas, intestine, and stomach of the gar (Lepisosteus osseus L.) Gen Comp Endocrinol. 1997;106:1–16. doi: 10.1006/gcen.1996.6842. [DOI] [PubMed] [Google Scholar]
- Hass N, Schwarzenbacher K, Breer H. A cluster of gustducin-expressing cells in the mouse stomach associated with two distinct populations of enteroendocrine cells. Histochem Cell Biol. 2007;128:457–471. doi: 10.1007/s00418-007-0325-3. [DOI] [PubMed] [Google Scholar]
- He W, Danilova V, Zou S, Hellekant G, Margolskee RF, Damak S. Partial rescue of taste response of alpha-gustducin null mice by transgenic expression of alpha-transducin. Chem Senses. 2002;27(8):719–727. doi: 10.1093/chemse/27.8.719. [DOI] [PubMed] [Google Scholar]
- Höfer D, Puschel P, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of α-gustducin. Proc Natl Acad Sci U S A. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfer D, Drenckhahn D. Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol. 1998;110:303–309. doi: 10.1007/s004180050292. [DOI] [PubMed] [Google Scholar]
- Höfer D, Asan E, Drenckhahn D. Chemosensory perception in the gut. News Physiol Sci. 1999;14:18–23. doi: 10.1152/physiologyonline.1999.14.1.18. [DOI] [PubMed] [Google Scholar]
- Holmgren S, Vaillant C, Dimaline R. VIP-, substance P-, gastrin/CCK-, bombesin-, somatostatin- and glucagon-like immunoreactivities in the gut of the rainbow trout, Salmo gairdneri. Cell Tissue Research. 1982;223:141–153. doi: 10.1007/BF00221505. [DOI] [PubMed] [Google Scholar]
- Holmgren S. Neuropeptide functions in the fish gut. Peptides. 1985;6:363–368. doi: 10.1016/0196-9781(85)90398-5. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/S0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Okada S, Naito H, Nagai T, Yasuoka A, Matsumoto I, Abe K. Two families of candidate taste receptors in fishes. Mech Dev. 2005;122:1310–1321. doi: 10.1016/j.mod.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y. Molecular mechanisms of taste transduction in vertebrates. Odontology. 2009;97:1–7. doi: 10.1007/s10266-008-0095-y. [DOI] [PubMed] [Google Scholar]
- Ku SK, Lee JH, Lee HS. Immunohistochemical study on the endocrine cells in the gut of the stomachless teleost, Zacco platypus (Ciprinidae) Anat Histol Embryol. 2004;33:212–219. doi: 10.1111/j.1439-0264.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- Kusakabe Y, Yamaguchi E, Tanemura K, Kameyama K, Chiba N, Arai S, Emori Y, Abe K. Identification of two alpha-subunit species of GTP-binding proteins, Galpha15 and Galphaq, expressed in rat taste buds. Biochim Biophys Acta. 1998;1403(3):265–72. doi: 10.1016/S0167-4889(98)00062-7. [DOI] [PubMed] [Google Scholar]
- Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci U S A. 2011;108:2094–2099. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- Manning AJ, Murray HM, Gallant JW, Matsuoka MP, Radford E, Douglas SE. Ontogenetic and tissue-specific expression of preproghrelin in the Atlantic halibut, Hippoglossus hippoglossus L. J Endocrinol. 2008;196:181–192. doi: 10.1677/JOE-07-0517. [DOI] [PubMed] [Google Scholar]
- Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- Mazzoni M, De Giorgio R, Latorre R, Vallorani C, Bosi P, Trevisi P, Barbara G, Stanghellini V, Corinaldesi R, Forni M, Faussone Pellegrini MS, Sternini C, Clavenzani P. Expression and regulation of α-transducin in the pig gastrointestinal tract. J Cell Mol Med. 2013 doi: 10.1111/jcmm.12026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- Ming D, Ninomiya Y, Margolskee RF. Blocking taste receptor activation of gustducin inhibits gustatory responses to bitter compounds. Proc Natl Acad Sci U S A. 1999;96:9903–9908. doi: 10.1073/pnas.96.17.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, Ionescu C, Bravo D, Shirazi-Beechey SP. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr. 2010;104:637–646. doi: 10.1017/S0007114510000917. [DOI] [PubMed] [Google Scholar]
- Muradov H, Kerov V, Boyd KK, Artemyev NO. Unique transducins expressed in long and short photoreceptors of lamprey Petromyzon marinus. Vision Res. 2008;48(21):2302–2308. doi: 10.1016/j.visres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/S0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Sheridan MA. Gastroenteropancreatic hormones and metabolism in fish. Gen Comp Endocrinol. 2006;148:116–124. doi: 10.1016/j.ygcen.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Ohmoto M, Okada S, Nakamura S, Abe K, Matsumoto I. Mutually exclusive expression of Gαia and Gα14 reveals diversification of taste receptor cells in Zebrafish. J Comp Neurol. 2011;519:1616–1629. doi: 10.1002/cne.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike H, Nagai T, Furuyama A, Okada S, Aihara Y, Ishimaru Y, Marui T, Matsumoto I, Misaka T, Abe K. Characterization of ligands for fish taste receptors. J Neurosci. 2007;27:5584–5592. doi: 10.1523/JNEUROSCI.0651-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Korsching SI. Shared and unique G alpha proteins in Zebrafish versus mammalian sense of taste and smell. Chem Senses. 2011;36:357–365. doi: 10.1093/chemse/bjq138. [DOI] [PubMed] [Google Scholar]
- Palmer JM, Greenwood-Van Meerveld B. Integrative neuroimmunomodulation of gastrointestinal function during enteric parasitism. J Parasitol. 2001;87:483–504. doi: 10.1645/0022-3395(2001)087[0483:INOGFD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pederzoli A, Bertacchi I, Gambarelli A, Mola L. Immunolocalisation of vasoactive intestinal peptide and substance P in the developing gut of Dicentrarchus labrax (L.) Eur J Histochem. 2004;48:179–184. doi: 10.4081/885. [DOI] [PubMed] [Google Scholar]
- Plisetskaya EM, Mommsen TP. Glucagon and glucagon-like peptides in fishes. Int Rev Cytol. 1996;168:187–257. doi: 10.1016/s0074-7696(08)60885-2. [DOI] [PubMed] [Google Scholar]
- Reinecke M, Muller C, Segner H. An immunohistochemical analysis of the ontogeny, distribution and coexistence of 12 regulatory peptides and serotonin in endocrine cells and nerve fibers of the digestive tract of the turbot, Scophthalmus maximus (Teleostei) Anat Embryol (Berl) 1997;195:87–101. doi: 10.1007/s004290050028. [DOI] [PubMed] [Google Scholar]
- Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- Ruiz-Avila L, McLaughlin SK, Wildman D, McKinnon PJ, Robichon A, Spickofsky N, Margolskee RF. Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature. 1995;376:80–85. doi: 10.1038/376080a0. [DOI] [PubMed] [Google Scholar]
- Sainz E, Cavenagh MM, Gutierrez J, Battey JF, Northup JK, Sullivan SL. Functional characterization of human bitter taste receptors. Biochem J. 2007;403:537–543. doi: 10.1042/BJ20061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwal MM, Sontag JM, Hoang L, Brenner S, Wilkie TM. G protein alpha subunit multigene family in the Japanese puffer fish Fugu rubripes: PCR from a compact vertebrate genome. Genome Re. 1996;6:1207–1215. doi: 10.1101/gr.6.12.1207. [DOI] [PubMed] [Google Scholar]
- Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) Clin Nutr. 2011;30:524–532. doi: 10.1016/j.clnu.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007;292:G457–G461. doi: 10.1152/ajpgi.00411.2006. [DOI] [PubMed] [Google Scholar]
- Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1420–G1428. doi: 10.1152/ajpgi.00504.2006. [DOI] [PubMed] [Google Scholar]
- Takechi R, Galloway S, Pallebage-Gamarallage MM, Johnsen RD, Mamo JC. Three-dimensional immunofluorescent double labelling using polyclonal antibodies derived from the same species: enterocytic colocalization of chylomicrons with Golgi apparatus. Histochem Cell Biol. 2008;129:779–784. doi: 10.1007/s00418-008-0404-0. [DOI] [PubMed] [Google Scholar]
- Terova G, Rimoldi S, Bernardini G, Gornati R, Saroglia M. Sea bass ghrelin: molecular cloning and mRNA quantification during fasting and refeeding. Gen Comp Endocrinol. 2008;155:341–351. doi: 10.1016/j.ygcen.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Visus IG, Abad ME, Garcia Hernández MP, Agulleiro B. Occurrence of somatostatin and insulin immunoreactivities in the stomach of sea bass (Dicentrarchus labrax L.): light and electron microscopic studies. Gen Comp Endocrinol. 1996;102:16–27. doi: 10.1006/gcen.1996.0041. [DOI] [PubMed] [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka A, Abe K. Gustation in fish: search for prototype of taste perception. Results Probl Cell Differ. 2009;47:239–255. doi: 10.1007/400_2008_6. [DOI] [PubMed] [Google Scholar]
- Yeung CM, Chan CB, Woo NY, Cheng CH. Seabream ghrelin: cDNA cloning, genomic organization and promoter studies. J Endocrinol. 2006;189:365–379. doi: 10.1677/joe.1.06593. [DOI] [PubMed] [Google Scholar]
- Zhang G, Deng S, Zhang H, Li H, Li L. Distribution of different taste buds and expression of alpha gustducin in the barbles of yellow catfish (Pelteobagrus fulvidraco) Fish Physiol Biochem. 2006;32:55–62. doi: 10.1007/s10695-006-6937-z. [DOI] [PubMed] [Google Scholar]
- Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–66. doi: 10.1016/S0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]


